
Diverted Total Synthesis in
Medicinal Chemistry Research
Luke Zuccarello
December 14, 2005
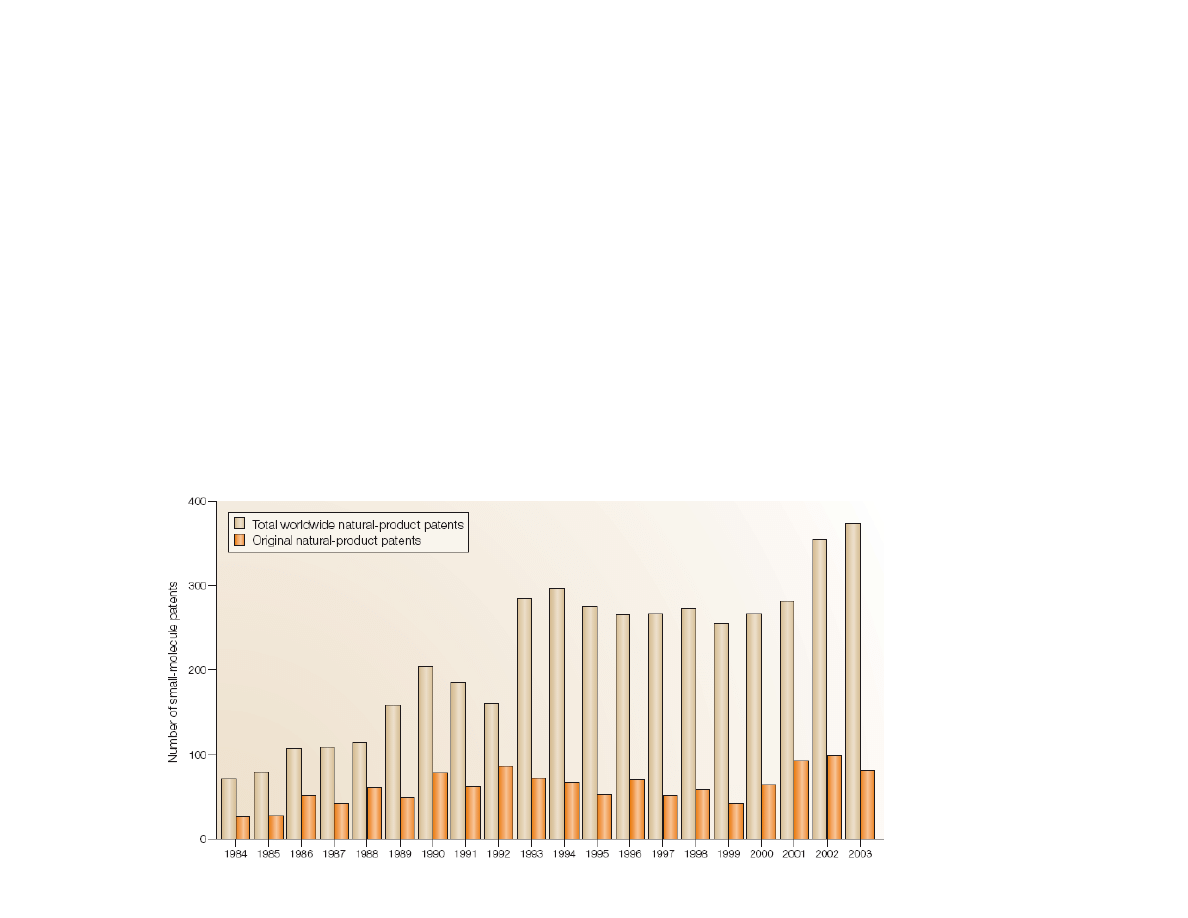
Natural Products in Drug Therapy
• About 50% of drugs in clinical use today are natural
products, or chemically modified natural products
• Sources for natural product-derived drugs: (a) directly
from plant/animal, (b) genetic engineering, (c) semi-
synthesis (d) total synthesis
• Pharmaceutical research involving natural products was
stagnant/declining in 1990s, but increasing again in the
current decade
Koehn, F. E.; Carter, G. T.
Nat. Rev. Drug Disc.
2005, 4, 206.
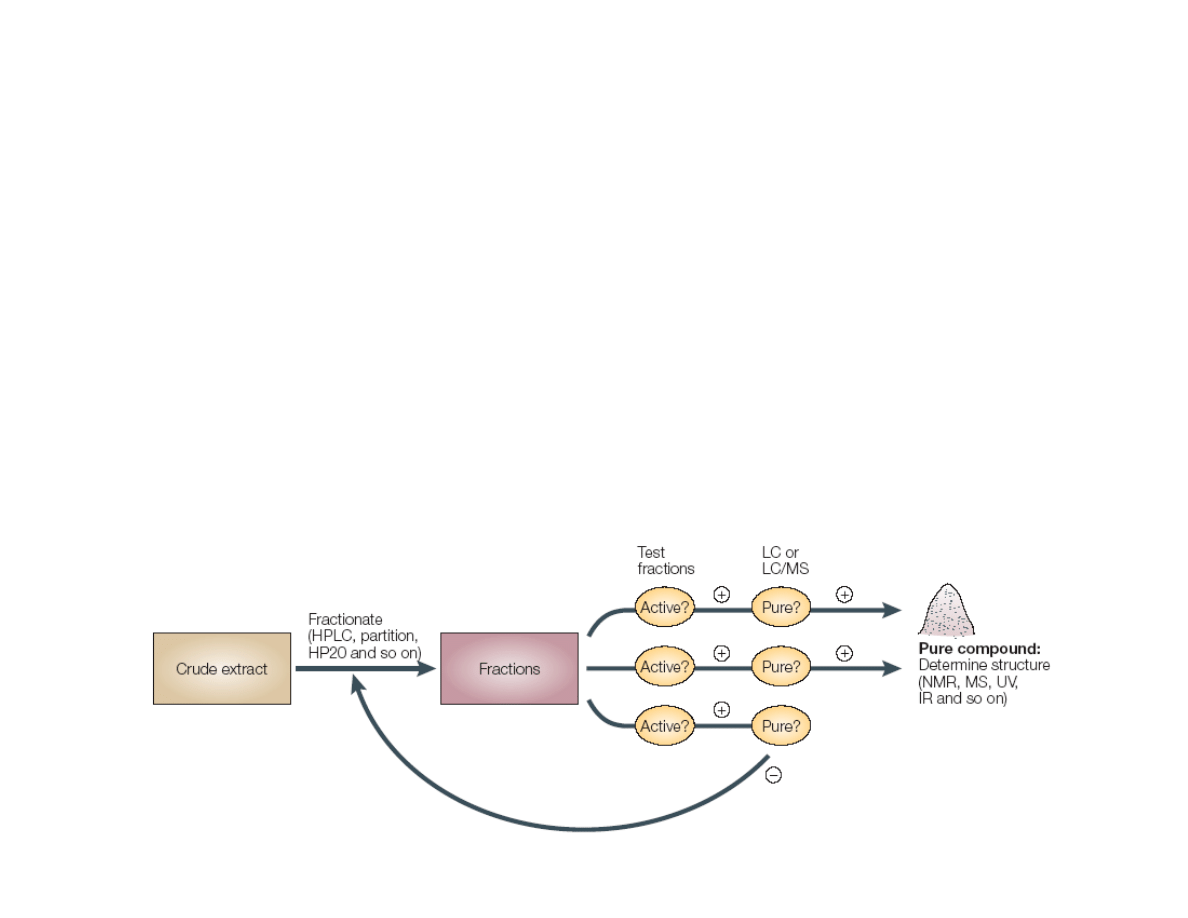
Why the Decline Natural Products Research
in the Pharmaceutical Industry?
• Advent of high-throughput screening (HTS) allows more compounds
to be assessed for hits
• Natural products are typically extracted as mixtures (10-100s) of
compounds with largely varying concentrations, which can (a) make
identifying actual active compound more difficult; (b) add more work
in additional purification; (b) give poor results with catalysis/binding
assays
• Combinatorial chemistry/synthetic libraries are better suited for HTS
than natural product extract libraries, leading to an industrial trend
toward purely synthetic libraries
• Major decline in “big pharma” research on infectious disease
therapy
Koehn, F. E.; Carter, G. T. Nat. Rev. Drug Disc. 2005, 4, 206.

Problems with Combinatorial Libraries in
Drug Screening
• Libraries designed to maximize number of compounds
screened (10
6
) may yield no hits due to lack of
biochemically relevant structure
• Compounds that are hits are often unselective in their
binding/activity
• Overall, R&D expectations have not been realized
Koehn, F. E.; Carter, G. T. Nat. Rev. Drug Disc. 2005, 4, 206.

Advantages of Natural Product Libraries in
Drug Screening
•
Recent advances in purification and analysis technology allows for natural
product library HTS
•
Natural products have been selected through evolution to interact with
macromolecules (eg proteins)
•
This includes natural selection of 3D structures and pharmacophores
•
Many natural products are “priveleged structures,” allowing them to interact
with multiple biological targets in various types of organisms
•
This is reinforced by research in the last 5-10 years which shows that the
protein fold space found in nature is smaller than previously predicted
•
At the same time, natural products are typically specific in their ability to
modulate protein-protein interactions (signal transduction, immune
response, mitosis, apoptosis)
•
Natural products typically do not violate Lapinski’s “Rule of Five”
Koehn, F. E.; Carter, G. T. Nat. Rev. Drug Disc. 2005, 4, 206.
Zhang, C.; DeLisi, C. J. Mol. Biol. 1998, 284, 1301.
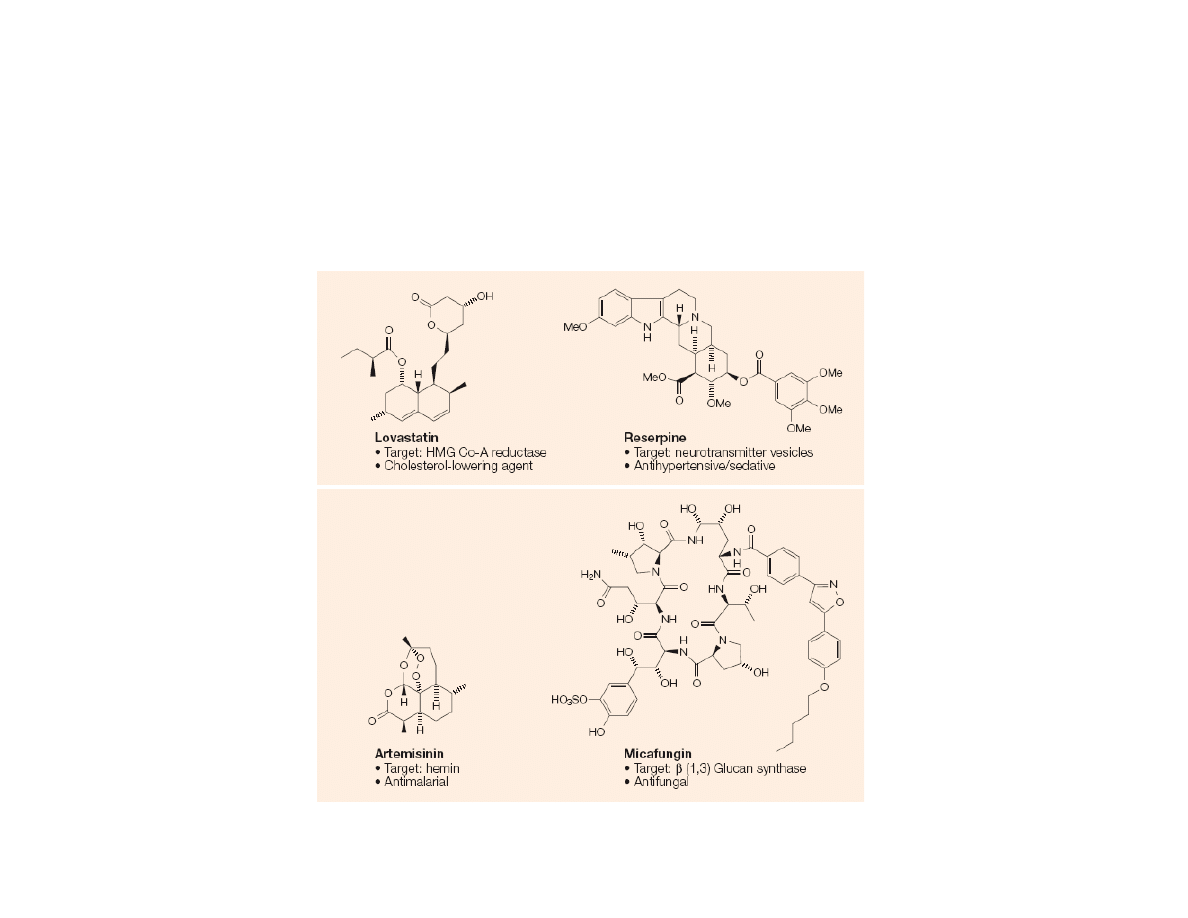
Types of Natural Product Derived
Therapeutics
1) Unaltered natural product as a drug
Koehn, F. E.; Carter, G. T. Nat. Rev. Drug Disc. 2005, 4, 206.

Types of Natural Product Derived
Therapeutics
2) Semi-synthetic analog: chemical manipulation
(typically functional group interconversion) of a natural
product
Example: exchanging a sugar on a natural product
Possible disadvantages: supply of natural product,
limitations to available analogues due to native
functionality
Paterson, I.; Anderson, E. Science 2005, 310, 451.
Njardarson, J. T. ; Gaul, C.; Shan, D.; Huang, X.-Y.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 1038.
.
