1
This work was supported by a cooperative agreement for Sub-
stance-Speci
7c Research Program Grant number U50/ATU398948
with the Agency for Toxic Substances and Disease Registry, CDC
and the Minority Health Professions Foundation but does not
necessarily represent the views of those organizations. This pro-
ject was approved by the King/Drew Medical Center Institutional
Review Board.
Environmental Research Section A 82, 81}90 (2000)
Article ID enrs.1999.4007, available online at http://www.idealibrary.com on
Maternal Bone Lead Contribution to Blood Lead
during and after Pregnancy
1
Stephen J. Rothenberg,*
,-,
?
Fuad Khan,*
,
A
Mario Manalo,*
,- Jian Jiang,- Rosa Cuellar,- Sergio Reyes,-
Susana Acosta,- Maritza Jauregui,- Maria Diaz,- Margarita Sanchez,-
Andrew C. Todd,A and Calvin Johnson*
,-
*Environmental Research Center and -Department of Anesthesiology, Drew University of Medicine and Science, 1621 East 120th Street,
Los Angeles, California 90059; ?Instituto Nacional de Salud PuHblica, Cuernavaca, MeHxico; and
A
Department of Community and Preventive Medicine, Mount Sinai School of Medicine, New York, New York
Received February 25, 1999
We examined bone lead contribution to blood lead
in a group of 311 immigrant women, 99% from Latin
America, during the third trimester of pregnancy
and 1 to 2 months after delivery. We measured
in
vivo tibia and calcaneus (heel) bone lead concentra-
tion in the postdelivery period with K shell X-ray
Buorescence. Prenatal and postnatal geometric
mean (range) blood lead level was 2.2
lg/dL (0.4 to
38.7) and 2.8
lg/dL (0.4 to 25.4), reBecting low cur-
rent exposure. Postnatal blood lead level was signif-
icantly higher than prenatal (
P < 0.0001). Mean
(range) tibia and calcaneus lead concentration was
6.7
lg/g (233.7 to 62.2) and 8.4 lg/g (2 30.1 to 66.4),
re
Becting varying but elevated past lead exposure.
Mean calcaneus lead concentration was signi
A-
cantly higher than mean tibia lead concentration
(
P
5 0.055). Variance-weighted multiple regression
and structural equation models showed that both
calcaneus and tibia lead were directly associated
with prenatal blood lead but only calcaneus lead
was associated with postnatal blood lead. Increas-
ing natural log years in the United States indepen-
dently predicted decreasing calcaneus and third-
trimester blood lead. The data suggest that while
some exogenous lead sources and modulators of
blood lead level, such as use of lead-glazed pottery
and calcium in the diet, control lead exposure dur-
ing and after pregnancy, endogenous lead sources
from past exposure before immigration continue to
in
Buence blood lead levels in this sample.
( 2000
Academic Press
Key Words : blood lead; bone lead; pregnancy; cal-
cium; Latina.
INTRODUCTION
Fetal development is altered by toxic exposure of
the mother during pregnancy. Controlling such ex-
posure before and during pregnancy can protect the
fetus from teratogenic effects. Bone is one of the
reservoirs for lead in the body. It has been suggested
that bone stores of lead might be readily mobilized
into circulation during pregnancy and lactation
(Manton, 1985; Silbergeld 1991). There is indirect
evidence that bone lead stores reenter circulation
and raise blood lead levels outside of pregnancy
(Gulson et al., 1995), raise circulating maternal
blood lead levels during pregnancy, raise blood lead
levels of the umbilical cord at term, and raise post-
partum blood lead of the mother (Manton, 1985;
Rothenberg et al., 1994; Rothenberg et al., 1996;
Gulson et al.,1998). Indirect evidence of the contri-
bution of bone lead to blood lead level in postpartum
lactating women has recently been published (Her-
nandez-Avila et al., 1996; Hu et al., 1996). Other
work using blood lead isotope ratios strongly impli-
cates bone lead as the source for more than 30% of
blood lead in a small group of pregnant women (Gul-
son et al., 1997). As lead in bone has a half-life of the
order of years to decades (Steenhout, 1982; Chris-
tofferson et al., 1987), its effects on health may far
outlast the end of signi
7cant exogenous lead expo-
sure.
81
0013-9351/00 $35.00
Copyright
( 2000 by Academic Press
All rights of reproduction in any form reserved.

2
Abbreviations used: SEM, structural equation modeling;
lg
pb
/g
bm
, micrograms of lead per gram of bone mineral;
lg
pb
/g
plaster
,
micrograms of lead per gram of plaster in phantoms; PbB, blood
lead level; PbBone, bone lead concentration; KDMC, King/Drew
Medical Center.
This study examines the contribution of postpar-
tum bone lead concentration in cortical and trabecular
bone measured by in vivo K shell X-ray
8uorescence
to both contemporaneous and third trimester blood
lead levels in a sample of 311 immigrant, predomi-
nantly Latina women in South Central Los Angeles.
MATERIALS AND METHODS
Subjects
We recruited subjects attending the outpatient
prenatal clinics at the King/Drew Medical Center
(KDMC)
2
in South Central Los Angeles. South Cen-
tral Los Angeles is one of the most economically
depressed areas in the state of California. KDMC is
a county hospital serving primarily low-income in-
ner-city minorities who lack the economic means to
purchase private medical care or medical insurance.
The Institutional Review Board (IRB) reviewed and
approved all procedures.
We contacted subjects in the third trimester
[mean (SE) number of days before delivery, 71.2
(1.5)] of pregnancy from June 1995 through July
1998. We distributed educational materials to sub-
jects and counseled them on the risks of lead expo-
sure and on how to reduce their exposure. Subjects
with greater than 10
lg/dL blood lead level (N"44)
were referred to the Los Angeles County Lead Pre-
vention treatment program for follow-up. The 2209
subjects who agreed to participate by giving an IRB-
approved informed consent represented 78% of total
available patients at the prenatal clinics.
Previously published data from this cohort
(Rothenberg et al., 1999) showed that immigrant
subjects were signi
7cantly older and had higher pre-
natal blood lead level than nonimmigrant subjects.
