
Original article
doi:10.1093/rheumatology/keu408
Non-steroidal anti-inflammatory drugs and risk of
venous thromboembolism: a systematic review and
meta-analysis
Patompong Ungprasert
1
, Narat Srivali
1
, Karn Wijarnpreecha
2,3
,
Prangthip Charoenpong
4
and Eric L. Knight
1
Abstract
Objective. The aim of this study was to integrate and examine the association between NSAID use and
venous thromboembolism (VTE).
Methods. We conducted a systematic review and meta-analysis of studies that reported odds ratios,
relative risks, hazard ratios or standardized incidence ratios for VTE among NSAID users compared
with non-users. Pooled risk ratios and 95% CIs were calculated using a random effects generic inverse
variance model.
Results. Six studies with 21 401 VTE events were identified and included in the data analysis. The pooled
risk ratio of VTE in NSAID users was 1.80 (95% CI 1.28, 2.52).
Conclusion. Our study demonstrated a statistically significant increased risk of VTE among NSAID users.
This finding has important public health implications given the prevalence of NSAID use in the general
population.
Key words: systematic review, epidemiology, meta-analysis, NSAIDs, respiratory.
Introduction
Venous thromboembolism (VTE), which includes deep
venous thrombosis (DVT) and pulmonary embolism (PE),
is a common illness with a reported annual incidence of
12 new cases per 1000 population [
]. Recognition of
its risk factors and appropriate preventive interventions
for this condition are crucial, as morbidity and mortality
from PE are high, with a reported 30-day case fatality
rate as high as 810% [
]. Several medical conditions,
including immobilization, surgery, pregnancy and cancer,
are recognized as risk factors for VTE.
NSAIDs, one of the most commonly used medications
around the world [
], are well known for their potential
adverse effects. For example, in the recent past, rofecoxib
was withdrawn from the market after a randomized pla-
cebo-controlled trial found an increased incidence of
myocardial infarction and sudden cardiac death among
rofecoxib users [
]. A subsequent meta-analysis con-
firmed this finding and also found an increased incidence
among other non-specific cyclooxygenase (COX) inhibitor
users [
]. Since arterial and venous thrombosis share sev-
eral pathophysiological mechanisms [
], NSAIDs might
increase the risk of VTE. However, the epidemiological
data on VTE risk among NSAID users is limited and con-
flicting. Thus, to further investigate this association, we
conducted a systematic review and meta-analysis of ob-
servational studies that compared the risk of VTE in
NSAID users vs non-users. We did not include rando-
mized controlled trials in this meta-analysis because
VTE is a less common adverse effect that generally re-
quires a larger sample size and a longer duration of
follow-up.
1
Department of Internal Medicine, Bassett Medical Center and
Columbia University College of Physicians and Surgeons,
Cooperstown, NY, USA,
2
Cardiac Electrophysiology Unit, Department
of Physiology,
3
Cardiac Electrophysiology Research and Training
Center, Faculty of Medicine, Chiang Mai University, Chiang Mai,
Thailand and
4
Department of Internal Medicine, Advocate Illinois
Masonic Medical Center, Chicago, IL, USA.
Correspondence to: Patompong Ungprasert, Department of Internal
Medicine, Bassett Medical Center and Columbia University College of
Physicians and Surgeons, 1 Atwell Road, Cooperstown 13326,
NY, USA. E-mail: p.ungprasert@gmail.com
Submitted 13 May 2014; revised version accepted 16 August 2014.
!
The Author 2014. Published by Oxford University Press on behalf of the British Society for Rheumatology. All rights reserved. For Permissions, please email: journals.permissions@oup.com
1
RHEUMATOLOGY
53
MET
A
-
ANALYSIS
Rheumatology Advance Access published September 24, 2014
at Warszawski Uniwersytet Medyczny on November 7, 2014
http://rheumatology.oxfordjournals.org/
Downloaded from

Materials and methods
Search strategy
Two investigators (P.U. and N.S.) independently searched
published studies indexed in Medline, Embase and the
Cochrane database from inception to December 2013.
The search terms were compiled from the names of indi-
vidual drugs, the therapeutic class and the mode of action
in conjunction with the terms pulmonary embolism, deep
venous thrombosis and venous thromboembolism. The
detailed search strategy is provided as
, available at Rheumatology Online. A manual
search of the references of selected retrieved studies
was also performed. Abstract and unpublished studies
were not included.
