Selective Functionalization of Amino Acids in Water:
A Synthetic Method via Catalytic C-H Bond
Activation
Brian D. Dangel, James A. Johnson, and Dalibor Sames*
Department of Chemistry, Columbia UniVersity
New York, New York 10027
ReceiVed May 25, 2001
ReVised Manuscript ReceiVed July 2, 2001
Amino acids are valuable building units and precursors for a
variety of organic compounds ranging from small molecules to
proteins. In living systems, the diversity of amino acid-derived
products is readily expanded by amino acid functionalization at
various stages of biosynthesis.
1
Accordingly, a synthetic meth-
odology that would allow for the direct and selective function-
alization of available amino acids would be of significant
importance. However, most metal reagents and catalysts for C-H
activation are sensitive to functional groups or an aqueous
environment, two typical features of amino acid chemistry.
2
Against such odds, there were two seemingly unrelated areas
which stimulated our investigation in this direction: first, the
original Shilov reaction (Pt(II)/Pt(IV)-mediated oxidation of
alkanes) is performed in aqueous acid solution,
3
second, the
coordination chemistry of Pt(II) salts with amino acids and
proteins has been intensely studied due to the clinical use of
cisplatin in tumor therapy.
4
In the process of merging these two
fields we discovered that the stoichiometric Shilov reaction was
compatible with amino acid substrates, and furthermore, we
developed a catalytic system capable of selective functionalization
of free R-amino acids in water.
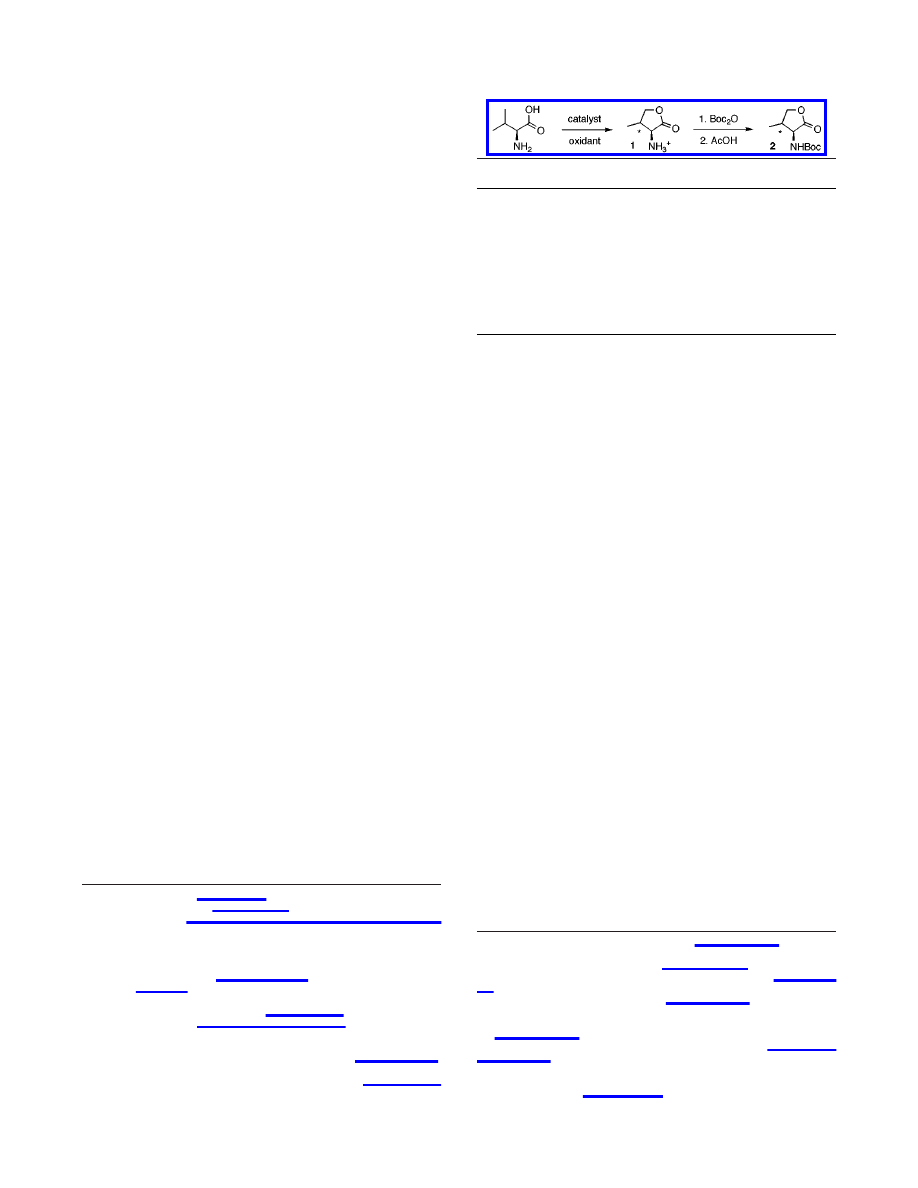
Initial experiments involved submitting
L
-valine to an aqueous
solution of K
2
PtCl
4
in the presence of K
2
PtCl
6
as the oxidant.