The same study also showed that predictors of pre-
natal blood lead level of immigrant and nonimmig-
rant subjects were signi
7cantly different. Lead level
of immigrant women was signi
7cantly associated
with duration of residence in the United States, age,
secular trend, seasonal trend, pica, coffee drinking,
dietary calcium, and use of folk remedies. Age, secu-
lar and seasonal trends, and folk remedy use were
also signi
7cantly associated with blood lead level in
nonimmigrant women but cigarette smoking was
the only other signi
7cant variable in this group. This
paper reports results only of immigrant subjects
(95.1% of total subjects returning for postnatal bone
lead measurements). Of recruited immigrant pa-
tients 1441 (68.6%) delivered at KDMC and 312
(14.9%) returned within 2 to 10 weeks for their
7rst
postpartum visit. All but 3 of these patients identi-
7ed themselves as Latina.
We administered a screening questionnaire and
drew a venous blood sample for blood lead analysis
at recruitment. At the postpartum visit [mean (SE)
number of days after delivery, 60.2 (2.1)] we admin-
istered a risk questionnaire, drew blood for blood
lead analysis and measured cortical (tibia) and tra-
becular (calcaneus) bone lead concentration. Bone
lead was not measured during pregnancy to avoid
exposure of the fetus to ionizing radiation.
Blood Lead Measurements
Blood samples were drawn into blue-top (trace
metal-free) Becton}Dickinson
Vacutainers with
heparin after thorough cleaning of the venipuncture
site. Blood samples were analyzed with a Perkin}
Elmer 4100ZL Zeeman atomic absorption spectrom-
eter with graphite furnace, correcting for back-
ground interference. Each sample was analyzed in
duplicate and the means of the duplicates were used
as data. Our laboratory has participated successfully
in both the Centers for Disease Control and Preven-
tion (now Wisconsin State Laboratory of Hygiene
Pro
7ciency Testing program) and the College of
American Pathology blood lead quality assurance
programs for 4 consecutive years without any out-of-
bounds measurements.
Accuracy of blood lead determinations was as-
sessed using blood lead values published by the ref-
erence laboratories of the CDC quality assurance
program during the period of data analysis. We
grouped the reference values into three ranges:
1}10
lg/dL (low), 11
}
20
lg/dL (medium), and 20
}
38
(high)
lg/dL. We calculated mean and standard er-
ror of unsigned deviations of our analyses from the
target values for each of these ranges. Mean (SE)
unsigned deviations were 0.4 (0.1)
lg/dL for low, 0.6
(0.1)
lg/dL for medium, and 1.2 (0.2) lg/dL for high
target ranges.
Precision of blood lead determination was as-
sessed using the unsigned difference of the duplicate
analysis for each sample processed during the data
analysis period. Mean (SE) unsigned difference be-
tween duplicate values for all samples was 0.2
(0.007)
lg/dL. We performed similar calculations on
samples whose mean values ranged between 2.0 and
3.0
lg/dL inclusive. This range encompassed the
mean, geometric mean, and median values for the
82
ROTHENBERG ET AL.

group. Mean (SE) unsigned difference between du-
plicate values for this restricted range of samples
was 0.2 (0.01)
lg/dL.
Blood samples were analyzed within 24 h of collec-
tion over the course of the study. Inspection of qual-
ity assurance and internal control data indicated no
systematic trend over the study period. Coefficients
of variation calculated from samples on a weakly
basis showed mean values that varied around 3.2%
across the study period.
Questionnaires
We used screening and risk-factor questionnaires
to gather basic socioeconomic and demographic in-
formation, medical and reproductive history, history
of lead exposure, and dietary habits. Risk factors
included work and hobby histories, use of leaded
paint and ceramic ware, pica, cigarette, alcohol, and
other drug use. Use of low-temperature ceramic
ware and folk remedy use was assessed by asking
about current use and coding the response as
a
dichotomous
variable.
Dietary
information
centered on consumption of high-calcium foods, oils,
and other fats. Physical activity levels were also
assessed. As part of this assessment, we asked the
subject how many hours/day in the past week were
spent in bed and in light, moderate, and heavy phys-
ical activity (all de
7ned by example), restricting the
sum of total hours to 24. Number of hours for each
activity was used in model testing. This question-
naire was used in a previously published study
(Rothenberg et al., 1999).
Questions regarding diet were based on frequency
of consumption over the past month. We coded die-
tary variables with a three point scale: never to less
than once per week, one to two times per week, and
greater than two times per week. We later recoded
these variables dichotomously into use less than
once per week (coded zero) and at least once a week
(coded one) categories, because few subjects reported
at the intermediate level. Most subjects either did
not use these dietary items at all or tended to use
them daily. Dietary variables related to calcium
(milk, yogurt, ice cream, and cheese) were similarly
combined. No subject used calcium supplements.
Years of education was a count variable, while work
history, hobby, medication, disease, and drug use
variables were dichotomous yes/no. Pregnancy and
lactation history variables were coded both as counts
(number of pregnancies, deliveries, number of chil-
dren breast fed) and as dichotomous variables (pre-
vious, coded one; no previous, coded zero).
We assessed pregnancy and lactaction history by
asking for the number of prior pregnancies, number
of known abortions, number of still births, if the
subject ever nursed, and if so how many children
were nursed for how many months. Variables tested
in models were the dichotomous ‘‘ever nursed’’, total
number of children nursed, nursed in the immedi-
ately past pregnancy (yes/no, and number of months
up to testing), total months of nursing, plus the
number of pregnancies, abortions, and still births.
Questionnaires were presented as structured in-
terviews by trained bilingual interviewers in the
language of the subject’s choice (Spanish or English).
Duplicate entry of the responses into the computer
data base minimized transfer error.
Bone Lead Measurements
We measured bone lead concentration at mid-tibia
(cortical bone) and mid-calcaneus (trabecular bone)
with a
109
Cd K-shell X-ray
8uorescence (KXRF) sys-
tem described previously (Todd et al., 1992, 1993).
The technique uses photons emitted from the decay
of
109
Cd (88 keV) to excite lead atoms within the bone
matrix, causing them to emit characteristic X rays.
The amount of coherent scattering of the 88-keV
109
Cd photons is proportional to the amount of bone
mineral. By normalizing the lead X-ray signal to the
coherent signal we can specify the measurment in
micrograms of lead per gram of bone mineral
(
lg
pb
/g
bm
). The technique allows estimation of the
error of measurement, which is equivalent to one
standard deviation.
