
Vaccine 20 (2002) S13–S17
Aluminum toxicokinetics regarding infant diet and vaccinations
L.S. Keith
∗
, D.E. Jones, C.-H.S.J. Chou
Agency for Toxic Substances and Disease Registry, Division of Toxicology, 1600 Clifton Road, NE, Mailstop E-29, Atlanta, GA 30333, USA
Received 4 June 2001; accepted 7 August 2001
Abstract
Some vaccines contain aluminum adjuvants to enhance the immunological response, and it has been postulated that this aluminum could
contribute to adverse health effects, especially in children who receive a vaccination series starting at birth. The pharmacokinetic properties
and end-point toxicities of aluminum are presented. In assessing the relevance of dietary and medical aluminum exposure to public health,
we estimated infant body burdens during the first year of life for breast milk and formula diets and for a standard vaccination schedule.
We then compared those body burdens with that expected for intake at a level considered safe for intermediate-duration exposure. The
methodology blends intake values and uptake fractions with an aluminum retention function derived from a human injection study using
radioactive
26
Al. The calculated body burden of aluminum from vaccinations exceeds that from dietary sources, however, it is below the
minimal risk level equivalent curve after the brief period following injection. Published by Elsevier Science Ltd.
Keywords: Aluminum; Vaccine; Diet
1. Introduction
Aluminum is the third most abundant element in the
earth’s crust. This status ensures that the element is present
in essentially all air, food, and water. Aluminum is not a
free metal in nature but is found concentrated in bauxite ore
deposits that are mined, dissolved in a high temperature and
pressure digestion process, crystallized and calcined to alu-
mina, and then reduced to metallic aluminum. The United
States produced 3.7 million metric tons in 1998 for use in
various industries, including building construction, baking
products, and medicine. Historical medical exposures to
aluminum in renally impaired individuals undergoing dial-
ysis produced a neurological condition in some individuals
known as dialysis dementia. Its medical use in vaccines
occasionally produces adverse reactions (granulomas, per-
sistent nodules, abscesses, or hypersensitivity) and has stim-
ulated interest by the World Health Organization [1] in the
toxicity of injected aluminum compounds, especially among
the infant population. This follows a similar effort regard-
ing thimerosal (a mercury-containing preservative which
has been eliminated from some vaccine formulations) and
reports of muscle nodules (termed macrophagic myofasci-
itis or MMF) observed primarily in immune-compromised
Frenchmen who had received aluminum adjuvated vacci-
∗
Corresponding author. Tel.:
+1-404-498-0734; fax: +1-404-498-0092.
E-mail address: skeith@cdc.gov (L.S. Keith).
nations several years before the nodules developed. The
pharmacokinetics and end point toxicity of aluminum are
described below. A method is presented for estimating infant
aluminum body burdens associated with dietary intake, vac-
cinations, and daily oral intake at a level considered safe by
the Agency for Toxic Substances and Disease Registry [2].
2. Uptake and distribution
2.1. Uptake
Aluminum and its compounds tend to solubilize into
trivalent Al
3
+
cations in acid environments below pH 5, a
phenomenon that makes exterior aluminum surfaces cor-
rodible in acid rain. In the same way, dietary aluminum
compounds dissociate in stomach acid to become unattached
ligands and free aluminum ions that subsequently hydrate
to form trivalent aluminum hexahydrate. A small portion
of the aluminum recomplexes with the original or another
available ligand in a manner that preferentially favors car-
boxylic acids, such as citrate or lactate. Dietary phosphorus
that attaches to the aluminum becomes unavailable for up-
take, leading in some cases (large quantity antacid use) to
hypophosphatemia with consequent skeletal implications.
The majority (>99%) passes unattached into the duodenum
where the increased alkalinity sequentially deprotonates
the aluminum hexahydrate ion into insoluble aluminum hy-
droxide, which is primarily excreted in the feces. A small
0264-410X/02/$ – see front matter. Published by Elsevier Science Ltd.
