TETRAHEDRON
LETTERS
Tetrahedron Letters 44 (2003) 3579–3580
Pergamon
A new direct synthesis of cinnamic acids from aromatic
aldehydes and aliphatic carboxylic acids in the presence of
sodium borohydride
Constantin I. Chiriac,* Fulga Tanasa and Marioara Onciu
Institute of Macromolecular Chemistry
‘Petru Poni’, Aleea Grigore Ghica Voda 41A, Iasi 6600, Romania
Received 23 September 2002; revised 4 February 2003; accepted 21 February 2003
Abstract—Cinnamic acids have been prepared in 59–86% yields by a new direct synthesis from aromatic aldehydes and aliphatic
carboxylic acids in the presence of sodium borohydride and N-methyl-2-pyrrolidinone (NMP) as solvent, at reflux (185–190°C),
for 9–12 hours. Without sodium borohydride, this reaction is not possible. © 2003 Published by Elsevier Science Ltd.
Cinnamic acids can be prepared from aromatic alde-
hydes and aliphatic anhydrides, in the presence of
bases, particularly with sodium or potassium salts of
the carboxylic acids corresponding to the anhydrides
used in the reaction (the Perkin reaction).
1
Thus, potassium acetate can be used for the reac-
tion between acetic anhydride and benzaldehyde,
yields are 70–72% at 180°C in 8 h. With sodium
acetate, the yields are lower under the same condi-
tions.
2
This reaction is not suitable for aliphatic alde-
hydes.
3
If the aliphatic carboxylic anhydrides are replaced by
the corresponding aliphatic carboxylic acids, the reac-
tion is not successful.
We have now found that this reaction is possible with
aliphatic carboxylic acids in the presence of sodium
borohydride.
It is known that the aliphatic carboxylic acids can react
with sodium borohydride resulting in different prod-
ucts. For example, when using acetic acid the following
reactions take place:
Scheme 1.
* Corresponding author.
0040-4039/03/$ - see front matter © 2003 Published by Elsevier Science Ltd.
doi:10.1016/S0040-4039(03)00529-X

C. I. Chiriac et al.
/
Tetrahedron Letters
44 (2003) 3579–3580
3580
Table 1. Cinnamic acids obtained by direct synthesis in the presence of sodium borohydride
Reaction time (h)
M.p.
c
(°C)
Yield
b
(%)
Literature m.p. (°C)
Cinnamic acids
a
10
176–177
III a
175–177
7
74
12
131–133
66
132–133
8
III b
9
248–250
III c
249–250
7
83
9
285–286
86
284–286
9
III d
81
III e
10
195–197
196–197
10
12
173–175
59
173–175
8
III f
10
105–107
III g
106–107
9
72
10
195–197
77
196–197
9
III h
a
The cinnamic acids obtained were identified by comparison of their m.p. and IR spectra with authentic samples.
b
Yields calculated based on the aromatic aldehydes I employed.
c
After recrystallization.
NaBH
4
(1 mole)+CH
3
COOH (1 mole)
Na
+
B
−
H
3
OOCCH
3
+H
2
Ref. 4
NaBH
4
(1 mole)+CH
3
COOH (3 mole)
Na
+
B
−
H(OOCCH
3
)
3
+3H
2
Ref. 5
NaBH
4
(1 mole)+CH
3
COOH (4 mole)
Na
+
B
−
(OOCCH
3
)
4
+4H
2
Ref. 6
We found that this last compound 3, prepared in situ in
acetic acid solution, can react with aromatic aldehydes
to give the corresponding cinnamic acids.