Study selection
The inclusion criteria were as follows: (i) casecontrol or
cohort studies published as original studies to evaluate
the association between use of NSAIDs and risk of VTE;
(ii) odds ratios (ORs), relative risks (RRs), hazard ratios
(HRs) or standardized incidence ratios with 95% CIs
were provided and (iii) random participants without VTE
were used as the reference group for casecontrol studies
and participants who did not use NSAIDs were used
as the reference group for cohort studies. Study eligibility
was independently determined by each investigator noted
above. Any disagreements were resolved by consensus.
The quality of the included studies was independently
evaluated by each investigator using the Newcastle
Ottawa quality assessment scale [
].
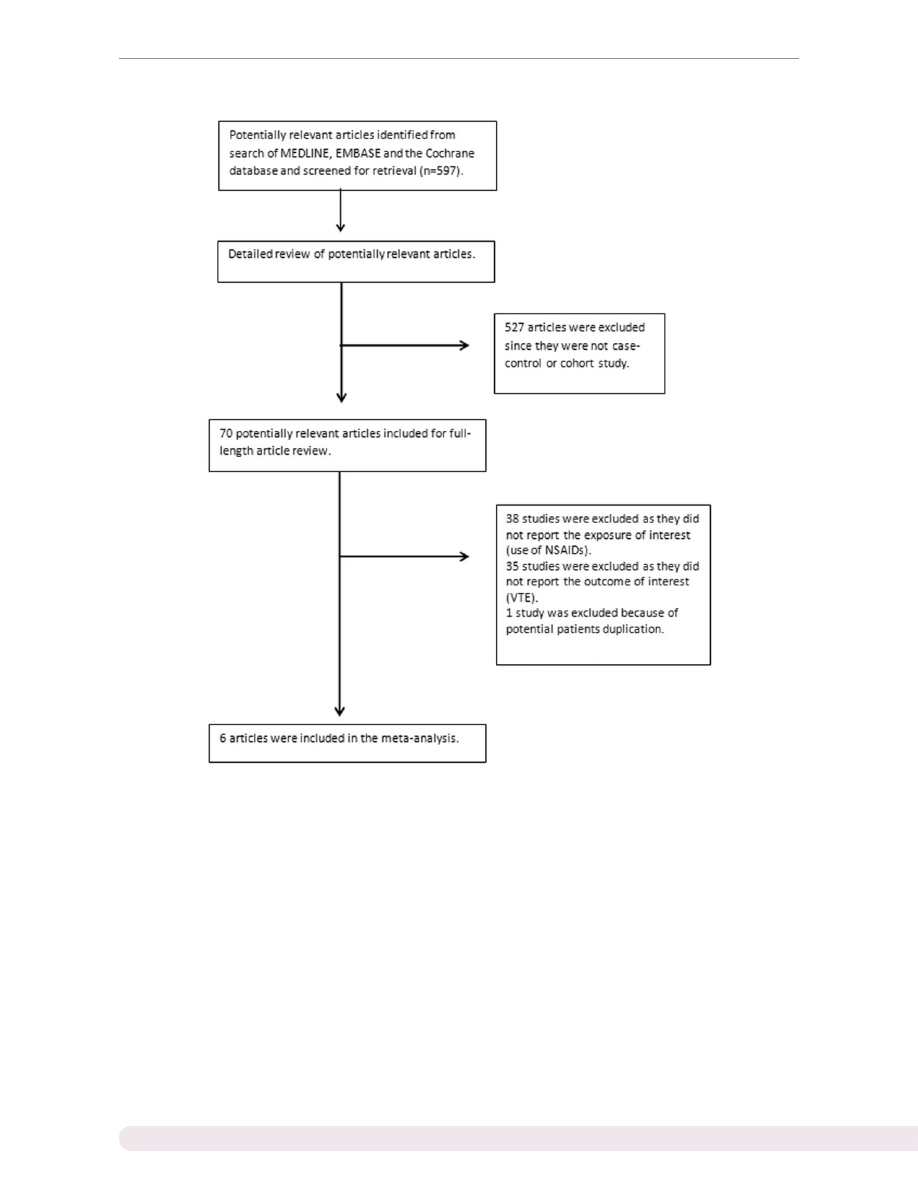
Our search strategy yielded 597 potentially relevant
articles. Five hundred and twenty-seven articles were
excluded because they were not casecontrol or cohort
studies. Seventy articles underwent full-length article
review. Thirty-eight of these were excluded because
they did not report the exposure of interest (use of
NSAIDs), 35 were excluded because they did not report
the outcome of interest (VTE) and 1 was excluded be-
cause it used the same database as was used by another
study. Six studies (one cohort study and five casecontrol
studies) with 21 401 VTE events met our eligibility criteria
and were included in the analysis [
].
outlines
our search methodology and selection process. The de-
tailed characteristics and quality assessment of these six
studies are described in
and
.