5
Surprisingly, heating the reaction mixture at 100
°
C for 12 h
yielded two major products identified as diastereomers of
γ-hy-
droxyvaline 1a and 1b in 5:1 ratio (anti/syn) (Table 1). Although
the conversion was low (<20% yield), this experiment demon-
strated that the chelation ability of the amino acid did not inhibit
the reaction but may in fact be responsible for the observed regio-
and stereoselectivity (see discussion below).
Encouraged by these results we focused our attention toward
the development of a catalytic system based on platinum in
combination with a practical oxidant. Copper(II) salts have been
used to oxidize Pd(0) in the Wacker process,
6
and Pt(0)/Pt(II) in
the Shilov and related oxidations.
7
Consequently, we treated
L
-valine with a catalytic amount of K
2
PtCl
4
(1-10 mol %) in the
presence of a stoichiometric amount of CuCl
2
in water. Remark-
ably, at temperatures >130
°
C, catalytic turnoVers were obserVed,
and the C-H bond functionalization occurred with regio- and
stereoselectiVity, affording lactones 1a and 1b in a 3:1 ratio (anti:
syn). Additionally, only limited racemization of the major products
and recovered starting material was found (<5% in 5 h, see
Supporting Information).
8
Hitherto, CuCl
2
and CuBr
2
proved to be the only oxidants
capable of regenerating the active platinum species, while other
metal salts were ineffective (CuSO
4
, Cu(OAc)
2
, Cu(OTf)
2
, Cu-
(OMs)
2
, Cu(O
3
SPh)
2
, FeCl
3
). Screening the 1-10 mol % range
of K
2
PtCl
4
in the presence of 1-10 equiv of CuCl
2
was conducted
(Table 1). In most cases maximum conversion was reached within
10 h at 160
°
C, whereas lower temperatures (
∼130
°
C) required
longer reaction times. The highest number of turnovers (20
turnovers based on the crude yield) was achieved in the presence
of 1 mol % of K
2
PtCl
4
and 5 equiv of CuCl
2
. After the reaction
yield was balanced with the amount of the platinum catalyst
required, the most practical conditions were determined to be 5
mol % of platinum catalyst and 7 equiv of copper chloride, to
furnish a 56% yield of hydroxyvaline isomers 1a and 1b. The
products were converted to N-Boc-lactones, obtained in 27%
overall yield.
Traditionally, C-H bond functionalization has been achieved
via radical processes where regioselectivity in complex substrates
has often been controlled through intramolecular abstraction of a
hydrogen atom by a proximal nitrogen or oxygen-centered radical
(e.g., Hoffmann-Lo¨ffler-Freytag reaction,
9
Barton reaction,
10
Breslow remote oxidation
11
). As an important control experiment,
we submitted
L
-valine to conditions known to generate a carboxyl-
centered radical (Na
2
S
2
O
8
, CuCl
2
or NaCl).
12
It was determined
that no hydroxyvaline 1 was formed, while simple carboxylic
acids yielded
γ-lactones in moderate yields (Table 1)! This
experiment strongly suggested that the catalytic process developed
herein did not proceed via a free radical mechanism, and it
(1) (a) Herbert, R. B. Nat. Prod. Rep. 1999, 16, 199-208. (b) Sahl, H.-G.;
Jack, R. W.; Bierbaum, G. Eur. J. Biochem. 1995, 230, 827-853.
(2) Jones, W. D. In ActiVation of UnreactiVe Bonds and Organic Synthesis;
Murai, S., Ed.; Springer: Berlin, 1999; pp 9-46.
(3) Shilov, A. E.; Shul’pin, G. B. ActiVation and Catalytic Reactions of
Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer
Academic Publishers: Dordrecht, 2000.
