
Shigella flexneri infection: pathogenesis and vaccine development
Amy V. Jennison, Naresh K. Verma
*
Faculty of Science, School of Biochemistry and Molecular Biology, The Australian National University, Canberra, ACT 0200, Australia
Received 1 May 2003; received in revised form 25 July 2003; accepted 30 July 2003
First published online 21 September 2003
Abstract
Shigella flexneri is a gram-negative bacterium which causes the most communicable of bacterial dysenteries, shigellosis. Shigellosis
causes 1.1 million deaths and over 164 million cases each year, with the majority of cases occurring in the children of developing
nations. The pathogenesis of S. flexneri is based on the bacteriaÕs ability to invade and replicate within the colonic epithelium, which
results in severe inflammation and epithelial destruction. The molecular mechanisms used by S. flexneri to cross the epithelial barrier,
evade the hostÕs immune response and enter epithelial cells have been studied extensively in both in vitro and in vivo models. Con-
sequently, numerous virulence factors essential to bacterial invasion, intercellular spread and the induction of inflammation have been
identified in S. flexneri. The inflammation produced by the host has been implicated in both the destruction of the colonic epithelium
and in controlling and containing the Shigella infection. The hostÕs humoral response to S. flexneri also appears to be important in
protecting the host, whilst the role of the cellular immune response remains unclear. The hostÕs immune response to shigellosis is
serotype-specific and protective against reinfection by the same serotype, making vaccination a possibility. Since the 1940s vaccines for
S. flexneri have been developed with little success, however, the growing understanding of S. flexneri’s pathogenesis and the hostÕs
immune response is assisting in the generation of more refined vaccine strategies. Current research encompasses a variety of vaccine
types, which despite disparity in their efficacy and safety in humans represent promising progress in S. flexneri vaccine development.
Ó 2003 Federation of European Microbiological Societies. Published by Elsevier B.V. All rights reserved.
Keywords: Shigella flexneri; Vaccine development; Pathogenesis
Contents
1.
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
44
2.
Pathogenesis of S. flexneri . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
44
2.1.
Crossing the colonic epithelial layer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
46
2.2.
Macrophage apoptosis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
46
2.3.
Adhesion to the basolateral membrane of colonic epithelial cells . . . . . . . . . . . . . . .
46
2.4.
Uptake by the epithelial cell . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
47
2.5.
Replication within the epithelial cell and intracellular and intercellular spread . . . . .
47
3.
The hostÕs immune response to S. flexneri . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
48
3.1.
Innate immunity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
48
3.2.
Cellular immunity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
48
3.3.
Humoral immunity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
49
4.
S. flexneri vaccine development . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
49
4.1.
Subunit vaccines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
50
4.2.
Killed oral vaccines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
50
FEMS Microbiology Reviews 28 (2004) 43–58
www.fems-microbiology.org
*
Corresponding author. Tel.: +61-2-6125-2666;
fax: +61-2-6125-0313.
E-mail address:
(N.K. Verma).
0168-6445/$22.00
Ó 2003 Federation of European Microbiological Societies. Published by Elsevier B.V. All rights reserved.
doi:10.1016/j.femsre.2003.07.002

4.3.
Non-invasive live vaccines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
50
4.4.
Invasive live vaccines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
50
4.5.
Hybrid vaccines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
51
4.6.
Multiple-serotype protection strategies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
51
5.
Conclusions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
53
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
53
1. Introduction
Members of the genus Shigella are gram-negative
facultative anaerobes that belong to the family, Enter-
obacteriaceae. They share common characteristics with
members of the genus, Escherichia and the genetic re-
latedness clearly suggests that they are a subtype of
E. coli [1,2]. The genus is divided into four species,
Shigella flexneri, Shigella boydii, Shigella sonnei and
Shigella dysenteriae. These species are further divided
into serotypes based on biochemical differences and
variations in their O-antigen. Based on this classification
scheme, Shigella flexneri is divided into 13 serotypes.
Shigella species invade the colonic and rectal epithe-
lium of primates and humans, causing the acute mucosal
inflammation characteristic of shigellosis. Infection is
usually confined to the superficial layer of the colonic
mucosa, where severe tissue damage leads to abscesses
and ulceration. Destruction of the epithelial layer leads
to the clinical symptoms of watery diarrhoea, severe
abdominal pain and cramping, eventuating in the
bloody mucoid stool characteristic of bacillary dysen-
tery. In the absence of effective treatments, shigellosis
patients may develop secondary complications such
as septicaemia, pneumonia and haemolytic uremic
syndrome [3].
Shigellosis occurs in an estimated 164.7 million people
per year, of which 1.1 million cases result in death. 163.2
million annual cases occur in developing countries and
69% of all patients are children under the age of five [4].
S. flexneri is endemic in most developing countries
and causes more mortality than any other Shigella spe-
cies [5]. The predominant serotypes of S. flexneri in
developing countries are serotypes 1b, 2a, 3a, 4a and 6,
whilst in industrialised countries most isolates are 2a [4].
The high incidence of Shigella in developing countries is
generally attributed to the lack of clean water, poor
sanitation, malnutrition and cost of antibiotic treat-
ment. Transmission is commonly via the faecal-oral
route, which is augmented by poor hygiene and close
personal contact.
Antibiotics can be used to treat shigellosis, reducing
the period of bacterial excretion from the patient.
However, S. flexneri is increasingly developing antibiotic
resistance [6]. This escalation in resistance to the com-
monly used, cheaper antibiotics adds increased strain to
the limited health services of developing countries.
Consequently, the World Health Organisation has pri-
oritised the development of a safe and effective vaccine
against S. flexneri [4].