Data extraction
A standardized data collection form was used to extract
the following information: last name of the first author, title
of the article, year of publication, country where the study
was conducted, study size, study population, definition of
NSAID exposure, verification of NSAID use, verification of
VTE, confounder assessed and adjusted effect estimates
with 95% CIs. The two investigators independently per-
formed this data extraction.
Statistical analysis
Review Manager 5.2 software (Cochrane Collaboration,
Oxford, UK) was used for the data analysis. We reported
the pooled effect estimate of VTE using the combination
of data from casecontrol and cohort analyses to increase
the precision of our estimates. We used the ORs of
casecontrol studies as an estimate of the RRs to pool
these data with the RR of the cohort study, as the out-
come of interest was relatively uncommon [
]. If the
cohort study provided a HR, the HR was used as an es-
timate of the RR. Adjusted point estimates and standard
errors were extracted from individual studies and were
combined by the generic inverse variance method of
DerSimonian and Laird [
]. Given the high likelihood of
between-study variance with the different study designs,
definitions of NSAID use and populations, we used a
random effects model rather than a fixed effects model.
All of the studies reported the VTE risk of all NSAID (non-
selective and selective COX-2 inhibitors) use while three
studies also provided data on the VTE risk of selective
COX-2 inhibitors [
]. Pooled RRs were calculated
for all NSAIDs and for selective COX-2 inhibitors.
Statistical heterogeneity was assessed using Cochran’s
Q test. This statistic was complemented with the I
2
stat-
istic, which quantifies the proportion of total variation
across studies that is due to heterogeneity rather than
chance. A value of I
2
of 025% represents insignificant
heterogeneity, 2550% low heterogeneity, 5075% mod-
erate heterogeneity and 75100% high heterogeneity [
].
This study was exempted from ethical approval by the
institutional review board of the Bassett Medical Center,
Cooperstown, NY, USA.
Results
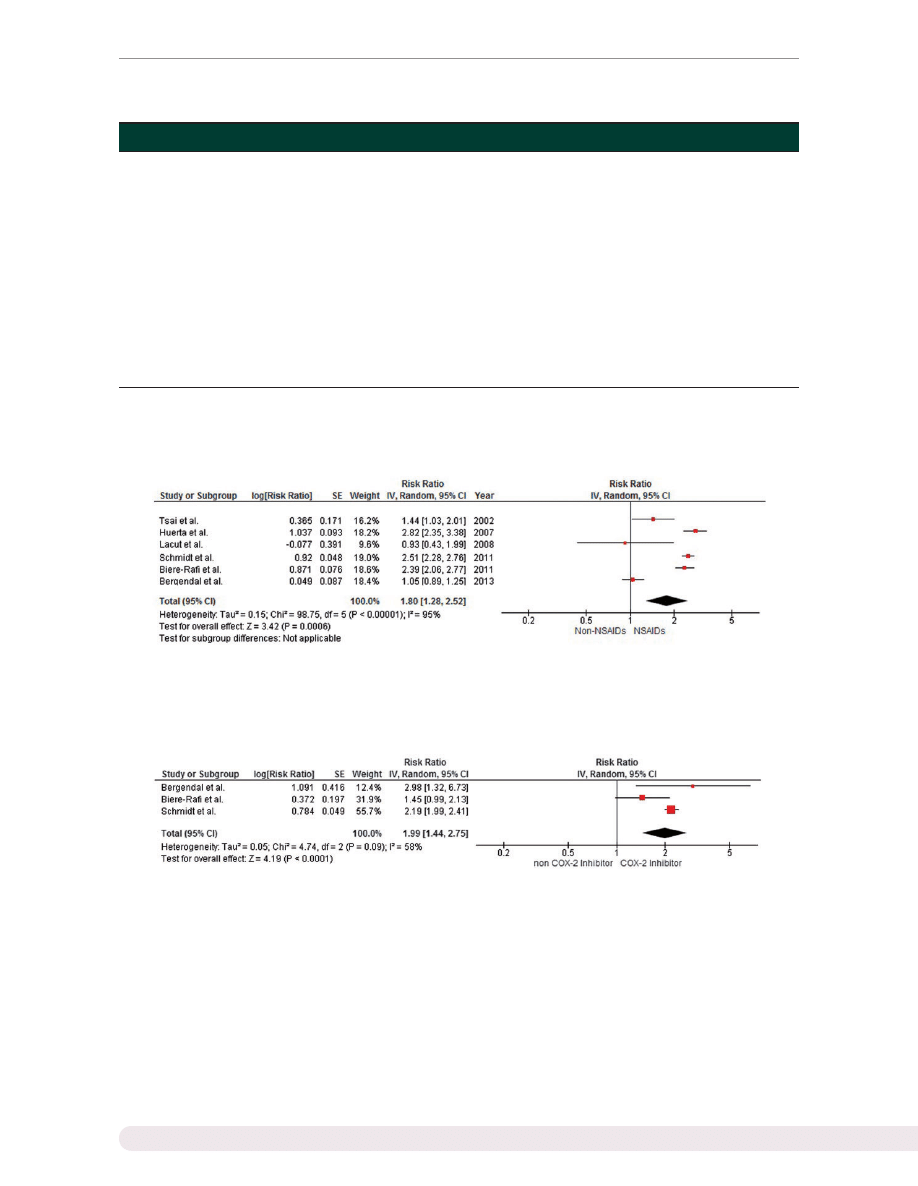
Our meta-analysis demonstrated a statistically signifi-
cantly increased VTE risk among subjects who used
NSAIDs with a pooled risk ratio of 1.80 (95% CI 1.28,
2.52). The statistical heterogeneity was high, with an I
2
of 95%. Three studies reported a risk ratio for participants
who used selective COX-2 inhibitors. The VTE risk among
selective COX-2 inhibitor users was also significantly ele-
vated, with a pooled risk ratio of 1.99 (95% CI 1.442.75).
