Total Syntheses of (-)-Terpestacin
Jason M. Stevens
05/02/2007
Me
Me
O
OH
HO
HO
Me
Me
Me
H
An Interesting History
1993: Isolation of terpestacin (Oka) reported [
α]
D
= +26
° (CHCl
3
) with the
absolute configuration as shown.
1998: First asymmetric synthesis of shown absolute configuration of
terpestacin by Tatsuda reports [
α]
D
= +27
° (CHCl
3
).
2001: Isolation of the enantiomer of terpestacin (Gräfe) reported [
α]
D
= -
16
° (CDCl
3
).
2002: Asymmetric synthesis of shown configuration of terpestacin by
Myers reported [
α]
D
= -17
° (CHCl
3
).
What’s going on???
Me
Me
O
OH
HO
HO
Me
Me
Me
H
Chloroform is the Culprit
Myers, A. G.; Siu, M.; Ren, F. J. Am. Chem. Soc. 2002, 124, 4230.
Me
Me
O
OH
HO
HO
Me
Me
Me
H
Me
O
HO
HO
Me
Me
Me
H
O
Cl
Me
"Cl
+
"
H
[
α]
D
= +33
o
Solutions of the natural product had been stored in chloroform prior to the
optical rotation measurments.
Note: CDCl
3
stored over K
2
CO
3
test positive for chlorine, although identical lots
of CDCl
3
stored over 4A mol. sieves did not.

Now that we know what we’re making…
Myer’s total synthesis 2002
Jamison’s total synthesis 2003, 2004
Trost’s total synthesis 2007
Myers Total Synthesis of (-)-Terpestacin
Myers, A. G.; Siu, M.; Ren, F. J. Am. Chem. Soc. 2002, 124, 4230.
Me
Me
O
OH
HO
HO
Me
Me
Me
H
Me
Me
OTBS
Me
Me
H
O
Me
OH
epoxide opening
cascade
enolate
alkylations
Me
Me
OTBS
Me
Me
TIPSO
O
I
Me
Me
O
Me
O
Br
O
Me
TIPSO
enolate
alkylation
The First Enolate Alkylation
OH
Ph
Me
N
Me
O
Me
I
2
, THF, H
2
O
96%
O
O
Me
I
O
O
Me
HO
O
CsO
CF
3
then
Et
2
NH
95%
O
O
Me
TIPSO
Me
Me
Me
O
Br
Me
O
Me
Me
O
Me
TIPSCl
imidazole
DMF, 98%
KHMDS
THF, -78
o
C
86%
no dr reported
TIPSO
O
Preparing for the Second Enolate
Alkylation
Me
O
Me
Me
O
Me
O
Me
HO
Me
Me
O
Me
O
TIPSO
OH
Me
MeO
Me
Me
O
Me
O
TIPSO
OH
KOH, EtOH
CH
2
N
2
pH = 5
TIPSO
Me
MeO
Me
Me
O
Me
O
TIPSO
O
NaH
DMF
then
ClCONMe
2
Me
Me
Me
O
Me
O
O
O
N
Me
Me
TIPSO
Dess-Martin
pyridine, CH
2
Cl
2
82%
Me
Me
Me
O
Me
HO
O
O
N
Me
Me
TIPSO
Me
Me
Me
O
Me
O
TIPSO
CH
3
Li
Et
2
O
-78
o
C to rt
DIBAL
THF, -78
o
C
85%
The Second Enolate Alkylation
Me
Me
Me
O
Me
O
TIPSO
Me
Me
OH
Me
Me
TIPSO
O
I
LiI, Sc(OTf)
3
THF, -25
o
C
92%
Me
Me
OTBS
Me
Me
TIPSO
O
I
TBSOTf
2,6-lutidine
THF, -78
o
C
97%
LiN(SiMe
2
Ph)
2
THF, 0
o
C
53%, 4.8:1 dr
Me
Me
OTBS
Me
Me
TIPSO
O
H
Installing the Side Chain
Me
Me
OTBS
Me
Me
H
OH
O
Me
Me
Me
OTBS
Me
Me
TIPSO
O
H
t
-BuO
O
Me
i
-Pr
2
NLi
THF, -78
o
C
94%
Me
Me
OTBS
Me
Me
TIPSO
H
OH
t
-BuO
2
C
Me
Red-Al, THF
-78 to 0
o
C
then HOAc
then
Red-Al, toluene
-78
o
C to rt
75%
Me
Me
OTBS
Me
Me
H
OH
O
HO
Me
OC(CF
3
)
2
Ph
Ph
2
S
OC(CF
3
)
2
Ph
Martin Sufurane
CH
2
Cl
2
, -78
o
C
89%
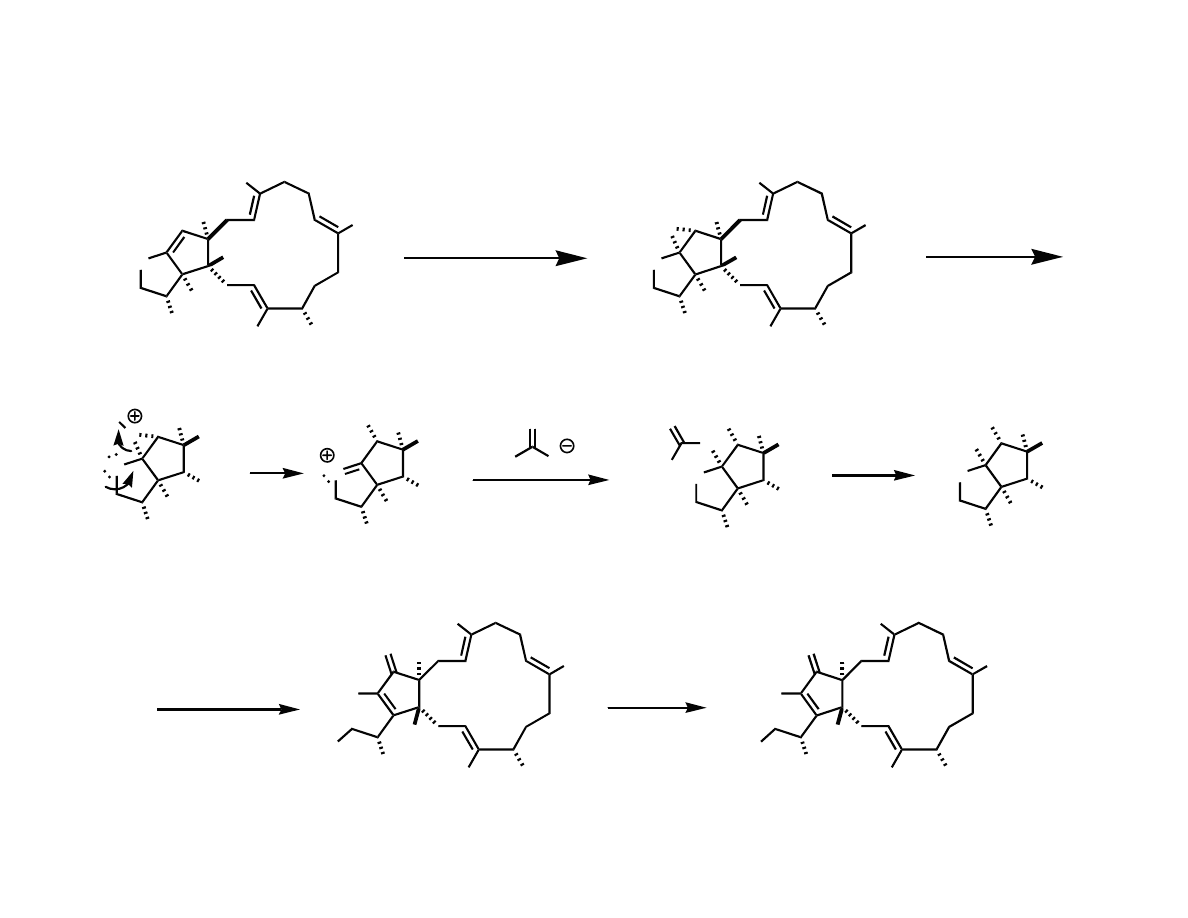
Completing the Total Synthesis
Me
Me
OTBS
Me
Me
H
OH
O
Me
DMDO
Acetone
"no need for the
McDougall procedure"
Me
Me
OTBS
Me
Me
H
OH
O
Me
O
CF
3
CO
2
H
Et
2
O
Me
OH
O
Me
O
H
R
R'
Me
OH
O
Me
HO
R
R'
O
F
3
C
O
Me
OH
O
Me
HO
R
R'
O
F
3
C
O
Et
2
NH
Me
OH
O
Me
HO
R
R'
HO
K
2
CO
3
, MeOH
Me
Me
O
OTBS
HO
HO
Me
Me
Me
H
1N HCl
Me
Me
O
OH
HO
HO
Me
Me
Me
H
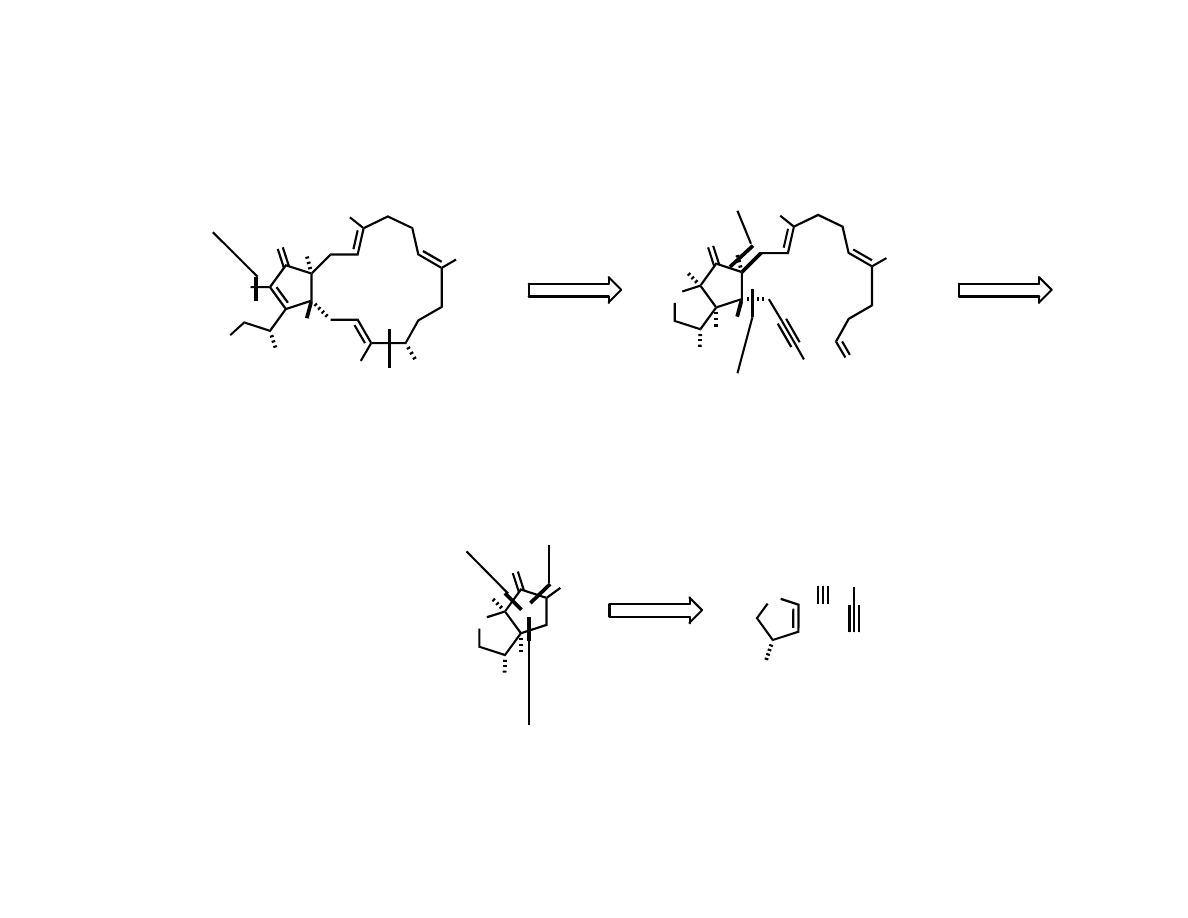
Jamison’s Total Synthesis
Chan, J.; Jamison, T. F. J. Am. Chem. Soc. 2004, 126, 10682.