PII: S 0 2 6 4 - 4 1 0 X ( 0 2 ) 0 0 1 6 5 - 2

S14
L.S. Keith et al. / Vaccine 20 (2002) S13–S17
fraction of the aluminum becomes systemic through pro-
cesses that have not yet been elucidated but are believed to
involve passive paracellular or transcellular diffusion. An
additional and unique carboxylic acid-mediated mechanism
enhances gastrointestinal tract absorption by more than an
order of magnitude. The resulting human uptake factors
range from 0.01% for the hydroxide to a maximum of around
1%, with lactate being measured at 0.78% [3–6]. Yokel and
McNamara [7] found the following similar relative uptake
of aluminum compounds in the rat: citrate
> lactate >
sucrose sulfate
> chloride > hydroxide > glycinate >
borate. Trivalent aluminum cations can block the uptake of
bioessential trivalent phosphorus or phosphate anions [8] so
effectively that a low aluminum diet and restricted antacid
use are recommended for hypophosphatemic individuals.
2.2. Transfer rate from blood
Systemic aluminum binds to serum proteins or anions and
is distributed rapidly to other tissues throughout the body.
Approximately, 89% of the aluminum reaching the blood
binds with transferrin, and the rest mainly attaches to cit-
rate [9]. The transfer rate from blood has been measured in
various studies. Sutherland and Greger [10] found an initial
half-time of 102–119 min, and transfer rates ranging from
0.003 h
−1
for a central compartment (which is likely bone)
up to 9 h
−1
for three peripheral compartments. In a more sen-
sitive study by Priest et al. [11], radioactive
26
Al citrate was
injected into a human volunteer and blood aluminum levels
were found to decrease by
>50% in 15 min and by >99% in
2 days. The advantages of using this accelerator-produced
radioisotope are high sensitivity using a small mass of alu-
minum and noninterference by the naturally present
27
Al.
2.3. Release from injection site
Aluminum adjuvants injected intramuscularly or subcuta-
neously in vaccines can experience some delay in entering
the bloodstream. Heimlich et al. [12] adsorbed a mock anti-
gen and each of several interstitial and serum proteins onto
an aluminum adjuvant. During an in vitro exchange reac-
tion test, free interstitial proteins were found to separate an
aluminum adjuvant from the mock antigen to which it was
bound. However, the free mock antigen could not separate
the interstitial protein-aluminum adjuvant complexes. Over
50% of the adjuvant transferred from the antigen to the in-
terstitial or serum protein within 15 min. This indicates that
the aluminum in vaccines may be readily mobilized from
the injection site. This rapid dissociation raises two distinct
but opposing possibilities: (1) that a smaller amount of ad-
juvant may be appropriate for some vaccines, or (2) that a
larger amount of aluminum may be needed to achieve max-
imum efficacy for some vaccines. Experiments to measure
antibody titers (as an indicator of vaccine efficacy) could be
devised using varying and precisely measured ratios of adju-
vant to antigen in order to identify an optimal concentration
of aluminum adjuvant for each vaccine type. Varying the
aluminum between initial and subsequent injections in a se-
ries could be considered.
2.4. Distribution pattern
Once aluminum is in the bloodstream, it distributes widely
to the various body tissues in a pattern that may parallel the
density of transferrin receptors within those tissues. Intra-
muscularly injected aluminum hydroxide in rabbits had the
following pattern of tissue redistribution: kidney
> spleen >
liver
> heart > lymph > brain [13]. The rat model followed
the same pattern, and the addition of bone analysis showed
that skeletal deposition greatly exceeds that of kidney, with
a value that doubled in uremic rats [14]. Based on these
studies, bone is the primary long-term reservoir for systemic
aluminum following either ingestion or injection in humans.
3. Retention
3.1. Elimination rates
The retention of aluminum is directly affected by excre-
tion, which has been studied in both rats and humans. Xu
et al. [15] found 66–70% of injected aluminum was excreted
in 24 h. In a human study, Priest et al. [11] injected a volun-
teer with 0.7
g of radioactive
26
Al as citrate and followed
blood levels and body elimination. They found that over 50%
of the aluminum distributed from blood to other body tissues
in 15 min. Long-term observation using excreta and whole
body monitoring found excretions of
>50% in 24 h, 85%
at 13 days, and 96% by 1178 days. Elimination followed a
power function featuring a rapid initial release followed by
successively longer-term components. The result is an over-
all slow buildup of aluminum in the body over a lifetime.