and
present the forest plots of our findings.
Sensitivity analysis
With the concern over high heterogeneity, we performed
jackknife sensitivity analysis by excluding one study at a
time. The results of this sensitivity analysis suggested that
our results were robust, as the pooled risk ratios remained
significantly elevated, ranging from 1.62 to 2.21, while the
corresponding 95% CI bounds remained >1.
Publication bias
Funnel plots to evaluate publication bias are shown in
. The graph is asymmetric, suggesting that publica-
tion bias in favour of positive studies may be present.
2
www.rheumatology.oxfordjournals.org
Patompong Ungprasert et al.
at Warszawski Uniwersytet Medyczny on November 7, 2014
http://rheumatology.oxfordjournals.org/
Downloaded from
Discussion
This is the first systematic review and meta-analysis of
published observational studies assessing the risk of
VTE among NSAID users. Our study demonstrates a sig-
nificant association between NSAID use and VTE, with an
overall 1.80-fold increased risk compared with subjects
who did not use NSAIDs. VTE risk appears to be even
higher among selective COX-2 inhibitor users, with a
1.99-fold increased risk, although the CI overlaps. The
VTE risk found in this study is slightly higher than the cor-
onary artery thrombosis risk seen in another meta-
analysis (ranging from 0.96 to 1.36) [
]. With the
widespread use of these medicines, this increased risk
may have important public health implications.
Heterogeneity between studies was present in this
meta-analysis. We suspect that differences in study
design, definitions of NSAID exposure and population
were the main source of heterogeneity. Three studies
were done in hospitalized subjects [
] and three
studies included both ambulatory and hospitalized sub-
jects [
,
]. One study included only patients with
PE [
], whereas the rest of the studies included patients
with DVT and/or PE. The definition and method of verifi-
cation for NSAID exposure also varied from study to
study, with some studies using a pharmacology-linked
F
IG
. 1
Identification of studies
VTE: venous thromboembolism.
www.rheumatology.oxfordjournals.org
3
NSAIDs and risk of VTE
at Warszawski Uniwersytet Medyczny on November 7, 2014
http://rheumatology.oxfordjournals.org/
Downloaded from
T
ABLE
1
Main
characteristics
of
case
control
studies
included
in
the
meta-analysis
Huerta
et
al.
[1
Lacut
et
al.
[12]
Biere-Rafi
et
al.
[13]
Schmidt
et
al.
[14]
Bergendal
et
al.