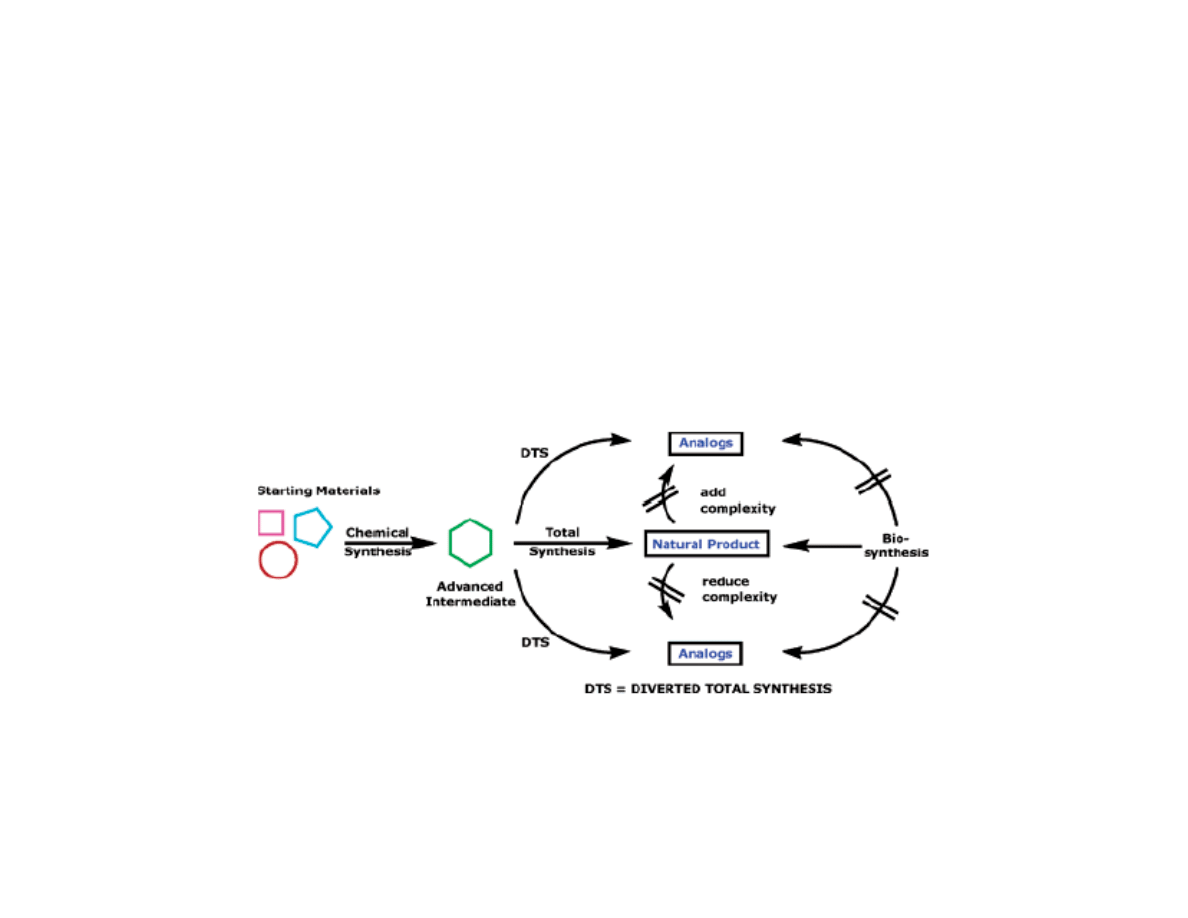
Types of Natural Product Derived
Therapeutics
3) Analogs from diverted total synthesis
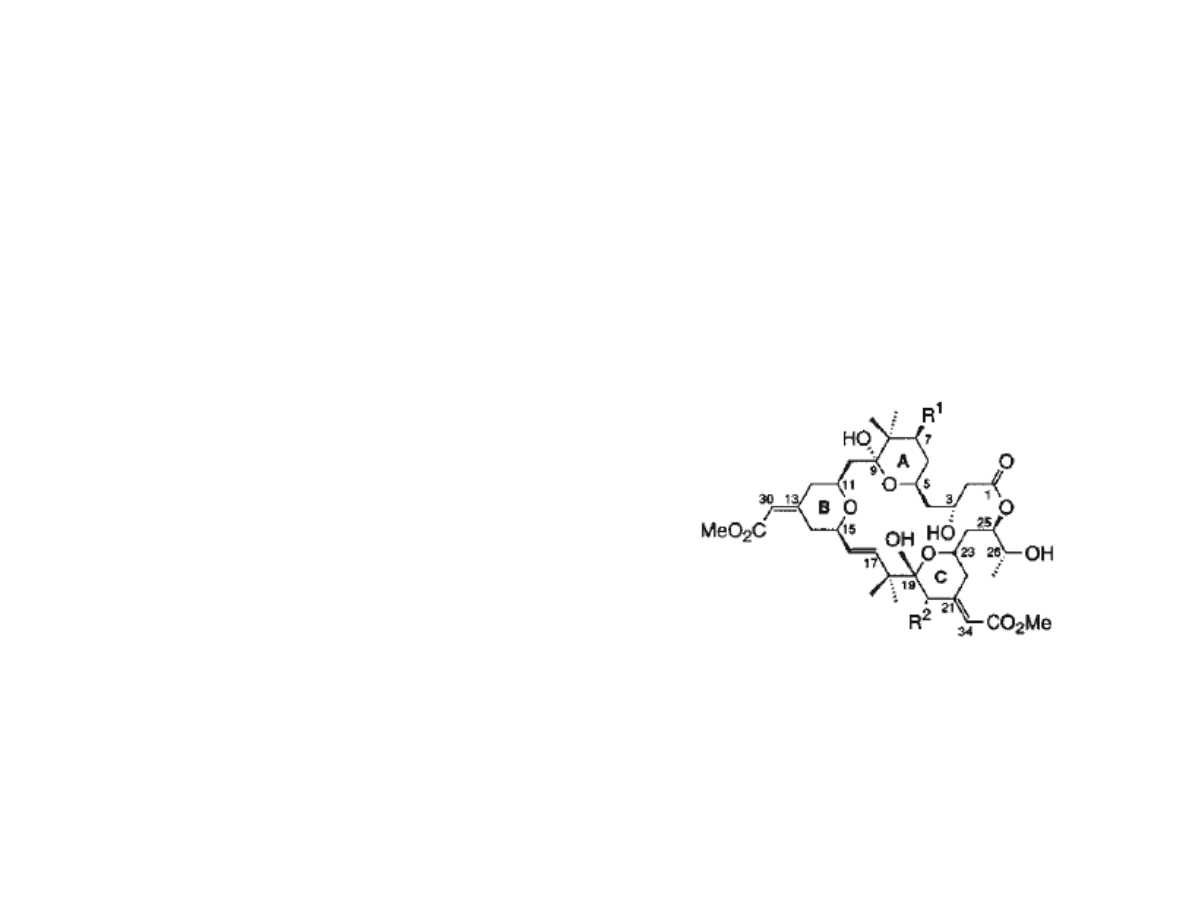
Bryostatin: Potent Anti-cancer Natural
Product
•Macrocyclic lactones from the marine invertebrate bugula neritina, first
isolated in 1968; fully characterized in 1982
•Bryostatins consist of at least 20 members, which vary at R
1
and R
2
•Isolated yields have varied between 10
-3
% to 10
-8
%
•Anti-cancer properties: apoptosis induction,
immune system booster, reverses multiple
drug resistance, synergistic with other drugs
•Currently in phase I and II clinical trials
Wender, P. A.; Hinkle, K. W.; Koehler, M. F. T.; Lippa, B. Med. Res. Rev. 1999, 19, 388.
Suffness M, Newman DJ, Snader K. In: Scheuer PJ, editor. Bioorganic marine chemistry 3. New York: Springer-
Verlag Publishers. pp. 131–168.
Pettit, G.R.; Herald, C.L.; Doubek, D.L.; Herald, D.L.; Arnold, E.; Clardy, J. J Am Chem Soc 1982, 104, 6846.
Pettit, G.R. J Nat Prod 1996, 59, 812.
Pettit GR. Fortschritte 1991, 57, 153.
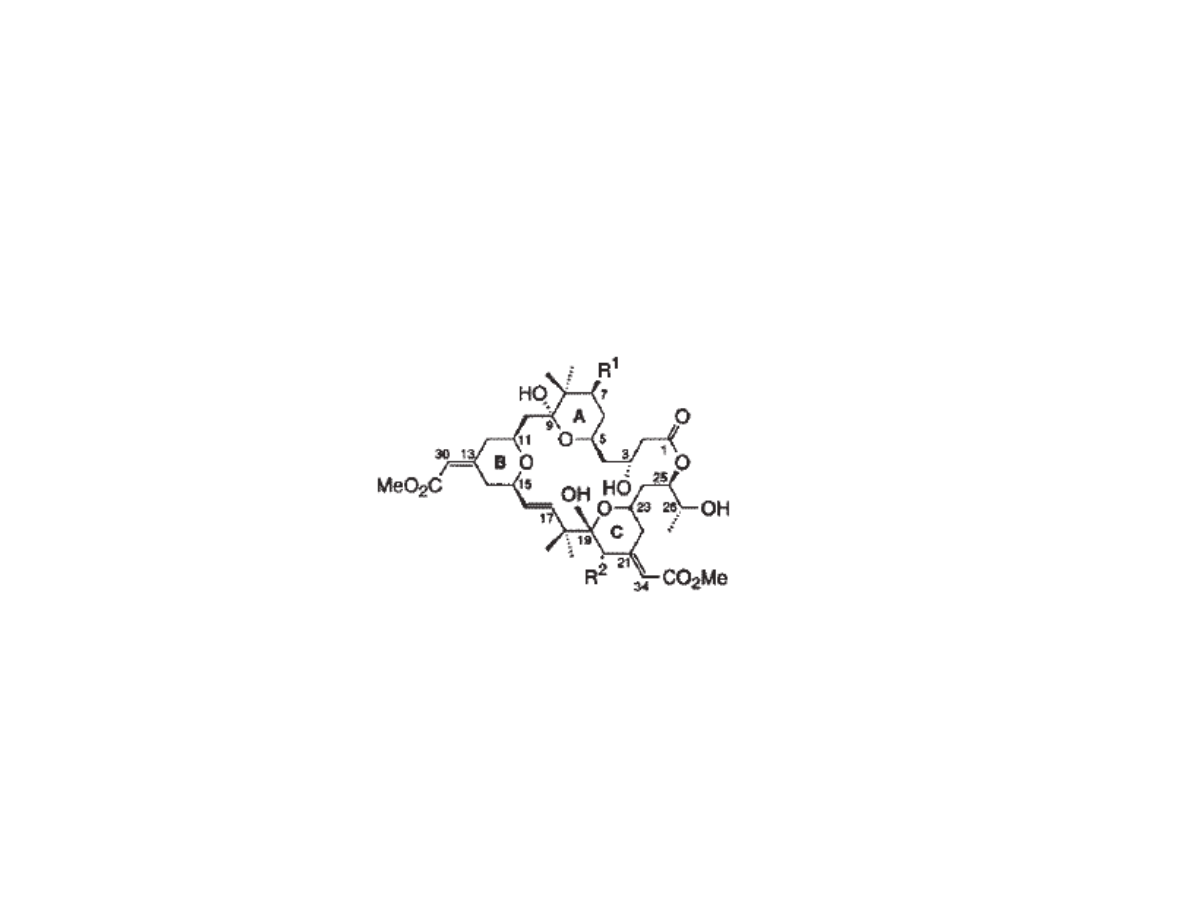
Bryostatin: Binding to PKC
Paul A. Wender, P. A.; De Brabander, J.; Harran, P. G.; Jimenez, J.-M.; Koehler, M. F. T.; Lippa, B.;
Park, C.-M.; Shiozaki, M. J. Am. Chem. Soc. 1998, 120, 4534.
Wender, P. A.; Cribbs, C. M.; Koehler, K. F.; Sharkey, N. A.; Herald, C. L.; Kamano, Y.; Pettit, G. R.;
Blumberg, P. M. Proc. Natl. Acad. Sci. U.S.A. 1988, 85, 7197.
http://www.stanford.edu/group/pawender/html/synth.html
•Protein Kinase C (PKC) family of serine/threonine kinases is involved in signal
transduction, and is important in the biochemistry of cancer
•Bryostatin binds to PKC w/ high affinity
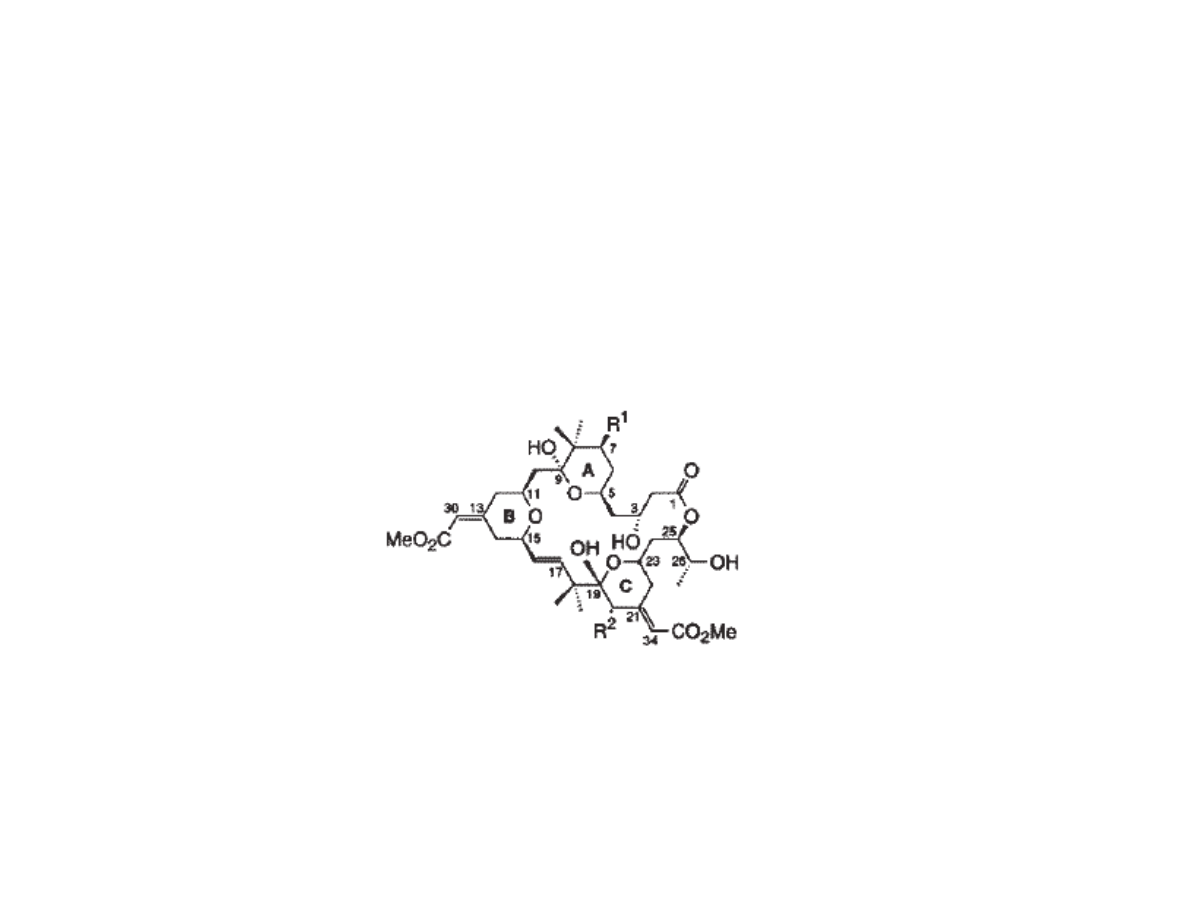
Bryostatin: Previous Total Synthesis
•Bryostatin 7 (R
1
=R
2
= OAc) : Masamune, 1990
•Bryostatin 2 (R
1
= OH, R
2
= O
2
CC
7
H
11
) : Evans, 1998
•Both total synthesis require >60 steps, making them untenable in process
Kageyama, M.; Tamura, T.; Nantz, M. H.; Roberts, J. C.; Somfai, P.; Whritenour, D. C.; Masamune, S.
J. Am. Chem. Soc.1990, 112, 7407.
Evans, D. A.; Carter, P. H.; Carreira, E. M.; Prunet, J. A.; Charette, A. B.; Lautens, M. Angew Chem Int.
Ed. 1998, 37, 2354.