Me
O
O
Me
H
O
Me
H
H
Me
Me
O
OH
HO
HO
Me
Me
Me
H
Me
O
O
Me
H
H
Me
O
Me
O
R
Pauson-Khand
Enolate
Alkylation
Reductive Aldehyde-
Alkyne Coupling
Pauson-Khand
Pauson-Khand
R
enolate
autoxidation
cuprate
conjugate
addition

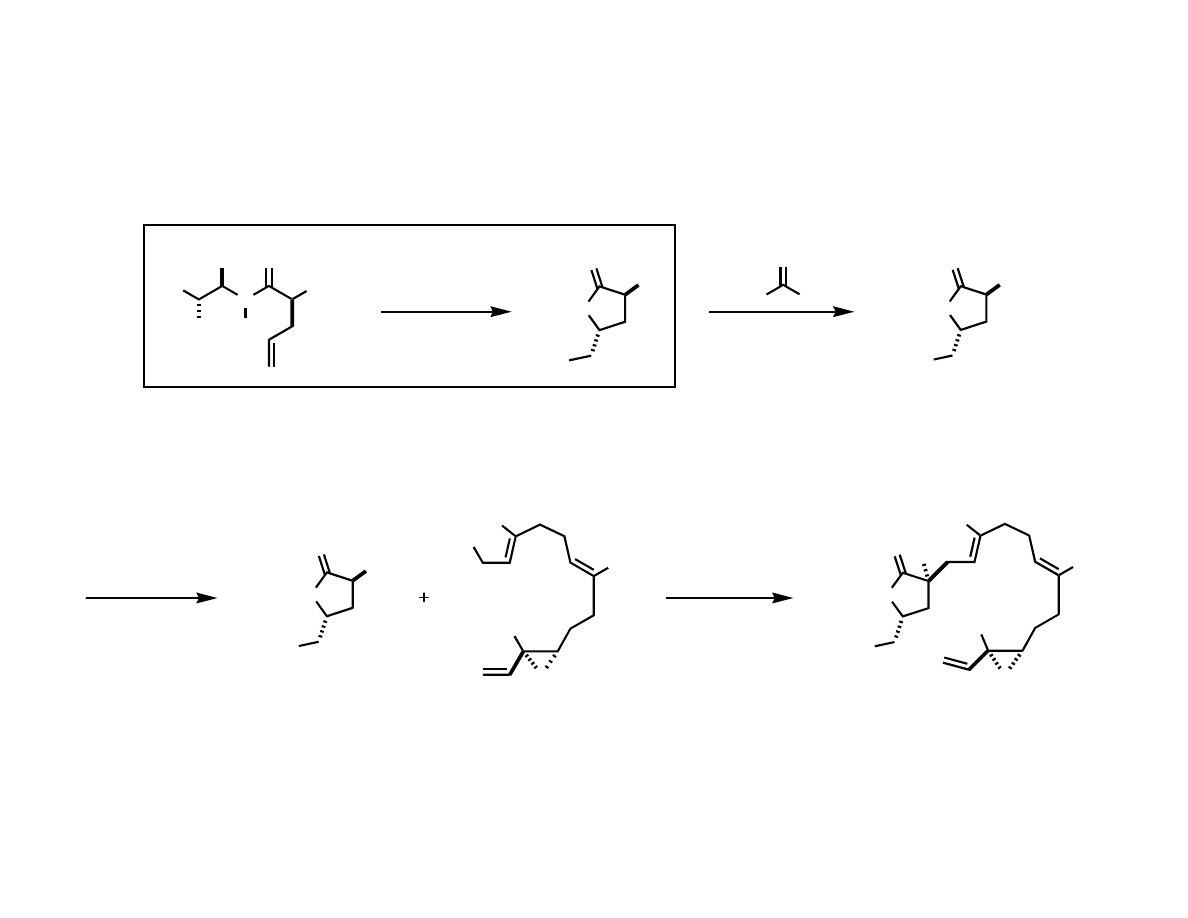
An Efficient preparation of the 5
Membered Ring
HO
Me
Me
Me
Me
n-BuLi, THF; MsCl
OH
Me
O
Me
> 95% ee
several hundred grams
Fs
(
CO
)
3
Co
(
CO
)
3
Co
n-BuSMe
75 - 77
o
C
DCE
40 - 60%
Fs
O
Me
H
H
O
Fs
only detectable isomer
Fs
(CO)
3
Co
(CO)
3
Co
MsO
Fs
Fs
CuBr Me
2
S
HCCMgBr
Co
2
(CO)
8
, Et
2
O
83% 2 steps
RhCl(PPh
3
)
3
H
2
:CO (1:1) 1200 psi
O
Me
OH
p -TSA
200
o
C
O
Me
(+)-(Ipc)
2
BH
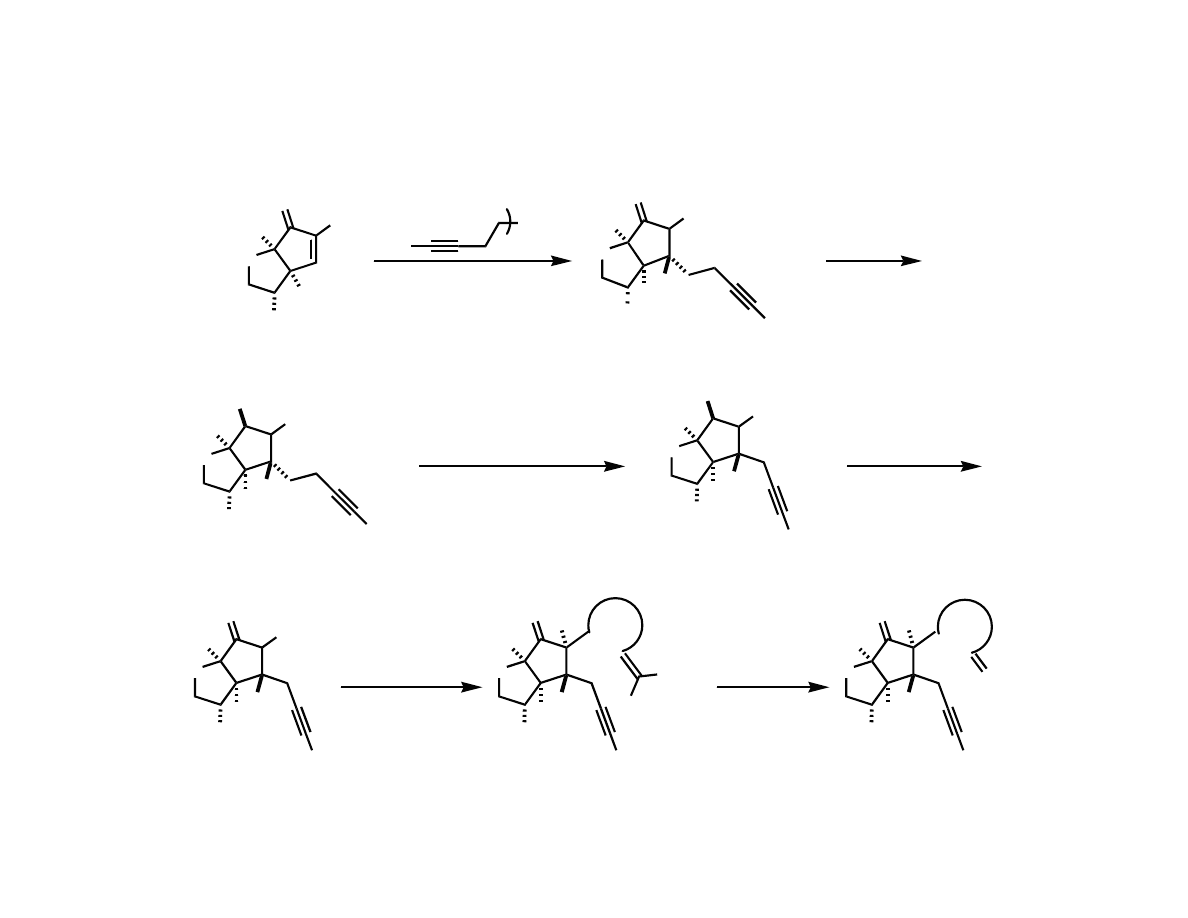
Preparing the Substrate for
Macrocyclization
H
O
Me
H
H
O
TIPS
Fs
1. NaBH
4
MeOH
2. TBAF
THF
O
Me
H
H
O
Fs
TIPS
CuLi
2
THF, Et
2
O
(1:1)
-78
o
C, 72%
> 95:5 dr
H
O
Me
H
H
HO
H
Fs
KOtBu(3 eq)
DMSO (~ 70 eq)
15 min
then 0.5M HCl work up
H
O
Me
H
H
Me
HO
Fs
TPAP, NMO
CH
2
Cl
2
57% (4 steps)
H
O
Me
H
H
Me
O
Fs
NaH, H
2
O
(really NaOH)
MeI
toluene
90%, 93:7 dr
H
O
Me
H
H
Me
O