3.2. Retention functions
Mature human tissue can contain aluminum concen-
trations of 20 mg/kg in lung, 5–10 mg/kg in bone, and
0.3–0.8 mg/kg in soft tissue. The body burden late in life
can be estimated to reach 20 mg in lung, 25–50 mg in bone,
and 9–24 mg in soft tissue, with the body burden totalling
approximately 50–100 mg Al. The Priest et al. [11] formulae
provide a method for assessing the fate of either injected or
dietary aluminum. The body burden from a single injection
followed a power function of the form,
R = 0.354dt
−0.32
,
(1)
where R is the retained fraction, d the uptake dose in mg Al,
and t the time in days following uptake.
Eq. (1) applies to a single oral dose or a single vaccination
injection, and may be summed for repetitive dietary intakes
or a multiple vaccination regimen. The body burden from
uniform daily dosing, such as may be assumed for adult
L.S. Keith et al. / Vaccine 20 (2002) S13–S17
S15
dietary sources, is obtained by integrating Eq. (1), resulting
in,
B = 0.52d(t
0
.68
− 1),
(2)
where B is the body burden based on constant uptake.
Uptake following injections is taken as 100%; dietary con-
tribution is considered to be the product of dietary intake
and the gastrointestinal tract uptake factor. Vaccinations typ-
ically begin early in infancy and are repeated several times
over the course of the first year of life, making this the focus
period of this article.
3.3. Infant dietary body burden
An infant’s general fluid consumption increases from
670 ml per day at birth to 900 ml per day at 6 months, with
aluminum intake depending on the dietary source. Breast
milk measurements cover the range of 5–380
g Al/l with
a central value around 40
g/l [16–20]; the high value is
associated with Croatian women, but the cause has not yet
been elucidated. Formula concentrations average around
225
g Al/l with a maximum of 1150 g/l. The higher lev-
els in formula over breast milk may be a result of food in-
dustry practices using aluminum components in processing
facilities and adding aluminum-containing compounds to
food ingredients to improve their blending and anti-caking
properties. The daily fluid volume coupled with the source
concentration gives the daily aluminum intake through 6
months of age. During the second 6 months, introduction of
semisolid food increases the aluminum intake to an average
0.7 mg per day [21]. An estimate of infant aluminum body
burden during year 1 was developed using a 0.78% uptake
factor and applying the Priest et al. [11] retention function