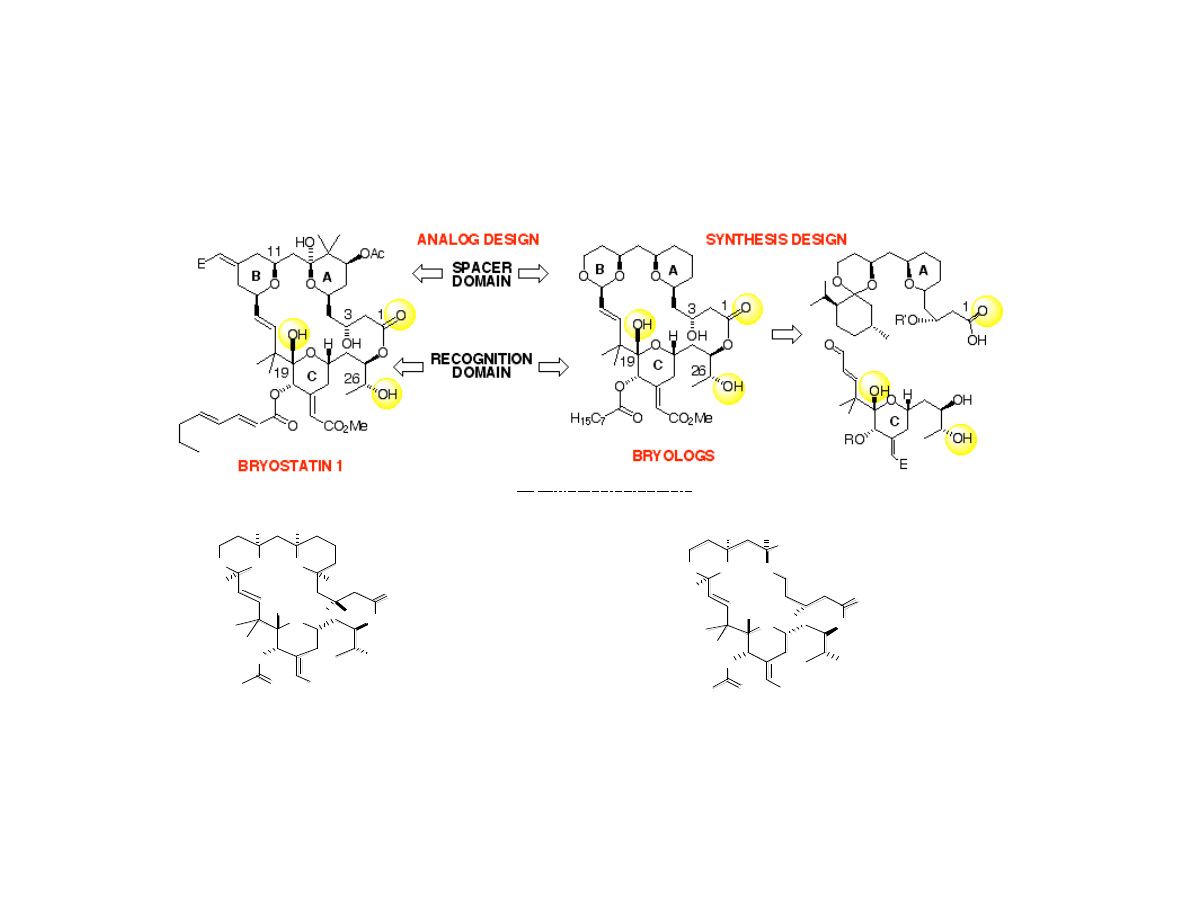
Wender’s Bryolog Targets
Wender, P. A.; Hinkle, K. W.; Koehler, M. F. T.; Lippa, B. Med. Res. Rev. 1999, 19, 388.
Paul A. Wender, P. A.; De Brabander, J.; Harran, P. G.; Jimenez, J.-M.; Koehler, M. F. T.; Lippa, B.;
Park, C.-M.; Shiozaki, M. J. Am. Chem. Soc. 1998, 120, 4534.
http://www.stanford.edu/group/pawender/html/synth.html
•Hypothesis by Wender et al. (1988): pharmacophore region of byrostatin include
C1, C19, and C26 oxygen atoms (bryostatin 1 K
i
= 1.35 nM)
O
O
O
H
H
H
H
O
O
O
O H
O
R
3
R
1
C
7
H
1 5
O
C O
2
Me
A) R
1
= OH , R
2
= H, R
3
= OH
B) R
1
= OH , R
2
= H, R
3
= OA c
C) R
1
= H, R
2
=OH , R
3
= O H
D) R
1
= H, R
2
= H , R
3
= OH
R
2
Sp ec ific A na log Ta rge ts
B
A
C
O
O
O
R
4
H
H
H
O
O
O
OH
O
OH
HO
C
7
H
1 5
O
C O
2
Me
B
C
E) R
4
= H
F) R
4
= t-B u
First Generation Sythesis of C Ring
O TBS
O
O
O
OP MB
OB n
+
4 ste ps
2 9%
fro m R -m e th y l la cta te
from me th yl i -p ro py l
ke ton e
O
OBn
OTB S
O H
H O
OM e
OPM B
1 ) Ph C OC l, D MA P; D MP
9 0 %
2 ) Sm I
2
9 0 %
O
OBn
OTB S
O
OM e
OPM B
1 ) L DA , CH OC O
2
M e
2 ) M sC l, TEA
3 ) D BU
7 0 %
O
OB n
OTBS
O
OMe
OP MB
C O
2
M e
1 ) Na B H
4
, C eC l
3 ,
-2 0 de g . C
2 ) C
7
H
1 5
CO
2
H , Ya m ag u ch i co n d' ns
9 3%
O
O Bn
OTB S
O
OM e
O PM B
CO
2
M e
C
7
H
15
O
1) H F/p yr.
2) D M P
86 %
O
OB n
O
O
OMe
OP MB
C O
2
M e
C
7
H
1 5
O
1 ) a ll yl -BE t
2
, E t
2
O, -1 0 de g . C
2 ) A c
2
O, DM AP
3 ) Os O
4
, N M O
4 ) P b( OAc )
4
, TE A; the n DB U
7 6 %
O
OB n
O
O Me
OP MB
C O
2
M e
C
7
H
1 5
O
C H O
1 ) D D Q
2 ) H F, CH
3
C N , H
2
O
7 5 %
O
OBn
O
OH
OH
C O
2
Me
C
7
H
1 5
O
C H O
Paul A. Wender, P. A.; De Brabander, J.; Harran, P. G.; Jimenez, J.-M.; Koehler, M. F. T.; Lippa, B.;
Park, C.-M.; Shiozaki, M. J. Am. Chem. Soc. 1998, 120, 4534.
First Generation Sythesis of “Spacer”
Region
Wender, P. A.; Hinkle, K. W.; Koehler, M. F. T.; Lippa, B. Med. Res. Rev. 1999, 19, 388.
Wender, P. A.; Baryza, J. L.; Bennett, C. E.; Bi, F. C.;. Brenner, S. E.; Clarke, M. O.; Horan, J. C.; Kan, C.; Lacôte, E.;
Lippa, B.; Nell, P. G.; Turner, T. M. J. Am. Chem. Soc. 2002, 124, 13648.
O
OH
O
1 ) S we rn
2 ) t-B uL i
3 ) D M P
4 ) L u ch e re d u ctio n
d r = 6 :1, 4 6%
O
OH
O
t-B u
1 ) t-Bu OK , a ll yl Br
2 ) 9- BBN ; H
2
O
2
, N a OH
3 )D MP
7 2%
O
O
O
t-Bu
O
1 ) (- )-Ip c
2
B-C H
2
C H=C H
2
2 ) TB SC l, i mi d.
3 ) ca t. KM nO
4
, N a IO
4
4 2 %
1 ) N a H, a ll yl Br 7 6%
2 ) 2 ) 9 -BB N ; H
2
O
2
, N a OH
3 )D M P
7 2 %
1 ) (-)- Ipc
2
B -C H
2
C H =C H
2
2 ) TBS Cl , im id .
3 ) ca t. K Mn O
4
, N aIO
4
4 2%
O
O
O
O
O
O
O
R
O
OH
TB SO
R = H , t-B u
6 0% fro m p e nta n etri o l
1 ) Sw e rn
2 ) D an is he fsk y's d i en e
O
O
N
Cr
Cl
O
O
O
O
th e n TFA
1 ) Lu ch e re du cti on