Me
Me
Me
H
O
Me
H
H
Me
O
Me
O
1. Sharpless
dihydroxylation
2. NaIO
4
MeOH:H
2
O (1:1)
25% (2 steps)
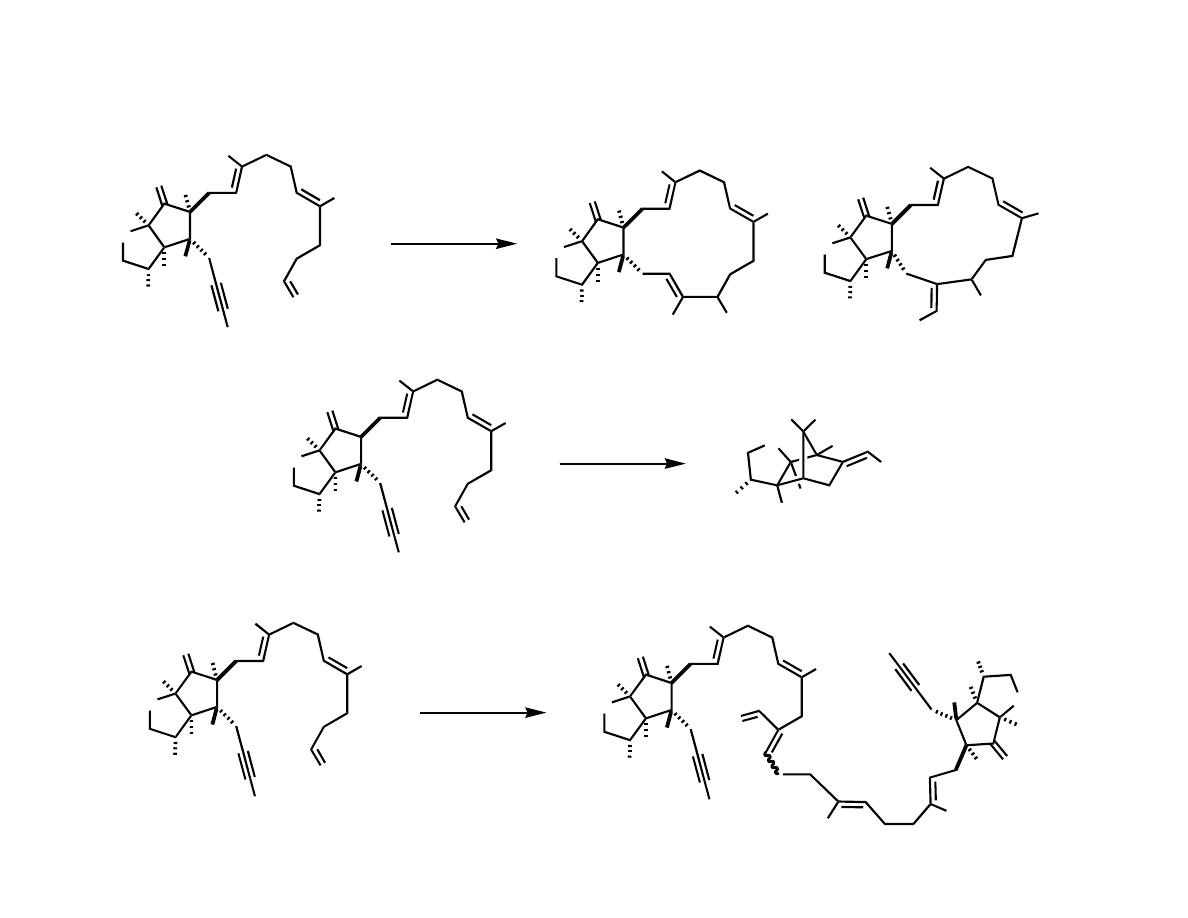
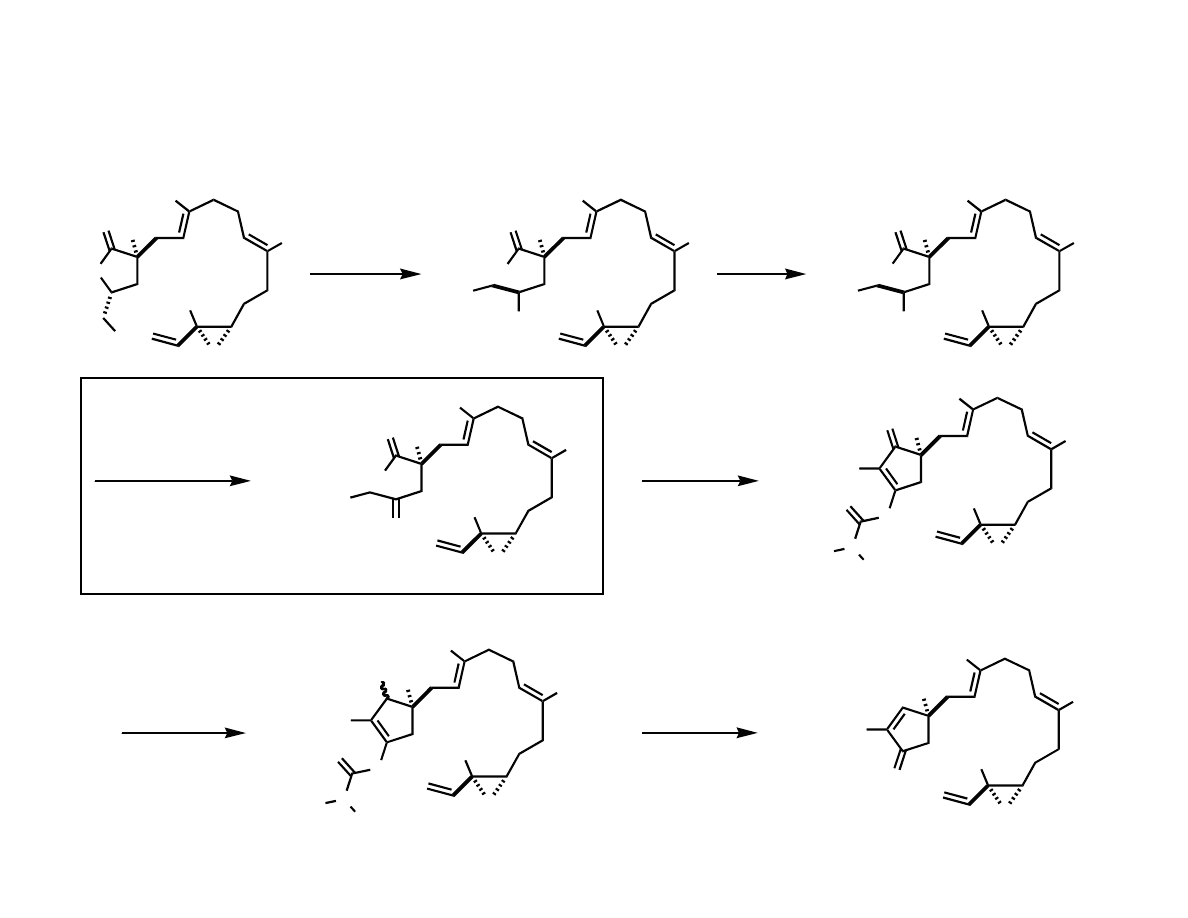
Intramolecular Coupling
Ni(cod)
2
PBu
3
BEt
3
, Toluene
45%
Me
Me
OH
Me
H
O
Me
H
H
Me
O
Me
H
O
Me
H
H
O
Me
Me
O
Me
desired product not observed
Me
Me
OH
Me
H
O
Me
H
H
O
Me
isolated product
10% Ni(cod)
2
20% PBu
3
BEt
3
, Toluene
65 - 75%
Me
O
Me
H
O
Me
H
H
O
Me
try deleting the C-19 methyl
O
H
H
Me
R
H
Me
OH
Me
O
Me
H
O
Me
H
H
O
SiMe
3
try altering the alkyne
Me
Ni(cod)
2
PBu
3
BEt
3
toluene, 110
o
C
Me
Me
H
O
Me
H
H
O
SiMe
3
Me
O
Me
Me
H
O
Me
H
H
O
Me
3
Si
Me
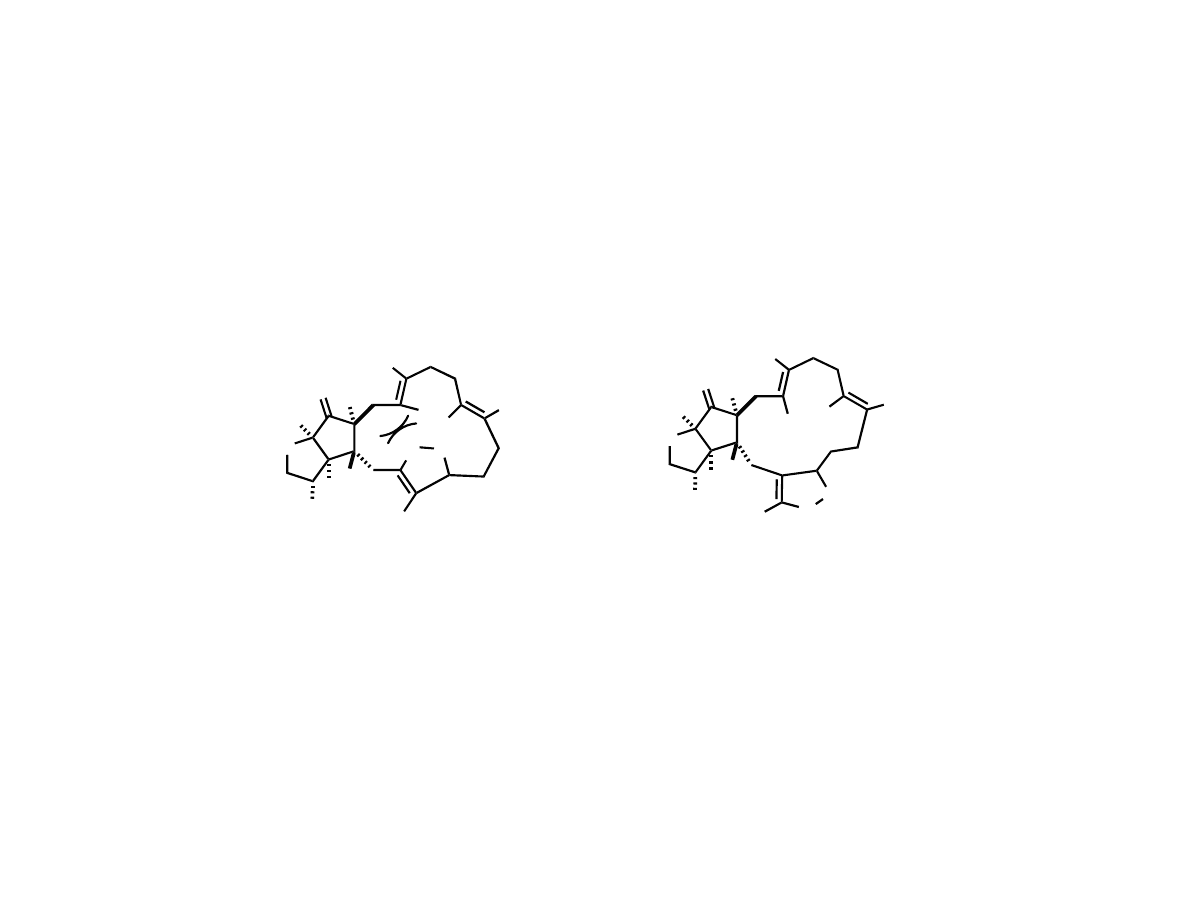
Possible Explanation for Selective
Formation of the 14-Membered Ring
Me
Me
O
Me
H
O
Me
H
H
O
Me
Me
Me
O
Me
H
O
Me
H
H
O
Me
L
N
M
M
L
N
Leads to 15 membered ring
Leads to 14 membered ring
H
H
H
H

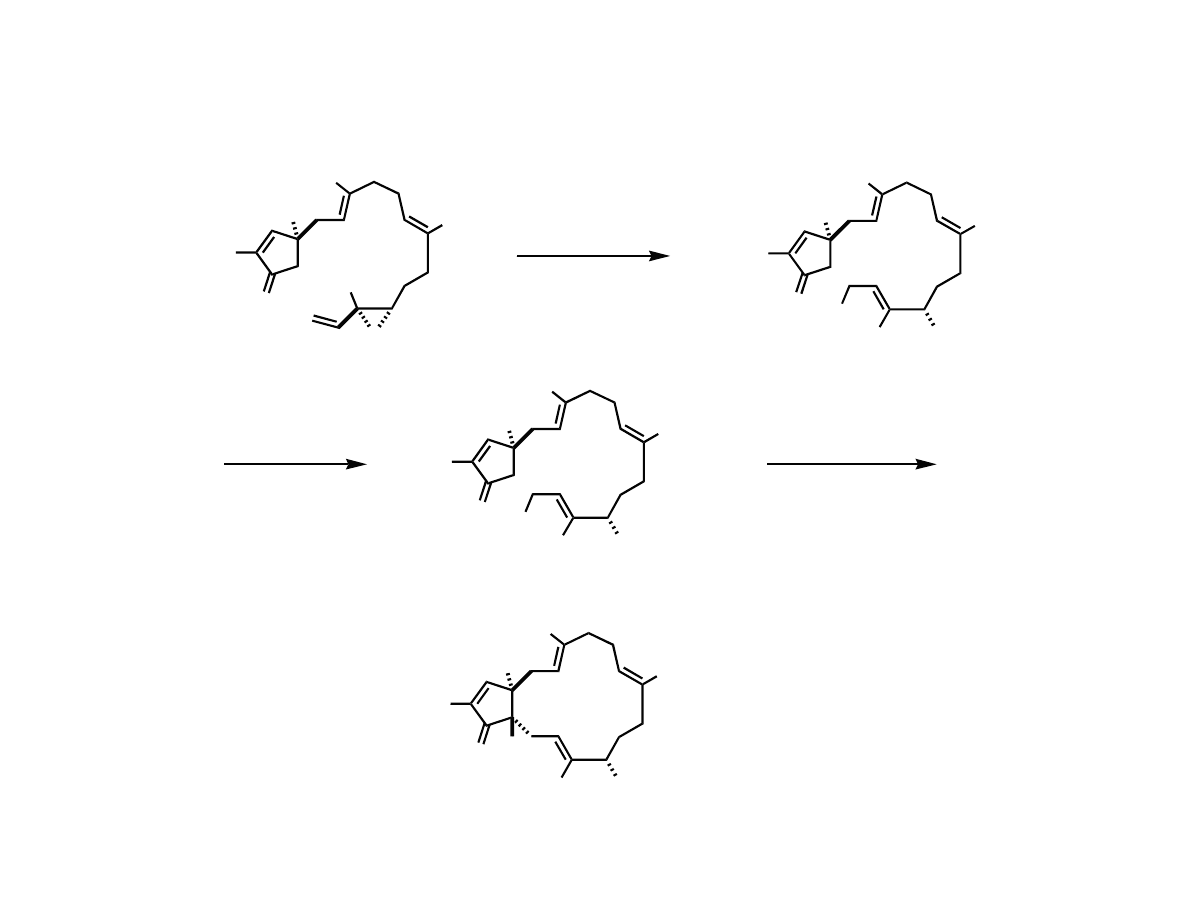
Try an Intermolecular Coupling
OH
Me
O
Me
> 95% ee
several hundred grams
SiMe
3
(
CO
)
3
Co
(
CO
)
3
Co
NMO
CH
2
Cl
2
51%
O
Me
H
H
O
SiMe
3