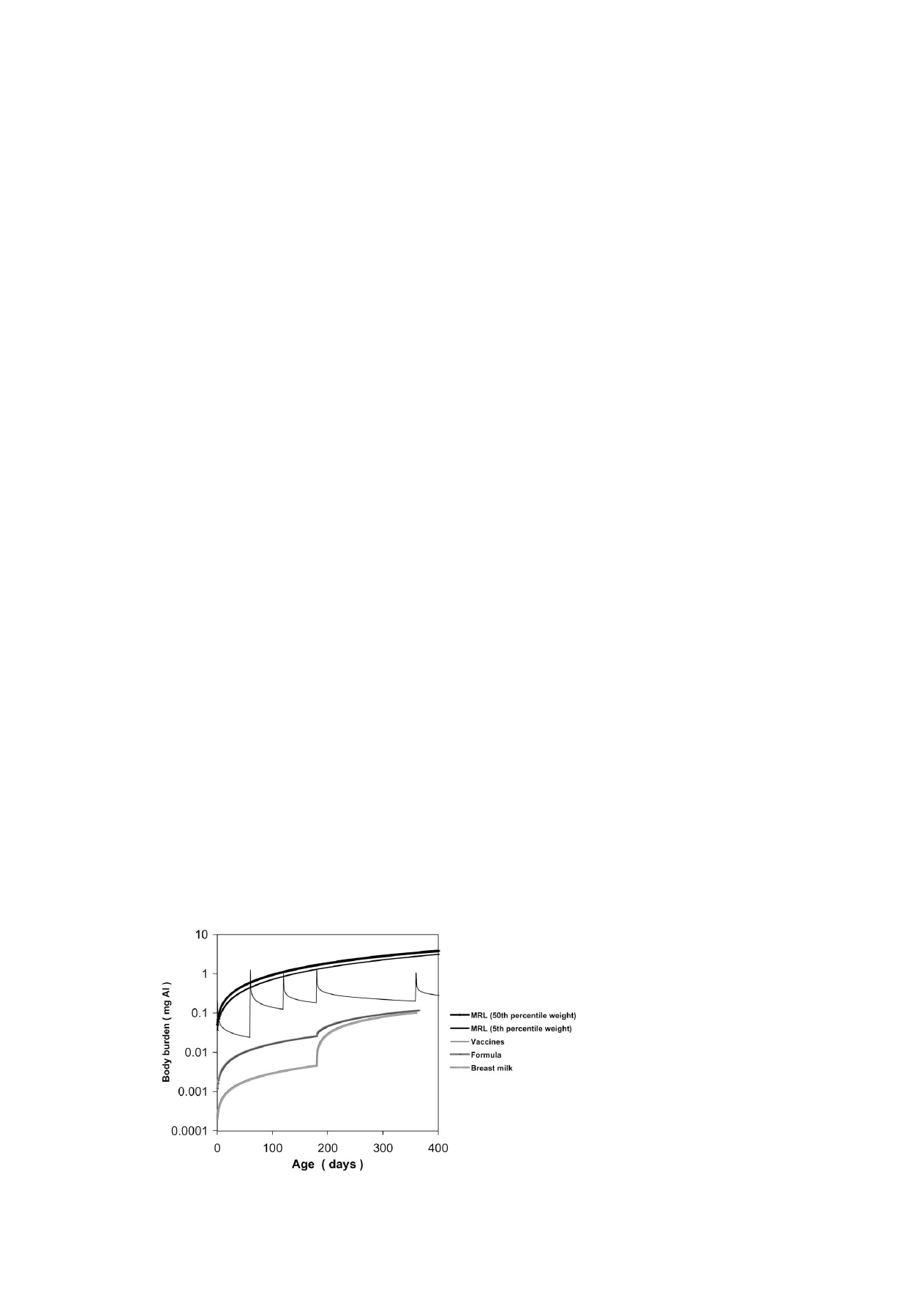
Fig. 1. Aluminum body burden contributions from diet and vaccines relative to MRL level intake.
to the daily aluminum intake, with the resulting dietary
curves shown in Fig. 1.
4. Toxicity summary
4.1. Historical toxicity observations
ATSDR has summarized the pharmacokinetics and end-
point toxicity of aluminum in its Toxicological Profile for
Aluminum [2]. The mechanism of aluminum toxic action is
currently unknown. The element may affect the phospho-
inositide second messenger-producing system, which mod-
ulates intracellular calcium concentrations [22]. It strongly
binds in a largely irreversible manner to large proteins, such
as nuclear components, which may relate to an inhibition
of neuronal microtubule formation. Neurotoxicity has been
identified as the most sensitive health end point for ingested
aluminum. Its effect on the nervous system was first recog-
nized in past decades in cases involving renal failure. The
dialysate solution for renal patients was made from tap wa-
ter, which naturally contained some aluminum. It was subse-
quently introduced into systemic circulation where it bound
with transferrin. This molecule was not filterable by the dial-
ysis equipment, resulting in an ever-increasing aluminum
body burden that was enhanced by elevated tap water lev-
els. A neurological disorder was produced in some patients
that was termed dialysis dementia. Investigation found ele-
vated serum aluminum levels, a condition that is now known
to be preventable by using water with low aluminum con-
tent. Other more subtle neurological effects that have been
induced in animal models or associated with human occu-
pational exposure include memory loss, fatigue, depression,
behavioral modifications, and learning impairment.

S16
L.S. Keith et al. / Vaccine 20 (2002) S13–S17
4.2. Inhalation exposure
Inhaled aluminum can be a respiratory toxicant causing
irritation and, ultimately, pulmonary fibrosis [23]. Such fi-
brosis has been produced by a pyrotechnic powder in com-
bination with a nonpolar oil, but has not been observed
since the manufacturing process adjusted to use another
type of lubricant. Steinhagen et al. [24] noted an increase
in the number of pulmonary macrophages following alu-
minum exposure. This outcome can also be induced by a
range of other inhaled species, and a review of the overall
inhalation database indicates that the effects are consistent
with pulmonary overload associated with diverse inorganic
dust.