2 ) i-B uO CH =C H
2
, H g (OA c)
2
3 ) De ca n e , 1 5 0 d e g. C
7 6%
O
O
O
1 ) H
2
, Pd (OH )
2
2 ) (-)- Ip c
2
B -C H
2
C H =C H
2
3 ) TBS Cl , im id .
4 ) KM nO
4
, N a IO
4
4 9%
O
O
O
C H O
O
OH
TBS O
Completion of Analogs
O
O
O
O
OH
TB SO
O
OBn
O
OH
OH
C O
2
Me
C
7
H
1 5
O
CH O
1 ) Ya ma g uc hi 81 %
2 ) HF -py r. 81 %
O
O
O
H O
O
O
O
OB n
O
OH
CO
2
M e
C
7
H
1 5
O
C H O
1) Am be rl ist- 15 , rt, d i lu te
2) Pd (OH )
2
, H
2
88 %
O
O
O
H O
O
O
O
OR
O
OH
C O
2
Me
C
7
H
1 5
O
A c
2
O, DM AP
8 5 %
Bry ol o g A
R = H
K
i
= 3 .4 n M
Bry ol o g B
R = A c
K
i
= 2 9 7 n M
O
O
O
R
O
OH
TBS O
1 ) Y am a gu ch i
2 ) H F-p yr .
3 ) A mb e rli st-1 5 , r t, d il ute
4 ) P d( OH )
2
, H
2
R = H , t-Bu
O
O
O
H O
O
O
O
OH
O
O H
C O
2
Me
C
7
H
1 5
O
t-Bu
O
O
O
H O
O
O
O
O H
O
OH
CO
2
M e
C
7
H
1 5
O
Bry ol o g F
K
i
= 8 .3 nM
B ryo lo g E
K
i
= 4 7 n M
Wender, P. A.; Hinkle, K. W.; Koehler, M. F. T.; Lippa, B. Med. Res. Rev. 1999, 19, 388.
Role of C3 Hydroxyl
O
O
HO
O
O
O
OH
O
OH
CO
2
Me
O
MeO
2
C
HO
OAc
Hydrogen bonding
stabilizing a conformation?
O
O
O
O
O
OR
O
OH
CO
2
M e
C
7
H
1 5
O
O
O
O
H O
O
O
O
OR
O
O H
C O
2
M e
C
7
H
15
O
O
O
O
O
O
O
OR
O
O H
C O
2
M e
C
7
H
15
O
O
O
O
HO
O
O
O
OH
O
OH
C O
2
Me
C
7
H
1 5
O
Br yo lo g A
K
i
= 3.4 nM
Br yo lo g C
K
i
= 28 5 nM
B ryo lo g D
K
i
= 2 97 n M
K
i
> 10 ,0 00 n M
Wender, P. A.; Hinkle, K. W.; Koehler, M. F. T.; Lippa, B. Med. Res. Rev. 1999, 19, 388.
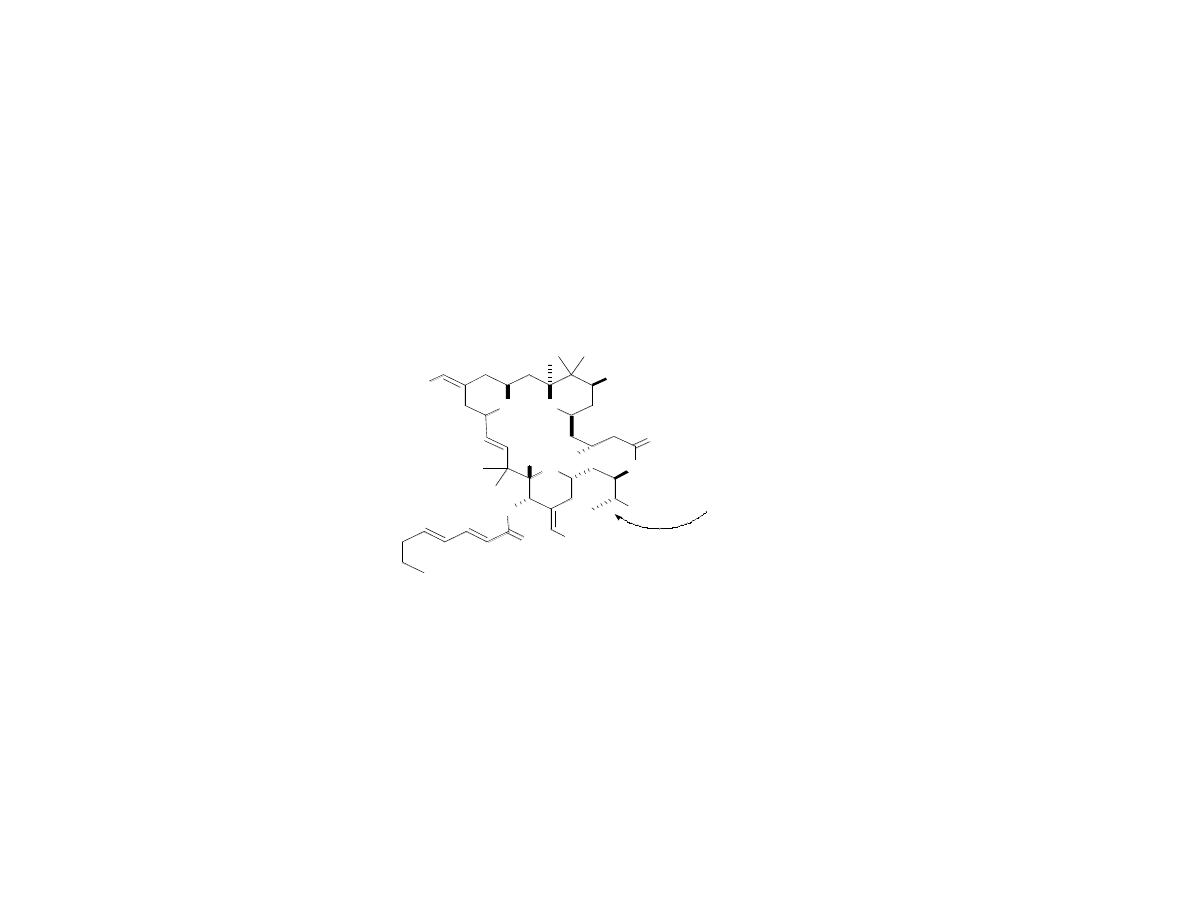
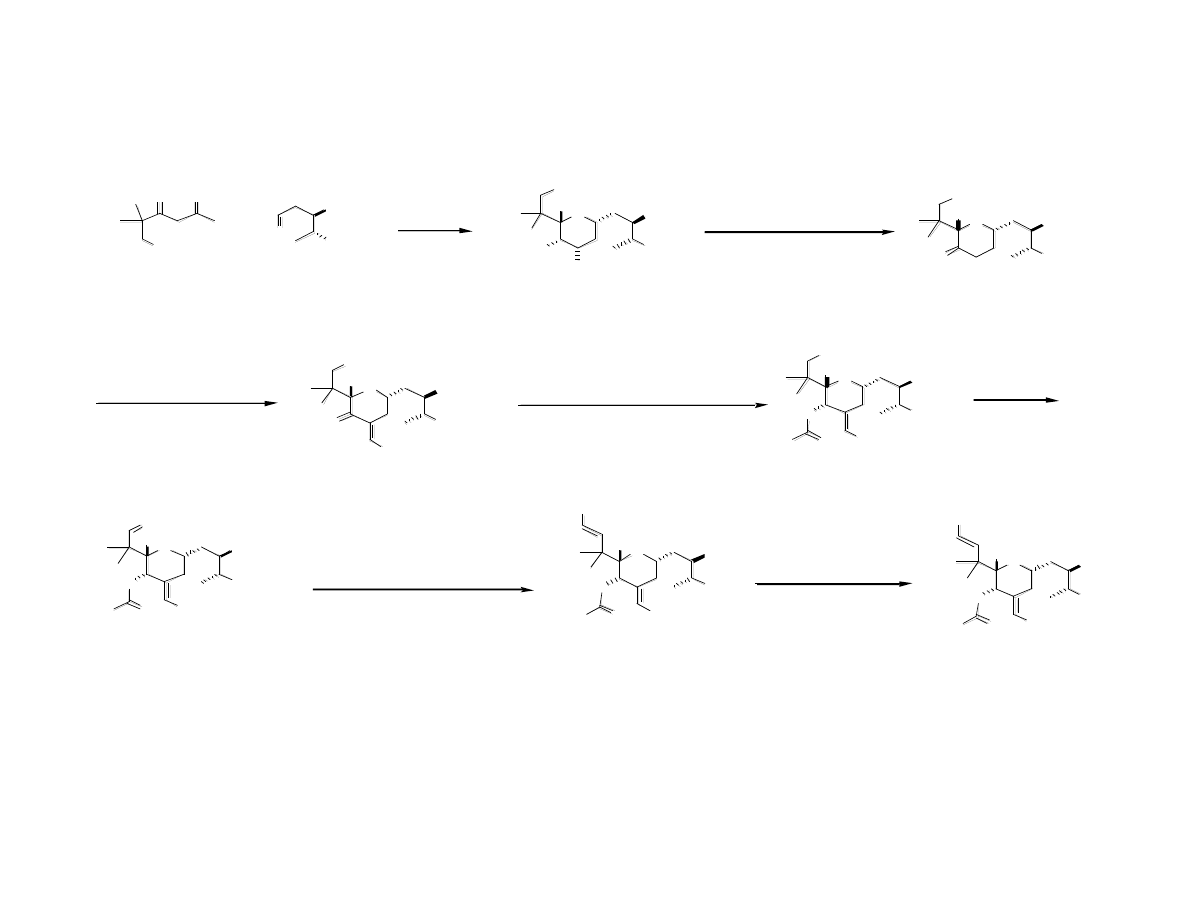
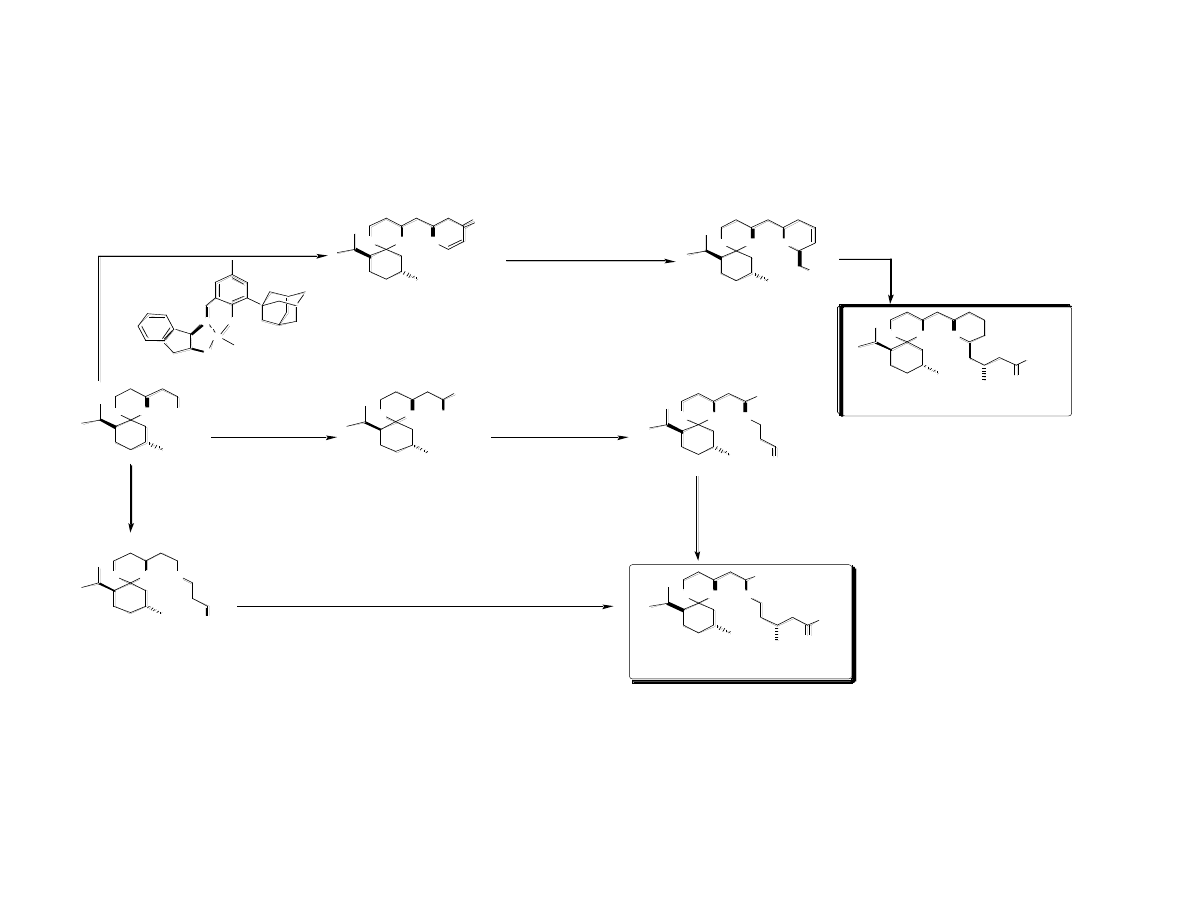
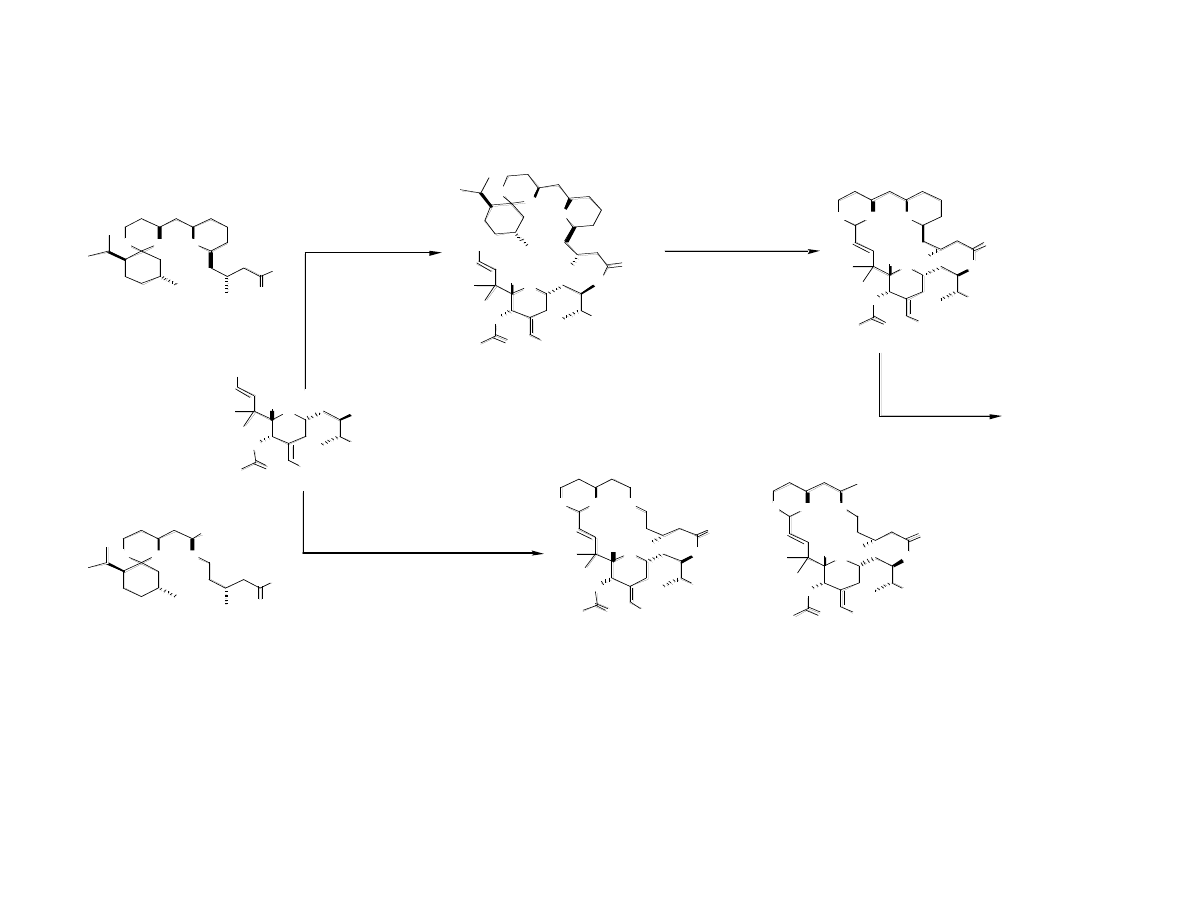
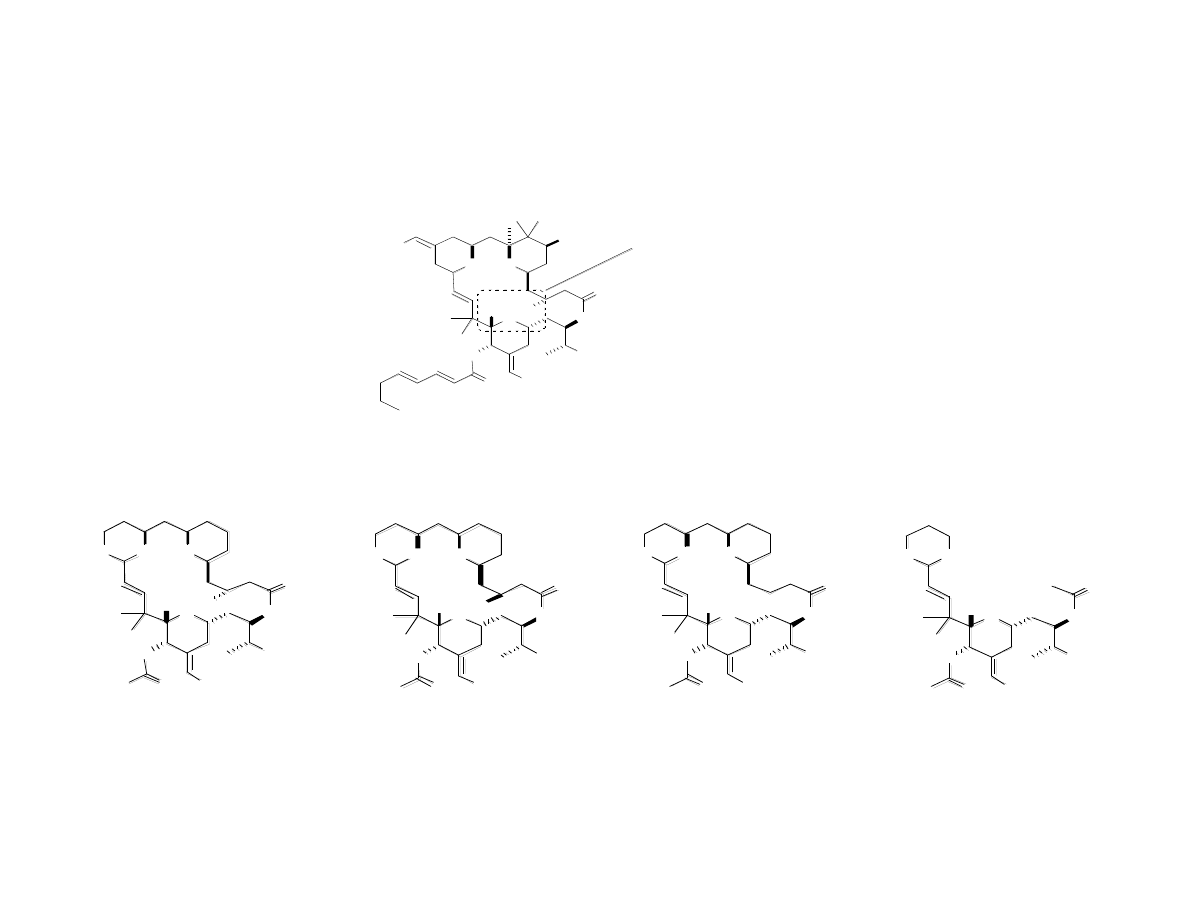
Fine-Tuning the Structure: Second
Generation Sythesis of C Ring
O
O
HO
O
O
O
OH
O
OH
CO
2
Me
O
MeO
2
C
HO
OAc
Are the methyl group
and the C26 stereocenter
needed for activity?
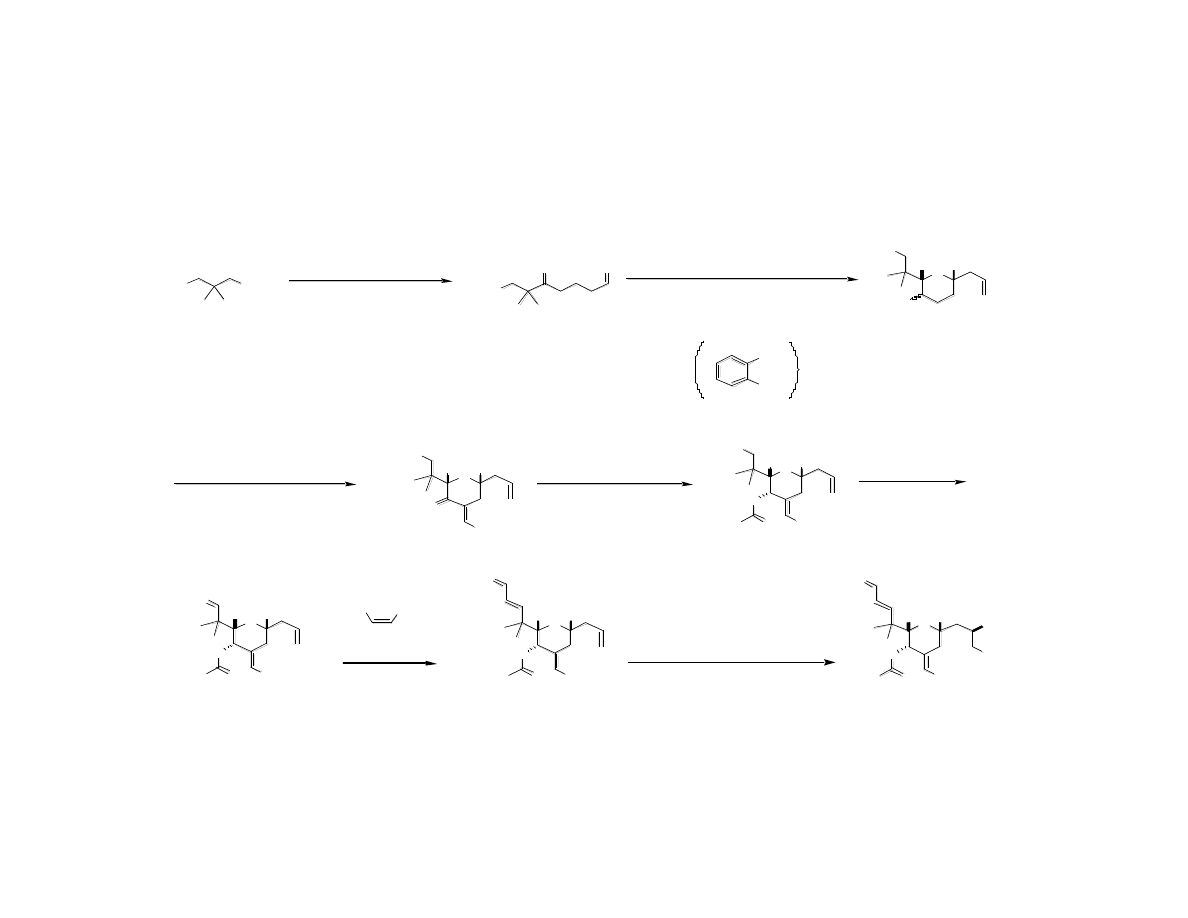
Fine-Tuning the Structure: Second
Generation Sythesis of C Ring
H O
OH
1 ) N aH , TBS C l
2 ) SO
3
- py r., T EA, D M SO
3 ) M gC l CH
2
C H
2
C H
2
OMg C l
4 ) Sw e rn
5 4 %
3 k g = $ 34 .8 0
TB SO
O
O
1 ) R -BIN OL , 4 A . M S, a ll ylS n Bu
3
T i(Oi Pr )
4
, B (OM e)
3
7 7%
2 ) ca t pTS A, 4 A . MS , P h Me 8 5 %
3 ) MM PP , Na H C O
3
M e OH /CH
2
C l
2
78 % , dr =4 :1
O
H O
OM e
T BSO
H
C O
3
-
C O
2
H
2
M g
2 +
M MP P
1 ) TP AP, N MO 7 8%
2 ) K
2
C O
3
, C HOC O
2
M e
Me OH 7 2%
O
O
OMe
TB SO
H
CO
2
M e
O
O
OM e
TBSO
H
C O
2
Me
1) L u ch e r ed u ctio n
2) C
7
H
1 5
C O
2
H , DIC , D MA P
93 %
O
C
7
H
1 5
1 ) 3 H F-TE A
2 ) D M P, N aH C O
3
8 7 %
O
O
OM e
O
H
CO
2
M e
O
C
7
H
1 5
t- Bu L i, Me
2
Zn ;
th e n 1 M HC l
90 %
O
O
OMe H
C O
2
M e
O
C
7
H
1 5
1 ) (D H QD )
2
PYR , K
2
Os O
2
(OH )
4
,
K
3
Fe( C N)
6
, K
2
C O
3
dr=2 .5 :1
2 ) p TSA
3 ) TBS C l, im id .