(4) (a) Appleton, T. G. Coord. Chem. ReV. 1997, 166, 313-359. (b)
Reedijk, J. Chem. ReV. 1999, 99, 2499-2510.
(5) (a) Labinger, J. A.; Herring, A. M.; Lyon, D. K.; Luinstra, G. A.;
Bercaw, J. E.; Horva´th, I. T.; Eller, K. Organometallics 1993, 12, 895-905.
(b) Kao, L.-C.; Sen, A. J. Chem. Soc., Chem. Commun. 1991, 1242-1243.
(6) Tsuji, J. Palladium Reagents and Catalysts; John Wiley and Sons:
Chichester, 1995; pp 19-124.
(7) (a) Lin, M.; Shen, C.; Garcia-Zayas, E. A.; Sen, A. J. Am. Chem. Soc.
2001, 123, 1000-1001. (b) VanKoten, G.; Terheijden, J.; van Beek, J. A.
M.; Wehman-Ooyevaar, I. C. M.; Muller, F.; Stam, C. H. Organometallics
1990, 9, 903-912.
(8) Smith, G. G.; Khatib, A. Reddy, G. S. J. Am. Chem. Soc. 1983, 105,
293-295.
(9) (a) Corey, E. J.; Hertler, W. R. J. Am. Chem. Soc. 1958, 80, 2903-
2904. (b) Buchschacher, P.; Kalvoda, J.; Arigoni, D.; Jeger, O. J. Am. Chem.
Soc. 1958, 80, 2905-2906.
(10) Barton, D. H. R.; Beaton, J. M. J. Am. Chem. Soc. 1961, 83, 4083-
4089.
(11) Breslow, R.; Baldwin, S.; Flechtner, T.; Kalicky, P.; Liu, S.; Washburn,
W. J. Am. Chem. Soc. 1973, 95, 3251.
(12) (a) Nikishin, G. I.; Svitanko, I. V.; Troyansky, E. I. J. Chem. Soc.,
Perkin Trans, II 1983, 595-601. For photochlorination and photobromination
of amino acids: (b) Kollonitsch, J.; Scott, A. N.; Doldouras, G. A. J. Am.
Chem. Soc. 1966, 88, 3624-3626. (c) Easton, C. J.; Hutton, C. A.; Tan, E.
W.; Tiekink, E. R. T. Tetrahedron Lett. 1990, 31, 7059-7062.
Table 1.
Catalytic Hydroxylation of
L
-Valine: Optimization
catalyst/oxidant
(mol%/equiv)
yield
a
(1, %)
anti/syn
1a/1b
TON
isolated
yield
b
(2, %)
mass balance
(%)
K
2
PtCl
4
/K
2
PtCl
6
c
16/0.33
21
5:1
0
91
K
2
PtCl
4
/CuCl
2
d
1/5
20
3:1
20
14
80
2.5/5
39
3:1
15
20
65
2.5/10
12
3:1
5
10
79
5/3
37
3:1
7
20
80
5/7
56
3:1
11
27
59
5/10
47
3:1
9
22
65
10/10
67
3:1
7
35
55
Na
2
S
2
O
8
/CuCl
2
e
0
a
Product/start. material ratio (
×100) determined by
1
H NMR of the
isolated crude mixture.
b
Isolated yields of 2 over three-step sequence
including hydroxylation, amino group protection and lactonization.
c
Conditions:
L
-valine, 0.33 M in H
2
O, 100
°
C, 10 h.
d
Conditions:
L
-valine, 0.33 M in H
2
O, 160
°
C, 10 h.
e
Conditions:
L
-valine, 0.91 M
in H
2
O, 1 equiv of Na
2
S
2
O
8
and 1 equiv of CuCl
2
(or NaCl), 90
°
C
(ref 12).
8149
J. Am. Chem. Soc. 2001, 123, 8149-8150
10.1021/ja016280f CCC: $20.00
© 2001 American Chemical Society
Published on Web 07/31/2001
highlighted the use of transition metal catalysts as a favorable
alternative to free radical reagents.
