Stereochemistry Tutorials: Classification of Isomers 1
Stereochemistry Tutorials: Classification of Isomers
Definitions for vocabulary words can be found in the Illustrated Glossary of Organic
Chemistry, available on the course web site.
The structures of organic molecules vary widely. Within this variety there are similarities
in structure that result in similarities in chemical, physical, and/or biological properties. If
one can understand the properties of a compound based on its structure, then we might be
able to predict properties of a compound with similar structure. For example, we know
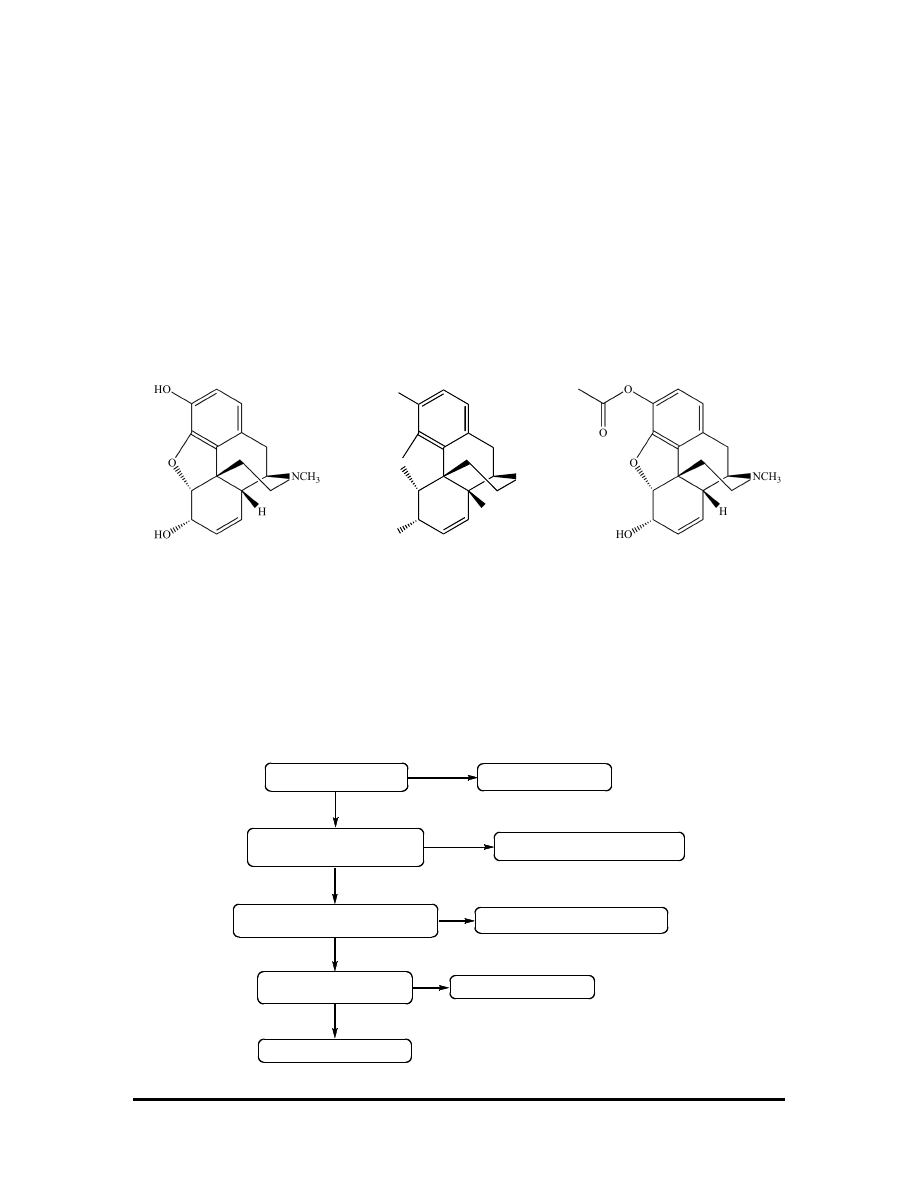
that the opiate alkaloid morphine is an analgesic, and so we expect (correctly, as it turns
out) that compounds with similar chemical structure might also have analgesic properties.
This is true for codeine and heroin; note their structural similarities to morphine.
O
H
3
CO
HO
NCH
3
H
Morphine
Codeine
Heroin
The power to predict the properties of a substance based on its similarity to other
substances of known structure is a powerful tool for organic chemists, especially so for
the discovery of new pharmaceuticals. It is useful, therefore, for you to learn to recognize
various categories of structural relationships, namely isomers, constitutional isomers,
conformational isomers, stereoisomers, enantiomers, and diastereomers. (This is a good
time to review definitions of these structural types.) A flowchart that shows the
relationship of these isomer types can be useful tool for their categorization.