Prior to measurement, the subject removed all
metal and plastic objects. We washed the skin above
each bone site and then thoroughly cleaned it with
4% glacial acetic acid to remove any super
7cial con-
tamination. The collimated beam of photons from
the
109
Cd source was directed orthogonally to the
bone surface at each site. We measured each bone
site for 30 min. Subjects were comfortably seated
with the leg restrained.
Nonlinear least squares
7tting (Marquardt algo-
rithm) of the spectra provided unbiased estimates of
lead concentration and of error of measurement. The
algorithm provides a
s
2
goodness of
7t statistic.
We calibrated the system every 2 weeks or after
any change to the system (e.g., detector warm-up,
change of spectroscopy settings) with a graded series
of plaster of paris tibia phantoms doped with known
amounts of lead. A complete line of phantom
measurements consisted of measuring the phan-
toms, ranging from a nominal zero
lg
pb
/g
plaster
to
220
lg
pb
/g
plaster
, a total of 20 times. Three quality
BONE LEAD AND BLOOD LEAD DURING PREGNANCY
83

TABLE 1
Variables Tested in Bone Lead Modeling
Age*
Years resident in United States*
Prenatal blood lead level*
Postnatal blood lead level*
Smoking
Alcohol use
Pica
Use of folk remedies*
Coffee drinking
Other caffeine beverages
Occupational exposure (various)
Hobby exposure (various)
Low temperature ceramic ware use
Currently use*
Frequency of use
Years of use
Use to prepare (a) food; (b) sauces; (c) drinks
Use to store foods
Used in childhood home
Diet
Frequency of fat intake
Frequency of calcium intake*
Activity
Hours in bed*
Hours in light activity
Hours in medium activity
Hours in heavy activity
Number of city blocks walked
Number of stairs climbed
Pregnancy of lactation history
Number of pregnancies
Number of abortions
Number of still births
Ever nursed
Number of children nursed
Total months nursed
Presently nursing
*Signi
7cant at P\0.10.
control phantoms were run during every subject
measurement day to detect out-of-control-range
peak ratios. Calibration lines were used for subjects
measured from the start of the collection of the line
until the start of the next line.
Data Analysis
We used Statgraphics Plus (Manugistics, Inc.,
Rockville, MD), SPSS (SPSS, Inc., Chicago, IL), Amos
(SmallWaters Corp., Chicago, IL), and Stata (Stata
Corp., East College Station, TX) for data manage-
ment and analysis. We transformed blood lead level
variables into their natural logarithms to reduce the
effects of outliers on subsequent statistical analyses.
Descriptive statistics and graphics provided quality
control for all variables. We removed one subject with
a blood lead level of 81
lg/dL, 5.8 geometric standard
deviations above the sample mean.
Univariate and bivariate analyses of a priori
selected predictor variables against the various lead
variables with P
\0.10 probabilities determined the
variables that were made available in multiple re-
gression analyses of the lead variables. We construc-
ted several models, including tibia and calcaneus
lead concentration with and without weighting for
bone lead error terms. Models were constructed by
forward stepwise and by backward elimination tech-
niques. The two models presented contained the
same terms using either elimination technique. We
used variance-weighted least squares regression
(Stata Corporation, 1997) for modeling bone lead
concentration, weighting each bone lead measure-
ment by its respective measurement error. Table 1
shows the variables considered for entry into mod-
els. Most variables tested were those previously
shown to be associated with blood lead levels or bone
lead concentration in the population from which this
sample was derived or in similar groups.
We used exploratory structural equation modeling
(SEM) with maximum likelihood estimation (Ar-
buckle, 1997) to determine if dietary calcium made
independent contributions to blood lead level and
bone lead concentration. We also used SEM to ex-
plore alternate models of the effect of bone lead
concentration on pre- and postnatal blood lead
levels. An acceptable model contained all signi
7cant
(P
\0.10) variables and had an overall nonsigni7-
cant
s
2
(P
[0.10).
RESULTS
Subjects ranged in age from 15 to 44 years, and
their education level ranged from 0 to 17 years.
Subjects returning for bone lead tests were signi
7-
cantly older, had spent signi
7cantly more years in
the United States, and were more likely to use folk
remedies than subjects not returning for bone lead
tests, though the differences between the two groups
were small (Table 2). There were no other differ-
ences between those returning and those not return-
ing.
Geometric mean (#/!geometric SD) postnatal
blood lead level was 2.8 (#4.9/!1.2)
lg/dL with
a range of 0.4 to 25.4
lg/dL. The increase in blood
lead from prenatal to postnatal was signi
7cant
(P
\0.0001). Prenatal and postnatal blood lead
(natural log transformed) were signi
7cantly corre-
lated (r"0.76, P
\0.0001).
84
ROTHENBERG ET AL.

TABLE 2
Subject Characteristics
Sample returning
Sample not returning
N"311
N"1697
Variable
Mean SD
Mean SD
Probability (t test)
Age
27.8
7.5
26.1
6.1
(
0.001
Education
8.5
3.5
8.6
3.2
'
0.10
*Prenatal blood Pb
2.2
#
4.8/!1.0
2.2
#
3.1/!1.4
'
0.10
*Years in United States
5.9
#
14.2/!2.4
5.4
#
13.8/!2.1
0.058
Hours in bed
8.6
2.2
8.8
2.0
'
0.10
%
Yes
%
Yes
s
2
Use folk remedies
6.4
4.3
0.078
Adequate calcium
54.5
52.0
'
0.10
Use clay pottery
7.0
7.9
'
0.10
*Geometric means and standard deviations.
TABLE 3
Variance-Weighted Multiple Regression Model of Calcaneus
Bone Lead Concentration (
lg
pb
/g
bm
) (
N
5 311)
Coef.
Prob.
95% CI
ln (years in United States) !2.10
0.001
!
3.32
!
0.89
Age (years)
0.14
0.067
!
0.01
0.30
Constant
8.11
(
0.001
3.70
12.51
TABLE 4
Variance-Weighted Multiple Regression Model of Tibia
Bone Lead Concentration (
lg
pb
/
g
bm
) (
N
5 311)
Coef.
