
Biomaterials 22 (2001) 2931}2936
The corrosion resistance of pure titanium in organic acids
Marie Koike*, Hiroyuki Fujii
Department of Removable Prosthodontics, Nagasaki University School of Dentistry, 1-7-1 Sakamoto, Nagasaki 852-8588, Japan
Received 15 September 2000; accepted 22 January 2001
Abstract
The purpose of this study was to assess the corrosive properties of titanium at various pHvalues. Cast pure titanium specimens
were immersed in 128 mmol/l of lactic and formic acids at pH1.0}8.5 for 3 weeks at 373C. The solubility, color, weight and chemical
binding state of specimens were observed. Titanium dissolved in all lactic acid. The amount of dissolved titanium tended to decrease
with a higher pH. In formic acid, the amount of dissolved titanium at pH 1.0 was larger than that in lactic acid at the same pH, but less
than the detectable limit at pH4.0 or higher. Signi"cant discoloration was macroscopically observed only in formic acid at pH2.5 and
4.0. The weight of the titanium samples immersed in lactic acid all decreased, but it was not a!ected by pH. In formic acid, the weight
decreased at pH1.0 and increased at pH2.5}5.5. Thickening of the TiO corresponding to that showing discoloration was observed in
the super"cial oxide "lm of the titanium samples. Our results show that the corrosive properties of titanium are markedly dependent
on pHin formic acid, and relatively less dependent on pHin lactic acid in which titanium is dissolvable at pH1.0}8.5.
2001
Elsevier Science Ltd. All rights reserved.
Keywords: Titanium; Organic acid; PH; Immersion; Corrosion
1. Introduction
Biocompatibility is one of the most important condi-
tions for biological materials, and since titanium is con-
sidered to have high biological a$nity, it is used as
a medical material. In fact, in patients with hypersensitive
reaction to the present dental alloys, pure titanium is one
of the metals that can be used for dental restoration.
However, there have been several studies describing pa-
tients who did not adapt to titanium [1,2] or patients
allergic to titanium [3}6].
We previously reported that titanium has a high resist-
ance to corrosion in physiological saline and arti"cial
saliva by immersion tests, and that it was dissolved and
became discolored when in contact with lactic acid or
formic acid, which are both produced in the mouth [7,8].
However, these results were obtained at pH 2.5 below
128 m
M
of acid solutions.
Based on the electric potential}pH "gure at 253C
[9], titanium is stable around the electric potential equi-
librium (passive state area), irrespective of pH, but it is
* Corresponding author. Tel.: #81-95-849-7693; fax: #81-95-849-
7694.
E-mail address: marie-k@net.nagasaki-u.ac.jp (M. Koike).
likely to corrode at electric potentials lower (strongly
acidic) and higher (strongly alkaline) than the equilib-
rium (corrosive area). The roughness and solidity of the
titanium surface varied by anodic oxidation in physiolo-
gical saline bu!ered at di!erent pHwith phosphate [10],
and titanium ions were more dissolved in culture solu-
tions at a lower pH[11]. Moreover, it has been hy-
pothesized that the dissolution of titanium varied in vivo
with complicated changes in the surrounding pHcaused
by organic and inorganic ions [12].
In the oral cavity, dental plaque, which includes or-
ganic acids such as lactic and formic acids, is more likely
to precipitate on the titanium surface, compared to other
dental metal alloys [13], and the kinds and concentra-
tions of organic acids can vary depending on whether the
environment is aerobic or anaerobic [14]. Furthermore,
the pHaround titanium was reduced in contact with
other metals such as amalgam [15].
The pHof dental plaque after consuming sugar is
about 4.0 [16], but it can range from 2.0 to 11.0 depend-
ing on the foods and beverages consumed [17]. Thus, it is
necessary to investigate the properties of titanium for
clinical use, especially under various conditions in the
mouth [7,8]. The present study investigated the e!ects of
organic acids at various pHon the chemical stability of
titanium.