Compare chemical formulas.
Not identical
Identical
Structures are not isomers.
Structures are
isomers
.
Compare order of atom attachment.
Not identical
Structures are
constitutional isomers
.
Identical
Can structures be made identical by
rotation around one or more single bonds?
Yes
Structures are
conformational isomers
.
No
Structures are
stereoisomers
.
Are structures mirror images?
Yes
No
Structures are
diastereomers
.
Structures are
enantiomers
.

2 Stereochemistry Tutorials: Classification of Isomers
Steroisomers are also called configurational isomers. This makes the process of
classifying isomers into a ‘con game’: The isomers may be constitutional,
conformational, or configurational.
Now let’s work a few examples to see how this ‘con game’ goes.
Example 1: Using the isomers classification flowchart above, categorize the following
molecules using all the terms that apply: Isomers, constitutional isomers, conformational
isomers, stereoisomers, enantiomers, and diastereomers. Molecular models can be very
useful for this task.
and
Solution: The molecules have the same chemical formula (C
4
H
10
O) but are not identical
so they are isomers. Examining the order of atom attachment we see they have the same
sequence of connectivity (a four-carbon chain with a hydroxyl group one the second
carbon), so they are not constitutional isomers. Experimenting with models reveals the
molecules cannot be made identical by rotation around bonds, so they are not
conformational isomers. By process of elimination, these molecules are stereoisomers.
When working with the models you may have noticed that the molecules are mirror
images, so they are enantiomers.
Example 2: Using the isomers classification flowchart above, categorize the following
molecules using all the terms that apply: Isomers, constitutional isomers, conformational
isomers, stereoisomers, enantiomers, and diastereomers. Molecular models can be very
useful for this task.