Prob.
95% CI
Daily hours in bed
0.51
0.033
0.04
0.99
Use folk remedy
7.18
0.003
11.96
2.40
Use leaded pottery
4.02
0.077
!
0.44
8.48
Constant
8.75
0.175
!
3.89
21.39
Mean (SD) [range] tibia lead concentration was
6.7 (12.5) [!33.7 to 62.2]
lg
pb
/g
bm
. Mean (SD) [range]
calcaneus lead concentration was 8.4 (13.2) [!30.1
to 66.4]
lg
pb
/g
bm
and was signi
7cantly higher than
tibia lead concentration (P"0.055); 3.5% of cal-
caneus and 4.8% of tibia measurements had uncer-
tainties greater than 15
lg
pb
/g
bm
. The two bone lead
measurements
were
signi
7cantly
correlated
(r"0.244, P
\0.001).
Tables 3 and 4 show the variance-weighted least
squares multiple regression models of tibia and cal-
caneus lead. Increased tibia lead in the sample was
related to lead-glazed ceramic ware use, use of folk
remedies, and daily number of hours spent in bed
during the pregnancy. Increased calcaneus lead was
related to higher maternal age and fewer years resi-
dent in the United States. (Fig. 1).
Table 5 shows the system of simultaneous equa-
tions used to build the structural equation model
shown in Fig. 2. The coef
7cients in Table 5 are based
on original measurement units. The coef
7cients
shown in Fig. 2 are normalized to allow direct-effect
size comparison among variables without reference
to measurement units. A distribution-free, 1000-
sample bootstrap estimation (not shown) revealed
negligible bias among coef
7cients and their standard
errors, justifying normalization.
Both tibia lead and calcaneus lead were positively
associated with third trimester blood lead, each with
approximately equal effect size, but only calcaneus
lead was associated with postnatal blood lead. In-
creasing years of residence in the United States was
associated with both decreasing calcaneus lead and
third trimester blood lead, though the effect size on
blood lead was twice the effect size on calcaneus
lead. Dietary calcium was associated only with third
trimester blood lead, and use of lead-glazed ceramic
ware was associated only with postnatal blood lead.
Increased time in bed was associated with increased
tibia lead but with decreased third trimester blood
lead.
It is noteworthy that neither pregnancy nor lacta-
tion history was associated with bone lead concen-
tration in this data set.
DISCUSSION
Published studies from Mexico City (Hernandez-
Avila et al., 1996) and Boston (Hu et al.,1996) have
BONE LEAD AND BLOOD LEAD DURING PREGNANCY
85
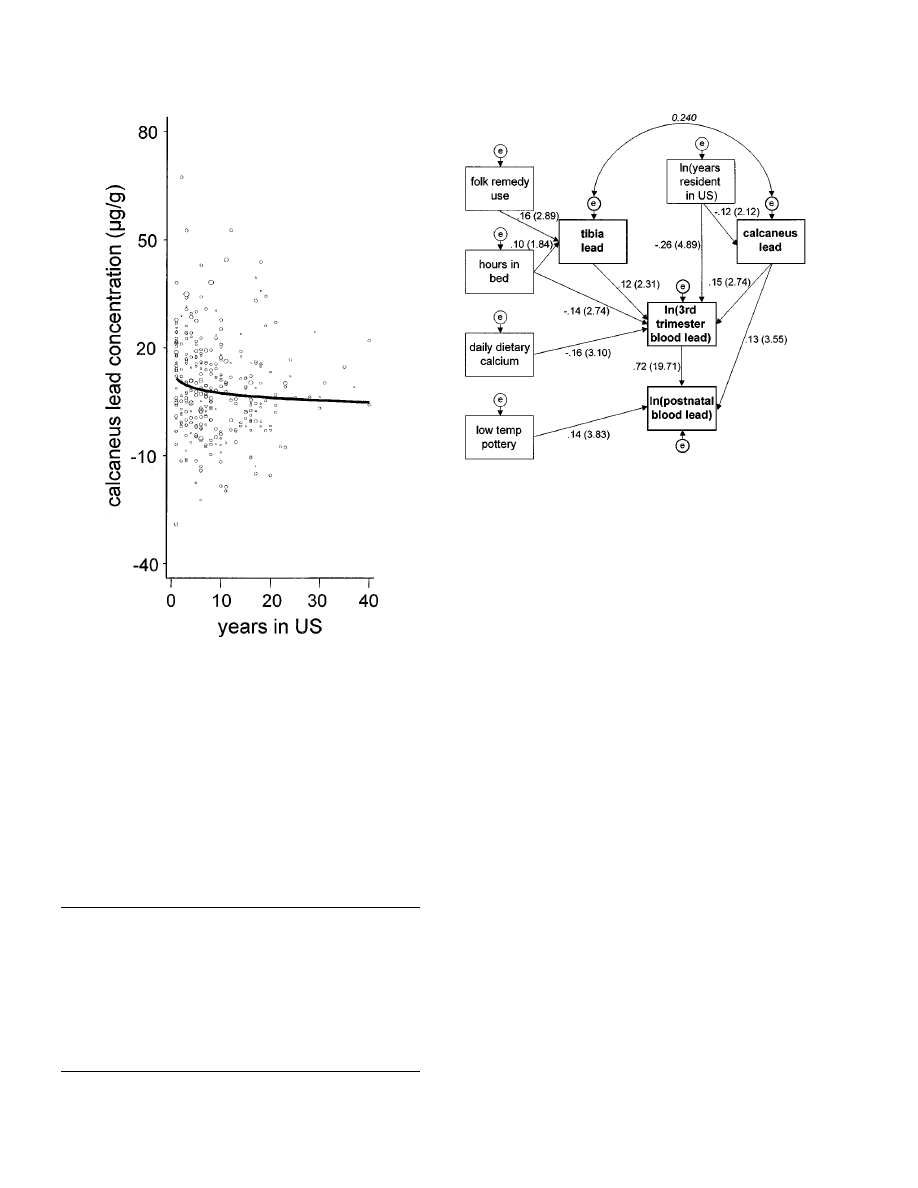
FIG. 1.
Natural logarithmic relationship between years resi-
dence in the United States and calcaneus lead concentration
adjusted for age in the variance-weighted multiple regression.