4 6%
Br
OE t
O
O
O
OH
H
C O
2
Me
O
C
7
H
1 5
O
OTBS
OH
Wender, P. A.; Baryza, J. L.; Bennett, C. E.; Bi, F. C.;. Brenner, S. E.; Clarke, M. O.; Horan, J. C.; Kan, C.; Lacôte, E.;
Lippa, B.; Nell, P. G.; Turner, T. M. J. Am. Chem. Soc. 2002, 124, 13648.
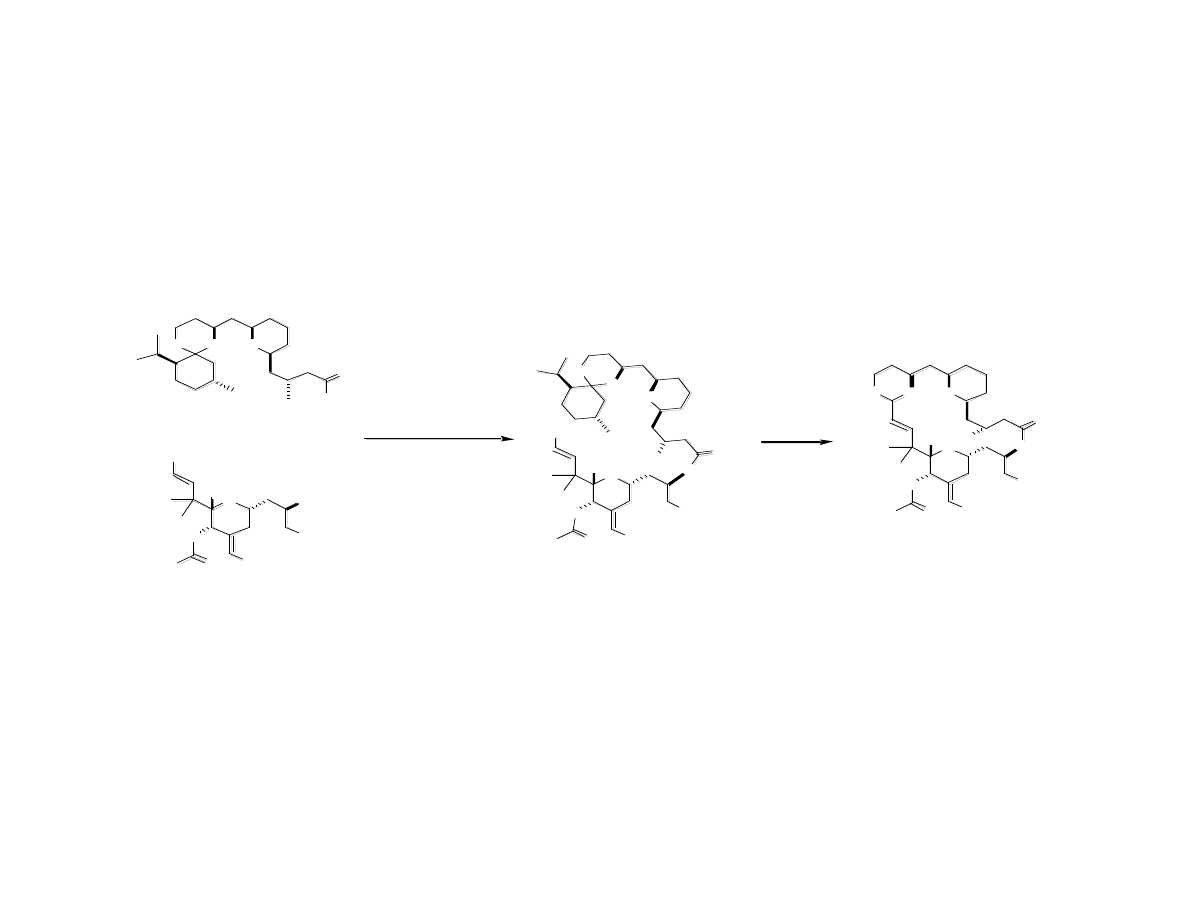
Completion of Bryolog G
O
O
O
O H
O
TBS O
O
OTB S
O
O H
OH
C O
2
M e
C
7
H
15
O
C H O
Y a ma g uc hi 8 7 %
O
O
O
TB SO
O
O
O
OTBS
O
OH
C O
2
Me
C
7
H
1 5
O
C HO
O
O
O
H O
O
O
O
OH
O
OH
C O
2
Me
C
7
H
15
O
H F-p yr
82 %
Bry ol o g G
K
i
= 0 .2 5 nM
Wender, P. A.; Baryza, J. L.; Bennett, C. E.; Bi, F. C.;. Brenner, S. E.; Clarke, M. O.; Horan, J. C.; Kan, C.; Lacôte, E.;
Lippa, B.; Nell, P. G.; Turner, T. M. J. Am. Chem. Soc. 2002, 124, 13648.
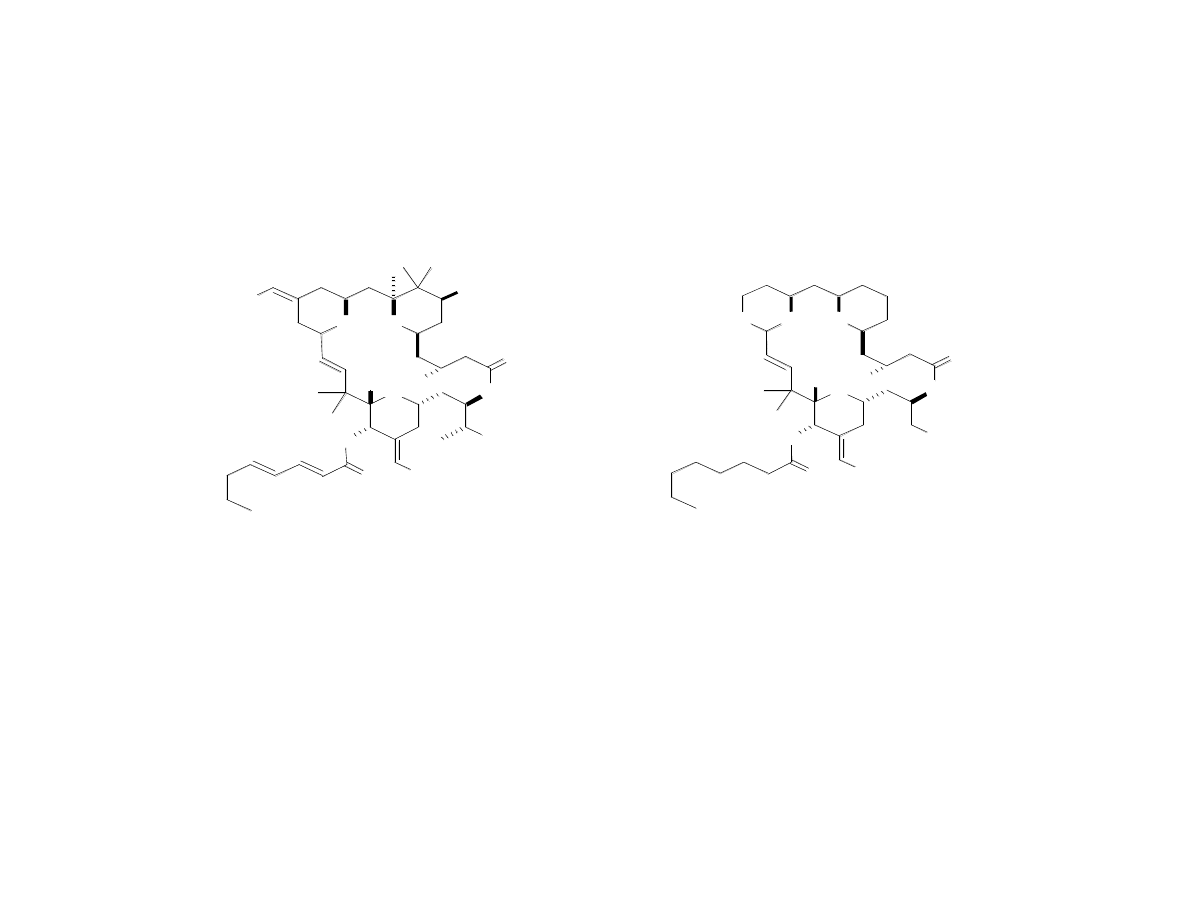
Comparison of Bryolog G to
Bryostatin 1
O
O
O
HO
O
O
O
OH
O
OH
CO
2
Me
O
Bryolog G
K
i
= 0.25 nM
O
O
HO
O
O
O
OH
O
OH
CO
2
Me
O
MeO
2
C
HO
OAc
Bryostatin 1
K
i
= 1.35 nM
•Over 60 steps in previous syntheses
of bryostatins
•In phase I and II clinical trials
•Cost (per previous synthesis): $2.3 million / g
•32 steps (longest linear = 20)
•As effective or more effective than byrostatin 1
as an anti-cancer agent in most cases
•Cost: $1400 / g
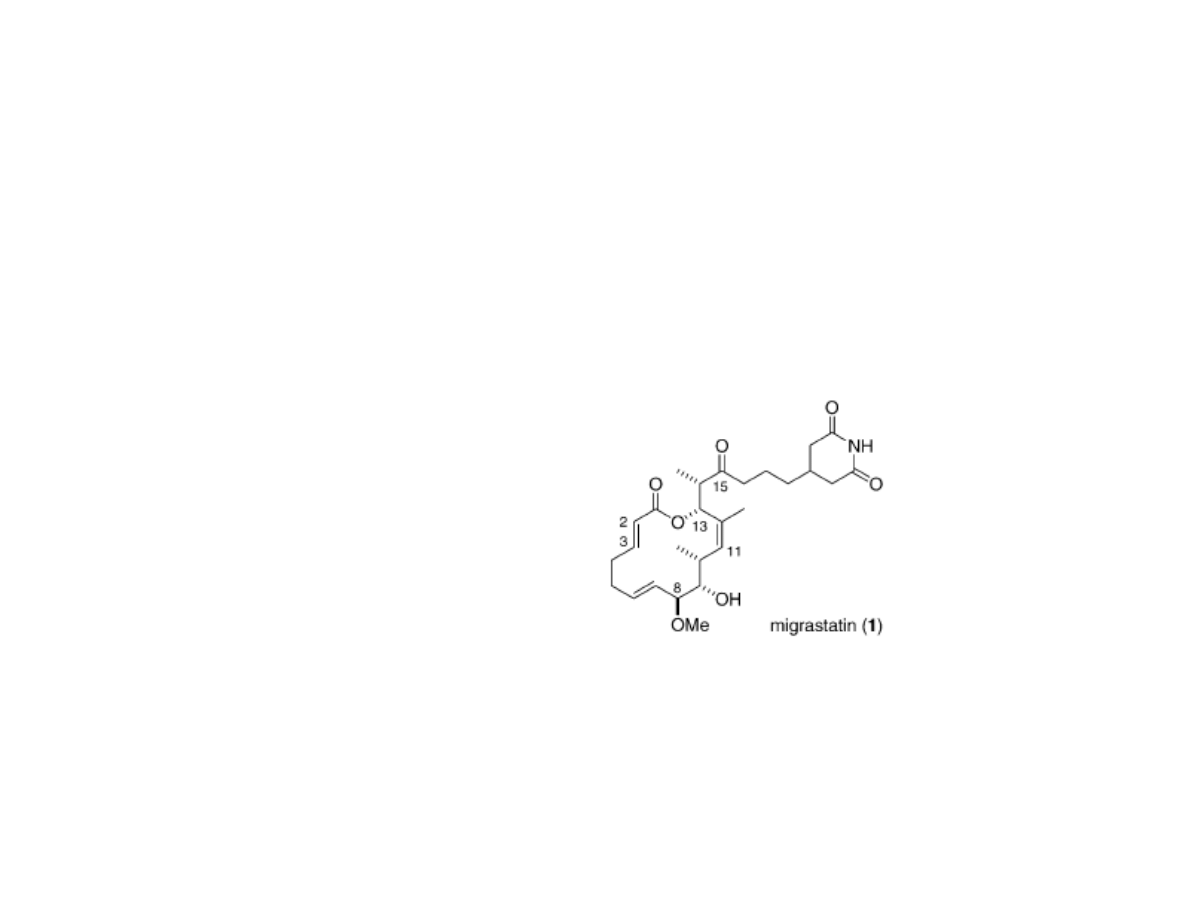
Migrastatin and Cell Migration
•Anti-cancer agents mode of action is typically cell death
•An alternative cancer therapy could rely on inhibition of cell migration
•Cell migration is observed in a number of normal physiological processes
(ovulation, wound healing, inflammation, embryonic development)
•Cell migration also observed in tumor angiogenesis, cancer
cell invasion, and metastasis