only detectable isomer
H
O
Me
H
H
TIPS
CuLi
2
THF, Et
2
O
then HCl
58%
O
NaBH
4
MeOH
H
O
Me
H
H
HO
TBAF
THF
77% (2 steps)
TIPS
TIPS
KOtBu(3 eq)
DMSO (~ 70 eq)
15 min
then 0.5M HCl work up
H
O
Me
H
H
HO
H
O
Me
H
H
Me
HO
H
TMSCl
THF
77%(2 steps)
H
O
Me
H
H
Me
TMSO
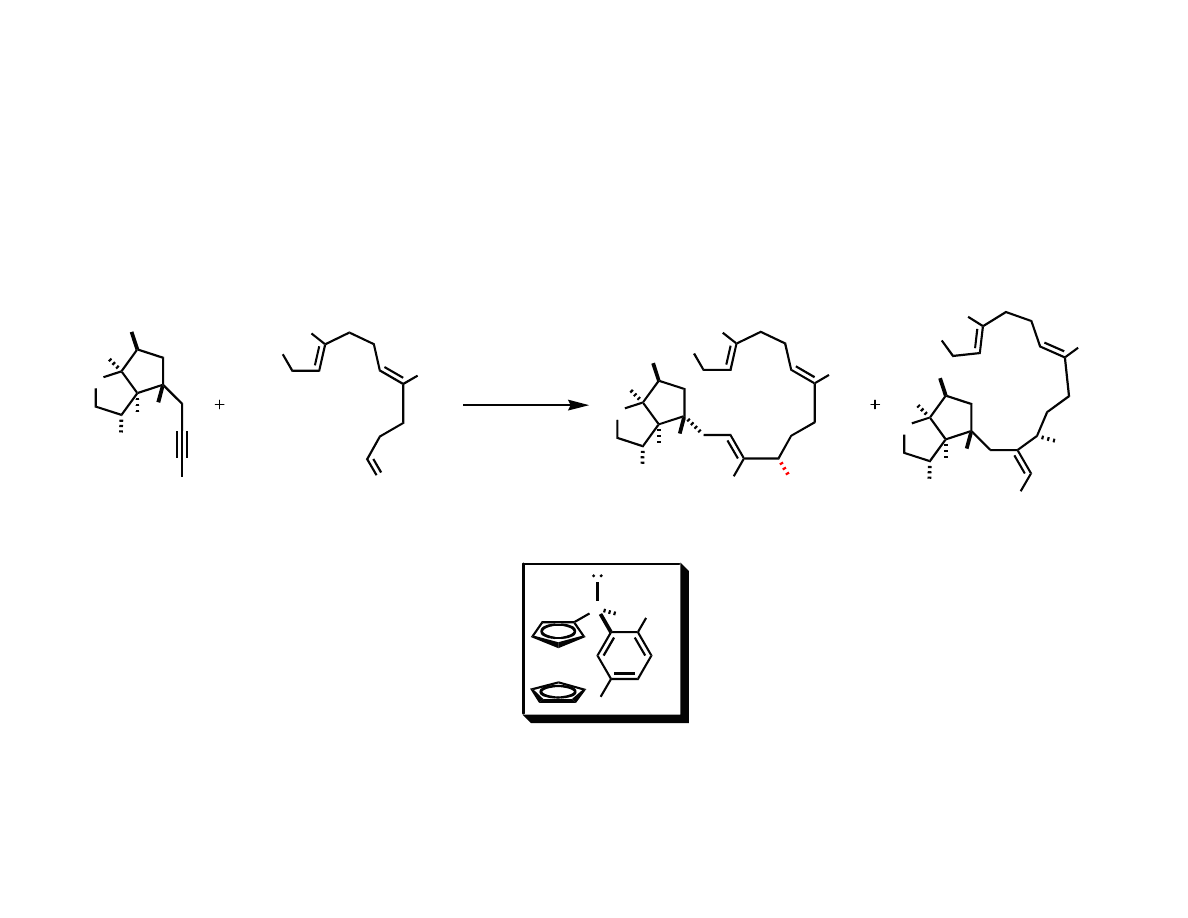
Intermolecular Coupling
Me
Me
TMSO
OH
Me
H
O
Me
H
H
TBSO
TMSO
H
O
Me
H
H
Me
Me
O
Me
TBSO
Ni(cod)
2
L*
BEt
3
, EtOAc
85%
2.6:1 rs
(15 vs 14)
Me
Me
TMSO
OH
Me
H
O
Me
H
H
TBSO
2:1 dr
Fe
P
Ph
Me
Me
L*

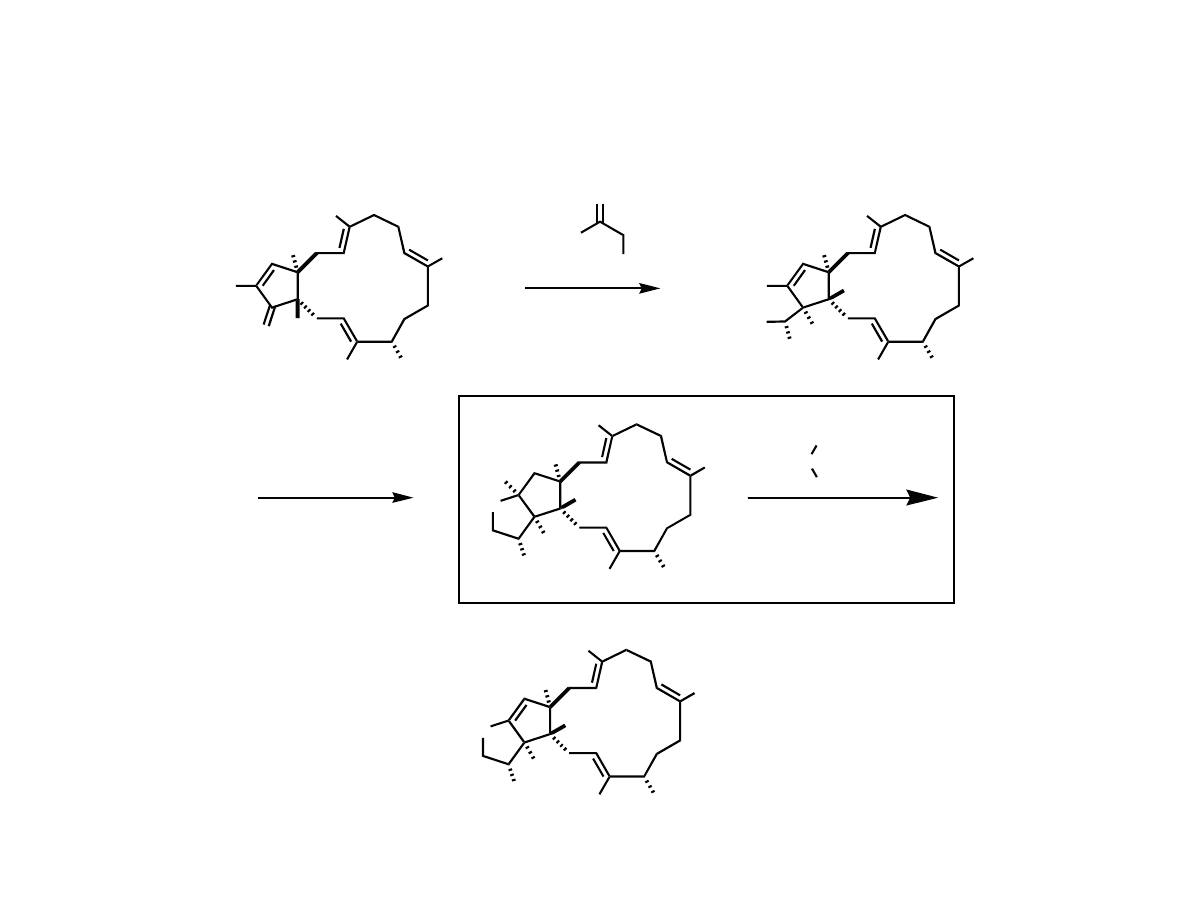
Assembling the Macrocycle
Me
Me
HO
OTIPS
Me
H
O
Me
H
H
TBSO
Me
Me
TMSO
OH
Me
H
O
Me
H
H
TBSO
1. TIPSOTf
2,6-lutidine
CH
2
Cl
2
2. 5% NaOH
MeOH, 0
o
C
57% (2 steps)
Me
Me
O
OTIPS
Me
H
O
Me
H
H
1. PPh
3
, I
2
,
imidazole
benzene:ether (2:1)
2. LiHMDS
THF, 0
o
C
22% (2 Steps)
stereochem not specified
as it will be destroyed later
Me
Me
O
OTIPS
Me
H
O
Me
H
H
HO
1. TPAP, NMO
CH
2
Cl
2
2. THF: 1M HCl
(1:1)
75% (2 steps)
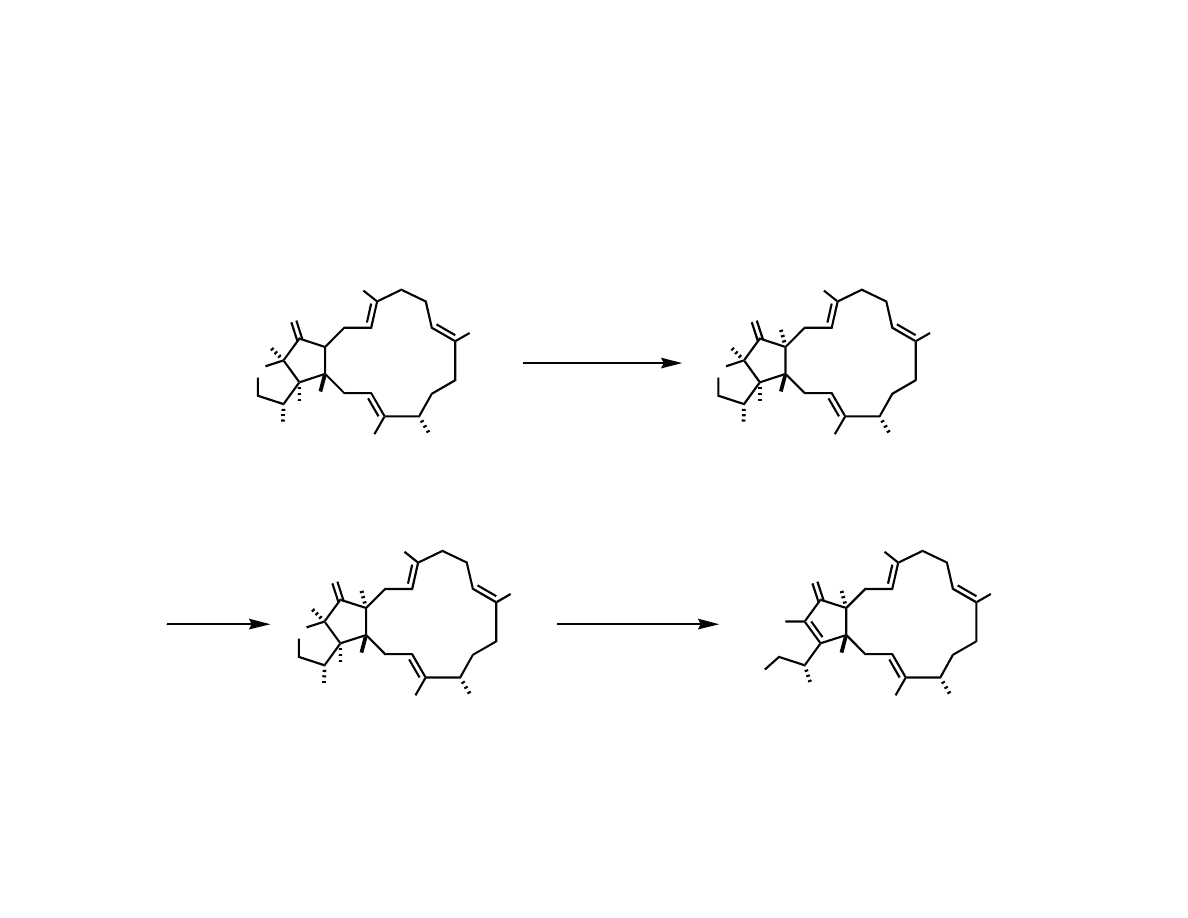
Completing the Total Synthesis
Me
Me
O
OTIPS
Me
Me
H
O
Me
H
H
NaH, H