Numerous virulence genes have been identified in S.
flexneri, with the majority of these genes being located
on a 220 kb plasmid known as the virulence plasmid. At
least three pathogenicity islands have also been located
on the S. flexneri chromosome, encoding important
virulence factors such as the lipopolysaccharide and
genes for temperature-dependant regulation of the ex-
pression of virulence genes on the plasmid [7–10]. Two
S. flexneri 2a genome sequences have recently been re-
leased, consisting of a chromosome of approximately
4 600 000 and 221 618 bp virulence plasmid [1,2]. These
sequences have confirmed that S. flexneri contains a
number of bacteriophage-related genes. Some of the
best-characterised S. flexneri phage genes are the sero-
type-conversion genes responsible for the serotype-spe-
cific modifications to the basic O-antigen structure [11].
The roles of many S. flexneri virulence genes have been
studied in a variety of cell culture experiments such as
invasion assays and plaque assays or through the use of in
vivo animal models such as the Sereny test in the guinea
pig, the mouse pulmonary model and rabbit ligated–
intestinal–loop model [12–15]. Continued research into
S. flexneri virulence and pathogenesis will yield further
understanding into the molecular basis of S. flexneri-
mediated invasion and destruction of the intestinal mu-
cosa, as well as the role of the hostÕs subsequent innate,
cellular and humoral immune responses. A comprehen-
sive understanding into S. flexneri’s disease-causing
mechanisms will assist in vaccine development.
2. Pathogenesis of S. flexneri
S. flexneri is highly infectious, requiring as little as
100 cells to cause disease in adult volunteers [16]. This
low infective dose is in part attributed to S. flexneri’s
ability to survive the low acidity of the hostÕs stomach,
via an up-regulation in acid resistance genes [17].
Once Shigella reach the colon, they begin to invade
the mucosa, penetrating, replicating within and spread-
ing between the mucosal epithelial cells. This behaviour
and the subsequent inflammatory response of the host
destroy the colonic epithelial layer generating the clini-
cal symptoms of shigellosis (Fig. 1) [18].
44
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
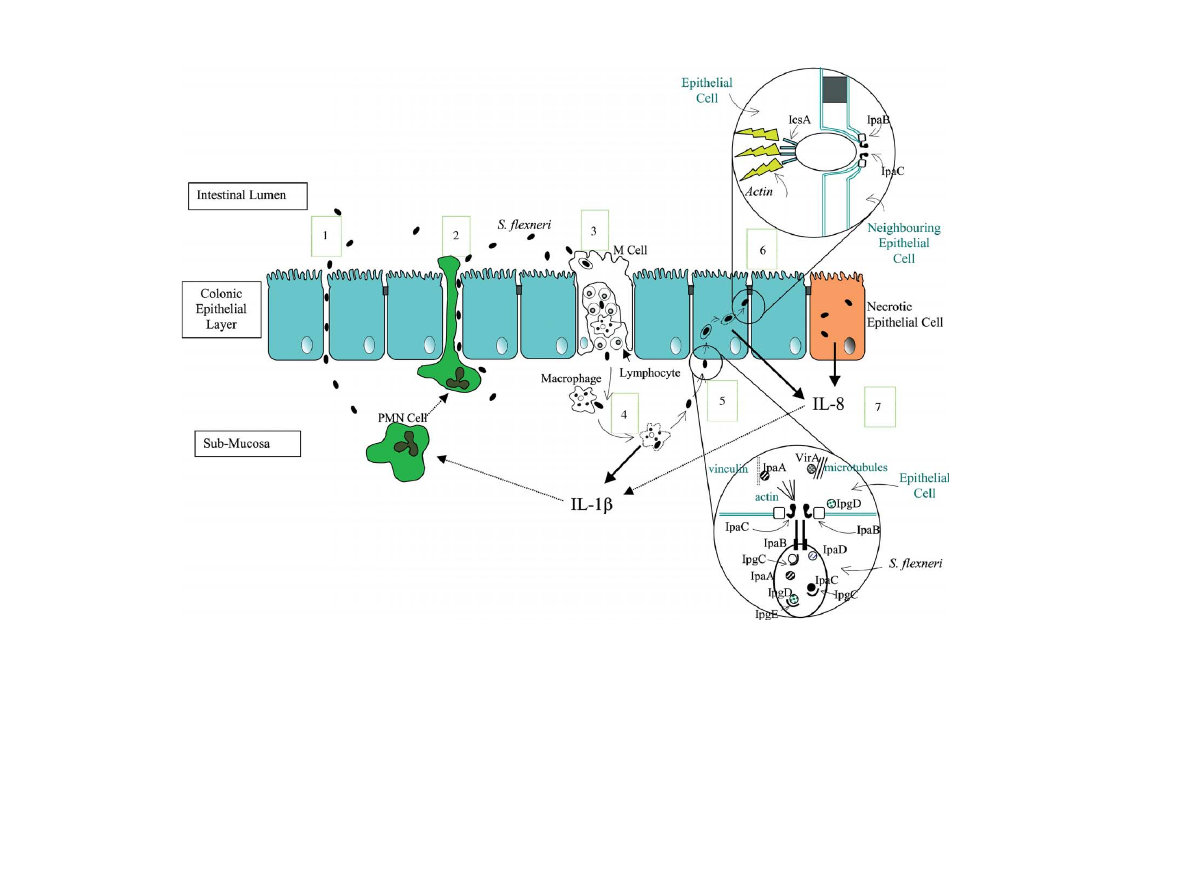
Fig. 1. Pathogenesis of S. flexneri. 1. Lumenal bacteria invade the colonic epithelial layer by three known mechanisms. S. flexneri can manipulate the tight junction proteins expressed by epithelial
cells, allowing paracellular movement of bacteria into the sub-mucosa. 2. PMN cells recruited by IL-8 and IL-1b produced in response to S. flexneri invasion create gaps between epithelial cells,
through which S. flexneri can transmigrate into the sub-mucosa. 3. Endocytic M cells transcytose bacteria, releasing them into an intraepithelial pocket filled with B and T lymphocytes and
macrophages. 4. Macrophages phagocytose the bacteria. S. flexneri escapes the phagosome and induces the macrophage to undergo apoptosis. The apoptotic macrophage releases IL-1b. 5. Sub-
mucosal S. flexneri contact the basolateral membrane of epithelial cells, activating secretion of proteins through their type-III secretion system. Proteins chaperoned in the cytosol of S. flexneri are
secreted into the epithelial cellÕs cytoplasm through a pore formed by IpaB and IpaC. IpaC polymerises actin, IpgD dissociates the plasma membrane from the actin cytoskeleton, VirA destabilises
microtubules and IpaA forms a complex with vinculin, depolymerising actin. This creates cell surface extensions which form around the bacterium, driving the epithelial cell to take up S. flexneri
into a vacuole. 6. IpaB and IpaC lyse the vacuole, releasing S. flexneri into the epithelial cellÕs cytoplasm. The S. flexneri protein, IcsA is displayed on only one pole of the bacterium, creating a
polymerised actin tail behind the bacterium. This propels S. flexneri through the cytoplasm until it contacts the plasma membrane, the force of the contact creates a protrusion into the
neighbouring epithelial cell. Both membranes are lysed by IpaB and IpaC, releasing S . flexneri into the neighbouring epithelial cell. 7. Intracellular S. flexneri induces the epithelial cell to release
IL-8. IL-8 and the IL-1b released from apopotic macrophages are chemotactic to PMN cells (represented by dotted arrows), attracting and inducing them to migrate through the epithelial layer to
the lumen. This epithelial disruption amplifies S. flexneri invasion of the epithelial layer.