•Migrastatin was isolated by Imoto and Kosan bioscience researchers in 2000
from Streptomyces bacteria
•Migrastatin has an IC
50
of 29 µM in wound healing assays
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang, X.-Y.;
Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
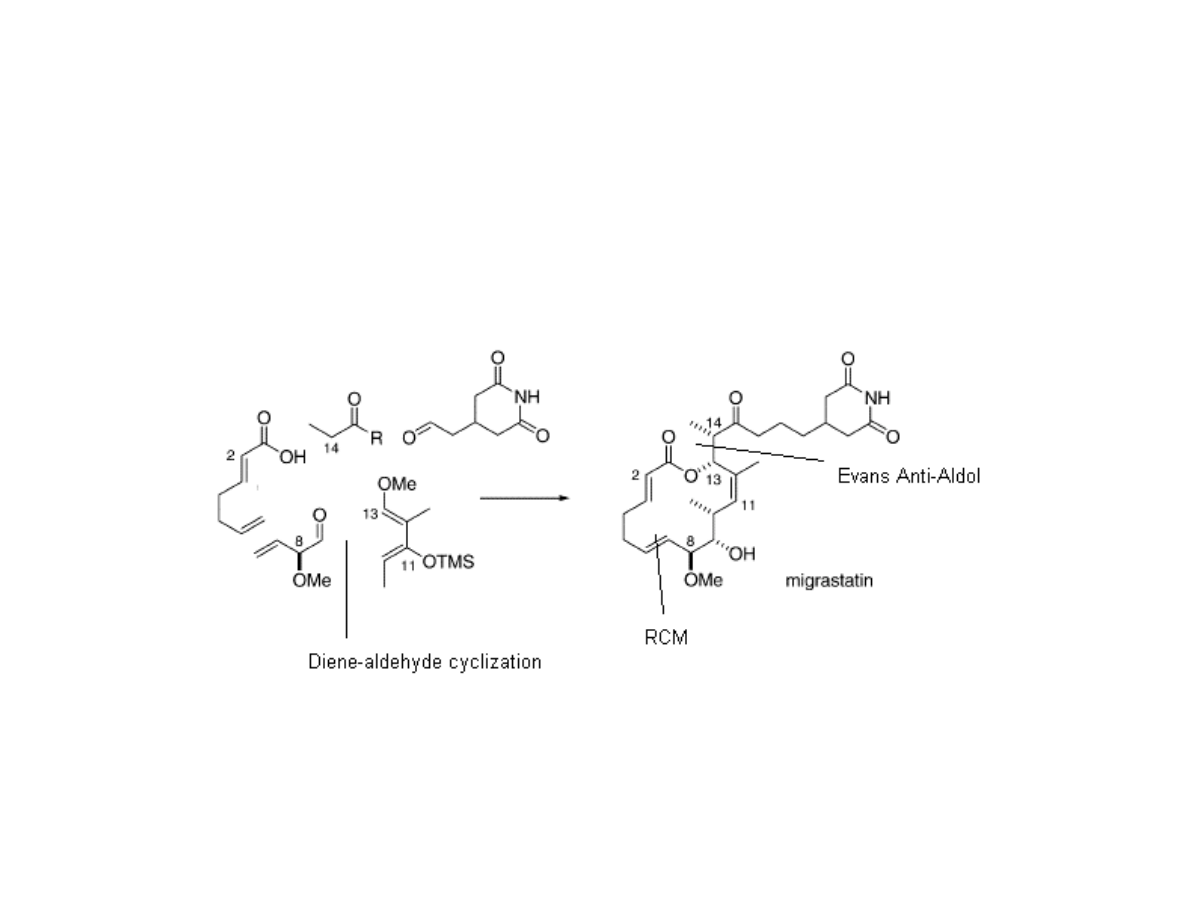
Retrosynthetic Analysis of
Migrastatin
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
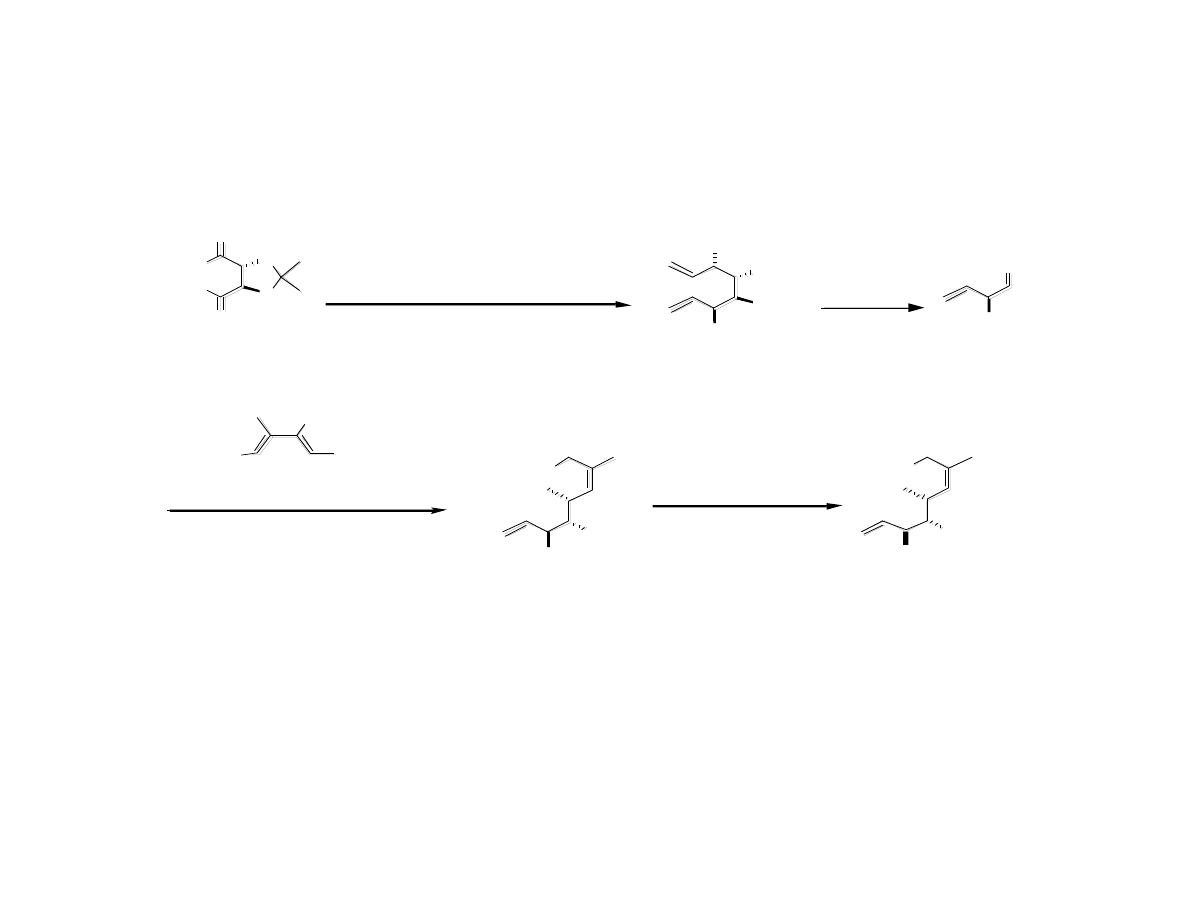
M eO
M eO
O
O
O
O
1 ) D IBAL -H , th en
ZnC l
2
, H
2
C CH M gB r 7 5 % , dr >9 :1
2 ) M eI, N aH ; the n
2M H Cl , Me OH 8 0 %
OMe
OMe
OH
OH
Pb (OA c)
4
Na
2
C O
3
OMe
O
1) T iC l
4
, -7 8 d e g. C ;
the n T FA, rt 8 7% (3 ste ps )
2) L i BH
4
3) C S A, H
2
O
4) L i BH
4
5 3% (3 ste p s)
Me O
OTM S
H O
OMe
OH
TB SOTf, 2 ,6-l u t.;
th en H OAc , H
2
O, TH F
8 0 %
H O
OM e
OTBS
Synthesis of C7 to C13 Fragment
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang, X.-Y.; Moore, M. A. S.;
Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
Jorgensen, M.; Iversen, E. H.; Paulsen, A. L.; Madsen, R. J. Org. Chem. 2001, 66, 4630.
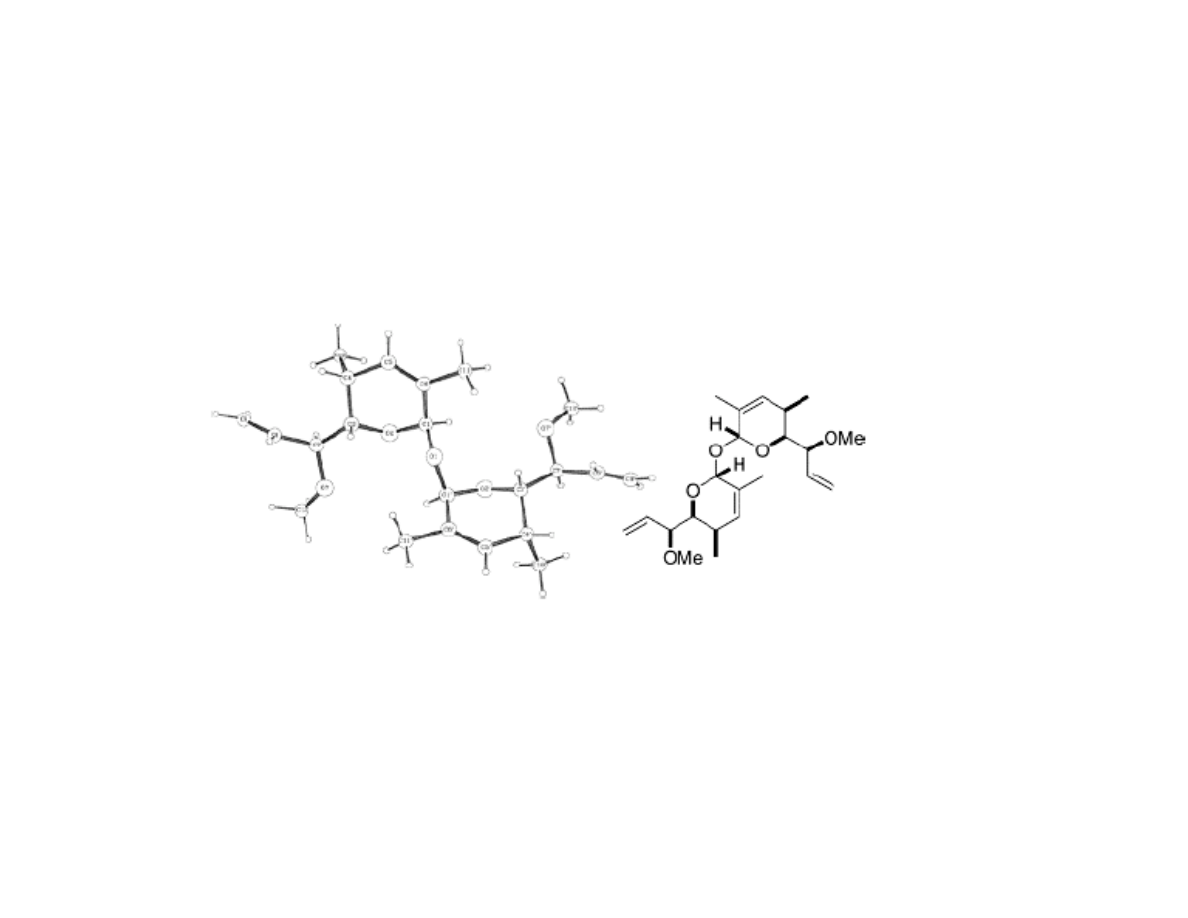
Serendipitous Biproduct
•15% yield of this dimer biproduct obtained during Ferrier rearrangement
when run at 0.3 M (scale-up conditions), though not significantly
observed at 0.1 M (small scale conditions)
•On the bright side, biproduct is crystalline
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
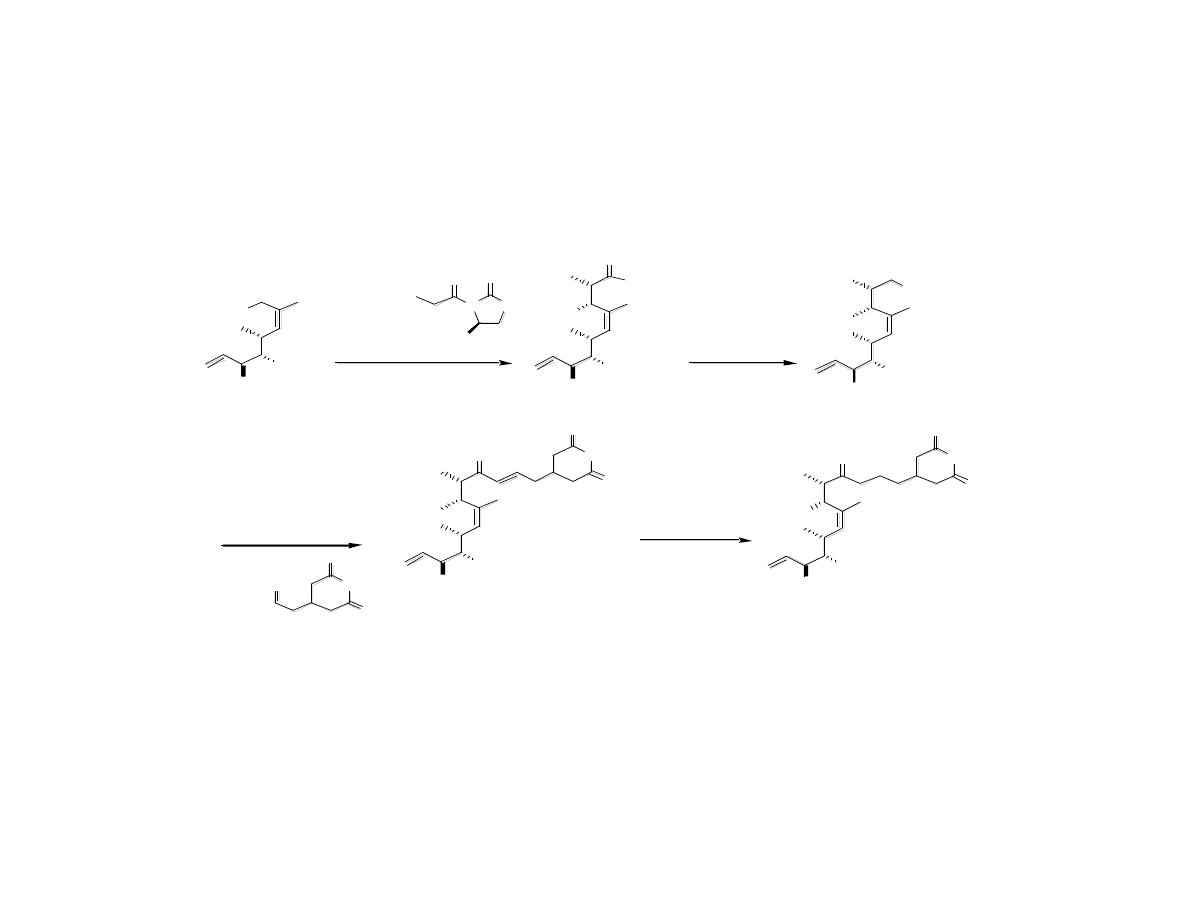
Attaching the Glutarimide Group
H O
OM e
O TBS
1) D M P
2) M g C l
2
TE A, TMS C l;
th en TFA , M e OH
67 %
N
O
O
Bn
O
H O
OM e
O TBS
X c
O
1) T ESC l , i mi d .