[1
]
Country
UK
France
The
Netherlands
Denmark
Sweden
Year
2007
2008
2011
2
011
2013
Cases
Hospitalized
or
non-
hospitalized
patients
who
were
diagnosed
with
DVT
and/or
PE
identified
from
the
G
eneral
Practice
Research
Database,
covering
3
million
p
eople
Patients
who
were
admitted
at
the
Brest
University
Hospital
with
a
d
iagnosis
of
DVT
and/or
PE
Hospitalized
patients
who
were
diagnosed
with
PE
identified
from
the
PHARMO
record
linkage
system,
covering
2
m
illion
people
Hospitalized
or
non-
hospitalized
patients
who
were
diagnosed
with
DVT
and/or
PE
identified
from
the
Danish
National
Patient
Registry
Female
patients
who
were
admitted
at
one
o
f
the
participating
hospitals
with
a
diagnosis
of
DVT
and/or
PE
Case
verification
P
atients
needed
to
receive
anticoagulant
NA
Confirmed
by
CT
or
V/Q
scan
NA
Radiological
evidence
of
VTE
+
patient
n
eeded
to
receive
anticoagulants
Controls
Sex-
and
age-matched
subjects
randomly
selected
from
same
database
Sex-
and
age-matched
hos-
pitalized
patients
ran-
domly
selected
from
the
same
hospital
Sex-,
age-
and
region-
matched
subjects
ran-
domly
selected
from
the
same
database
Sex-
and
age-matched
sub-
jects
randomly
s
elected
from
the
same
database
Sex-
and
age-matched
hospitalized
patients
randomly
selected
from
the
Swedish
population
register
Period
of
inclusion
1994
2000
2000
2004
1990
2006
1999
2006
2003
2009
Age
range,
years
20
79
18
96
18
96
NA
18
64
Female,
%
N
A
58.5
57.0
53.7
100
Cases,
n
6550
402
4433
8
368
1433
Controls,
n
10
000
402
16
802
8
2
2
18
1402
Definition
of
NSAID
exposure
Most
recent
p
rescription
lasted
until
the
index
d
ate
or
ended
in
the
30
days
before
the
index
date
Current
use
of
NSAIDs
at
the
time
o
f
admission
Most
recent
prescription
lasted
until
the
index
date
or
ended
in
the
90
days
before
the
index
date
Most
recent
prescription
lasted
until
the
index
date
or
ended
in
the
60
days
before
the
index
date
Use
of
NSAIDs
during
the
90
days
before
the
index
date
Verification
o
f
NSAIDs
use
Pharmacy
record
from
the
same
database
Structured
interview
and
validation
from
information
provided
by
the
National
Health
Service
of
France
Pharmacy
record
from
the
same
database
Pharmacy
record
from
the
same
database
Structured
phone
interview
Confounder
assessed
Sex,
age,
BMI,
s
moking,
fracture,
surgery,
cancer,
visits
to
the
family
phys-
ician
last
year
None
Hospitalization
Hospitalization,
pregnancy,
fracture,
surgery,
cancer,
diabetes,
CAD,
stroke,
PVD,
diabetes
mellitus,
RA,
COPD,
CHF,
OA,
obesity,
medication,
liver
disease,
renal
failure,
osteoporosis
Age
Quality
assessment
(Newcastle
Ottawa
scale)
Selection:
4
stars;
compar-
ability:
1
star;
exposure:
2
stars
Selection:
2
stars;
compar-
ability:
1
star;
exposure:
2
stars
Selection:
4
stars;
compar-
ability:
2
stars;
exposure:
2
stars
Selection:
3
s
tars;
compar-
ability:
2
star;
exposure:
2
stars
Selection:
3
stars;
com-
parability:
1
star;
expos-
ure:
2
stars
CAD:
coronary
artery
disease;
CHF;
congestive
heart
failure;
COPD:
chronic
obstructive
pulmonary
disease;
DVT;
deep
venous
thrombosis;
NA:
not
ava
ilable;
PE:
pulmonary
embolism;
PHARMO:
PHARMO
Institute,
Utrecht,
The
Netherlands;
PVD:
peripheral
vascular
disease;
V/Q
scan:
ventilation-perfusion
scan;
VTE:
veno
us
thromboembolism.
4
www.rheumatology.oxfordjournals.org
Patompong Ungprasert et al.
at Warszawski Uniwersytet Medyczny on November 7, 2014
http://rheumatology.oxfordjournals.org/
Downloaded from
database [
,
], while other studies used a struc-
tured or phone interview [
,
]. Controlling for confoun-
ders might also contribute to this heterogeneity, as it was
done differently between studies, from virtually no correc-
tion to control for a large number of confounders. It should
be noted that, from our sensitivity analysis, exclusion of
the study by Bergendal et al. [
], the only study that
exclusively included only female participants, noticeably
decreased the statistical heterogeneity, with a reduction in
I
2
from 95% to 78%.
Why NSAIDs may increase the risk of VTE is unclear.