0142-9612/01/$ - see front matter
2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 0 4 0 - 0

Table 1
Test material and the chemical composition of mass %
Material
Ti
Fe
Others
Pure titanium A
99.49
0.25
0.26
2. Materials and methods
2.1. Preparation of samples
According to the manufacturer's instructions, cast
samples measuring about 15 mm
;20 mm;1 mm or
5 mm
;5 mm;1 mm were made from pure titanium
(equivalent to ASTM grade 2, described simply as tita-
nium hereafter) (Table 1) [18]. The surfaces of the cast
samples were air-abraded using
50 m alumina pow-
der, and then the samples were "nished with ASTM
C600 wet silicon carbide paper.
During the process of abrasion, surface thickness of the
samples was reduced by 250}300
m, and the reduced
thickness was more than the thickness of the reaction layer
[19]. Subsequently, the samples were subjected to ultra-
sonic cleaning in acetone for 15 min to remove any grease,
then stored in a desiccator. Solubility, color, and weight
changes were examined using the large samples, and the
chemical binding states of the surface were examined using
small samples. Experiments were performed in duplicate.
2.2. Immersion of samples
As immersion solutions, lactic acid and formic acid at
pH2.5 were diluted with distilled and deionized water to
128 m
M
(equivalent to lactic acid at 1%), and were ad-
justed to pH1.0, 4.0, 5.5, 7.0, and 8.5 using 1
N
hydrochlo-
ric acid or 1
N
sodium hydroxide. After pHadjustment,
the concentration of hydrochloric acid and sodium hy-
droxide in the immersion solutions was 159 m
M
(0.58%)
and 105 m
M
(0.42%), respectively.
A titanium sample was entirely immersed in 10 ml of the
immersion solution in a 50 ml centrifugal tube, and the
tube was covered with a lid to prevent evaporation. For
each immersion solution, 4 tubes containing a large
sample and 1 tube containing a small sample were pre-
pared. These tubes were shaken at 80 rpm in an isothermal-
shaking bath at 373C. Three weeks later, the amounts of
dissolved metal ions were measured, then the samples were
removed from the tubes, and subjected to ultrasonic clean-
ing in distilled water for 15 min, then in acetone for 15 min.
The samples were dried in a desiccator, and the color,
weight, and properties of the surface were examined.
2.3. Determination of dissolved ion concentration
The amount of ions dissolved in the immersion solu-
tion of each tube was measured 4 times in an Inductively
Coupled
Plasma-Atomic
Emission
Spectrometry
(SPS1700HVR, Seiko Instruments Inc., Chiba, Japan;
ICP-AES hereafter). Measurements below the detectable
limit (0.005 ppm) were regarded as 0 ppb. The standard
reagent was titanium standard solution for atomic ab-
sorption spectrometry diluted to 1 ppm with unused im-
mersion solution.
The amount per unit area of dissolved ions was deter-
mined by dividing the measured amount by the surface
area calculated using the outer size of samples.
2.4. Discoloration of the surface of samples
The color of the surface of the dried samples was
visually examined, the color at 5 randomly chosen sites in
each sample was measured using a spectrophotometer
(CM-503i, MINOLTA Co. Ltd., Tokyo, Japan), and the
mean of measured values were regarded as the color
value of that sample.
Measurements were performed under the conditions of
a light source of D65, a visual "eld angle of 103, and
a diameter of 3 mm. The color di!erence (
E*ab) be-
tween the standard color (color value of the samples
before immersion) and the measured color value
was determined according to the L*a*b* colorimetric
system [20].
2.5. Weight changes of samples
Samples were weighed using an electronic analytical
balance (AEM-5200, Shimadzu Co., Kyoto, Japan, read-
ability"0.001 mg).
The weights of polished, defatted, and dried samples
before immersion and washed and dried samples after
immersion were measured twice, and the mean was deter-
mined. The weight change per unit area was determined
by dividing the di!erence in the mean weight before and
after immersion by the surface area of samples.
2.6. Observations of the chemical binding state
on the titanium surface
To examine the changes in the properties of the tita-
nium surface, the chemical binding state of the sample
surface were observed using an X-ray photoelectron
spectrometer (ESCA-850, Shimadzu Co., Kyoto, Japan,
described simply as ESCA hereafter), and the depth was
measured by repeated etching using argon ions in the
apparatus. Based on the reported binding energy of tita-
nium and measurement of O1s, the binding energies of Ti
and TiO at 2p1/2 and 2p3/2 were calculated to be 459.9
and 464.8 eV, respectively, and 453.8 and 459.1 eV, re-
spectively [21,22].