A.V.
Jennison,
N.K.
Verma
/
FEMS
Mircobiolo
gy
Reviews
28
(2004)
43–58
45

2.1. Crossing the colonic epithelial layer
Experiments using polarised cell lines have demon-
strated that the majority of S. flexneri epithelial cell
invasion occurs through the basolateral pole of colonic
epithelial cells [19]. The epithelial layer acts as a barrier
to pathogens found in the gut lumen. S. flexneri is able
to penetrate the epithelial lining through the follicular
associated epithelium (FAE). This is the epithelial layer
found above the mucosa-associated lymph nodes, which
contains highly endocytic M cells (Membranous epi-
thelial cells).
M cells sample and transport lumenal antigen across
the epithelial barrier, releasing it into an intraepithelial
pocket formed by the basolateral membrane of the M cell.
This pocket is filled with lymphocytes and macrophages
waiting to take up any delivered lumenal antigen and
initiate a mucosal immune response [20]. The transcytotic
properties of M cells are exploited as a route for invasion
of the impermeable epithelial lining by a number of
pathogens, including S. flexneri (Fig. 1) [15].
Shigella appear to enter M cells by the same mem-
brane ‘‘ruffling’’ seen in epithelial cell invasion [21].
Once internalised in an endocytic vacuole by the M cell,
shigellae are moved rapidly through the cell and released
into the intraepithelial pocket. Once the FAE is crossed,
Shigella can access the basolateral membrane of the
epithelial cells.
In the later stages of a Shigella infection, shigellae
exploit the hostÕs inflammatory response in order to
amplify bacterial penetration of the colonic epithelium.
Macrophages infected by S. flexneri are induced to
undergo apoptosis, releasing large amounts of IL-1,
which is important in inducing inflammation and re-
cruiting PMN cells to the site of infection. Additionally,
the invasion of epithelial cells by Shigella activates the
transcription and secretion of IL-8. IL-8 is chemotactic
for PMN cells and plays a significant role in recruiting
PMN cells to the infected subepithelial area, where they
transmigrate through the epithelial lining to reach lu-
menal bacteria (Fig. 1) [22].
The influx of PMN cells across the epithelial layer in
response to Shigella disrupts the integrity of the epi-
thelium allowing lumenal bacteria to cross into the sub-
mucosa in an M-cell independant mechanism [23]. This
PMN recruitment has been demonstrated to be crucial
for the generation of the inflammation and tissue de-
struction typical of shigellosis in a number of studies.
Experiments in the rabbit ligated–intestinal–loop model
of Shigella infection where either IL-1 or IL-8 is inhib-
ited almost abolished inflammation, tissue destruction
and notably decreased the amount of bacterial invasion
[24,25]. Additionally, the blocking of CD18, an adhesion
molecule used by PMNs during migration, in the same
animal model also diminished tissue damage and bac-
terial invasion [26].
Ironically, PMN-mediated interruption of the barrier
function of the epithelial layer promotes the local spread
of Shigella, whilst the same PMN cells appear to be
responsible for restricting the infection to the submu-
cosa and preventing systemic dissemination [25].
Recent research has revealed that the S. flexneri is
capable of manipulating the tight-junction associated
proteins of human intestinal epithelial cells, allowing
bacterial paracellular movement through a model in-
testinal barrier. These results suggest that shigellae are
also capable of penetrating the colonic epithelium via an
M cell or PMN independant mechanism (Fig. 1) [27].
2.2. Macrophage apoptosis
Once released into the intraepithelial pocket of the M
cell, bacteria are engulfed by resident macrophages,
possibly through a bacterial driven macropinocytic
event similar to Shigella entry of epithelial cells [28].
S. flexneri is able to evade the killing mechanisms of the
macrophage by IpaB-mediated lysis of the phagocytic
vacuole (Fig. 1). The membrane lysing properties of the
virulence plasmid IpaB invasin allows the bacteria to
gain free access to the cytoplasm [29]. Once in the
macrophage cytosol, secreted IpaB binds and activates
caspase-1, a member of the pro-apoptotic cysteine pro-
teases [30]. Caspase-1 dependant apoptosis is not an
immunologically silent cell death, as activated caspase-1
cleaves and activates the pro-inflammatory cytokines
IL-1b and IL-18 [31]. Macrophage apoptosis occurs
within four hours of in vivo Shigella infection, releasing
bacteria into the sub-mucosa [32].