To gain more insight into this catalytic process in terms of
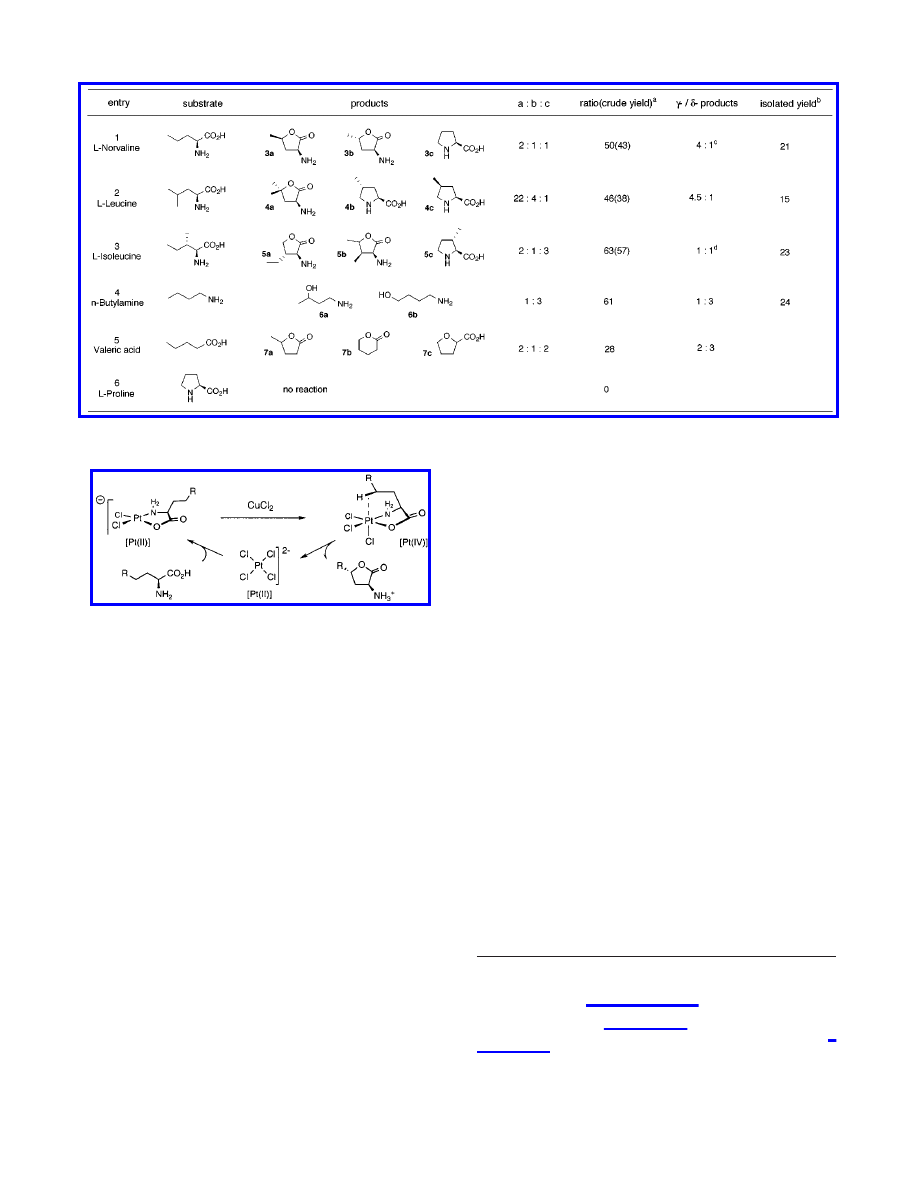
generality and mechanism, we applied the selected conditions (5
mol % of K
2
PtCl
4
, 7 equiv of CuCl
2
) to other amino acid
substrates (Table 2). In the case of
L
-norvaline, oxidation of the
γ-methylene unit was the predominant pathway yielding γ-lac-
tones 3a and 3b in 32% crude yield and 2:1 ratio, as well as
γ-ketone in 7% crude yield. The minor δ-selective pathway
provided
L
-proline, presumably originating from chlorination of
the terminal methyl group, followed by intramolecular substitution
of the chloride by the amino group.
L
-Leucine also provided
γ-lactone 4a as the major product (γ-/δ-products, 4.5:1) together
with methylpyrrolidines 4b and 4c as the minor component (4b/
4c, 4:1).
L
-Isoleucine provided three major products, 5a and 5b
from hydroxylation of the
γ-methyl and γ-methylene group,
respectively, while pyrrolidine 5c was formed through function-
alization of the
δ-methyl group. The γ- and δ-functionalized
products were obtained in an approximately 1:1 ratio. Simple
aliphatic amines were also hydroxylated under the catalytic
conditions. In sharp contrast to
L
-norvaline, n-butylamine showed
preference for
δ-hydroxylation, yielding products 6a and 6b in a
1:3 ratio. In the case of valeric acid, the
δ-position was
hydroxylated with minor preference (3:2) over the
γ-position,
affording three products, namely, lactones 7a and 7b, and the
unexpected 2-oxolanecarboxylic acid 7c.