Mg K
(acceleration voltage, 8 kV; ion current, 30 mA)
was used as the excited X-ray source for ESCA. Etching
was performed in 90 layers at 1 kV, 20 mA for 30 s. The
2932
M. Koike, H. Fujii / Biomaterials 22 (2001) 2931}2936
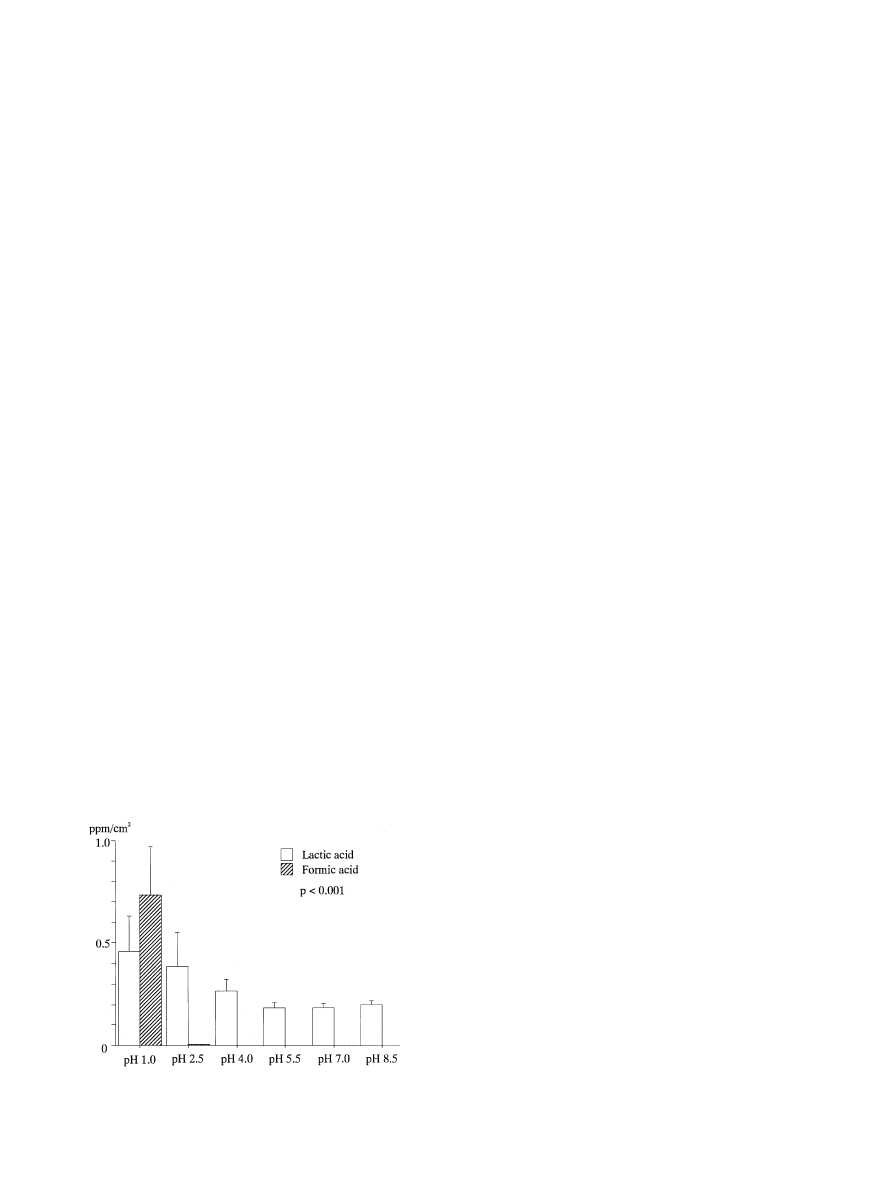
Fig. 1. Dissolution of titanium ion after 3-week immersion test (mean
and SD).
degree of vacuum was about 2
;10\ Pa at the time of
examination
of
the
sample
surface
and
about
6.5
;10\ Pa at the time of etching. By using the formula
for calculation, etching depth per etching was calculated
to be approximately 18 A
s [23].