4.3. Dermal exposure
Aluminum compounds can also cause dermal irritation
or an immunologic response. Antiperspirants containing
aluminum chlorhydrate can cause a localized underarm
irritation in some individuals [25]. Aluminum adjuvated
vaccines have produced a permanent and decentralized sen-
sitivity in those individuals who experience injection site
granulomatous nodules that last more than several weeks,
a phenomenon that is reportedly quite rare [26]. Similar le-
sions have been observed in hilar and peribronchial lymph
nodes following inhalation exposure.
4.4. Oral exposure
Musculoskeletal toxicity is atypical among those with
normal renal function. However, trivalent aluminum ions
have been found to pharmacokinetically compete with di-
valent magnesium and calcium ions during hydroxyapatite
osteogenic formation. This occurs despite the ionic valence
disparity that makes aluminum an unexpected competitor
based on valence considerations alone. Osteomalacia in ure-
mic individuals involves systemic aluminum buildup that
correlates with aluminum content of bone [27,28]. Con-
versely, osteomalacia and rickets in otherwise healthy indi-
viduals involve phosphate depletion rather than aluminum
buildup in bone. These effects are observed among chronic
antacid users in whom the aluminum binds with intestinal
phosphorus and prevents its uptake [29,30]. Either can ulti-
mately lead to pathological fractures.
4.5. Minimal risk level (MRL)
ATSDR reviewed a large body of literature and con-
cluded that neurotoxicity is the most sensitive health end
point for ingested aluminum compounds. The agency
used this end point in developing an oral MRL, or
dose which is expected to be safe for human exposure.
The basis was an intermediate-duration study in which
mice were fed a diet containing aluminum lactate. They
experienced spontaneous motor activity interference with a
no-observed-adverse-effect level (NOAEL) of 62 mg Al/kg
per day [31]. Applying uncertainty factors of 3 for extrapo-
lation to humans and 10 for human variability produced an
MRL of 2 mg Al/kg per day.
5. MRL body burden
The MRL curves in Fig. 1 are based on low and average
weight infants consuming aluminum at an amount equiva-
lent to the MRL each day starting from birth. The curves
are adjusted for increasing daily body mass and food intake
using an intestinal uptake factor of 0.78% and excretion ac-
cording to Eq. (1). Since the MRL is a function of body
weight, which significantly changes during year 1 for the in-
fant, it was necessary to first modify Eq. (1) by an appropri-
ate body weight function before integration. Functions were
derived for standard 5th and 50th percentile females using a
standard chart of monthly infant body weights [32], and are
of the form,
BW
(5th percentile female) = 2.36 + 10(1 − e
−0.0023×A
)
and
BW
(50th percentile female) = 3.23 + 10(1 − e
−0.0028×A
),
(3)
where BW is the body weight (kg) and A is age (days).
The MRL curves are the upper two on Fig. 1, and the
curve for 5th percentile infants is below that for the 50th
percentile group. The dietary curves for ingested breast milk
and formula are the lower two in Fig. 1, and fall well below
both MRL curves.
6. Vaccine body burden
The systemic aluminum body burden from vaccine injec-
tions can be estimated using the previous Priest equation
and the injection dose and schedule information presented in
Table 1. A simplified dosing schedule was used that reduced
the CDC-recommended time windows [33] to specific time
Table 1
Vaccination schedule [33]
Age
Vaccination(s)
a
Aluminum content (mg) [34]
Birth
Hep B
0.25
2 months
Hep B
+ DTP
0.50–1.10
4 months
DTP
0.25–0.85
6 months
Hep B
+ DTP
0.50–1.10
12 months
DTP
0.25–0.85
a
Hep B: hepatitis B; DTP: diphtheria
+ tetanus toxoids + acellular
pertussis.

L.S. Keith et al. / Vaccine 20 (2002) S13–S17
S17
points, including birth and 2, 4, 6, and 12 months. Hepati-
tis B and DTP were selected since they are the two primary
aluminum adjuvated vaccines given to infants during their
first year of life. Aluminum hydroxide is the adjuvant for
hepatitis B, whereas DTP can be adjuvated with the hydrox-
ide, phosphate, or potassium sulfate. The US formulations
require 0.25 mg of aluminum in the hepatitis B vaccine and
0.25–0.85 mg in the various DTP versions [34]. Table 1 iden-
tifies the range of aluminum content for relevant injections
by age, and the vaccination curve in Fig. 1 applies to the
maximum aluminum doses. That curve is below the MRL
and above the dietary intake curves, and shows spikes on
the injection day followed by rapid elimination during the
first few days. Overlaps occur between the MRL and vac-
cine curves during the first 1–3 days postinjection.