The pathophysiology of increased arterial thrombosis risk
(and thus coronary artery events) is explained by a
thromboxaneprostacyclin imbalance. Inhibition of the
T
ABLE
2
Main characteristics of the cohort study included in the meta-analysis
Tsai et al. [10]
Country
USA
Study design
Prospective cohort
Year
2002
Cohort
Residents of four US communities. Follow-up included semi-annual
contacts, alternating between phone calls and clinic visits
Definition of NSAID use
NA
Event verification
Duplex US scan or venography or CT angiography or autopsy
Follow-up
From 1987 until death, migration from the system or 31 December 1999
Age, mean, years
59.0
Female, %
55.0
Cohort, n
19 293
Events, n
215
Average follow-up, years
7.8
Confounder assessed
Sex, age, race
Quality assessment (NewcastleOttawa scale)
Selection: 3 stars; comparability: 1 star; outcome: 3 stars
F
IG
. 2
Forest plot of all NSAIDs
IV: inverse variance; SE: standard error.
F
IG
. 3
Forest plot of selective COX-2 inhibitors
COX: cyclooxygenase; IV: inverse variance; SE: standard error.
www.rheumatology.oxfordjournals.org
5
NSAIDs and risk of VTE
at Warszawski Uniwersytet Medyczny on November 7, 2014
http://rheumatology.oxfordjournals.org/
Downloaded from
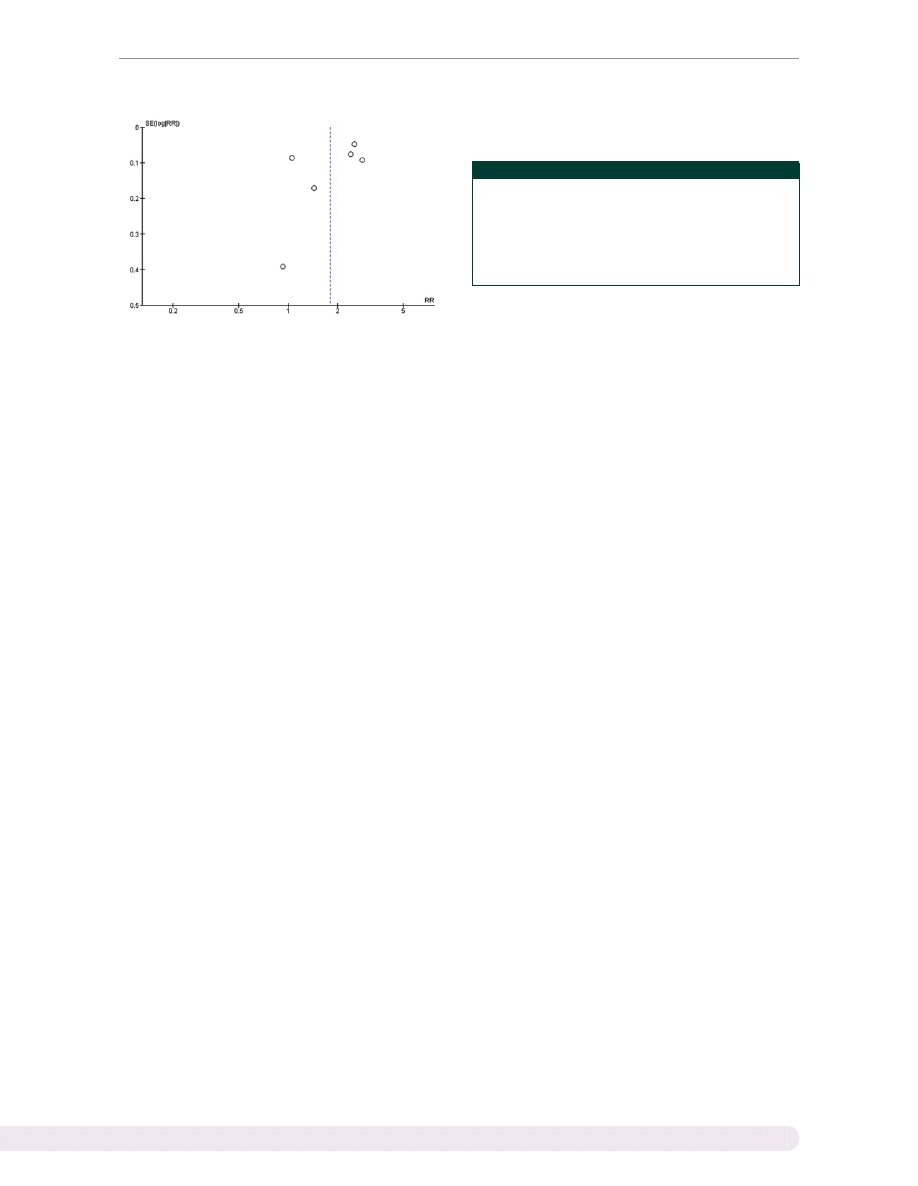
COX-2 enzyme has been shown to inhibit the synthesis of
prostacyclins, potent platelet activation inhibitors, while
stimulating the release of thromboxane, a potent platelet
aggregation facilitator, from the activated platelets. The
activation and aggregation of platelets might, in turn,
induce a coagulation cascade and clotting [
,
,
This mechanism might explain the increased risk of
venous thrombosis we observed in this study. In fact,
the VTE risk of selective COX-2 inhibitors appears to be
higher than overall NSAIDs, although without statistical
significance, as the CI overlaps.