2.3. Adhesion to the basolateral membrane of colonic
epithelial cells
It remains unclear exactly why S. flexneri preferen-
tially invades epithelial cells thorough their basolateral
membrane. The apical membrane of the colonic epi-
thelial cells is covered with glycolipids which form a
mucin layer. This layer may act as a physical barrier
preventing S. flexneri access to the apical membrane,
interfering with the type-III secretion systems delivery of
the invasion plasmid antigens (Ipa), required for
Shigella entry into epithelial cells [33].
Additionally the basolateral membrane of epithelial
cells may display cellular components utilised by Shigella
as cell adhesion receptors. The role of receptor-mediated
epithelial cell adhesion in Shigella infection is incom-
pletely understood. However, it is known that the Shigella
Mxi/Spa secreton system requires contact with a host cell
to trigger the secretion of the Ipa invasins [34]. A number
of basolateral receptors capable of binding Shigella
components have been identified. The Ipa proteins are
capable of interaction with a5b1 integrin, a basolateral
receptor which binds the extracellular matrix located
46
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58

beneath the epithelium [35]. IpaB is also able to bind the
membrane receptor, CD44, which is the major cell surface
receptor for hyaluronic acid and is found on the baso-
lateral membrane of epithelial cells [36,37]. Both a5b1
integrin and CD44 can act as cytoskeleton linkers, sug-
gesting that upon the binding of Shigella they may con-
tribute to the cytoskeletal reorganisation seen during
epithelial invasion [38].
More recently, bacterial adherence to epithelial cells in
a polarised model epithelium was shown to be dependant
on the length and presence of the O-antigen [39]. These
results suggest that the S. flexneri LPS may play a role in
cell-bacteria interactions during epithelial cell invasion. A
number of cell receptors capable of binding LPS have
been characterised, including CD14 and the Toll family of
receptors (TLRs), which are found on the basolateral
membrane of epithelial cells [40,41].
2.4. Uptake by the epithelial cell
S. flexneri invades epithelial cells through a macr-
opinocytic process, where S. flexneri-induced rear-
rangements of the host cell cytoskeleton engulf the
bacterium into a vacuole (Fig. 1).
The virulence plasmid of S. flexneri encodes two loci
crucial to this invasive phenotype, the ipa locus and the
mxi-spa locus. The ipa operon encodes the ‘‘invasion
plasmid antigens’’, IpaA, IpaB, IpaC and IpaD, which
are the effectors of bacterial entry into the host cell. The
mxi-spa operon encodes the components of a type-III
secretion system, which is a flagella-like structure used
to deliver proteins, such as the Ipa proteins, from the
bacterial cytoplasm to the cytoplasmic membrane or
even cytosol of the host cell [42]. The mxi-spa operon
and IpaB, IpaC and IpaD are essential for in vitro epi-
thelial cell invasion [43,44].
The detailed mechanisms by which the Ipa proteins
generate Shigella invasion are not completely under-
stood. The Ipa proteins are synthesised and stored
within the bacteria, where they are associated with
chaperone proteins until secretion is activated by con-
tact with a host cell [45,46]. A complex formed by IpaB
and IpaD may play a role in the regulation of this se-
cretion [34]. Once secretion is activated by contact with
an epithelial cell, the N-terminus of IpaC binds IpaB
[47]. Both proteins are hydrophobic allowing this com-
plex to insert into the membrane of the host cell to form
a pore [48]. It is presumed that the other effector mole-
cules, delivered by the type III secreton, are able to ac-
cess the host cytoplasm through this pore.
The C-terminal domain of IpaC activates host cell
Rho GTPases, triggering actin polymerisation and fi-
lopodial extensions in the vicinity of the bacteria [49].
IpaA is secreted into the cytosol of the host epithelial
cell where it binds the cytoskeleton-associated protein
vinculin. The IpaA–vinculin complex depolymerises ac-
tin filaments, organising an entry foci around the bac-
terium [50,51]. IpgD is injected into the epithelial cell by
the S. flexneri type III secretion system, where it acts as
a phosphoinositide phosphatase, uncoupling the plasma
membrane from the actin cytoskeleton, allowing mem-
brane extensions to form [52].
VirA has recently been identified as an additional
effector molecule of S. flexneri epithelial cell invasion.
An interaction between VirA and tubulin within the
host cytosol destabilises microtubules around the bac-
terial site of entry. It is proposed that this destabilisation
could stimulate Rac1, a Rho family GTPase, creating
lamellipodial extensions in the host cell [53].
The cytoskeletal rearrangements induced by the
Shigella effector proteins results in the bacterium being
internalised by the epithelial cell within a macropino-
cytic vacuole.
2.5. Replication within the epithelial cell and intracellular
and intercellular spread
The macropinocytic vacuole containing the Shigella
bacterium is rapidly lysed by the IpaB invasin, which
acts as membranolytic toxin in the phagosome mem-
brane, releasing Shigella into the host cell cytoplasm
[29]. The lysis of the phagosome may also involve IpaC,
which is able to disrupt phospholipid membranes upon
insertion of its hydrophobic regions [54,55].
S. flexneri can replicate inside the cytoplasm of epi-
thelial cells in vitro with a doubling time of 40 minutes.