2.7. Statistical analysis
The amount of dissolved titanium ions, discoloration
of the surface of samples, and changes in the sample
weight were statistically examined by one-way analysis of
variance (ANOVA). If a di!erence appeared to be signi"-
cant, the parameter was further examined by multivariate
variance analysis [24].
3. Results
3.1. Dissolution of titanium ions
The pHof both lactic acid and formic acid signi"cantly
a!ected the dissolution of titanium ions and the e!ect of
pHwas signi"cantly higher in formic acid than in lactic
acid (p(0.001).
Fig. 1 shows the amount of dissolved titanium ions in
immersion solutions. In lactic acid, the amount of dis-
solved titanium ions decreased with a higher pH, within
a range of 1.0
GpHG7.0. The amounts of dissolved ions
at pH1.0 and 2.5 were signi"cantly larger than those at
pH4.0 or higher (p(0.015), but there was no di!erence
among pHvalues greater than pH4.0. The amount of
dissolved ions was slightly larger at pH8.5, but the
di!erence was not signi"cant. In formic acid, the e!ects of
pHon dissolution were very signi"cant. A large amount
of titanium (0.735 ppm/cm
) was dissolved at pH1, and
this was 1.6-fold larger than the amount of ions dissolved
in lactic acid at the same pH. However, the amount of
dissolved ions markedly decreased at a pHhigher than
1.0, and was below the detectable limit at pH4.0 or
higher.
3.2. Discoloration
Fig. 2 shows the characteristic cases of titanium
samples before and after immersion. Analysis of the
color di!erence revealed that the e!ects of pHon dis-
coloration di!ered between lactic acid and formic acid,
and color di!erences between lactic acid and formic acid
were observed only for pH2.5, 4.0 and 5.5 (Fig. 3)
(p(0.0001). In lactic acid, the color di!erences of
samples were considered
`slighta according to the
criteria proposed by the International Lighting Com-
mittee
, but it was not signi
"cant between di!erent pH
(p'0.72).
However, the e!ects of pHin formic acid on discolora-
tion were signi"cant (p(0.001), and marked discolora-
tion of the samples immersed in formic acid at pH2.5 and
pH4.0 was visually observed. The color di!erence value
(
E*ab) was 13.8 at pH2.5 and 8.3 at pH4.0, which were
considered to be
`very mucha and `mucha, respectively,
according to the above criteria.
E*ab of the sample immersed in formic acid at pH
1.0, 7.0 and 8.5 were less than 1.37, which was not
signi"cantly di!erent from those in lactic acid (p'0.44).
3.3. Weight changes
The e!ect of pHon weight varied with the solution
used (Fig. 4, p"0.003). The weight of all samples immer-
sed in lactic acid decreased by 1.2}3.0
g/cm. However,
the di!erences in the decreased weight were not signi"-
cant between di!erent pH(p'0.05).
The changes in the weight of the samples immersed in
formic acid varied with pH(p(0.001); weight decreased
by 8.4
g/cm at pH1.0, while weight slightly increased at
pH2.5}5.5 (1.9}0.9
g/cm). There were no signi
"cant
changes in weight at pH7.0 and 8.5.
3.4. Changes in chemical binding state of surface
Fig. 5 shows the result of analysis with ESCA. Before
immersion, peaks corresponding to the binding energy of
TiO were observed on the "rst layer (super"cial layer) of
the samples, and the "rst peak corresponding to the
binding energy of Ti (metal titanium) was observed on
the 10th layer (Fig. 5).
All peaks observed on the "rst layer of the samples
after immersion corresponded to those of the binding
energy of TiO. In the samples immersed in lactic acid,
there were no di!erences in the layers showing these
peaks between di!erent pH. However, the TiO layers of
the specimen surface were thinner after immersion than
before immersion.