2) L i BH
4
83 %
OM e
OTB S
OH
TE SO
OM e
O TBS
TES O
1 ) D MP ; th e n
Li C H
2
P (O)(O Me )
2
;
th e n D M P
2 ) L iC l, D BU , Me C N
5 7 %
NH
O
O
O
O
NH
O
O
Stry ke r Rg t; the n
HO Ac, H
2
O, THF
82 %
H O
OM e
O TBS
O
N H
O
O
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
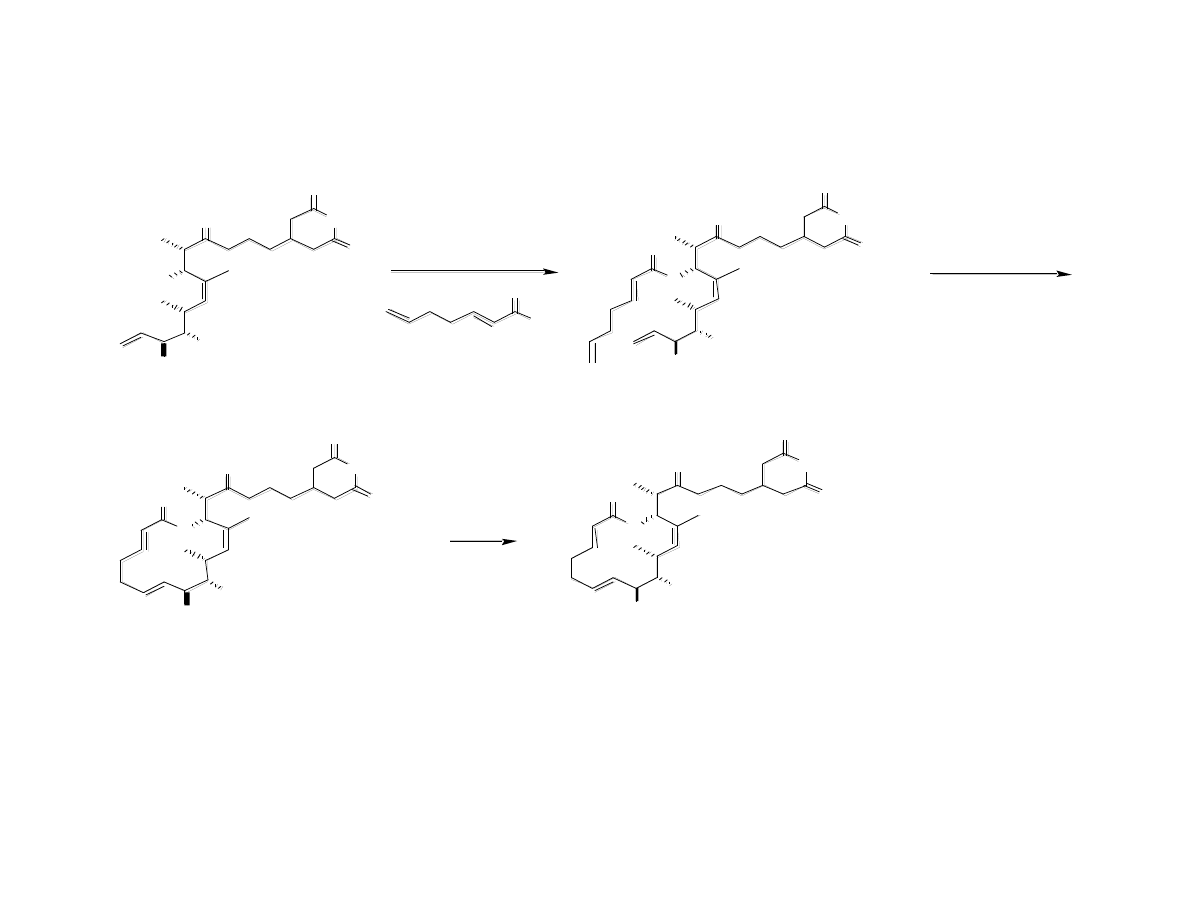
Completion of Migrastatin
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
H O
OM e
OTBS
O
NH
O
O
Ya m ag u ch i (u si ng p yr.
in ste a d o f DM AP )
67 %
OH
O
O
O
N H
O
O
O
OTB S
OMe
G2 (2 0% ), 0 .5 m M
Ph Me , re flu x
69 %
O
O
N H
O
O
O
OT BS
OM e
H F-p y r.
8 5 %
O
O
N H
O
O
O
OH
OM e
m igra s ta tin
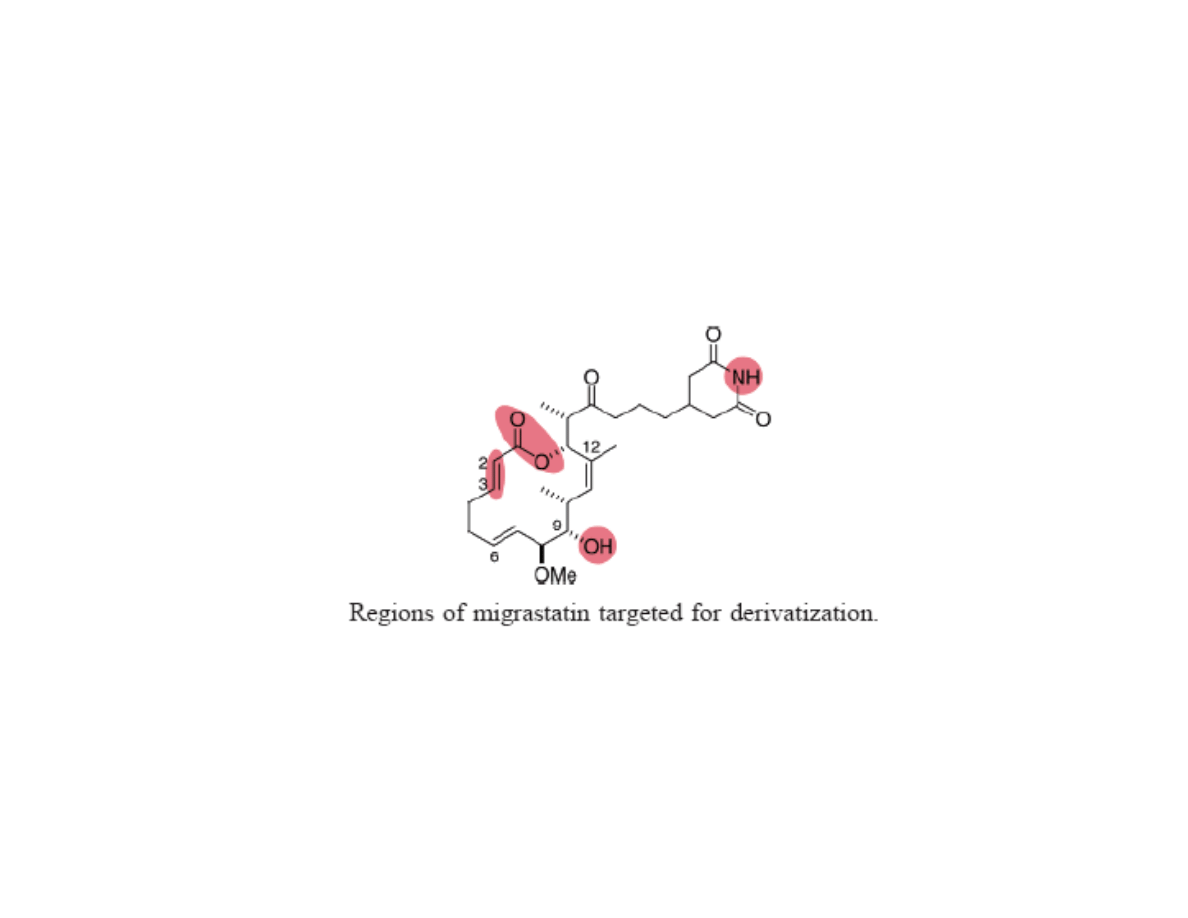
Derivitization of Migrastatin through
Diverted Total Synthesis
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
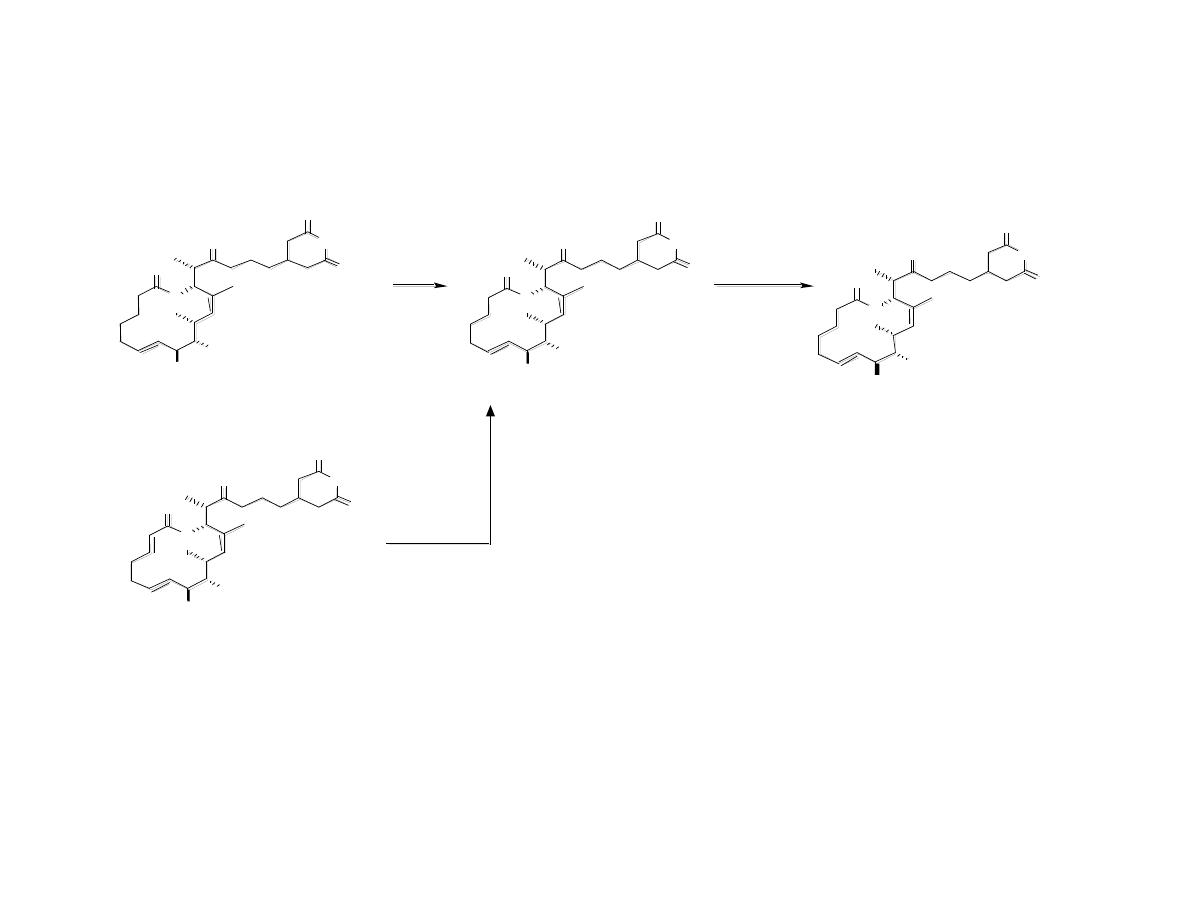
Synthesis/Evaluation (Cell Migration
Assay) of Migralogs A and B
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
O
O
NH
O
O
O
OTB S
O Me
H F-p yr.
8 1%
M eI, C s
2
C O
3
a ce to ne
85 %
O
O
NH
O
O
O
OH
O Me
O
O
N Me
O
O
O
O H
OM e
O
O
NH
O
O
O
OH
O Me
mi gr as ta ti n
IC
5 0
= 2 9 µM
Sta bl e i n m o us e
b l oo d pl as ma
X
S tr yke r R g t
M ig ra lo g A
IC
5 0
= 1 0 µM
Sta b le in mo u se
b lo od p la sm a
M i gra l og B
IC
5 0
= 7 µM
S tab l e i n m ou se
b l oo d p l as ma
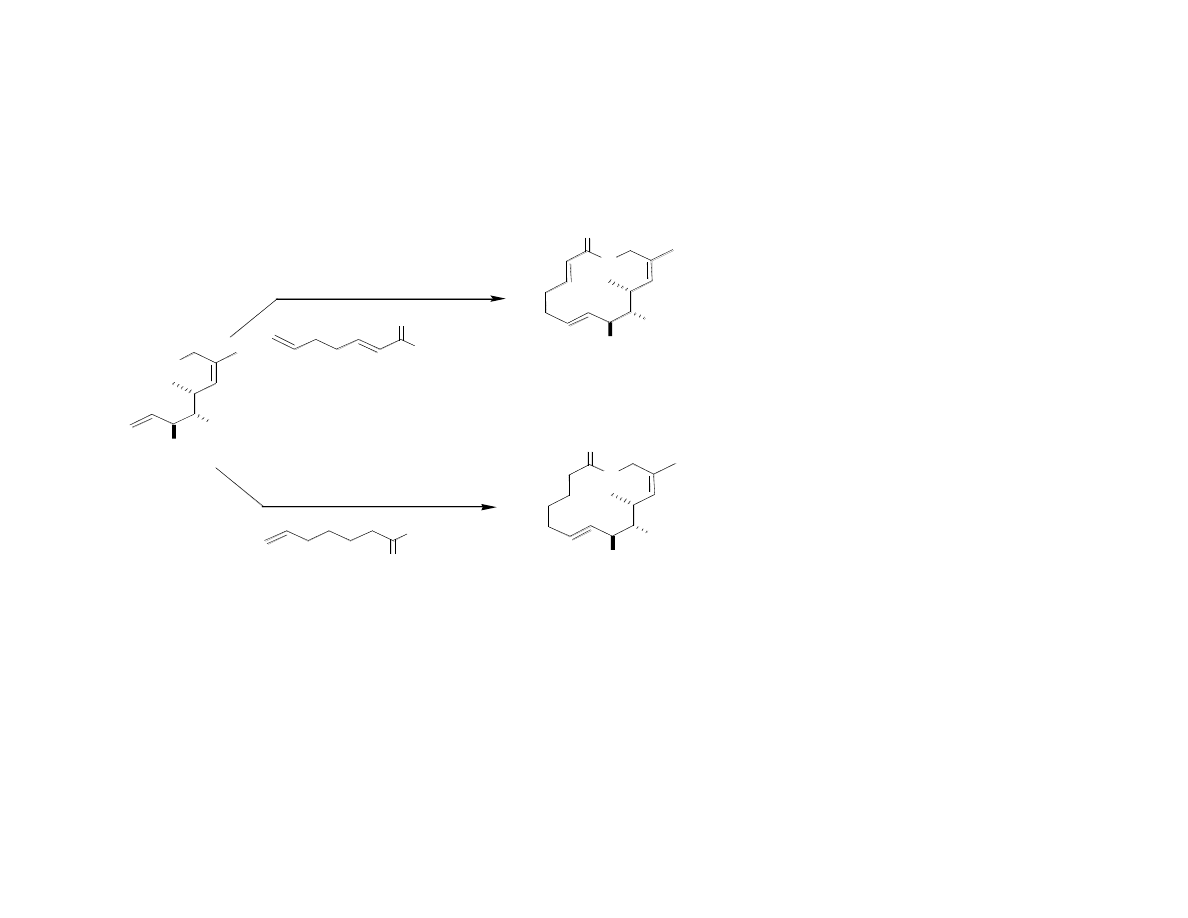
Simplified Migralogs C and D
Have Improved Activity…
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
HO
Me
OTBS
Cl
O
1) DMAP
82%
2) G2 (20%), 0.5 mM
PhMe, reflux 76%
3) HF-pyr. 94%
1) Yamaguchi (using pyr.