Epithelial cells are observed undergoing necrotic-like
death during shigellosis (Fig. 1) [56]. Although it was
initially proposed that Shigella multiplication within the
cytosol was the cause of epithelial cell lysis, it seems
more likely that the cells are being destroyed by the
hostÕs inflammatory response [26]. In fact, Shigella
would gain little advantage from killing the epithelial
cell as whilst the bacteria are contained within the epi-
thelial cell they are protected from immune cells and are
in a favourable environment for replication [56].
mxiE, a gene located within the mxi/spa locus has
recently been identified as a transcriptional regulator for
a number of putative virulence factors required for vir-
ulence in the Sereny test. mxiE is only activated when
the bacterium is within the epithelial cell cytosol sug-
gesting that its role is to regulate virulence genes used in
the post-invasion steps of infection [57].
Shigella is able to exploit the host cells actin assembly
machinery to move through the host cell cytoplasm and
into adjacent epithelial cells. This intra and intercellular
spread is a crucial step in the virulence of Shigella and is
driven by the outer membrane protein, IcsA (VirG)
[58–60]. IcsA is expressed in a unipolar fashion on the
bacterial surface, with the greatest concentration local-
ised to the old pole of the bacterium [61]. Newly syn-
thesised IcsA appears to be directly targeted to the old
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
47

pole by two internal regions, where it is autotransported
to the outer membrane [62,63]. The maintenance of IcsAÕs
unipolar localisation is essential for intracellular move-
ment and appears to be dependant on the structure of the
LPS. Mutant S. flexneri strains missing, expressing partial
O-antigenÕs or lacking a modal distribution of O-antigen
chain length display non-polar surface localisation of
IcsA and are unable to spread from cell to cell [64–66]. It is
possible that the LPS maintains IcsA polarity by forming
interlocking microdomains with its O-antigen side chains
on the surface of the bacterium, which would prevent IcsA
from diffusing away from the old pole [67]. A recent study
has revealed the need for S. flexneri to display short length
O-antigen chains in order to prevent the blocking of Ic-
sAÕs active sites by very long O-antigen chains. This
finding suggests that S. flexneri has evolved to express two
O-antigen chain lengths with each contributing to the
virulence of the strain; short chains which allow IcsA to
function and long chains which confer resistance to serum
[68].
The Shigella protein DegP also appears to be re-
quired for efficient intracellular spread and polarised
expression of IcsA. The exact role of DegP is unknown
but it may be important in the delivery of IcsA to the
bacterial surface [69].
IcsA at the bacterial pole interacts with the host protein
neural Wiskott–Aldrich syndrome protein (N-WASP)
and possibly with vinculin [70–72]. IcsA specifically binds
N-WASP and not other members of the WASP family,
which stimulates actin-related protein (Arp) 2/3 complex-
mediated actin polymerisation [73,74]. This ligand speci-
ficity of IcsA–N-WASP may determine which host cells
allow Shigella to use actin-based motility [74].
Actin polymerisation at the pole of the bacterium
creates propulsive force, which drives the bacterium
through the cytoplasm of the cell until it contacts the host
cell membrane, forming a protrusion into the neigh-
bouring epithelial cell [75]. The protrusion is actively en-
docytosed by the neighbouring cell in a myosin light chain
kinase dependant mechanism, which also requires cad-
herin expression [76,77]. The bacteria are then sur-
rounded by two cellular membranes, which are lysed by
secreted IpaB and IpaC [78]. Another protein, VacJ, has
also been shown to be essential for freeing Shigella into
the cytoplasm of the next cell [79]. Thus, Shigella is able
to replicate and spread within the intestinal epithelial
layer whilst avoiding exposure to the extracellular envi-
ronment and its circulating immune cells.
3. The hosts immune response to S. flexneri
3.1. Innate immunity
The severe inflammation generated by shigellosis can
persist in the gut for over a month, with a general up-
regulation of a variety of cytokines (IL-1, TNF-a, IL-6,
IFN-c, TNF-b, IL-4, IL-10, TGF-b and IL-8) [80]. Al-
though some of the clinical symptoms of shigellosis may
actually be a direct consequence of the cytokines, they
also assist in controlling and containing the infection.
Resident macrophages and infiltrating monocytes are
unable to efficiently kill S. flexneri in their phagosomes
and instead succumb to apoptosis [81,82]. The IL-18
released by apoptotic macrophages can target NK cells
and T lymphocytes, inducing production of IFN-c [83].
IFN-c deficient mice are five times more susceptible to a
Shigella infection, as IFN-c activates macrophages and
fibroblast cells, which promote bacterial clearance and
possibly inhibit bacterial replication within epithelial
cells [84].
The most important consequence of the hostÕs innate
immune response appears to be the cytokine induced
migration of PMN cells. The transcription factor NF-j
B is activated in Shigella-infected epithelial cells in an
LPS-dependant mechanism, leading to the production
and secretion of IL-8 by the infected cells [39]. IL-8 is a
potent chemoattractant for PMN cells, as is the IL-1
released from apoptotic macrophages.
Shigella is unable to escape the phagocytic vacuole of
PMN cells and are killed inside the phagosome [85].
Recent research has implicated the human neutrophil
elastase (NE) as a key host defence protein of the neu-
trophil, capable of degrading Shigella virulence proteins
within 10 min of Shigella infecting the neutrophil [86].
PMN cells ultimately play a crucial role in controlling
the Shigella infection, confining extracellular bacteria to
the mucosa, preventing deeper tissue invasion and sys-
temic spread [25,87].