M. Koike, H. Fujii / Biomaterials 22 (2001) 2931}2936
2933
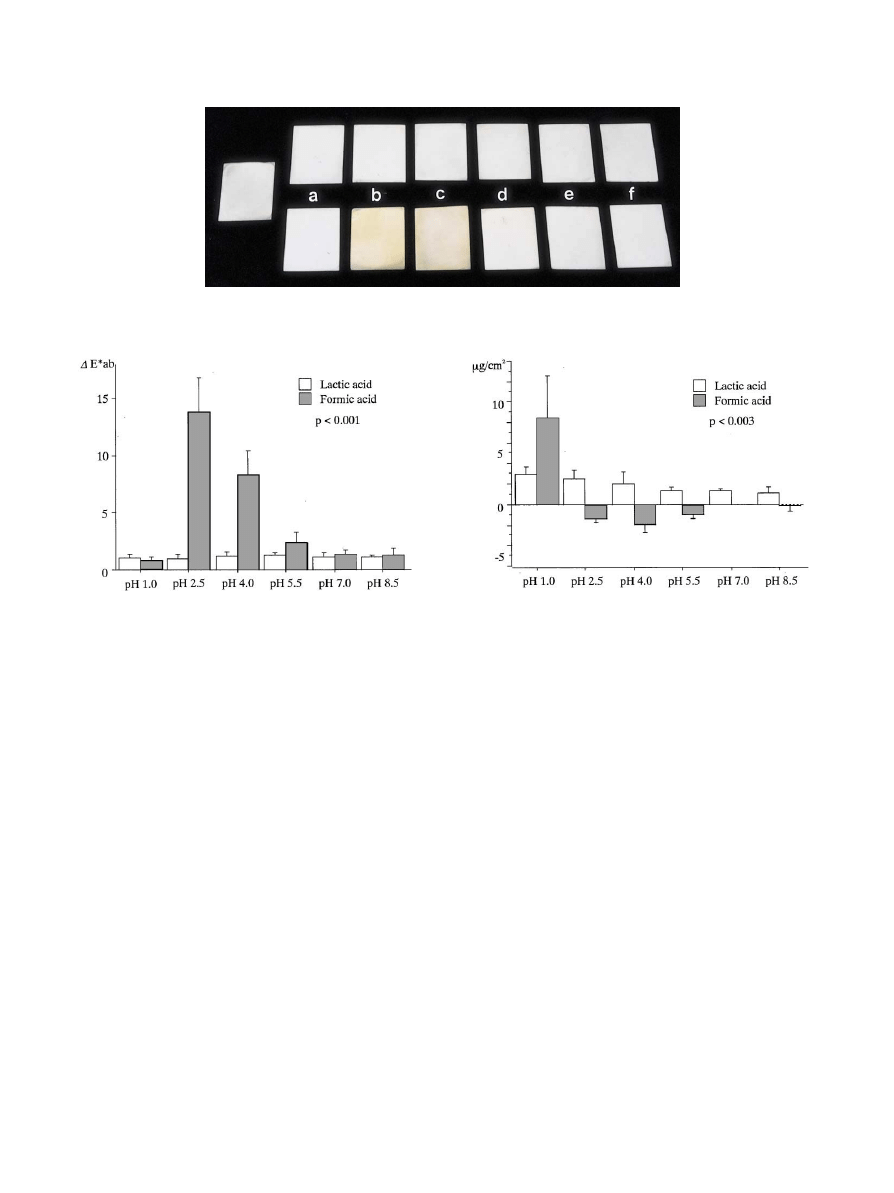
Fig. 2. Titanium specimens before [control (left)] and after 3-week immersion tests in lactic acid (upper step) and formic acid (lower step). a: pH1.0, b:
pH2.5, c: pH4.0, d: pH5.5, e: pH7.0, f: pH8.5.
Fig. 3. Color di!erence of titanium specimens after 3-week immersion
test (mean and SD).
Fig. 4. Weight changes of titanium specimens after 3-week immersion
test (mean and SD).
In the samples immersed in formic acid at pH1.0, there
were no di!erences in the layer of the "rst peak corre-
sponding to the binding energy of Ti between before and
after immersion (Fig. 5). However, the TiO layer was
thickened in the samples immersed in formic acid at pH
2.5 or higher. Moreover, the peaks of Ti did not appear
until the 35th layer at pH2.5, the 55th layer at pH4.0 and
the 20th layer at pH5.5 or higher.