Also, aspirin, a specific and irreversible COX-1 inhibitor,
has proved effective for VTE prevention [
]. This
might provide further evidence that the increased VTE
risk comes primarily from COX-2 inhibition.
Even though the six studies included in this meta-
analysis were of high quality, there are some limitations
and thus our results should be interpreted with caution.
First, we cannot exclude the possibility of publication bias
in favour of positive studies, as the funnel plot is asym-
metric. Second, the statistical heterogeneity in this study
is high and thus the data from individual studies might
be too heterogeneous to combine. Third, most of the
included studies were conducted using a medical regis-
trybased database, raising the possibility of coding
inaccuracy. Fourth, this is a meta-analysis of observa-
tional studies and thus can only demonstrate an associ-
ation, not establish cause and effect, so we cannot be
certain that NSAIDs themselves or other potential con-
founders increase the risk of VTE. For example, patients
may have been prescribed NSAIDs for underlying ill-
nesses causing pain and immobility or for chronic inflam-
matory disorders, which are linked to a higher VTE risk
compared with the general population [
]. We could
not perform a meta-regression to adjust for these poten-
tial confounders as the primary studies do not provide
sufficient data to do so. Furthermore, all NSAIDs were
evaluated as one group in this study, but not all individual
NSAIDs may increase VTE risk.
In conclusion, the results of our meta-analysis demon-
strate a statistically significantly increased VTE risk among
NSAID users. Physician should be aware of this associ-
ation and NSAIDs should be prescribed with caution,
especially in patients at high baseline risk of VTE.
Rheumatology key messages
.
This
is
the
first
meta-analysis
to
investigate
the
association
between
NSAIDs
and
venous
thromboembolism.
.
This study demonstrated a statistically significant
increased risk of venous thromboembolism among
NSAID users.
Funding: No specific funding was received from any fund-
ing bodies in the public, commercial or not-for-profit
sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no
conflicts of interest.
Supplementary data
are available at Rheumatology
Online.
References
1
Naess IA, Christiansen SC, Romundstad P et al. Incidence
and mortality of venous thrombosis: a population-based
study. J Thromb Haemost 2007;5:6929.
2
Cushman M, Tsai AW, White RH et al. Deep venous
thrombosis and pulmonary embolism in two cohorts: the
longitudinal investigation of thromboembolism etiology.
Am J Med 2004;117:1925.
3
Stein PD, Kayali F, Olson RE. Estimated case fatality rate
of pulmonary embolism, 19791998. Am J Card 2004;93:
11979.
4
Ungprasert P, Kittanamongkolchai W, Price CD et al. What
is the ‘‘safest’’ non-steroidal anti-inflammatory drugs? Am
Med J 2012;3:11523.
5
Bresalier RS, Sandler RS, Quan H et al. Cardiovascular
events associated with rofecoxib in a colorectal adenoma
chemoprevention trial. N Engl J Med 2005;352:1092102.
6
McGettigan P, Henry D. Cardiovascular risk and inhibition
of cyclooxygenase: a systematic review of the observa-
tional studies of selective and nonselective of cyclooxy-
genase 2. JAMA 2006;296:163344.
7
Franchini M, Mannucci PM. Venous and arterial throm-
bosis: different sides of the same coin? Eur J Intern Med
2008;19:47681.
8
Prandoni P. Link between arterial and venous disease.
J Intern Med 2007;262:34150.
9
Stang A. Critical evaluation of the Newcastle-Ottawa
scale for the assessment of the quality of nonrandomized
studies in meta-analyses. Eur J Epidemiol 2010;25:6035.
10 Tsai AW, Cushman M, Rosamond WD et al.
Cardiovascular risk factors and venous thromboembolism
incidence: the longitudinal investigation of thrombo-
embolism etiology. Arch Intern Med 2002;162:11829.
F
IG
. 4
Funnel plot of all included studies
RR: risk ratio; SE: standard error.
6
www.rheumatology.oxfordjournals.org
Patompong Ungprasert et al.
at Warszawski Uniwersytet Medyczny on November 7, 2014
http://rheumatology.oxfordjournals.org/
Downloaded from

11 Huerta C, Johansson S, Wallander MA, Garcia-
Rodriguez LA. Risk factors and short-term mortality of
venous thromboembolism diagnosed in primary care set-
ting in the United Kingdom. Arch Intern Med 2007;167:
93543.