Another host defence mechanism directed against
Shigella has recently been discovered. The glycoprotein,
lactoferrin, present in mucosal secretions, breast milk
and phagocytic cells can impair the ability of S. flexneri
to invade HeLa cells, exposing IpaB–IpaC complexes to
protease degradation by disrupting the bacterial surface
[88]. Additionally, a study in transgenic mice expressing
a human intestinal defensin has demonstrated an im-
portant role for intestinally-secreted antibiotic peptides
in controlling a Salmonella typhimurium enteric infection
[89]. It is highly likely that intestinal defensins would
display similar antibiotic properties against enteric
S. flexneri.
3.2. Cellular immunity
Very little data is available on the hostÕs cellular im-
mune response to S. flexneri, especially in comparison to
other intracellular bacteria. Studies have shown in-
creased T cell activation in shigellosis patients and T cell
clones have been isolated which proliferate in response
to S. flexneri antigen [90–93]. The cytokines induced by
Shigella antigens in vaccine studies are suggestive of Th1
48
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58

and Th2 lymphocyte responses [94,95]. Additionally, the
increased susceptibility of AIDS patients, deficient in
CD4
þ
T cells, to shigellosis could suggest that cell-
mediated immunity can play a protective role in shig-
ellosis [96].
However, the contribution of T lymphocytes to the
hostÕs protective immunity to Shigella was studied in the
mouse pulmonary model where mice deficient in T cells
were vaccinated with attenuated S. flexneri. These mice
were suitably protected from challenge with wild type
bacteria despite their deficiency in T lymphocytes, sug-
gesting that even if T cell responses develop to Shigella
they are not essential for protection [97].
3.3. Humoral immunity
Information about the hostÕs humoral response to
Shigella infection has been collected from numerous se-
rological studies of infected humans and experiments
performed in animal models. The data suggests that the
humoral immune response is a major component of
protective immunity to shigellosis with both systemic and
mucosal responses activated against the LPS and some
virulence plasmid encoded proteins, including the Ipa
proteins. The serotype-specific structure of the LPS is
assumed to be the major target of the hostÕs immune re-
sponse as natural and experimental infections with Shi-
gella confer serotype-specific immunity, where previous
infection or vaccination provides little to no protection
against heterologous serotypes [98–100]. However, anti-
bodies directed against epitopes shared between certain
O-antigen structures do appear to show some cross-re-
activity [101]. The protective significance of these cross-
reactive antibodies is incompletely understood and is
discussed in more detail in Section 4.6. However, the
overall importance of an antibody response to Shigella
infection has been confirmed in a study which showed that
a reduced and delayed humoral immune response in
comparison to adult patients is the likely cause of the in-
creased susceptibility of children to shigellosis [102].
It appears that both the systemic and mucosal arms
of the humoral response are activated as serum IgG,
IgM and secretory IgA have all been implicated in the
generation
of
serotype-specific
immunity
against
S. flexneri.
Secretory IgA (sIgA) is made up of 2 IgA units and
two polypeptides, the J chain and the secretory com-
ponent (SC). sIgA transcytoses into the lumenal cavity
of the intestine where the secretory component binds the
mucosal coating of the epithelial cells, forming an an-
tibody shield over the cells [103]. sIgA can also coat the
outer membrane of lumenal bacteria, impeding invasion
by preventing their attachment to the mucosal surfaces,
mediate antibody-dependant cell-mediated cytotoxicity
and interfere with bacterial utilisation of growth factors
[104].
IgA, especially anti-LPS IgA have been detected in
humans suffering natural shigellosis in a number of
studies and is thought to play an important role in im-
munity to re-infection [105–109]. Anti-LPS secretory
IgA antibodies in the breast milk of mothers exposed to
shigellosis appear to be responsible for the decreased
severity of shigellosis in Shigella-infected infants [110].
Additionally, the implantation of a serotype-specific
sIgA hybridoma on the back of mice protected them
against intranasal challenge with a lethal dose of
S. flexneri organisms [111]. This experiment suggests
that a mucosal antibody directed against a single LPS
epitope of Shigella could be sufficient for protective
immunity against re-infection by the homologous sero-
type.
Despite shigellosis generally being a localised mucosal
infection, serum antibodies IgG and IgM are detected in
natural human infections directed against the LPS and
virulence plasmid antigens [105–107,112,113]. IgG and
possibly IgM directed against the LPS have been shown
to play a protective role in immunity to Shigella in mice
studies. IgA deficient vaccinated mice are fully protected
against pulmonary Shigella challenge, suggesting that
IgG or IgM are able to provide immunity [114]. Im-
munised mice deficient in all T lymphocytes were pro-
tected
from
wild-type
Shigella
challenge
by
a
predominantly anti-LPS IgM response [97]. It is still
unclear what role serum antibodies directed against the
LPS of S. flexneri are playing in the generation of se-
rotype-specific immunity, although they may be stimu-
lating complement killing of the bacteria or mediating
antibody-dependant cellular cytotoxicity in the mucosal
area [115–117]. However, it must be stressed that the
protective role of serum antibodies in controlling
Shigella infection has been predominately characterised
in mice and warrants further investigation as human
vaccination data suggests that the parenteral stimula-
tion of serum Ig does not correlate with protection
[118–121].
4. S. flexneri vaccine development
The cost of treating shigellosis with antibiotics, es-
pecially in the developing world, is unrealistic. The se-
rotype-specific immunity generated by S. flexneri
provides protection against reinfection by the homolo-
gous serotype, making vaccination a viable option for
controlling shigellosis.
A suitable vaccine for shigellosis must fulfil certain
requirements: the mucosal immune system must be
activated and this immunity should be long-lasting,
the vaccine must be cheap to manufacture, induce
minimal side effects and be simple to administer, as
children in developing countries will be the main
recipients.
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
49

Since the 1940s a number of candidate vaccines for S.
flexneri have been developed but as yet none have been
successful enough for field release. Early attempts to
develop S. flexneri vaccines consisted of inactivated
bacteria delivered parenterally, which failed to induce a
protective immune response, despite inducing a high
titre of serum anti-LPS antibody [118–121]. The lack
of protection was most likely due to the failure of
the parenteral vaccine in inducing a mucosal immune
response.
Consequently, many recent vaccine strategies have
concentrated on developing live vaccine strains which
can be administered orally and will activate the effectors
of mucosal immunity.