and
Solution: The molecules have the same chemical formula (C
4
H
6
O
6
) but are not identical
so they are isomers. Examining the order of atom attachment we see they have the same
sequence of connectivity (carbon with H, COOH, and OH attached to another carbon
with H, COOH, and OH), so they are not constitutional isomers. Experimenting with
models reveals the molecules cannot be made identical by rotation around bonds, so they
are not conformational isomers. By process of elimination, these molecules are
stereoisomers. When working with the models you may have noticed that the molecules
are not mirror images, so they are diastereomers. (When examining models to detect
mirror image relationships, it is often necessary to consider different conformations. Try
to rotate the molecule and its bonds so that obvious groups can be moved into a mirror
image if possible. In this case you might use the carboxylic acid groups for this purpose.)
Stereochemistry Tutorials: Classification of Isomers 3
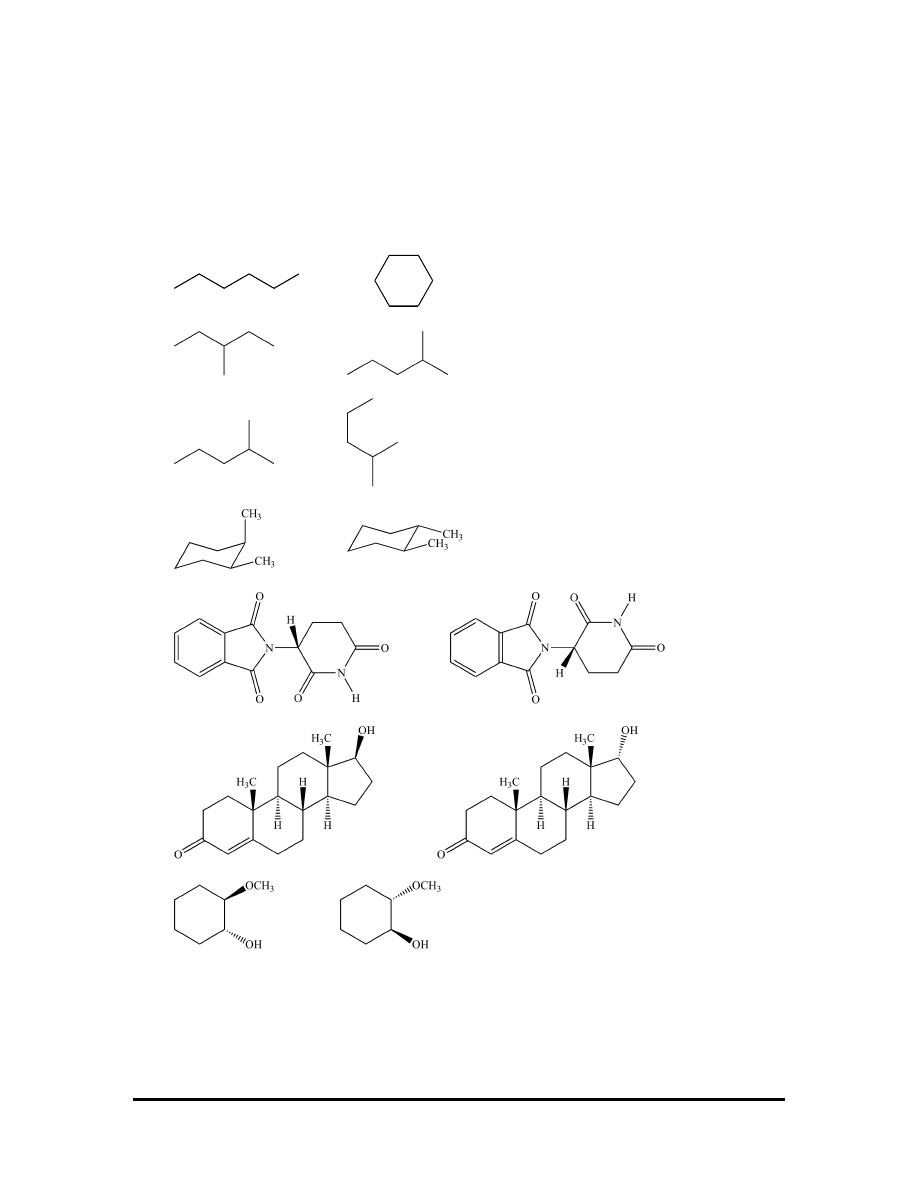
Exercises
Using the isomers classification flowchart given above, categorize each pair of structures
using all the terms that apply: Identical, isomers, constitutional isomers, conformational
isomers, stereoisomers, enantiomers, and diastereomers. Molecular models can be very
useful for this task.
(a)
and
(b)
and
(c)
and
(d)
and
(e)
and
(f)
and
(g)
and
Solutions to Exercises
(a) These molecules do not have the same chemical formula (C
6
H
14
and C
6
H
12
) so they
are not isomers.
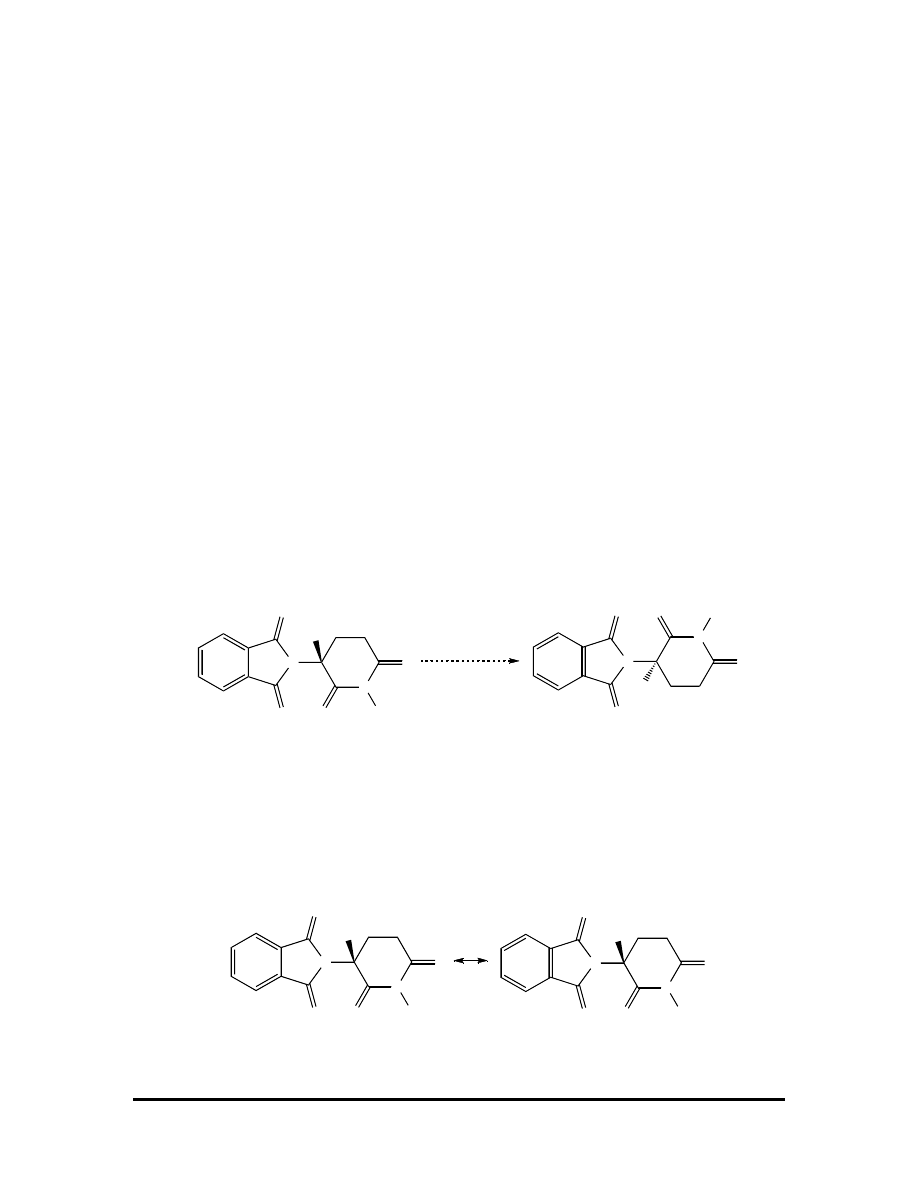
4 Stereochemistry Tutorials: Classification of Isomers
(b) These structures have the same chemical formula (C
6
H
14
) so they might be isomers
(we have to verify that they are not identical before we can conclude they are
isomers). They do not have the same sequence of atom connections (the methyl group
on the third carbon versus the second carbon) so they are constitutional isomers.