4. Discussion
The pHin human saliva is reported to be 6.2}7.6
(mean, 6.7), usually 6}7 in plaque deposits 2}2.5 h after
food intake (carbohydrate), and 7}8 with starved plaque
8}12 h after intake of carbohydrate [25]. It has also been
reported that pHdecreases to almost 4 when sucrose is
being eaten [16], and that the pHof foods and beverages
can range from 2.0 to 11.0 [17]. However, the reduction
of oral pHby drinking and eating is not considered to
persist for a prolonged period because of the bu!ering
action of components in saliva and plaque.
Titanium is reported to have corrosion resistance to
hydrochloric acid at 3% or lower and sodium hydroxide
at 40% or lower [26]. In this study, the concentration of
hydrochloric and sodium hydroxide in immersion solu-
tion were 0.58% and 0.42%, respectively. Thus, our re-
sults were considered to re#ect the e!ects of pH, not of
the pH-adjust itself.
In lactic acid, dissolution of titanium ions and weight
loss of the samples were observed at every pHtested,
although they slightly decreased at a higher pH(Figs. 1
and 4). On the other hand, there were no di!erences
in the color di!erences and corrosion depth between
di!erent pH(Figs. 3 and 5). These results reveal that
hydrogen evolution type corrosion occurred on the sur-
face of titanium at every pHtested between 1.0 and 8.5 in
lactic acid.
However, in formic acid, corrosion of the samples
was highly dependent on pH; at pH 1.0, the amount of
dissolved titanium ions and the weight loss were
0.7 ppm/cm
and 8 g/cm, respectively, which were
higher than those of the samples immersed in lactic acid
at the same pH. Nevertheless, discoloration and the
thickness of the super"cial TiO layer were very similar
to those of the samples immersed in lactic acid. These
"ndings
indicate that hydrogen evolution type cor-
rosion also occurred on the surface of titanium samples
2934
M. Koike, H. Fujii / Biomaterials 22 (2001) 2931}2936
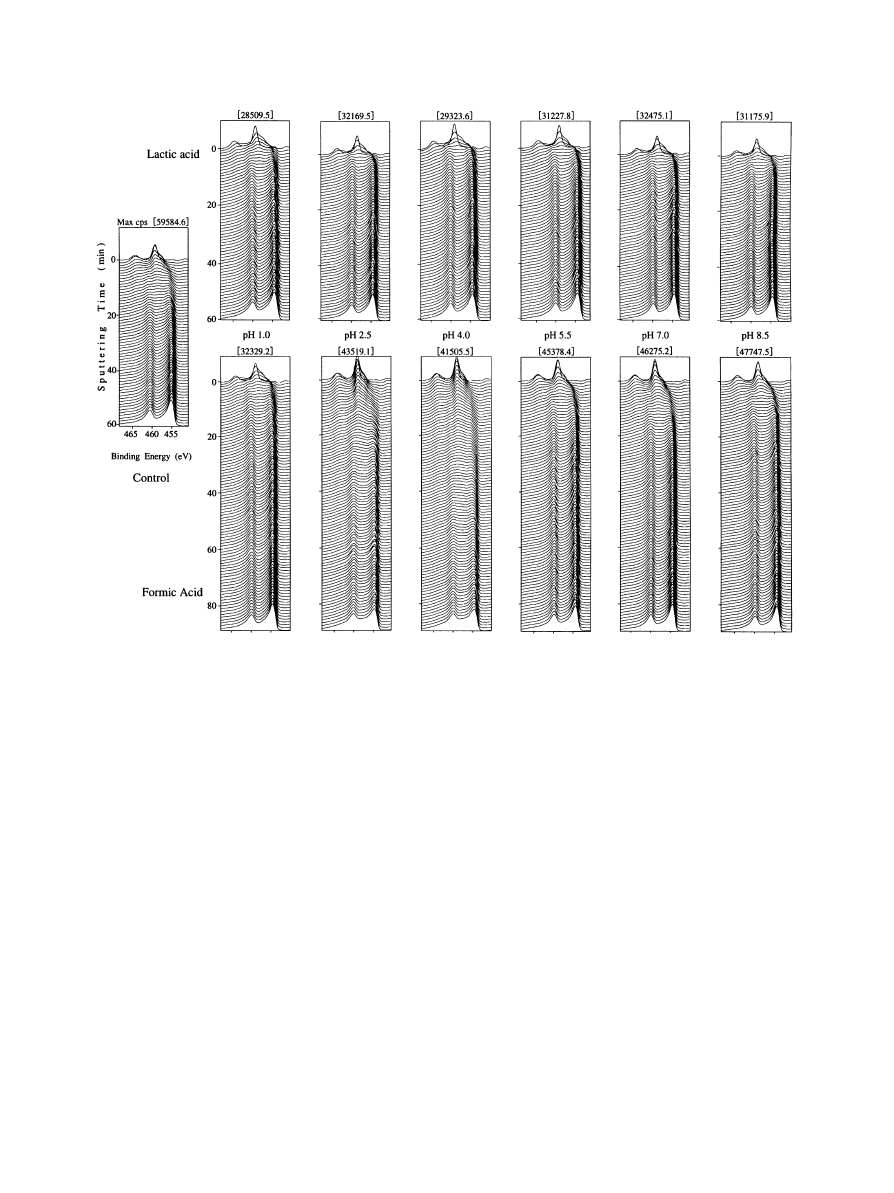
Fig. 5. The results of depth analysis with ESCA before (left) and after 3-week immersion test in lactic (upper) and formic (lower) acid (mean and SD).
immersed in formic acid at pH1.0, and that this cor-
rosion was more active in formic acid than in lactic acid
at the same acid concentration.
However, in the samples immersed in formic acid, the