12 Lacut K, van der Maaten J, Le Gal G et al. Antiplatelet
drugs and risk of venous thromboembolism: result from
the EDITH case-control study. Haematologica 2008;93:
11178.
13 Biere-Rafi S, Di Nisio M, Gerdes V et al. Non-steroidal anti-
inflammatory drugs and risk of pulmonary embolism.
Pharmacoepidemiol Drug Saf 2011;20:63542.
14 Schmidt M, Christiansen CF, Horvath-Puho E et al. Non-
steroidal anti-inflammatory drug use and risk of venous
thromboembolism. J Thromb Haemost 2011;9:132633.
15 Bergendal A, Adami J, Bahmanyar S et al. Non-steroidal
anti-inflammatory drug use and venous thromboembolism
in women. Pharmacoepidemiol Drug Saf 2013;22:65866.
16 Davies HT, Crombie IK, Tavakoli M. When can odds ratio
mislead? BMJ 1998;316:98991.
17 DerSimonian R, Laird N. Meta-analysis in clinical trials.
Control Clin Trial 1986;7:17788.
18 Higgins JP, Thompson SG, Deeks JJ, Altman DG.
Measuring inconsistency in meta-analyses. BMJ 2003;
327:55760.
19 Graff J, Skarke C, Klinkhardt U et al. Effect of selective
COX-2 inhibition on prostanoids and platelet physiology in
young healthy volunteers. J Thromb Haemost 2007;12:
237685.
20 McGettigan P, Henry D. Cardiovascular risk and inhibition
of cyclooxygenase: a systematic review of the observa-
tional studies of selective and nonselective inhibitors of
cyclooxygenase 2. JAMA 2006;296:163344.
21 Anderson DR, Dunbar MJ, Bohm ER et al. Aspirin
versus low-molecular-weight heparin for extended
venous thromboembolism prophylaxis after total hip
arthroplasty: a randomized trial. Ann Intern Med 2013;158:
8006.
22 Larocca A, Cavallo F, Bringhen S et al. Aspirin or
enoxaprarin thromboprophylaxis for patients with newly
diagnosed multiple myeloma treated with lenalidomide.
Blood 2012;119:9339.
23 Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C,
Knight EL. Risk of venous thromboembolism in patients
with rheumatoid arthritis: a systematic review and
meta-analysis. Clin Rheumatol 2014;33:297304.
24 Johannesdottir SA, Schmidt M, Horvath-Puho.,
Sorensen HT. Autoimmune skin and connective tissue
diseases and risk of venous thromboembolism: a popu-
lation-based case-control study. J Thromb Haemost 2012;
10:81521.
25 Ungprasert P, Sanguankeo A. Risk of venous thrombo-
embolism in patients with idiopathic inflammatory
myositis: a systematic review and meta-analysis.
Rheumatol Int 2014. [Epub ahead of print].
www.rheumatology.oxfordjournals.org
7
NSAIDs and risk of VTE
at Warszawski Uniwersytet Medyczny on November 7, 2014
http://rheumatology.oxfordjournals.org/
Downloaded from
Wyszukiwarka
Podobne podstrony:
Consumption of cocoa, tea and coffee and risk of cardiovascular disease
J L Allen Jr Foreign priests and risk of plunder
Effect of Drugs and Alcohol on Teenagers
Describe the role of the dental nurse in minimising the risk of cross infection during and after the
Encyclopedia of Mind Enhancing Foods, Drugs and Nutritional Substances ~ [TSG]
FALLS, INJURIES DUE TO FALLS, AND THE RISK OF ADMISSION
Psychedelic Drugs And The Awakening Of Kundalini Donald J
(Ebook) Charles Tart Sex, Drugs And Altered States Of Consciousness
The Risk of Debug Codes in Batch what are debug codes and why they are dangerous
MENDOZA LINE, THE Full of Light and Full of Fire CD (Misra) MSR37 , Non Exportable to UK, Europe an
Study Flavanoid Intake and the Risk of Chronic Disease
Assessment of balance and risk for falls in a sample of community dwelling adults aged 65 and older
Harris, Sam Drugs and the Meaning of Life
Congressional Budget Office Federal Debt and the Risk of a Fiscal Crisis
6 6 Detection and Identification of Drugs; Summary
Cancer Risk According to Type and Location of ATM Mutation in Ataxia Telangiectasia Families
05 DFC 4 1 Sequence and Interation of Key QMS Processes Rev 3 1 03
więcej podobnych podstron