Size of the circle is directly proportional to the measurement error
of calcaneus lead concentration.
TABLE 5
Simultaneous Equations for Structural Equation Model
Calcaneus Pb
"!
1.63* ln (years in United States)#C
Tibia Pb
"
0.62* (hours in bed)
#
8.80* (use folk remedy)#C
ln (blood Pb pre) "0.009* (calcaneus Pb)#0.008* (tibia Pb)
!
0.51* (daily Ca)
!
0.21* ln (years in United States)
!
0.06* (hours in bed)#C
ln (Blood Pb post) "0.60* ln (blood Pb pre)
#
0.006* (calcaneus Pb)
#
0.35* (lead-glazed pottery)#C
FIG. 2.
Structural equation model of bone lead and blood lead.
Each unidirectional arrow represents a directional effect of one
variable (tail) on another variable (head). The numbers beside
each arrow represent the standardized regression weight and, in
parentheses, the z score of the regression weight in the model.
A z score of 1.96 is equivalent to a probability of approximately
P"0.05, assuming a very large N. The curved two-headed arrow
represents a covariance relationship and the number in italics
above it is the correlation coef
7cient. Each ‘‘e’’ represents an
unobserved error term,
7xed at 1. The model s
2
df"24
"
30.24,
P"0.18.
examined the relationship between bone lead con-
centration and blood lead levels in postpartum
women. The Mexico City study measured blood lead
in lactating women 1 month after delivery at a mean
level of 9.6
lg/dL. The mean tibia and patella bone
lead concentrations were 12.5 and 13.3
lg
pb
/g
bm
, re-
spectively. These data suggest moderate past and
continuing lead exposure.
The Boston study examined women 3 to 6 months
postpartum and found a geometric mean blood lead
level of 3.0
lg/g. Mean tibia and patella bone lead
concentrations were 4.5 and 5.8
lg
pb
/g
bm
, respective-
ly, These data suggest low past and present lead
exposure.
The geometric mean blood lead level from our
study group is nearly the same as that of the Boston
group but mean bone lead concentrations fall mid-
way between the Boston and the Mexico city study
groups. Our data suggest moderate past lead expo-
sure but low current exposure, congruent with the
immigrant status of our study group.
Blood Lead and Bone Lead
Prenatal blood lead level is a strong predictor of
postnatal blood lead level. The signi
7cant increase
86
ROTHENBERG ET AL.

in blood lead level between prenatal and postnatal
measurements may be explained by changes in he-
matological dilution, body weight, and organ size
after delivery. In the
7rst months after pregnancy
these variables return to near prepregnancy values,
the fetus is no longer a sink for lead in the mother,
and blood lead levels, assuming lead sources are
constant, are expected to rise.
We have previously suggested (Rothenberg et al.,
1999) that the immigrant subjects in this study have
higher third trimester blood lead levels than the
nonimmigrant subjects due to past exposure before
immigration. The immigrants, almost all from Latin
America (and principally from Mexico), have lived
much of their lives in environments in which their
exposure was higher than it presently is in the
United States. Thus, most subjects would experience
a sharp drop in exogenous lead exposure upon
immigration. With the short half-life of lead in blood
(around 30}36 days), a sustained elevation in blood
lead after immigration would require continued ex-
posure to exogenous or endogenous sources of lead.
In the previous study (Rothenberg et al., 1999) we
documented the in
8uence of exogenous factors, such
as use of folk remedies, pica, and dietary calcium on
increased prenatal blood lead level of immigrants.
However, we found that the strongest in
8uence on
prenatal blood lead came from maternal age and
years resident in the United States. We suggested
that both factors were surrogates for mobilization of
lead from bones. As lead in bone has a half-life
measured in years, lead tends to accumulate in bone
with age (Drasch et al., 1987; Manea-Krichten et al.,
1991; Kosnett et al., 1994; Hu et al., 1996), even with
very low daily exposure. Similarly, after the end of
elevated lead exposure, lead in bone decreases with
time (Borjesson et al., 1997). The
7ndings of positive
association of prenatal blood lead level with age and
negative association of prenatal blood lead level with
natural log years residence in the United States lend
support to the hypothesis that maternal bone lead is
an endogenous source of lead in blood.
The multiple regression model for calcaneus lead
concentration shows the pattern that one would ob-
serve if lead in bone increases with age and de-
creases with time after the end of a period of
elevated exposure. The investigation of postpartum
mothers in Mexico City showed a similar in
8uence of
time from the start of high exposure (number of
years resident in Mexico City) on lead in the patella
(trabecular bone) (Hernandez-Avila et al., 1996). The
lack of effect of dietary calcium on calcaneus lead in
the present study, however, contrasts with the sig-
ni
7cant effect of calcium on patella lead in the
Mexico City study. This could be due to varying
contributions of different levels of baseline dietary
calcium in the two countries, differences in current
exogenous lead exposure, and possibly the difference
in trabecular bone measured. Though both cal-
caneus and patella are trabecular bone, the cal-
caneus is subject to considerably more physical
stress than would be expected for patella. The re-
peated stress incurred by the calcaneus during walk-
ing or running might well produce metabolic
differences between calcaneus and patella (Felson et
al., 1993). As the present study had over three times
the number of subjects as the Mexico City study, it is
unlikely that low power in the present study led to
insigni
7cant results.
The Mexico City study found that only dietary
calcium was associated with tibia bone lead. We
found a different pattern of results. Folk remedy use,
hours in bed, and use of lead-glazed pottery were all
associated with increased tibia lead. The use of lead-
containing folk remedies by people of Latin Ameri-
can origin is well documented (Baer et al., 1988;
Fernandez et al., 1997; Rothenberg et al., 1999), as is
the contribution to blood lead of using lead-glazed
pottery (Hernandez Avila et al., 1991; Rothenberg et
al., 1992, 1996, 1998; Matte et al., 1994; Rojas Lopez
et al., 1994; Romieu et al., 1994; Fernandez et al.,
1997). The number of hours in bed variable, on the
other hand, requires additional comment. Bone
metabolism is altered by physical stress and bone
mineral loss increases with inactivity. If time spent
in bed re
8ects inactivity and lack of bone stress, the
altered metabolic activity induced with changes
associated with pregnancy might lead to increased
accumulation of bone lead. Though the question sol-
iciting number of hours spent in bed directed the
subject to respond based on her past week’s history,
the response could also re
8ect long-term activity
levels, perhaps even predating the study pregnancy.