7. Conclusions
Children are born with a systemic aluminum body burden,
which is increased throughout life by the inhalation and di-
etary intake of aluminum compounds as well as by injections
of vaccines and allergy treatments containing aluminum ad-
juvants. Those injections may produce localized reactions
without systemic impact. The body burden associated with
dietary uptake from either breast milk or formula during the
first several months of life and from semisolid food during
the remainder of that first year is estimated to reach approx-
imately 0.1 mg. This value is lower than the estimated body
burden of approximately 4 mg that would result from con-
suming aluminum at a rate equal to the MRL of 2 mg/kg per
day. The body burden attributable to vaccines may be ex-
pected to fall between the two except for a period of a few
days following individual vaccinations.
References
[1] World Health Organization. Vaccine safety. Weekly epidemiological
record. 1999; 74:337–48.
[2] Toxicological profile for aluminum. Atlanta, GA: US Department
of Health and Human Services, Public Health Service. Agency for
Toxic Substances and Disease Registry, 1999.
[3] Day JP, Barker J, Evans LJ, Perks J, Seabright PJ, Ackrill P, et al.
Aluminum absorption studied by
26
Al tracer. Lancet 1991;337:1345.
[4] DeVoto E, Yokel RA. The biological speciation and toxicokinetics
of aluminum. Environ Health Perspect 1994;102:940–51.
[5] Ganrot PO. Metabolism and possible health effects of aluminum.
Environ Health Perspect 1986;65:363–441.
[6] Greger JL, Baier MJ. Excretion and retention of low or moderate
levels of aluminum by human subjects. Food Chem Toxicol
1983;21:473–7.
[7] Yokel RA, McNamara PJ. Influence of renal impairment, chemical
form, and serum protein binding on intravenous and oral aluminum
kinetics in the rabbit. Toxicol Appl Pharmacol 1988;95:32–43.
[8] Handbook of chemistry and physics. 80th ed. Boca Raton, FL: CRC
Press, 1999.
[9] Öhman L-O, Martin RB. Citrate as the main small molecule binding
Al
3
+
in serum. Clin Chem 1994;40:598–601.
[10] Sutherland JE, Greger JL. Kinetics of aluminum disposition after
ingestion of low to moderate pharmacological doses of aluminum.
Toxicology 1998;126:115–25.
[11] Priest ND, Newton D, Day JP, Talbot RJ, Warner AJ. Human
metabolism of aluminum-26 and gallium-67 as citrates. Hum Exp
Toxicol 1995;14:287–93.
[12] Heimlich JM, Regnier FE, White JL, Hem SL. The in vitro
displacement of adsorbed model antigens from aluminium-containing
adjuvants by interstitial proteins. Vaccine 1999;17(22):2873–81.
[13] Flarend RE, Hem SL, White JL, Elmore D, Suckow MA, Rudy AC,
et al. In vivo absorption of aluminum-containing vaccine adjuvants
using
26
Al. Vaccine 1997;15:1314–8.
[14] Walker VR, Sutton RAL, Meirav O, Sossi V, Johnson R, Klein J, et
al. Tissue disposition of
26
Al in rats measured by accelerator mass
spectrometry. Clin Invest Med 1994;17:420–5.
[15] Xu ZX, Pai SM, Melethil S. Kinetics of aluminum in rats. Part
II: dose-dependent urinary and biliary excretion. J Pharm Sci
1991;80(10):946–51.
[16] Koo WWK, Kaplan LA, Krug-Wispe SK. Aluminum contamination
of infant formulas. J Parenteral Nutr 1988;12:170–3.
[17] Weintraub R, Hams G, Meerkin M, Rosenberg AR. High aluminum
content of infant milk formulas. Arch Dis Child 1986;61:914–6.
[18] Simmer K, Fudge A, Teubner J, James SL. Aluminum concentrations
in infant formulae. J Paediatr Child Health 1990;26:9–11.
[19] Hawkins NM, Coffey S, Lawson MS, Delves HT. Potential aluminum
toxicity in infants fed special infant formula. J Pediatr Gastroenterol
Nutr 1994;19:377–81.