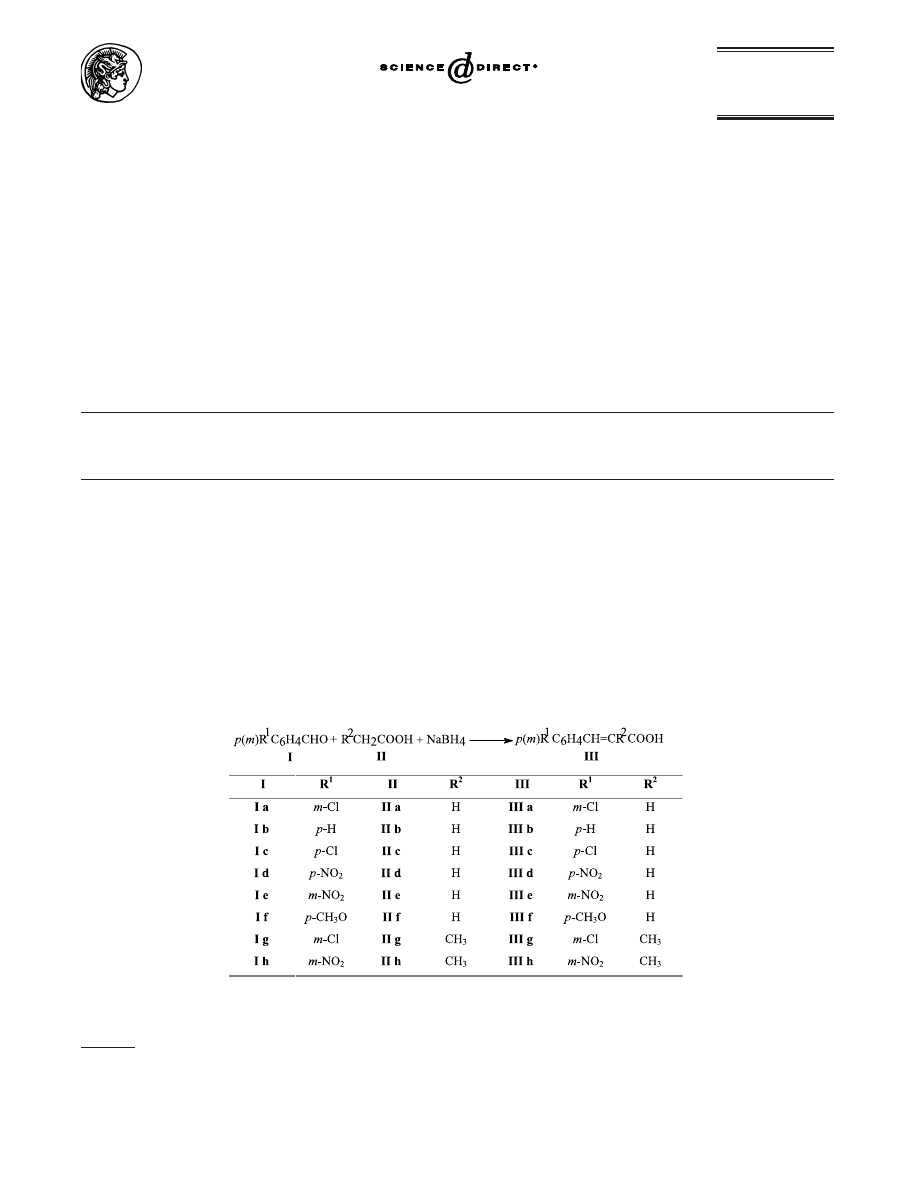
By stepwise investigations, we established that aromatic
aldehydes I can react with aliphatic carboxylic acids II
in the presence of sodium borohydride, in the mole ratio
1: excess: 1.33, resulting in cinnamic acids III, as presented
in Scheme 1.
Using a mole ratio between the aldehyde I and NaBH
4
of 1:1.33, good yields for this reaction were obtained.
Without a suitable solvent, this reaction is difficult to
perform. We tested many solvents, such as DMSO, DMF,
N-methyl-2-pyrrolidinone (NMP), HMPTA etc. From
these solvents, we selected NMP because it is a good
solvent for our products, is stable under the reaction
conditions and has a high boiling point (202°C).
The synthesis necessitates high temperatures (reflux at
180–190°C), during 9–12 h (Table 1). At lower temper-
atures, the yields decrease. For example, the yield for
product IIIa decreased to 45–48% when the reaction was
performed at 145–150°C, for 6 h.