13
The results described herein uncovered regioselectivity trends
for R-amino acids that were distinctly different from those for
simple aliphatic amines and carboxylic acids. Therefore, we
propose that functionalization of R-amino acids proceeds Via a
mechanism based on chelate-directed C-H bond actiVation
(Figure 1). The resistance of proline to oxidation under these
conditions supports the proposed hypothesis, as its cyclic nature
prevents an intramolecular collision between the Pt(IV) metal and
a C-H bond.
In summary, we have developed a catalytic process for the
selective functionalization of R-amino acids in water. Although
catalytic directed functionalization of arene rings has previously
been achieved, the discovery of the corresponding process for
unactivated alkane segments has been prevented owing to the
greater difficulty of metal-mediated cleavage of alkane C-H
bonds.
14
This report has demonstrated a very rare, if not the first,
example of catalytic heteroatom-directed functionalization of
remote alkyl groups in complex substrates.
Acknowledgment.
We dedicate this paper to Professor Ronald
Breslow on the occasion of his 70th birthday. D. S. is a Cottrell Scholar
of Research Corporation and a recipient of the Camille and Henry Dreyfus
New Faculty Award. J. A. J. received the BMS Fellowship in Synthetic
Chemistry. We thank Dr. L. J. Williams for stimulating discussions and
Dr. J. B. Schwarz for editorial assistance.
Supporting Information Available: Detailed experimental proce-
dures, spectral characterization of products (PDF). This material is
available free of charge via the Internet at http://pubs.acs.
JA016280F
(13) The product 7c was generated by copper chloride-mediated oxidation
of
δ-lactone 7b, γ-lactone 7a was resistant to this oxidative process (see
Supporting Information).
(14) (a) Dyker, G. Angew. Chem., Int. Ed. 1999, 38, 1698-1712. (b)
Catalytic N-directed functionalization of activated sp
3
C-H bonds: Murahashi,
S.-I.; Hirano, T.; Yano, T. J. Am. Chem. Soc. 1978, 100, 348-350. Chatani,
N.; Asaumi, T.; Ikeda, T.; Yorimitsu, S.; Ishii, Y.; Kakiuchi, F.; Murai, S. J.
Am. Chem. Soc. 2000, 122, 12882-12883.
Table 2.
Catalytic Functionalization of Selected Substrates
a
Product to starting material ratio (
×100), determined by
1
H NMR of isolated crude mixtures. Crude yields were determined by NMR and
recovered crude mass.
b
Combined isolated yields of Boc-lactones (a three-step sequence including functionalization, amino group protection, and
lactonization), and Boc-pyrrolidines (two steps).
c
γ-ketone was also formed in 7% crude yield.
d
5b is a 1:1 mixture of two stereoisomers.
Figure 1.
Chelate-directed C-H bond functionalization. Proposed
catalytic cycle.
8150 J. Am. Chem. Soc., Vol. 123, No. 33, 2001
Communications to the Editor
Wyszukiwarka
Podobne podstrony:
Determination of carbonyl compounds in water by derivatizati
chemical behaviour of red phosphorus in water
Changes in Brain Function of Depressed Subjects During
Penier, Izabella What Can Storytelling Do For To a Yellow Woman The Function of Storytelling In the
Highly selective synthesis of menthols from citral in a one step process
Functional and Computational Assessment of Missense Variants in the Ataxia Telangiectasia Mutated (A
bukowski, charles burning in water drowning in flame selected poems 1955 1973 (1997) Notepad
Short term effect of biochar and compost on soil fertility and water status of a Dystric Cambisol in
Dance, Shield Modelling of sound ®elds in enclosed spaces with absorbent room surfaces
Attitudes toward Affirmative Action as a Function of Racial ,,,
Proteomics of drug resistance in C glabrata
Estimating Temperatures in a Water
Microstructures and stability of retained austenite in TRIP steels
MMA Research Articles, Risk of cervical injuries in mixed martial arts
Development of financial markets in poland 1999
Antigone Analysis of Greek Ideals in the Play
Analysis of Police Corruption In Depth Analysis of the Pro
więcej podobnych podstron