[20] Mandic ML, Grgic J, Grgic Z, Seruga M, Hasenay D. Aluminum
levels in human milk. Sci Total Environ 1995;170:165–70.
[21] Pennington JAT, Schoen SA. Estimates of dietary exposure to
aluminum. Food Addit Contam 1995;12:119–28.
[22] Jope RS, Johnson GVW. Neurotoxic effects of dietary aluminum.
In: Aluminum in biology and medicine. Chichester, England: Wiley,
1992. p. 254–67.
[23] McLaughlin AIG, Kazantzis G, King E, Teare D, Porter RJ, Owen
R, et al. Pulmonary fibrosis and encephalopathy associated with the
inhalation of aluminum dust. Br J Ind Med 1962;19:253–63.
[24] Steinhagen WH, Cavender FL, Cockrell BY. Six month inhalation
exposures of rats and guinea pigs to aluminum chlorhydrate. J
Environ Pathol Toxicol 1978;1:267–77.
[25] Brusewitz
S.
Aluminum.
Stockholm,
Sweden:
University
of
Stockholm, Institute of Theoretical Physics, 1984; 203:138.
[26] Fiejka M, Aleksandrowicz J. Aluminum as an adjuvant in vaccines
and post-vaccination reactions. Rocz Panstw Zakl Hig 1993;44(1):73–
80.
[27] Andreoli SP, Bergstein JM, Sherrard DJ. Aluminum intoxication from
aluminum-containing phosphate binders in children with azotemia
not undergoing dialysis. N Engl J Med 1984;310:1079–84.
[28] Wills MR, Savory J. Aluminum and chronic renal failure: Sources,
absorption transport, and toxicity. CRC Crit Rev Clin Lab Sci
1989;27:59–107.
[29] Carmichael KA, Fallon MD, Dalinka M, Kaplan FS, Axel L, Haddad
JG. Osteomalacia and osteitis fibrosa in a man ingesting aluminum
hydroxide antacid. Am J Med 1984;76:1137–43.
[30] Pivnick EK, Kerr NC, Kaufman RA, Jones DP, Chesney R. Rickets
secondary to phosphate depletion. Clin Pediatr 1995;34:73–8.
[31] Golub MS, Donald JM, Gershwin ME, Keen CL. Effects of aluminum
ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol
1989;11:231–5.
[32] Behrman RE, Kliegman RM, Jenson HB, editors. Nelson’s textbook
of pediatrics, 16th ed. Philadelphia: W.B. Saunders Co., 2000.
[33] Centers for Disease Control and Prevention. Notice to readers:
recommended childhood immunization schedule, US, 2000. MMWR
Weekly. January 21, 2000/49(02):35–38,47.
[34] Physicians’ desk reference. 52nd ed. Montvale, NJ: Medical Econo-
mics Company, 1998.
Document Outline
- Aluminum toxicokinetics regarding infant diet and vaccinations
Wyszukiwarka
Podobne podstrony:
Fat Burning Furnace Diet and Weight Loss Secrets
Food and eating Diet and health (24)
2005 Diet and Age Affect Intestinal Morphology and Large Bowel Fermentative End Product Concentratio
diabetes and vaccines fact sheet
Organic Law 8 2000 of 22 December, Reforming Organic Law 4 2000, of 11 January, Regarding the Rights
Shigella flexneri infection pathogenesis and vaccine development
Food and eating Diet and health (tłumaczenie)
Self Image Psychology Secrets The Hidden Reasons Why You Sabotage Your Diet And Fitness Efforts And
Diet, Weight Loss and the Glycemic Index
68 979 990 Increasing of Lifetime of Aluminium and Magnesium Pressure Die Casting Moulds by Arc Ion
Enzyme Systems that Metabolise Drugs and Other Xenobiotics Current Toxicology
Lead in food and the diet
Greenhill Fighting Vehicles Armoured Personnel Carriers and Infantry Fighting Vehicles
Information and History regarding the Sprinter
Dental Pathology and Diet at Apollonia, a Greek Colony on the Black Sea
2011 6 NOV Companion Animal Medicine Evolving Infectious, Toxicological, and Pa
Diet, Weight Loss and the Glycemic Index
68 979 990 Increasing of Lifetime of Aluminium and Magnesium Pressure Die Casting Moulds by Arc Ion
więcej podobnych podstron