2
O
(really just NaOH)
MeI
toluene
65%, > 95:5 dr
Me
Me
O
OTIPS
Me
H
O
Me
H
H
Me
Me
O
OH
HO
HO
Me
Me
Me
H
Me
Me
O
OH
Me
Me
H
1. KHMDS, O
2
P(OEt)
3
THF, -78
o
C
2. K
2
CO
3
, MeOH
48% (2 steps)
O
Me
H
H
TBAF
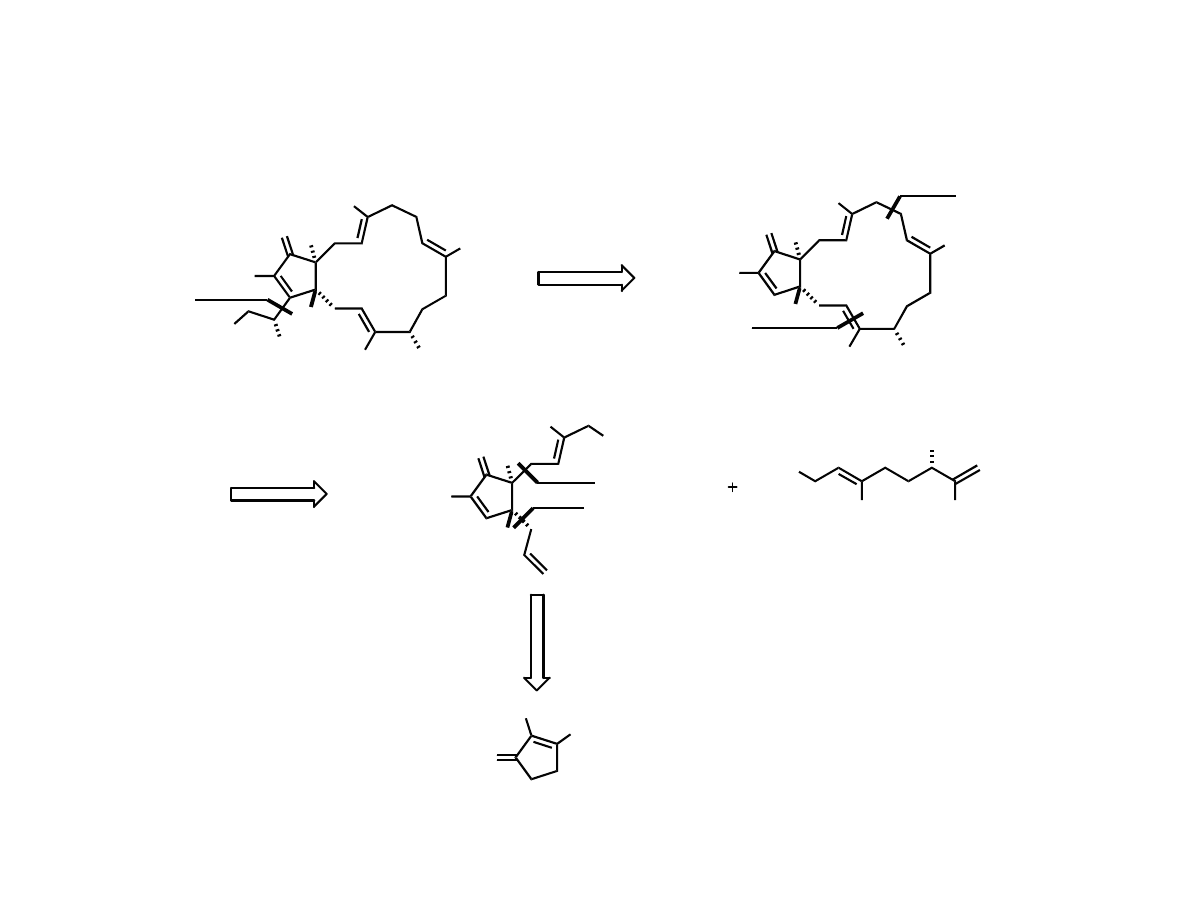
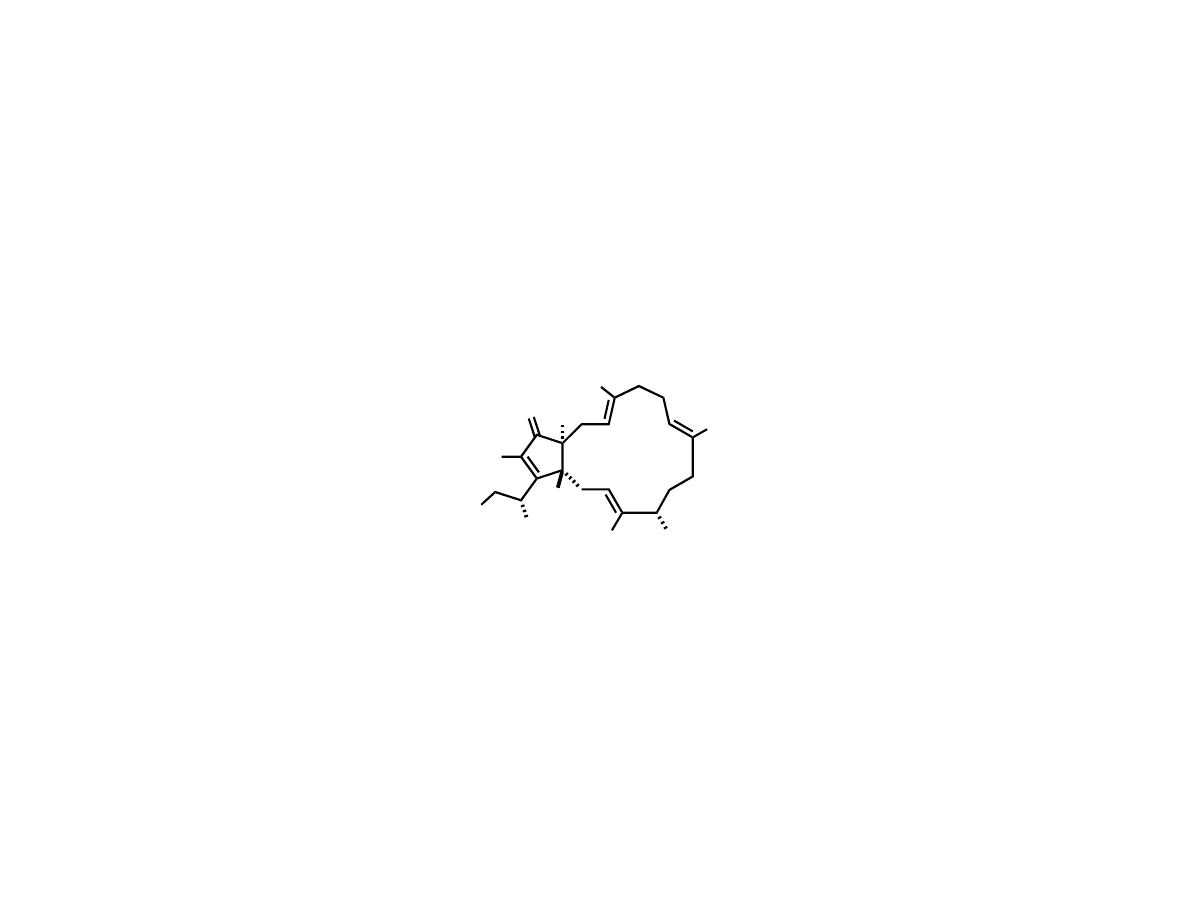
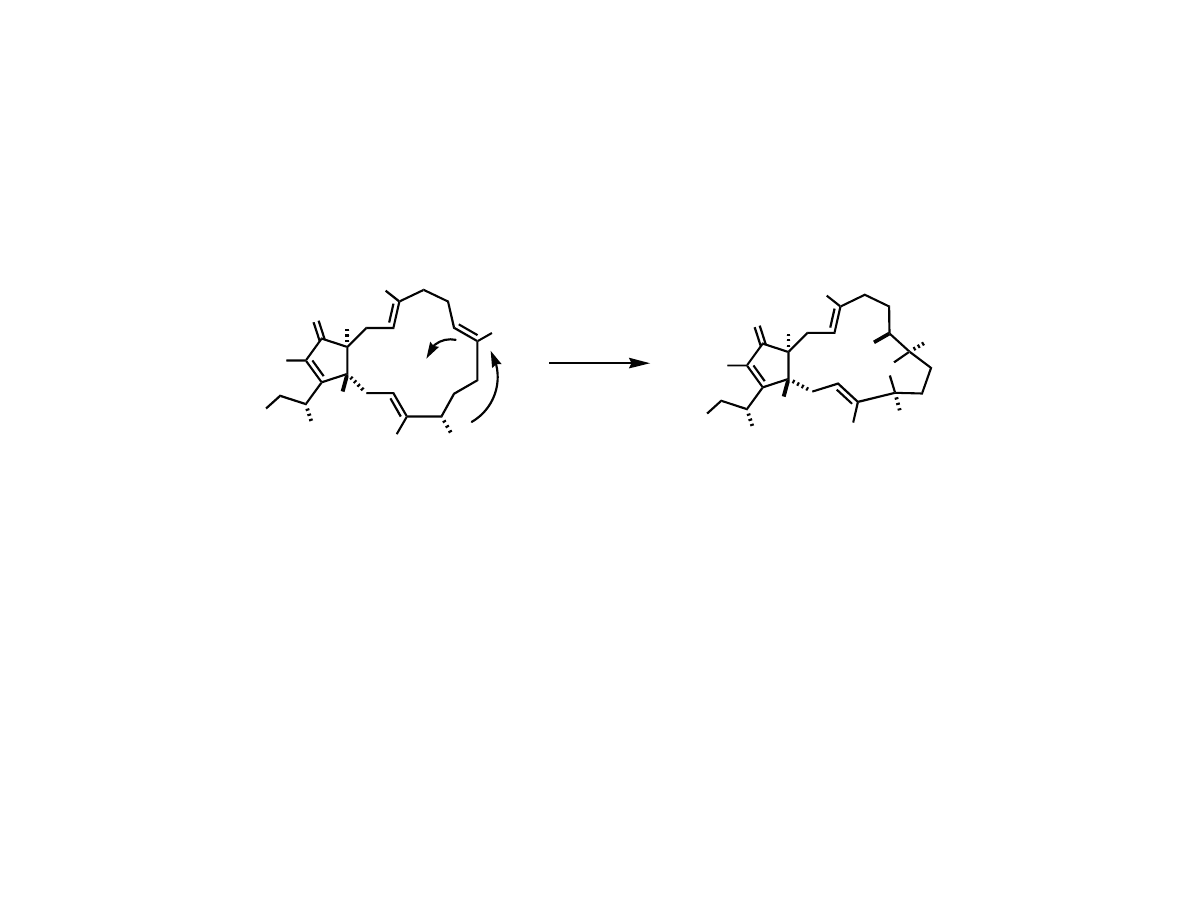
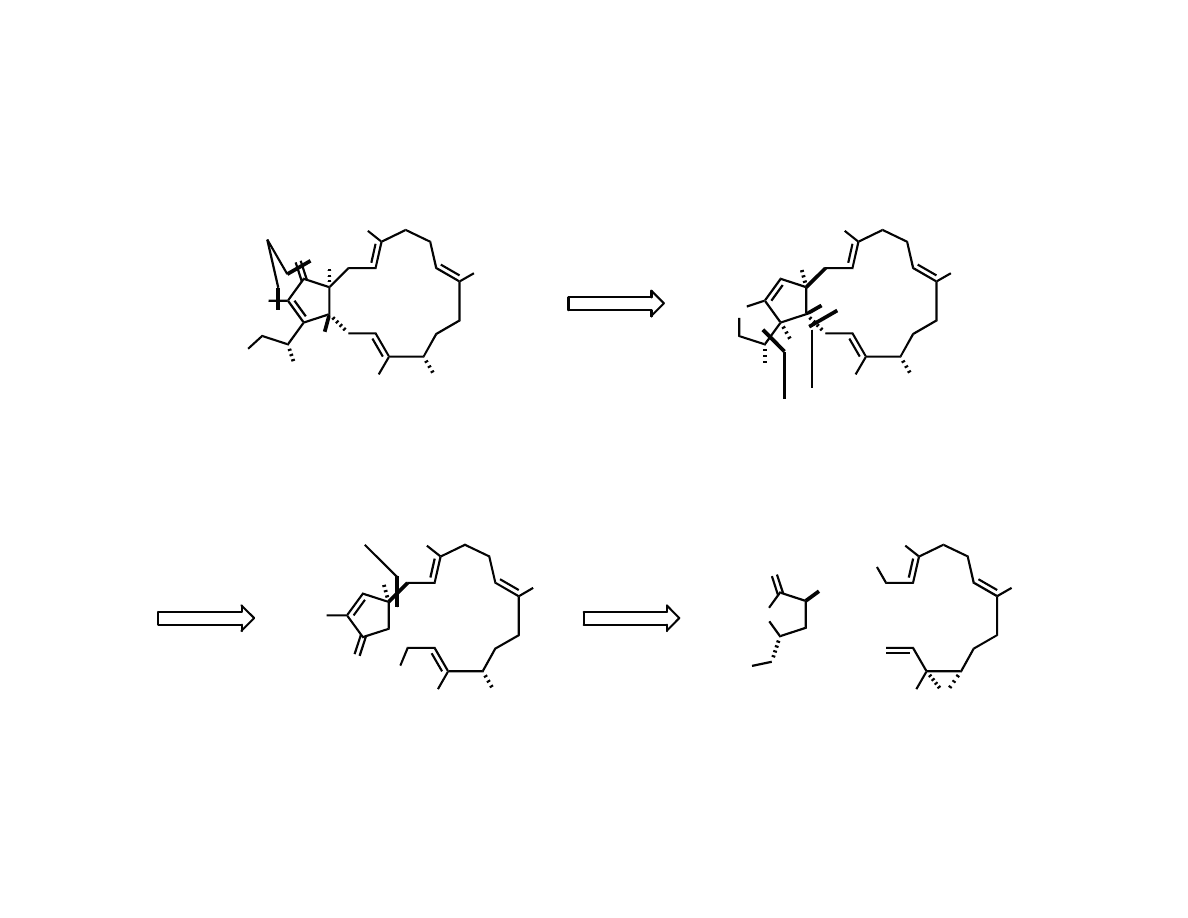
Trost’s Total Synthesis of (-)-Terpestacin
Trost, B. M.; Dong, G.; Vance, J. A. J. Am. Chem. Soc. 2007, 129, 4540.
Me
Me
O
OH
HO
HO
Me
Me
Me
H
Me
Me
O
OH
HO
Me
Me
H
RCM
sulfone mediated
alkylation
Me
OH
Me
PhO
2
S
O
HO
Me
Me
H
Br
Sakurai
allylation
Pd-AAA & Claisen
Pd-AAA
& Claisen
HO
O
Me
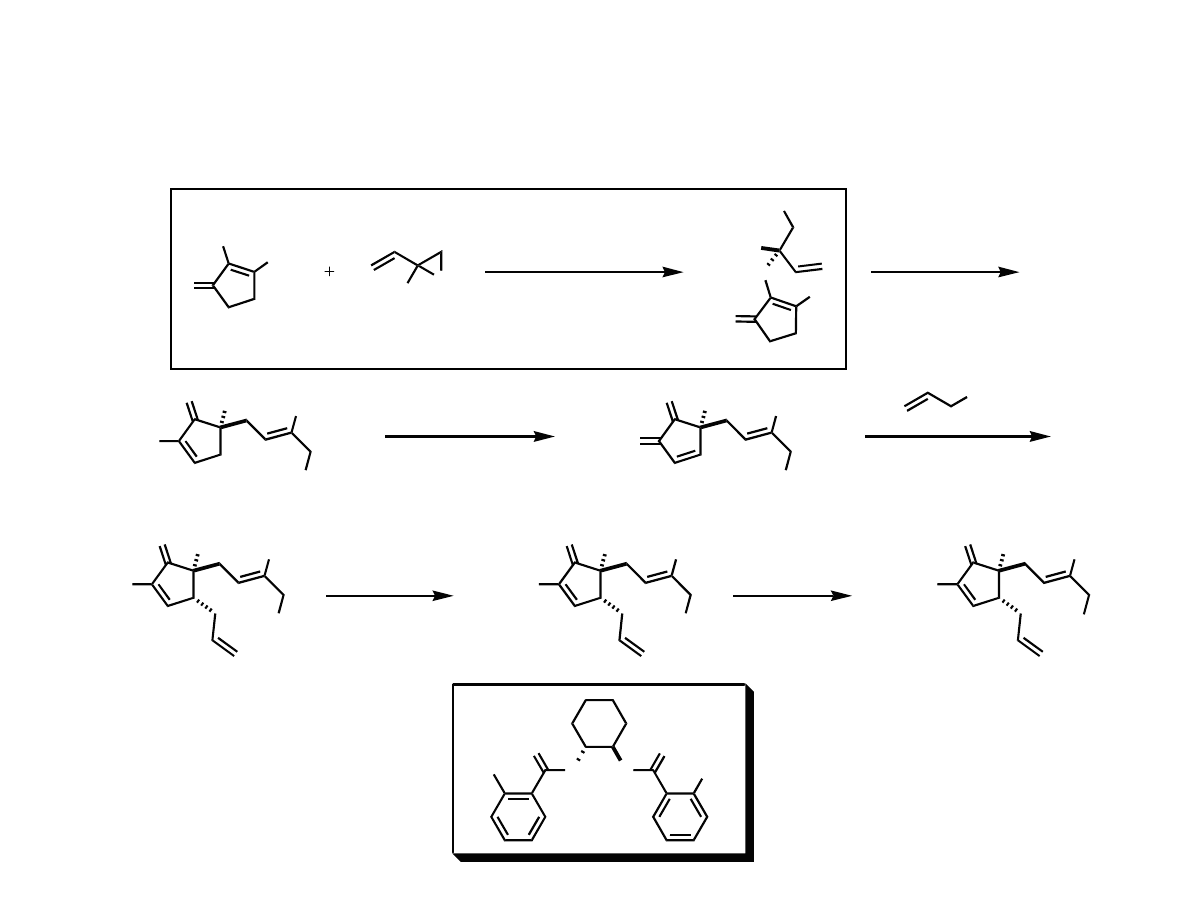
Preparation of the Advanced
Cyclopentenone
HO
O
Me
O
Me
1 mol% Pd(dba)
3
2.6 mol% L*,
50 mol% Bu
4
N
+
Cl
-
;
CH
2
Cl
2
then
TIPSOTf, 2,6-lutidine
95%, 88-96%ee
O
O
Me
Me
TIPSO
CHCl
3
,
μW
100
o
C, 15 min
120
o
C, 15 min
O
HO
Me
Me
Pd(OAc)
2
, Cs
2
CO
3
CH
3
CN, rt
78% (2 steps)
O
O
Me
Me
MgBr
2
Et
2
O
CH
2
Cl
2
, -78
o
C to rt
86%, dr 5.7:1
SiMe
3
TIPSO
TIPSO