(c) These structures have the same chemical formula (C
6
H
14
) so they might be isomers.
They have the same sequence of atom connections (a five-carbon chain with a methyl
group attached to the second carbon) so they are not constitutional isomers. They
can be interconverted (i.e., made identical) by rotation around a single bond, followed
by rotation of the entire structure, so they are conformational isomers. (Verify this
with a model). Some textbooks do not treat conformational isomers as a type of
isomers, and will therefore label these molecules as identical.
(d) These structures have the same chemical formula (C
8
H
16
), so they are might be
isomers. They have the same sequence of atom connections (a cyclohexane ring with
methyl groups on adjacent carbons) so they are not constitutional isomers. The
structures cannot be interconverted by rotation around one or more single bonds
(verify this with a molecular model) so they are stereoisomers (configurational
isomers). They are not mirror images in any conformations (verify with molecular
models) so they are diastereomers.
(e) At first inspection, we see the molecules are very similar. Rotation around one bond
makes them even more similar:
N
N
O
O
O
O
H
H
N
N
O
O
O
H
O
H
180
o
bond rotation
The molecules have the same chemical formula (C
13
H
10
N
2
O
4
) so they might be
isomers. The sequence of atom attachments is identical so they are not
constitutional isomers. They cannot be interconverted by rotation around one or
more single bonds, so they are stereoisomers. The molecules are nonsuperposable
mirror images, so they are enantiomers.
Don’t be fooled by the positions of the double bonds (i.e., resonance contributors).
N
N
O
O
O
O
H
H
N
N
O
O
O
O
H
H

Stereochemistry Tutorials: Classification of Isomers 5
Remember that resonance contributors are alternate ways of representing the same
structure. It does not matter which resonance structure you use when analyzing
isomer types.
(f) These molecules have the same chemical formula (C
19
H
28
O
2
) so they might be
isomers. The sequence of atom attachment is identical so they are not constitutional
isomers. The molecules cannot be interconverted by rotation around one or more
single bonds, so they are not conformational isomers. The molecules are not mirror
images or identical so they are diastereomers.
(g) These molecules have the same chemical formula (C
7
H
14
O
2
) so they might be
isomers. They have the same sequence of atom attachment (a cyclohexane ring with
OH and OCH
3
on adjacent carbons) so they are not constitutional isomers. They
cannot be interconverted by rotation around one or more single bonds (carefully
verify this with molecular models) so they are not conformational isomers. The
same molecular models will reveal that these structures are nonsuperposable mirror
images, and therefore they are enantiomers.
Wyszukiwarka
Podobne podstrony:
2b classification of consonants tables (1)
The problems in the?scription and classification of vovels
25 Oxford's classification of Language Learning (Teaching) strategies
Legg Calve Perthes disease The prognostic significance of the subchondral fracture and a two group c
1961 The Classification of Clusters of Galaxies Morgan
Classification of Packed Executables for Accurate Computer Virus Detection
Fitzcarraldo starring Klaus Kinski Claudia Cardina A True Classic Of World Cinema
BIBLIOGRAPHY #2 Classics of Medieval Spirituality
test the classification of languages
On the definition and classification of cybercrime
Generic Detection and Classification of Polymorphic Malware Using Neural Pattern Recognition
From the design of a generic metamorphic engine to a black box classification of antivirus detection
The Classics of Weiqi in Thirteen Chapters
A Classification of Design Patterns
A Classification of Viruses through Recursion Theorems
Classification of Computer Viruses Using the Theory of Affordances
Osho Tao The Golden Gate vol 2, Discourses on Ko Hsuan’s The Classic of Purity
P1 Classification of costs and mathematics for budgets
P1 Classification of costs and mathematics for budgets
więcej podobnych podstron