4.1. Subunit vaccines
Subunit Shigella vaccines may avoid the safety issues
associated with live vaccines.
LPS can be complexed to proteosomes and delivered
intranasally to humans. Clinical trials have revealed that
a S. flexneri 2a LPS–proteosome vaccine is capable of
generating a serotype-specific immune response in hu-
mans [122]. S. flexneri LPS has also been attached to
proteins and delivered parenterally to volunteers as
potential vaccines. These vaccines were safe in humans
and induced strong serum antibody responses [123–126].
Other subunit vaccines are yet to be evaluated in
humans. Mice and guinea pigs were protected from
S. flexneri challenge by mucosal immunisation with a
purified complex of IpaB, IpaC, IpaD and LPS [127].
Ribosomal preparations from Shigella delivered paren-
terally can generate protective immunity in guinea pigs
and monkeys [128]. The immune response is directed
against O-antigen polysaccharides (
L
L
-hapten) purified
with the ribosomal preparation. However, the O-antigen
content in ribosomal preparations varies, making a
consistent vaccine difficult to manufacture [129].
4.2. Killed oral vaccines
Early challenge experiments in monkeys revealed that
orally administered acetone-killed and dried Shigella
was unable to protect monkeys from infection [130].
More recently however, an oral heat-killed S. flexneri
vaccine evaluated in a rabbit model was shown to be
100% protective [131]. Thus, further studies are required
to determine the protective capabilities of killed oral
vaccines for S. flexneri in humans.
4.3. Non-invasive live vaccines
Mutations in either the S. flexneri chromosome or the
virulence plasmid have been used to generate non-in-
vasive live vaccine strains. Most of these strains were
safe in humans and were able to induce some degree of
protective immunity in volunteers (Table 1). Probably
the most successful of these vaccines is the invasion
plasmid mutant, S. flexneri 2a Istrati T
32
which is 100%
safe in humans and provides up to an 85% protective
efficacy. However, it must be administered in large
(1
10
11
CFU) multiple doses every six months which is
expensive and difficult to implement in developing
countries [132].
4.4. Invasive live vaccines
Invasive oral Shigella vaccine strain strategies are
increasingly being explored as invasive strains deliver
antigen to the mucosal immune system, provoking a
strong immune response. As the genetic understanding
of S. flexneri virulence has improved so have the strat-
Table 1
Live non-invasive oral S. flexneri vaccines which have been assessed in monkeys or humans
Vaccine
Description
Safety
Efficacy
Comment
References
S. flexneri 2a 2457O
Spontaneous
avirulent mutant, virF
inactivated by
insertion
Reverts to virulence
in humans
Monkeys are
protected. Caused
dysentery in 34% of
human volunteers
Reactogenic in
humans
[130,146,147]
S. flexneri streptomy-
cin dependant strains
Spontaneous
mutants incapable of
growing in the
absence of
streptomycin
Reversion to
streptomycin
independence in
volunteers. Diarrhoea
and vomiting in
15–35% of volunteers
(5
10
10
CFU)
Up to 90% protection
in field trials with
multiple doses. US
trials found marginal
protection
Unstable
phenotype and
inconsistent
protective efficacy
[98,148,149]
S. flexneri 2a Istrati
T
32
Spontaneous deletion
of ipaBCDA, invA
and icsA (virG) from
the virulence plasmid
Safe in humans, mild
adverse effects in very
few volunteers at
2
10
11
CFU
Around 80%
protection in humans
when administered in
5 doses
Protection lasts 6
months. Reduces
attack rate of
heterologous
Shigella serotypes
[132,150]
50
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58

egies to construct safe invasive vaccines. Invasive vac-
cine strains are generally attenuated by mutations in
either virulence genes necessary for pathogenesis after
cell entry or in metabolic genes which prevent the bac-
teria from replicating and spreading in the host after
invasion.
Mutations in either icsA and/or in a variety of met-
abolic genes have produced attenuated invasive vaccine
strains which are safe and capable of up to 100% pro-
tection with multiple doses in monkeys (Table 2). A
number of auxotrophic vaccine strains, some also car-
rying mutations in virulence genes, have been assessed
for their safety and ability to induce a serotype-specific
immune response in human volunteers in phase 1 clini-
cal trials [94,133–135]. These strains vary in the levels of
their attenuation and their immunogenicity (Table 2).
The S. flexneri 2a vaccine strain, SC602 has pro-
ceeded to phase 2 clinical trials in humans. This strain
carries deletions in icsA as well as the aerobactin iuc
locus, which is involved in iron transport. SC602 is safe
in humans at low doses (1
10
4
CFU) and capable of
providing protection to immunised humans challenged
with wild type 2a S. flexneri. However, the vaccine is
only weakly attenuated causing symptoms such as di-
arrhoea and fever when administered in doses higher
that 1
10
4
CFU [136]. Thus, despite promising results
with S. flexneri invasive vaccine candidates, further
work is required to achieve a balance between immu-
nogenicity and safety in humans.
4.5. Hybrid vaccines
E. coli vaccine candidates have also been used to
develop hybrid vaccines expressing Shigella antigens.
Early attempts using Shigella–E. coli hybrid vaccines
developed invasive vaccines which caused symptoms
in human volunteers or which were not protective
[137,138]. Strains based on E. coli K12 carrying the
group- and type-specific antigen of S. flexneri 2a and the
virulence plasmid from S. flexneri 5 were unable to in-
duce significant protection in immunised volunteers
[139,140].
Additionally, S. flexneri candidate vaccine strains are
being engineered to express the O-antigenÕs of other
Shigella species. The S. flexneri 2a vaccine strain T
32
carrying a plasmid containing the gene cluster coding
for S. sonnei O-antigen, was capable of providing 100%
protection to mice against challenge with both virulent
S. flexneri and S. sonnei [141]. The S. dysenteriae O-
antigen biosynthesis genes were integrated into the
SFL124 (serotype Y) vaccine strain, generating strains
able to induce antibodies specific to both homologous
and heterologous O-antigen structures in mice [142].