Integrated Structural Equation Model of Blood
Lead and Bone Lead
Single-equation regression models typically de
7ne
a single dependent variable and one or more inde-
pendent variables. With SEM the investigator uses
a system of simultaneous equations in which the
dependent variable of one equation can appear as an
independent variable of another. A system of equa-
tions allows testing hypotheses of effect direction
that is not possible in single-equation models. Sev-
eral recent publications address these and other
issues relevant to SEM and related techniques
(JoKreskog et al., 1989; Bentler et al., 1996).
BONE LEAD AND BLOOD LEAD DURING PREGNANCY
87

We used SEM analysis to quantify directional re-
lationships between blood lead and bone lead, ac-
counting for other explanatory variables that might
affect one or the other lead variable or both simulta-
neously. We used bone lead measurements 2 months
after pregnancy as a surrogate for bone lead during
pregnancy. The long half-life of lead in bone and the
limited precision of the bone lead measurement
makes it unlikely that there were measurable cha-
nges in bone lead between the third trimester and
the postpartum period.
SEM gives the size, direction, and signi
7cance of
proposed linkages among variables. The model
s
2
goodness of
7t statistic evaluates how well the pro-
posed model
7ts the data set (i.e., if the model s
2
is
signi
7cant, then the proposed model does not 7t
the data, no matter how signi
7cant each of the
coef
7cients describing linkages among variables
may be).
In developing the model shown in Fig. 2, we
started with one strong assumption: blood lead mea-
sured earlier in time controls blood lead measured
later in time and not the reverse. With this direc-
tional relationship
7xed, we tested models in which
bone lead contributed only to postnatal blood lead,
only to prenatal blood lead, or to both. Although
coefficients describing the relationship between
bone lead and postnatal blood lead alone were con-
sistently larger and more signi
7cant than coeffi-
cients between bone lead and prenatal blood lead
alone or both prenatal and postnatal blood lead, the
model
s
2
in the
7rst scenario was highly signi7cant
(P
\0.001), indicating a poor 7t (signi7cant differ-
ence) of model to data.
We modeled the relationship between calcaneus
and tibia lead concentration as a nondirectional
covariance term. The shorter half-life of calcaneus
lead suggests that calcaneus lead may be more labile
than tibia lead. Thus, calcaneus lead might be more
easily liberated from the bone and so contribute to
tibia lead. Uptake of lead by calcaneus from
exogenous and endogenous sources may be more
ef
7cient than uptake by tibia. We tested alternate
models in which we speci
7ed each directional rela-
tionship between calcaneus and tibia lead conentra-
tion. The two directional models and the covariance
model gave equivalent results, both in terms of size
and signi
7cance of relationships among other vari-
ables, as well as in the size of the model
s
2
. Failing to
specify any relationship between the two bone lead
measures resulted in a model with a signi
7cant s
2
(P"0.003). As there was no clear advantage gained
by selecting a directional relationship between the
two bone lead measures and little literature to guide
us, we performed all further modeling with the non-
directional covariance relationship.
The most interesting relationships among vari-
ables described by SEM are those in which one
variable is signi
7cantly associated with two other
signi
7cantly associated variables. These relation-
ships can be found in Fig. 2 by looking for variables
whose relationships form triangles. We will discuss
two of these relationships in the integrated model:
those involving years in United States, calcaneus
lead, and prenatal blood lead and those involving
calcaneus lead and prenatal and postnatal blood
lead.
We hypothesize that many immigrants in the
study experienced a step decrease in environmental
lead exposure upon moving to the United States. If
this were so, immigration was clearly not accom-
panied by a step decrease in blood lead. Instead,
prenatal blood lead level decreased as a function of
log years in the United States. Calcaneus bone lead
in the variance-weighted multiple regression model
decreased with the natural logarithm of years in the
United States. Thus, calcaneus lead contribution to
blood lead level might explain the effect of years
residence on blood lead. In the SEM we observe
a signi
7cant path from the years variable to cal-
caneus lead and thereafter to third trimester blood
lead, as predicted. However, we see that the original
relationship between years of residence and third
trimester blood lead is still signi
7cant, despite hav-
ing accounted for the contribution of calcaneus lead
to blood lead. Removing any leg of this triangular
relationship results in signi
7cant model s
2
s, without
substantial change in the remaining coefficients.
This pattern of results suggests that not all of the
effect of years residence on third trimester blood
lead can be due to the effect of decreasing bone lead
after immigration. Some signi
7cant part of the per-
sistence and slow decrease in prenatal blood lead
level after immigration must be due to one or more
unobserved variables, perhaps acculturation that
slowly reduces exposure to culturally determined
lead sources.
Calcaneus lead concentration independently ac-
counts for some variance in prenatal and postnatal
blood lead level, despite the strong direct association
between the two blood lead measures. However,
tibia lead concentration is signi
7cantly associated
only with prenatal blood lead level. The Mexico City
study (Hernandez-Avila et al., 1996) found a signi
7-
cant association between their measure of trabecu-
lar bone lead (patella) and postnatal blood lead also,
without a signi
7cant association between tibia lead
and blood lead. To directly compare results between
88
ROTHENBERG ET AL.

the two studies, we provisionally constructed a mul-
tiple regression model for nontransformed postnatal
blood lead (the variable used in the Mexico City
study) with calcaneus lead and lead-glazed ceramic
ware use as independent variables. We obtained
a calcaneus coef
7cient of 0.071, compared to their
patella coef
7cient of 0.06. The coef7cients of trabecu-
lar lead on postnatal blood lead in the two studies
are the same.