As can be seen in Table 1, cinnamic acids III were obtained
in yields which ranged from 59 to 86% depending on the
reaction conditions and structure of the aldehyde. The
cinnamic acid IIIf was obtained with the lowest yield.
Cinnamic acids with electron-withdrawing groups were
obtained in good yields.
11
The detailed mechanism of this reaction will be discussed
in a separate communication.
In conclusion, we have found a new synthesis for the direct
preparation of cinnamic acids from aromatic aldehydes
and aliphatic carboxylic acids in the presence of sodium
borohydride. This method is a very effective alternative
to the classical Perkin synthesis. Without the sodium
borohydride, the synthesis is not possible.
References
1. (a) Johnson, J. R. Org. React. 1942,
1, 210; (b) Johnson,
J. R. Org. Syn. Coll. 1955,
3, 426.
2. Kalnin, P. Helv. Chim. Acta 1928,
11, 977.
3. Crawford, M.; Little, W. T. J. Chem. Soc. 1959, 722.
4. Reetz, T. J. Am. Chem. Soc. 1960,
82, 5039.
5. Hutchins, R. O. J. Org. Chem. 1978,
43, 2301.
6. Markini, P. J. Org. Chem. 1975,
40, 3455.
7. Cleland, G. H. J. Org. Chem. 1961,
26, 3362.
8. Fedorov, B. S. Prom. Org. Sin. Akad. Nauk SSSR 1967,
173 (Chem. Abstr. 1968,
68, 77903h).
9. Urushibara, Y.; Hirota, M. Nippon Kagaku Zasshi 1961,
82, 351 (Chem. Abstr. 1962, 56, 10025g).
10. Zimmerman, H. J. Am. Chem. Soc. 1959,
81, 2091.
11. General procedure for the synthesis of cinnamic acids: In
a 100 mL three-necked Claisen flask fitted with a mechanical
stirrer, 0.14 mole (excess) of aliphatic carboxylic acid II was
added. Then, 1 g (0.0266 mole) of sodium borohydride was
added slowly in small portions, under stirring and cooling
with ice, in order to maintain the temperature in the flask
at 20–30°C. Then, the solution obtained was stirred for 1
h at room temperature, and then for 1 h at 90–100°C. To
this solution, at 70–90°C, 0.02 mole of aromatic aldehyde
I was added and then 2mL of NMP as solvent. The solution
obtained was stirred for 2–3 min. The mechanical stirrer
was replaced with a condenser and the excess of compound
II was removed by distillation, until the temperature in the
flask increased to 185–187°C. Then, the distillation set was
replaced with an air-cooled reflux condenser 30 cm long and
2.9 cm in diameter, which was extended with a water-cooled
reflux condenser. This solution was heated under reflux, at
185–190°C, for 9–12 h (see Table 1). At the end of the
reaction, the final solution was treated with 70–80 mL water
and then with NaOH solution 20%, to pH=9–10. From
this solution, the unreacted aromatic aldehyde I was
distilled with water under vacuum (30–40 mm Hg), until
the distillate was no longer cloudy. The solution was diluted
with water until a volume of 80–90 mL was obtained which
was then filtered at 30–40°C. The filtrate was treated with
HCl solution 15–20%, until pH=1–2, when cinnamic acid
III precipitated. After 2–3 h of stirring under cooling with
ice, the final product III obtained was filtered, washed with
15–20 mL cold water and dried. Yields ranged from 59 to
86% (Table 1).
Document Outline
Wyszukiwarka
Podobne podstrony:
rotter cat
Hyd prd cat 2009 english webb
A32 Ziyang J Cat 2001 id 49773 Nieznany (2)
podrecznikII 1 cat id 365892 Nieznany
CAT BĘBEN
cat patterns sept02
cat today 75 2002 459
Benzaldehyd
Benzaldehyd
Twisty Cat, Dokumenty Textowe, Koty, Rasy kotów
CAT 330D
Cat Schield Zawrót głowy
cat comm 8 2007 1621
C7 200611 CAT PL
Cat Illusion
cat 6AD en id 108772 Nieznany
Benzalkonii chloridum
więcej podobnych podstron