O
HO
Me
Me
1. TBAF
THF, 86%
2. CBr
4
, PPh
3
CH
3
CN
88%
O
PMBO
Me
Me
PMBCl
cat. Cs
2
CO
3
DMF, Bu
4
N
+
I
-
79%
O
PMBO
Me
Me
TIPSO
TIPSO
Br
NH
HN
O
O
Ph
2
P
PPh
2
L*
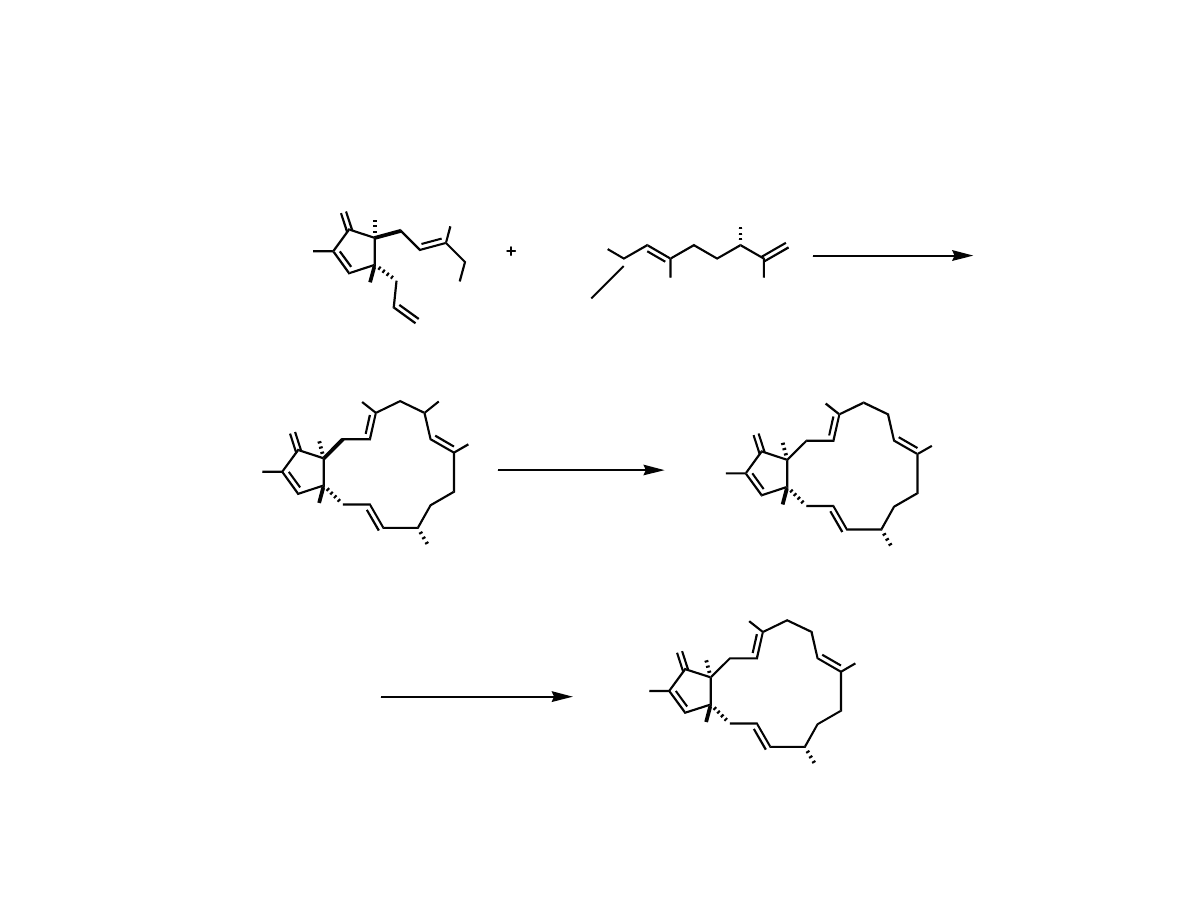
Sulfone Mediated Alkylation and RCM
O
PMBO
Me
Me
Br
Me
OH
Me
PhO
2
S
allows alkylation
α to
the sulfone
LHMDS (2 eq)
THF/HMPA (3:1)
-40
o
C
74-85%
Me
O
OH
PMBO
Me
Me
H
H
SO
2
Ph
20 mol %Pd(OAc)
2
25 mol % DPPP
NaBH
4
, DMSO
77%
Me
O
OH
PMBO
Me
Me
H
10 mol% G2
benzene 0.001M
35-44%of the desired
E isomer
Me
O
OH
PMBO
Me
Me
H
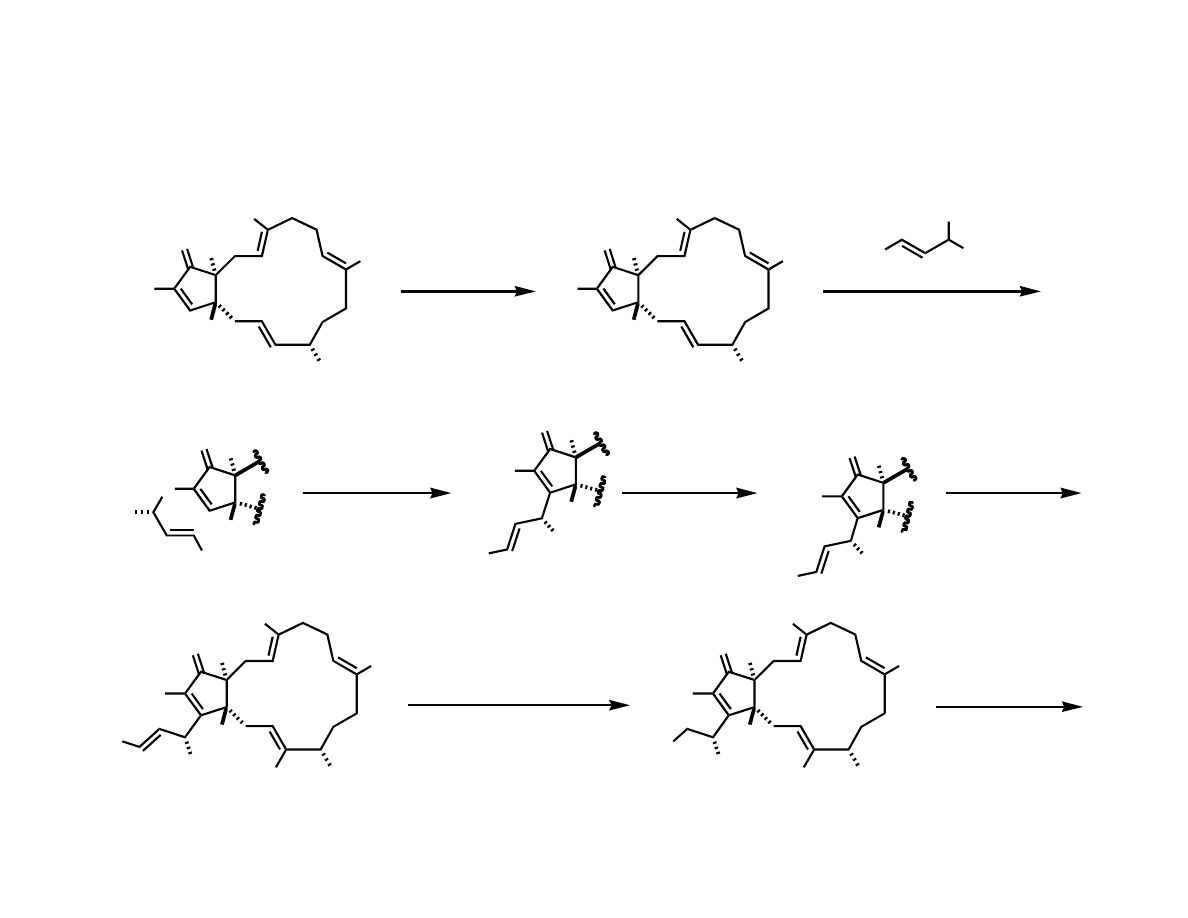
Completing the Total Synthesis
Me
O
OH
PMBO
Me
Me
H
PMBCl
cat. Cs
2
CO
3
DMF, Bu
4
N
+
I
-
MgBr
2
Et
2
O
Me
2
S
CH
2
Cl
2
-78
o
C to rt
93%
Me
O
OH
HO
Me
Me
H
O
O
Me
H
OBoc
Me
Me
2 equiv
2.5% Pd
2
(dba)
3
, 7.5%L*
CH
2
Cl
2
89%, 15:1 dr
O
PMBO
Me
H
Me
Me
CHCl
3
,
μW
100
o
C, 15 min
120
o
C, 15 min
O
HO
Me
H
Me
Me
Me
Me
Ac
2
O
pyridine
69% (3 steps)
Me
Me
O
OAc
PMBO
HO
Me
Me
Me
H
Me
Me
O
OAc
PMBO
Me
Me
Me
H
Me
1. Sharpless dihydroxylation
65%, 80% brsm
2. NaIO
4
THF/H
2
O (4:1)
3. NaBH
4