When we simultaneously account for the in
8uence
of prenatal blood lead on postnatal blood lead the
relationship between calcaneus lead concentration
and postnatal blood lead level remains. The different
patterns of effect of tibia and calcaneus lead on pre-
and postnatal blood lead might be due to the relative
lability of the two bone lead sources. During the last
trimester of pregnancy the fetus experiences rapid
skeleton growth. The high calcium demand on the
mother may serve to mobilize lead from both cortical
and trabecular bones to a similar degree. The effect
of dietary calcium on prenatal blood lead level but
not postnatal blood lead level also suggests that high
calcium demand in the absence of adequate dietary
calcium may augment release of blood from bone
during the last trimester of pregnancy. We found no
effect of nursing history, nor of presence or absence
of nursing after the studied pregnancy, on either
bone lead or blood lead. Although precision of bone
lead measurement, discussed above, may have
played a role in our failure to
7nd signi7cant effects
of pregnancy and lactation history on bone lead con-
centration, it is possible that there was signi
7cantly
more calcium stress during pregnancy than during
lactation in our sample. If calcium demand and sub-
sequent lead mobilization from bone was less after
pregnancy than during pregnancy in our sample, the
more easily mobilized trabecular bone lead might be
the only measurable bone lead effect on postnatal
blood lead level.
Although other authors have found increasing age
associated with increasing bone lead, as did we in
the variance-weighted regressions above, we could
not place age into the SEM model. Every alternate
model with age associated with either or both of the
bone lead or the blood lead variables had a signi
7-
cant
s
2
value (all probabilities
\0.006), indicating
that the data did not
7t the hypothesized models.
Some of the problem may reside in the nature of our
sample, in which 23.5% of the subjects were 21 years
of age or younger. In another study sample with
a large age range, the authors found that the age
effect on bone lead was limited to subjects over 21
years (Kosnett et al., 1994). Another in
8uence might
be that years in the United States and age are highly
correlated. Despite accounting for that covariance in
the test SEM models, the collinearity between the
two variables might have been responsible for the
poor model
7t. It is likely that a bone lead model
of nonimmigrants, in which time resident in the
United States is not a factor, will contain a signi
7-
cant age term.
CONCLUSIONS
Bone lead is a signi
7cant source of lead in preg-
nant immigrant women and remains so in the early
postnatal period. As long-term lead exposure elev-
ates bone lead, and lead exposure before immigra-
tion remains beyond control of national health
authorities, the bone lead contribution to circulating
lead may be dif
7cult to control. As long as population
lead exposure remains unregulated in other parts of
the world, endogenous stores of lead will continue to
in
8uence circulating blood lead levels during and
after pregnancy in women of all nations. It is clear,
however, that other sources, both observed in this
study and not observed, play an important role in
continuing lead exposure during and after preg-
nancy. Dietary calcium and use of lead-glazed ce-
ramic ware in
8uence prenatal and postnatal blood
lead level, respectively, and these modulators of
blood lead level are amenable to control. Further
study of the relationship between years of residency
in the United States and prenatal blood lead level
might reveal other controllable factors contributing
to maternal lead exposure during pregnancy.
ACKNOWLEDGMENTS
We thank Thomas Yoshikawa, MD; Teiichiro Fukushima, MD;
Tom Carter; Vincent de Ciutiis, MD; the staff of the prenatal
clinics and delivery service at the King/Drew Medical Center; and
the women who served as subjects, all of whom contributed to the
success of this project.
REFERENCES
Arbuckle, J. (1997). ‘‘Amos Users’ Guide, Version 3.6,’’ Small-
Waters Corp. Chicago, IL.
Baer, R. D., and Ackerman, A. (1988). Toxic Mexican folk reme-
dies for the treatment of empacho: The case of azarcon, greta,
and albayalde. J. Ethnopharmacol. 24, 31}39.
Bentler, P., and Dudgeon, P. (1996). Covariance structure analy-
sis: Statistical practice, theory, and directions. Annu. Rev.
Psychol. 47, 541}570.
BONE LEAD AND BLOOD LEAD DURING PREGNANCY
89

Borjesson, J., Gerhardsson, L., Schutz, A., Mattsson, S., Sker
fving, S., and Osterberg, K. (1997). In vivo measurements of
lead in
7ngerbone in active and retired lead smelters. Int. Arch.
Occup. Environ. Health 69, 97}105.
Christofferson, J. O., Schuts, A., Skerving, S., Ahlgren, L., and
Mattson, S. (1987). Decrease of skeletal lead after end of occu-
pational exposure. Arch. Environ. Health 41, 312}318.
Drasch, G. A., Bohm, J., and Baur, C. (1987). Lead in human
bones. Investigations on an occupationally non-exposed popula-
tion in southern Bavaria (F.R.G.). I. Adults. Sci. Total Environ.
64, 303}315.
Felson, D. T., Zhang, Y., Hannan, M. T., and Anderson, J. J.
(1993). Effects of weight and body mass index on bone mineral
density in men and women: The Framingham study. J. Bone
Miner. Res. 8, 567}573.
Fernandez, G. O., Martinez, R. R., Fortoul, T. I., and Palazuelos,
E., (1997). High blood lead levels in ceramic folk art workers in
Michoacan, Mexico. Arch. Environ. Health 52, 51}55.
Gulson, B. L., Jameson, C. W., Mahaffey, K. R., Mizon, K. J.,
Korsch, M. J., and Vimpani, G. (1997). Pregnancy increases
mobilization of lead from maternal skeleton J. Lab. Clin. Med.
130, 51}62.
Gulson, B. L., Mahaffey, K. R., Jameson, C. W., Mizon, K. J.,
Korsch, M. J., Cameron, M. A., and Eisman, J. A. (1998).
Mobilization of lead from the skeleton during the postnatal
period is larger than during pregnancy. J. Lab. Clin. Med. 131,
324}329.
Gulson, B. L., Mahaffey, K. R., Mizon, K. J., Korsch, M. J.,
Cameron, M. A., and Vimpani, G. (1995). Contribution of tissue
lead to blood lead in adult female subjects based on stable lead
isotope methods. J. Lab. Clin. Med. 125, 703}712.
Hernandez Avila, M., Romieu, I., Rios, C., Rivero, A., and
Palazuelos, E. (1991). Lead-glazed ceramics as major determi-
nants of blood lead levels in Mexican women. Environ. Health
Perspect. 94, 117}120.
Hernandez-Avila, M.,
Gonzalez-Cossio, T.,
Palazuelos, E.,
Romieu, I., Aro, A., Fishbein, E., Peterson, K. E., and Hu, H.