instead of DMAP)
48%
2) G2 (20%), 0.5 mM
PhMe, reflux 55%
3) HF-pyr. 66%
OH
O
O
O
OH
OMe
Migralog C
IC
50
= 0.022 µM
Migralog D
IC
50
= 0.024 µM
O
O
OH
OMe
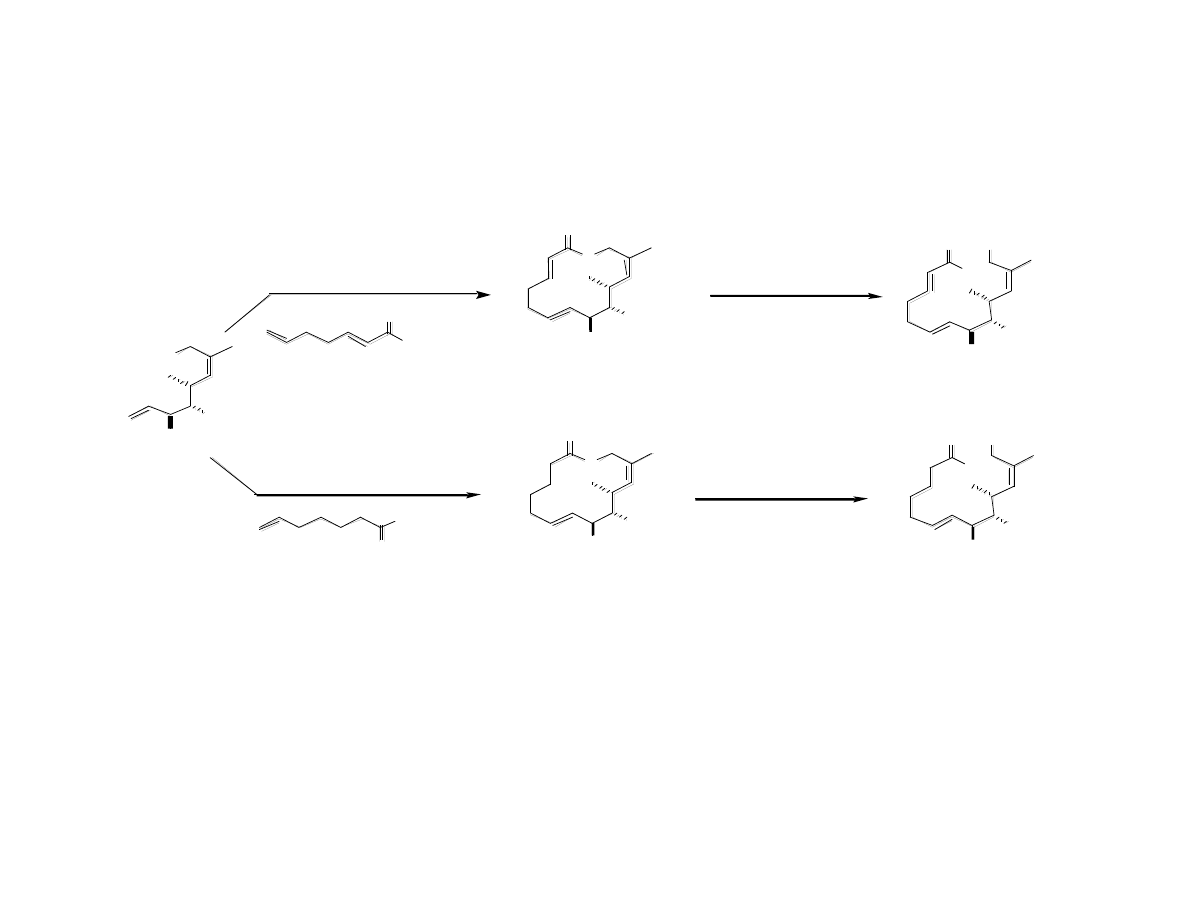
…But Are Quickly Hydrolyzed In Vivo
H O
Me
OTBS
C l
O
1 ) D MA P
8 2%
2 ) G2 (2 0% ), 0 .5 m M
Ph Me , re fl u x 7 6%
3 ) H F-p yr. 9 4 %
1 ) Ya m ag u ch i (u si ng p yr.
i n stea d of D M AP)
4 8 %
2 ) G2 (2 0% ), 0 .5 m M
Ph Me , re flu x 5 5 %
3 ) H F-p yr. 6 6 %
OH
O
O
O
OH
O Me
Mi gr al og C
IC
5 0
= 0 .0 22 µM
Mi gr al og D
IC
5 0
= 0 .0 24 µM
O
O
OH
OM e
20 mi n . i nc ub a tio n
in mo u se b l oo d pl as ma
5 m in . in cu ba tio n
in mo u se b l oo d pl as ma
O H
O
O H
OMe
M ig ra lo g E
IC
50
n ot re p or ted
M ig ra lo g F
IC
50
= 0.3 7 8 µM
O H
O
OH
OM e
OH
OH
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
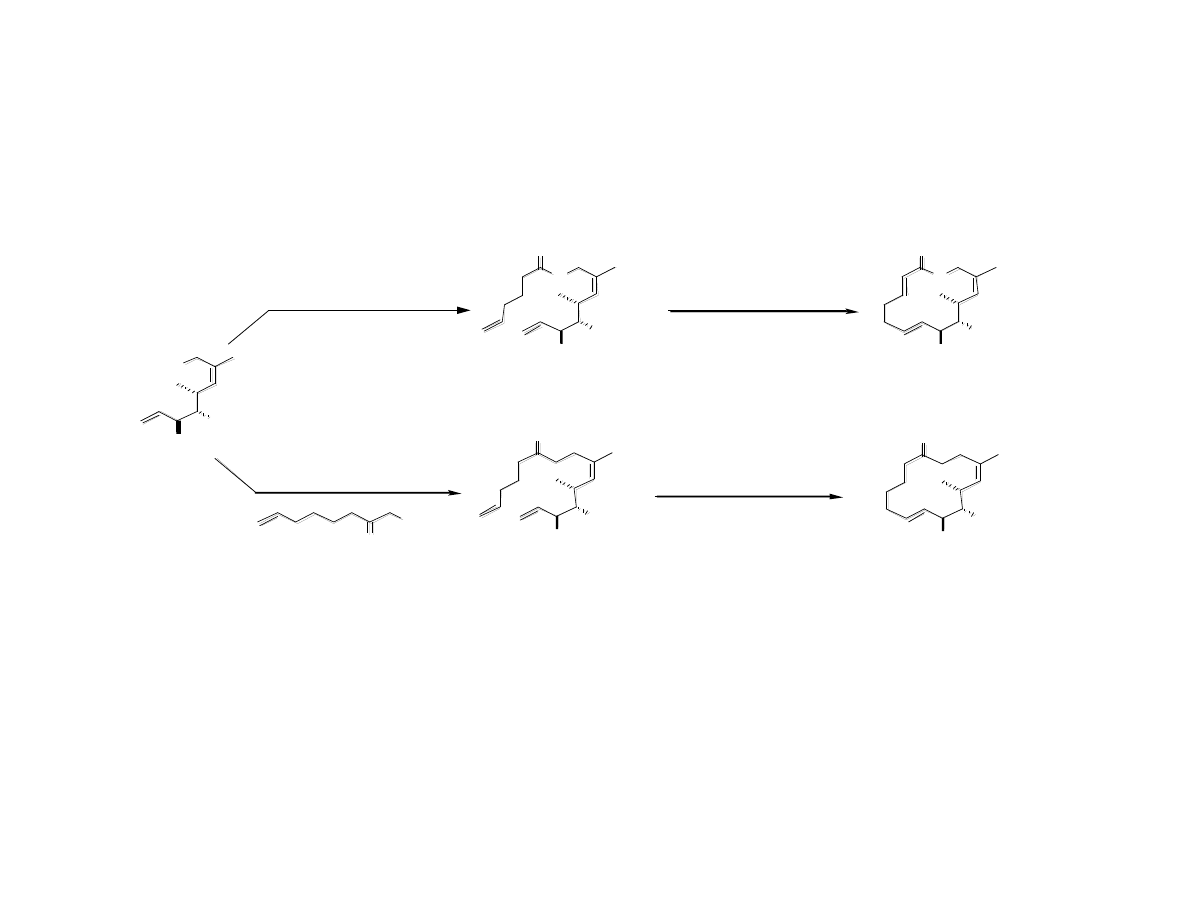
Stabilizing the Cyclic Core
H O
Me
O TBS
O
1 ) C Br
4
, P h
3
P ( so lid su p p.)
2 ) D BU
the n N a/H g ,
N a
2
H PO
4
, M eO H 6 1 %
N
O
OTBS
OM e
Mi g ra lo g G
IC
50
= 0.2 5 5 µM
Sta b le in mo u se
bl o od p la sm a
O
OT BS
OMe
1 ) (Ph O)
2
P (O)N
3
D B U, P hM e 8 7%
2 ) Ph
3
P, H
2
O ; th e n E DC ,
DIE A, 6 -h ep te no ic ac id 9 2 %
1 ) G2 (2 0 % ), 0.5 mM
Ph M e, re flu x 8 1 %
2 ) H F-p yr . 9 0 %
SO
2
P h
1 ) G2 (2 0 %) , 0 .5 mM
Ph M e, re flu x 6 0 %
2 ) H F-p yr . 81 %
N
O
OH
OMe
O
OH
OMe
M ig ra lo g H
IC
5 0
= 0 .1 00 µM
S tab l e i n m ou se
b lo o d p l as ma
Gaul, C.; Njardarson, J. T.; Shan, D.; Dorn, D. C.; Wu, K.-D.; Tong, W. P.; Huang,
X.-Y.; Moore, M. A. S.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 11326.
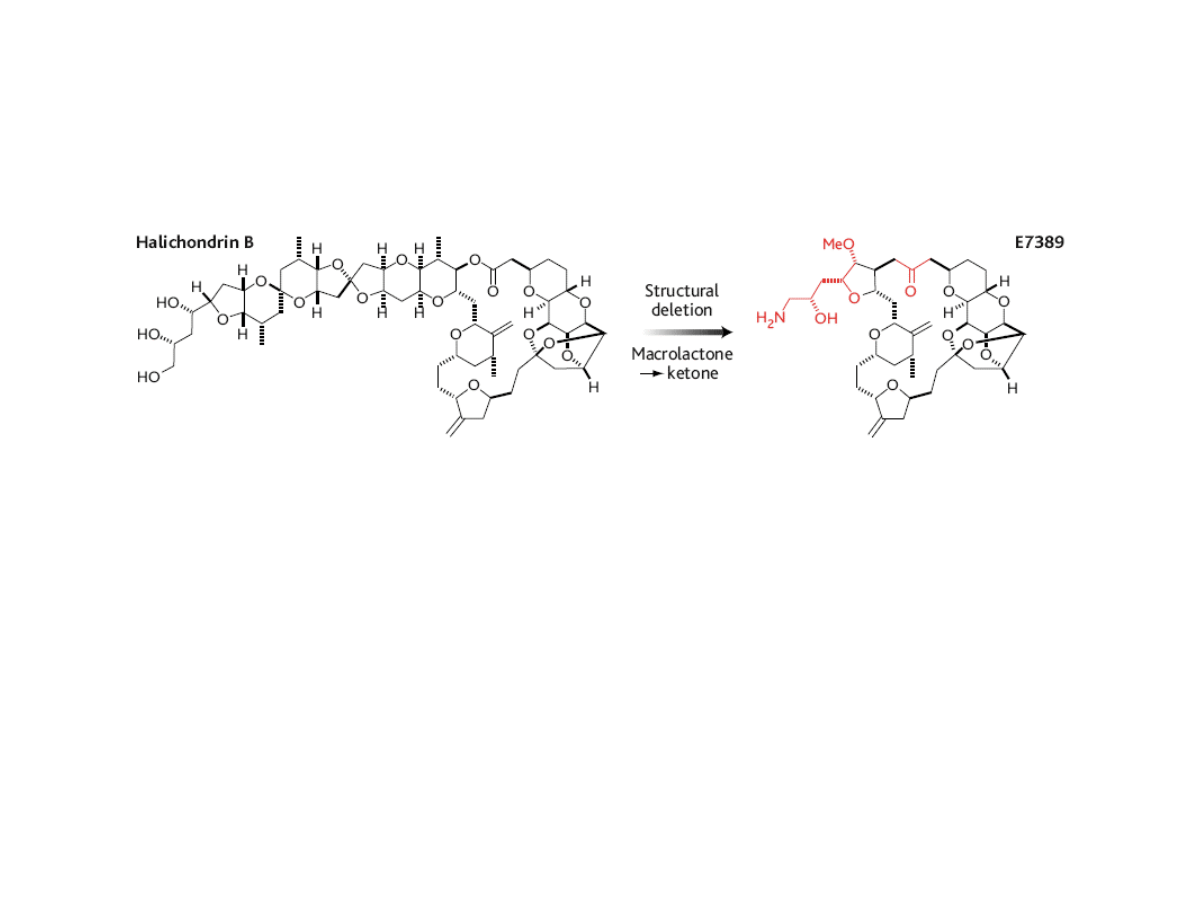
Additional Reading:
Halichondrin B and E7389
Aicher, T. D.; Buszek, K. R.; Fang, F. G.; Forsyth, C. J.; Jung, S. H.; Kishi, Y.; Matelich, M. C.;
Scola, P. M.; Spero, D. M.; Yoon, S. K. J. Am. Chem. Soc. 1992, 114, 3163.
Zheng, W. J.; Seletsky, B. M.; Palme, M. H.; Lydon, P .J.; Singer, L .A.; Chase, C. E.; Lemelin, C.
A.; Shen, Y. C.; Davis, H.; Tremblay, L.; Towle, M. J.; Salvato, K. A.; Wels, B. F.; Aalfs, K. K.;
Kishi ,Y.; Littlefield, B. A.; Yu, M. J. Bioorg. Med. Chem. Lett. 2004, 14, 5551.
Paterson, I.; Anderson, E. Science 2005, 310, 451.
•Halichondrin B is a highly cytotoxic (antimitotic) marine natural product.
Total synthesis: Kishi, 1992
• Recent diverted total synthesis has led to simplified analog E7389 with
similar antimitotic activity. Also, replacement of lactone with ketone
has made E7389 more robust in vivo
•E7389 currently in phase I clinical trials
Wyszukiwarka
Podobne podstrony:
2002 3 MAY Lasers in Medicine and Surgery
In vivo MR spectroscopy in diagnosis and research of
Introduction to multivariate calibration in analytical chemistry
Ethics in Global Internet Research
Our World In Medicine
2002 3 MAY Lasers in Medicine and Surgery
In vivo MR spectroscopy in diagnosis and research of
Titanium in medicine
Total synthesis of batrachotoxinin A
Current Clinical Strategies, History and Physical Exam in Medicine (2005) 10Ed; BM OCR 7 0 2 5
Reedy&Reedy Statistical Analysis in Art Conservation Research
optimization in organic chemistry
Total syntheses of ( ) terpestacin
Open Problems in Computer Virus Research
Instruments used in medicine
the silence in medicine
więcej podobnych podstron