, CH
2
Cl
2
/MeOH
-78
o
C
78% (2 steps)
1. LiOH,
THF/MeOH/H
2
O
(3:1:1), 89%
2.MgBr
2
Et
2
O
Me
2
S, CH
2
Cl
2
-78
o
C to rt
74%

Document Outline
- Total Syntheses of (-)-Terpestacin
- An Interesting History
- Chloroform is the Culprit
- Now that we know what we’re making…
- Myers Total Synthesis of (-)-Terpestacin
- The First Enolate Alkylation
- Preparing for the Second Enolate Alkylation
- The Second Enolate Alkylation
- Installing the Side Chain
- Completing the Total Synthesis
- Jamison’s Total Synthesis
- An Efficient preparation of the 5 Membered Ring
- Preparing the Substrate for Macrocyclization
- Intramolecular Coupling
- Possible Explanation for Selective Formation of the 14-Membered Ring
- Try an Intermolecular Coupling
- Intermolecular Coupling
- Assembling the Macrocycle
- Completing the Total Synthesis
- Trost’s Total Synthesis of (-)-Terpestacin
- Preparation of the Advanced Cyclopentenone
- Sulfone Mediated Alkylation and RCM
- Completing the Total Synthesis
Wyszukiwarka
Podobne podstrony:
Total synthesis of batrachotoxinin A
efficient synthesis of tryptamine heterocycles 6 (8) 1167 1171 (1977) [R 1977 08 1167]
Diverted Total Synthesis in Medicinal Chemistry Research
Total eclipse of the heart Bonnie Tyler
efficient synthesis of tryptamine heterocycles 6 (8) 1167 1171 (1977) [R 1977 08 1167]
w sapx363 Managing the Total Cost of Ownership of Business Intelligence
ashel 13 Total Eclipse of the Heart
Total number of students in tertiary education, as a percentage of the population aged 20–24, by EU
Crystal Jordan [In the Heat of the Night 01] Total Eclipse of the Heart [pdf](1)
Highly selective synthesis of menthols from citral in a one step process
980 Bonnie Tyler Total eclipse of the heart
Concise Large Scale Synthesis of Psilocin and Psilocybin (Shirota, Hakamata & Goda)
brainwashing a synthesis of the russian textbook on psychopolitics
Synthesis of alpha aminoacids
Syntheses, structural and antimicrobial studies of a new N allylamide
Synthetic Drugs of?use
Effects of preoperative physiotherapy in hip osteoarthritis patients awaiting total hip replacement
więcej podobnych podstron