(1996). Dietary and environmental determinants of blood and
bone lead levels in lactating postpartum women living in
Mexico City. Environ. Health Perspect. 104, 1076}1082.
Hu, H., Hashimoto, D., and Besser, M. (1996). Levels of lead in
blood and bone of women giving birth in a Boston hospital.
Arch. Environ. Health 51, 52}58.
Hu, H., Payton, M., Korrick, S., Aro, A., Sparrow, D., Weiss, S. T.,
and Rotnitzky, A. (1996). Determinants of bone and blood lead
levels among community-exposed middle-aged to elderly men.
The normative aging study. Am. J. Epidemiol. 144, 749}759.
JoKreskog, K., and SoKrbom, D. (1989). ‘‘LISREL 7: A guide to the
program and applications,’’ SPSS Inc., Chicago, IL.
Kosnett, M. J., Becker, C. E., Osterloh, J. D., Kelly, T. J., and
Pasta, D. J. (1994). Factors in
8uencing bone lead concentration
in a suburban community assessed by noninvasive K X-ray
8uorescence. JAMA 271, 197
}
203.
Manea-Krichten, M., Patterson, C., Miller, G., Settle, D., and
Erel, Y. (1991). Comparative increase, of lead and barium with
age in human tooth enamel, ribs and ulna. Sci. Total Environ.
107, 179}203.
Manton, W. I. (1985). Total contribution of airborne lead to blood
lead. Br. J. Ind. Med. 42, 168}172.
Matte, T. D., Proops, D., Palazuelos, E., Graef, J., and Hernandez
Avila, M. (1994). Acute high-dose lead exposure from beverage
contaminated by traditional Mexican pottery. Lancet 344,
1064}1065.
Rojas Lopez, M., Santos Burgoa, C., Rios, C., Hernandez Avila, M.
and Romieu, I. (1994). Use of lead-glazed ceramics is the main
factor associated to high lead in blood levels in two Mexican
rural communities. J. Toxicol. Environ. Health 42, 45}52.
Romieu, I., Palazuelos, E., Hernandez Avila, M., Rios, C., Munoz,
I., Jimenez, C., and Cahero, G. (1994). Sources of lead exposure
in Mexico City. Environ. Health Perspect. 102, 384}389.
Rothenberg, S., Manalo, M., Jiang, J., Khan, F., Cuellar, R.,
Reyes, S., Sanchez, M., Reynoso, B., Aguilar, A., Diaz, M.,
Acosta, S., Jauregui, M., and Johnson, C. (1999). Maternal
blood lead level during pregnancy in South Central Los
Angeles. Arch. Environ. Health 54, 151}157.
Rothenberg, S. J., Karchmer, S., Schnaas, L., Perroni, E., Zea, F.,
and FernaHndez Alba, J. (1994). Changes in serial blood lead levels
during pregnancy. Environ. Health Perspect. 102, 151}160.
Rothenberg, S. J., Karchmer, S., Schnaas, L., Perroni, E., Zea, F.,
Salinas, V., and Fernandez Alba, J. (1996). Maternal in
8uences
on cord blood lead levels. J. Expo. Anal. Environ. Epidemiol. 6,
211}227.
Rothenberg, S. J., Schnaas, L., Perroni, E., Hernandez, R., and
Karchmer, S. (1998). Secular trend in blood lead levels in a co-
hort of Mexico City childern. Arch. Environ. Health 53, 231}235.
Rothenberg, S. J., Schnaas}Arrieta, L., Ugartechea, J. C., Per-
roni-Hernandez, E., Perez-Guerrero, I. A., Cansino-Prtiz, S.,
Salinas, V., Zea-Prado, F., and Chicz-Demet, A. (1992).
A documented case of perinatal lead poisoning. Am. J. Public
Health 82, 613}614.
Silbergeld, E. K. (1991). Lead in bone: Implications for toxicology
during pregnancy and lactation. Environ. Health Perspect. 91,
63}70.
Stata Corporation (1997). Variance weighted least squares. In
‘‘Stata Reference Manual,’’ Vol. 3, pp. 523}527. Stata Press,
College Station, TX.
Steenhout, A. (1982). Kinetics of lead storage in teeth and bones:
An epidemiologic approach. Arch. Environ. Health 37, 224}231.
Todd, A. C., and McNeill, F. E. (1993). In vivo measurements of
lead in bone using a 109Cd ‘spot’ source. Basic Life Sci. 60,
299}302.
Todd, A. C., McNeill, F. E., Palethorpe, J. E., Peach, D. E.,
Chettle, D. R., Tobin, M. J., Strosko, S. J., and Rosen, J. C.
(1992). In vivo X-ray
8uorescence of lead in bone using K X-ray
excitation with 109Cd sources: Radiation dosimetry studies.
Environ. Res. 57, 117}132.
90
ROTHENBERG ET AL.
Document Outline
Wyszukiwarka
Podobne podstrony:
Describe the role of the dental nurse in minimising the risk of cross infection during and after the
During and After WWII
Cadmium, chromium, lead, manganese and nickel concentrations in blood of women in non polluted areas
Elaine Bergstrom Blood to Blood (v1 0) (doc)
Glińska, Sława i inni The effect of EDTA and EDDS on lead uptake and localization in hydroponically
Rueda Contribution to inertial mass by reaction of the vacuum to accelerated motion (1998)
AN Increased Osteoprotegerin Serum Release Characterizes The Early Onset of Diabetes Mellitus and Ma
the garden and story a contribution to the theory of garden narrative Content File PDF
Freies Deutschland Guerrilla Warfare in East Prussia, 1944 1945; A Contribution to the History of
Vinay B Kothari Executive Greed, Examining Business Failures that Contributed to the Economic Crisi
The Coptic Contribution to Christian Civ Aziz S Atteya
Brzechczyn, Krzysztof Unsuccessful Conquest and Successful Subordination A Contribution to the Theo
Elaine Bergstrom Blood To Blood
J R R Tolkien Some Contributions to Middle English Lexicography
Russell, Bertrand Has Religion Made Useful Contributions to Civilization
Loyalty in National Socialism, A Contribution to the Moral History of the National Socialist Period
Chomsky N Linguistic Contributions to the Study of Mind
więcej podobnych podstron