amount of dissolved titanium was low at pH2.5 or
higher. Furthermore, at pH2.5 and pH4.0, a signi"cant
discoloration and weight gain were observed (Figs. 2}4),
and the super"cial TiO layer was thickened 4}4.5-fold
(Fig. 5). These results demonstrate that oxygen pen-
etrated the surface of titanium and di!used (oxygen di!u-
sion type corrosion) at pHranged from 2.5 to 4.0 in
formic acid. The surface color of titanium varies with the
thickness of the super"cial oxide "lm [27]. At a pH
higher than 5.5, corrosion of titanium would be very
low even though corrosion does occur under these
conditions.
In conclusion, the results of the present study reveal
that both hydrogen evolution type corrosion and oxygen
di!usion type corrosion can occur on the surface of
titanium in the oral cavity, and that corrosive properties
were markedly dependent on pHin formic acid, but less
dependent in lactic acid.
Acknowledgements
This study was partly supported by Grant-in-aid for
Scienti"c Research (C) No. 10671837 from The Japanese
Ministry of Education, Science, Sports and Culture.
References
[1] Merritt K, Rodrigo JJ. Immune response to synthetic materials.
Sensitization of patients receiving orthopaedic implants. Clin
Orthop 1996;326:71}9.
[2] Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J,
Peoch M. Dissemination of wear particles to the liver, spleen, and
abdominal lymph nodes of patients with hip or knee replacement.
J Bone Joint Surg Am 2000;82:457}76.
[3] Lalor PA, Revell PA, Gray AB, Wright S, Railton GT, Freeman
MAR. Sensitivity to titanium, a cause of implant failure? J Bone
Joint Surg Br 1991;73-B:25}8.
[4] Abdallah HI, Balsara RK, O'Riordan AC. Pacemaker contact
sensitivity: clinical recognition and management. Ann Thorac
Surg 1994;57:1017}8.
[5] Yamauchi R, Morita A, Tsuji T. Pacemaker dermatitis from
titanium. Contact Dermatitis 2000;42:52}3.
M. Koike, H. Fujii / Biomaterials 22 (2001) 2931}2936
2935

[6] Basketter DA, Whittle E, Monk B. Possible allergy to complex
titanium salt. Contact Dermatitis 2000;42:310}1.
[7] Koike M, Nakamura S, Fujii H. In vitro assessment of release
from titanium by immersion tests. J Jpn Prosthodont Soc 1997;
41:675}9.
[8] Koike M, Fujii H. In vitro assessment of corrosive properties of
titanium as a biomaterial. J Oral Rehabil, in press.
[9] Pourbaix M. Electrochemical corrosion of metallic biomaterials.
Biomaterials 1984;5:122}34.
[10] Khan MA, Williams RL, Williams DF. In-vitro corrosion and
wear of titanium alloys in the biologic environment. Biomaterials
1996;17:2117}26.
[11] Doi H, Takeda S. In vitro metal dissolution under various extrac-
tion conditions. J J Dent Mater 1990;9:375}86.
[12] Strietzel R, HoKsch A, Kalb#eisch H, Buch D. In vitro corrosion of
titanium. Biomaterials 1998;19:1495}9.
[13] Simonis A, KruKaKmer A, Netuschil L, Schlachta T, Geis-Gerstorfer
J.
In-vivo-Korrosionsuntersuchungen
an
ZahnuKaKrztlichen
Legierungen unter BeruKcksichtigung des Speichel-pH-Wertes.
Dtsch ZahnuKaKrztl Z 1990;45:485}9.
[14] Suga S, editor. Illustrated cariology. Tokyo: Ishiyaku Publishers
Inc., 1990. p. 120}127.
[15] Ravnholt G. Corrosion current and pHrise around titanium
coupled to dental alloys. Scand J Dent Res 1988;96:466}72.
[16] Imfeld Th. Evaluation of the cariogenicity of confectionery by
intra-oral wire-telemetry. Schweiz Monatsschr Zahnheilkd 1977;
87:437}64.
[17] McCabe JF, editor. Applied dental materials. 8th ed. London:
Butler & Tanner Ltd., 1990. p. 1}28.
[18] J. Morita Corp. Instruction manual of pure titanium A.
1989.
[19] Miyakawa O, Watanabe O, Okawa S, Nakano S, Kobayashi M.
Reaction layers of titanium cast in molds containing spinel.
J J Dent Mater 1995;14:560}8.
[20] Commission internationale de l'eclairage. Technical report
COLORIMETRY.
Vienna:
Commission
internationale
de
l'eclairage, 1986.
[21] Ramqvist L, Hamrin K, Johansson G, Fahlman A, Nordling C.
Charge transfer in transition metal carbides and related com-
pounds studied by ESCA. J Phys Chem Solids 1969;30:
1835}47.
[22] Porte LD, Demosthenous M, Duc TM. EEtude ESCA de
l'interaction oxyge`ne-titane*. J Less-common Met 1977;56:
183}91.
[23] Briggs D, Seah MP, editors. Practical surface analysis by auger
and X-ray photoelectron. Sussex: Wiley, 1983.
[24] Erwin K, editor. Introductory mathematical statistics. Principles
and methods. New York: Wiley, 1970.
[25] Edgar WM, Mullane DMO, editors. Saliva and oral health.
London: British Dental Association, 1996.
[26] Ito G, editor. Corrosion science and engineering. Tokyo: Corona
Publishing Co. Ltd., 1979.
[27] Cotton JB, Hay"eld PCS. Decorative "nishes on titanium. Trans
Inst Met Finish 1967;45:48}52.
2936
M. Koike, H. Fujii / Biomaterials 22 (2001) 2931}2936
Wyszukiwarka
Podobne podstrony:
In vitro corrosion resistance of titanium made using differe
5 49 62 The Influence of Tramp Elements on The Spalling Resistance of 1 2343
The corrosion behaviour of Ti
Corrosion behaviour of commercially pure titanium shot blast
72 1031 1039 Influence of Thin Coatings Deposited by PECVD on Wear and Corrosion Resistance
25 339 348 Development Trends of Corrosion Resistant Plastic Mould Steels
Freies Deutschland Guerrilla Warfare in East Prussia, 1944 1945; A Contribution to the History of
the other side of change resistance
Resistance and the background conversations of change
Corrosion behavior of titanium nitride
mapi com The Ayurvedic View of Marijuana
Interruption of the blood supply of femoral head an experimental study on the pathogenesis of Legg C
Ebsco Gross The cognitive control of emotio
37 509 524 Microstructure and Wear Resistance of HSS for Rolling Mill Rolls
Bo Strath A European Identity to the historical limits of the concept
Betsy Powell Bad Seeds, The True Story of Toronto's Galloway Boys Street Gang (2010)
więcej podobnych podstron