Similar approaches are also being used to generate
vaccines protective against multiple S. flexneri serotypes
and will be discussed below.
4.6. Multiple-serotype protection strategies
Because immunity to S. flexneri is serotype-specific,
vaccination against one serotype will only provide pro-
tection to infection by the homologous serotype. The
serotypes of S. flexneri differ in their distribution with up
to four different serotypes prevalent in an endemic area.
Thus, the ideal S. flexneri vaccine would provide pro-
tection to all prevalent serotypes of a particular geo-
graphical region.
All S. flexneri serotypes, with the exception of sero-
type 6, share a common O-antigen backbone. The ad-
dition of glucosyl and/or O-acetyl groups to the sugars
of the backbone generates the type (I, II, IV, V and X)
and group (3, 4, 6 and 7, 8) antigens that define the
serotypes. Consequently, some serotypes share type and/
or group antigens on their LPS [11]. Because the im-
mune response is primarily directed against the LPS,
some antibodies generated against the group or type
antigen of one serotype should be cross-reactive to other
serotypes. For example, antibody in human sera raised
against S. flexneri 2a has been shown to cross-react with
LPS from heterologous serotypes 1a, 2b, 5a and Y,
which share type or group antigens with the serotype 2a
O-antigen structure [101]. As cross-reactivity of the hu-
man sera to all of the different serotypes was not ob-
served, it appears that the common group 1 antigen,
which is shared by all S. flexneri serotypes [143] was not
able to induce any sufficiently cross-reactive antibodies.
This suggests that the group 1 antigen is poorly immu-
nogenic and may not have a role in inducing protective
antibodies against heterologous serotypes. It also re-
mains unclear whether the cross-reactive antibodies di-
rected against the other group and type antigens of the
LPS, as mentioned above, can provide any protection
against infection by heterologous serotypes. Therefore,
further research is required to adequately establish the
role of the cross-reactive O-antigen epitopes in human
immunity against shigellosis.
However, animal studies have shown that mixing a
number of S. flexneri vaccines of different serotypes into
a vaccine cocktail can invoke an immune response with
cross-reactive potential. A vaccine cocktail containing
serotype 2a and 3a S. flexneri strains was administered
to guinea pigs in the Sereny test, conferring significant
protection to challenge by serotypes 1b, 2b, 5b and Y
[144]. Thus, by combining a selection of S. flexneri ser-
otypes into a vaccine cocktail, it may be possible to
cross-protect against most S. flexneri serotypes.
Alternatively, single S. flexneri vaccine strains can be
engineered to express the O-antigen of more than one
serotype. Such strains should be capable of generating a
protective immune response in the host directed against
each of the serotype specific O-antigen structures. This
lab has previously reported the serotype-conversion of
the serotype Y S. flexneri candidate vaccine strain,
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
51

Table 2
Live invasive oral S. flexneri vaccines which have been assessed in monkeys or humans
Vaccine
Description
Safety
Efficacy
Comment
References
S. flexneri 5a SC5700
Attenuated by insertions in icsA
and iuc
Mucoid diarrhoea in some
monkeys with 5
10
10
CFU
About 70% protection in
monkeys after 3 doses
Slightly reactogenic in monkeys
[151]
S. flexneri 5a SC560
Deletion in icsA
Mucoid diarrhoea and mild
clinical symptoms in some
monkeys with 5
10
10
CFU
100% protective in monkeys
after 3 doses
Slightly reactogenic in monkeys
[60]
S. flexneri 5a SC433
Deletion in envZ and ompR
Mucoid diarrhoea and mild
clinical symptoms in some
monkeys with 5
10
10
CFU
100% protective in monkeys
after 3 doses
Slightly reactogenic in monkeys
[60]
S. flexneri 5a SC445
Deletion in envZ, icsA and
ompR
100% safe in monkeys at
5
10
10
CFU
One out of five monkeys
challenged became sick
Level of attenuation may be too severe
for consistent protection
[60]
S. flexneri 2a vc77
Auxotrophic for purine (Pur
)
and rifampicin resistant
No symptoms in adults or
children up to 3
10
9
CFU
Dysentery in 2 out of 4 adults
challenged
Poor protective capabilities
[152–154]
S. flexneri Y TSF-21
Thymine requiring (Thy
) and
temperature sensitive (Ts
)
No symptoms in monkeys at
1
10
11
CFU
100% protection in monkeys
after 2 doses
Temperature sensitivity
mutation has a high rate of reversion
[155,156]
S. flexneri Y SFL114
Insertional inactivation of aroD
No symptoms in monkeys at
2–3
10
10
CFU
100% protection in monkeys
after four doses
Some reversion of phenotype observed
in laboratory
conditions
[157]
S. flexneri Y SFL124
Deletion of aroD
Very mild symptoms in some
human volunteers at 2
10
9
CFU and children at up to
1
10
9
CFU
100% protection in monkeys
after three doses of 1
10
11
CFU
Strain is advantageous as it can be
converted to new serotypes
[135,158–160]
S. flexneri 2aSFL1070
Deletion of aroD
Mild symptoms in volunteers at
1
10
7
– 1
10
8
CFU. Increased
clinical symptoms at 1
10
9
CFU
About 85% protection in
monkeys after 4 doses 1
10
11
CFU
Further attenuation may be necessary
[133,161]
S. flexneri 2a
CVD1203
Deletions in icsA and aroA
No symptoms in humans when
administered in a single dose of
1
10
6
CFU
Challenge only performed in
guinea pigs, provided 83%
protection
Strain may require further
attenuation in order to reduce
reactogenicity in humans
[134,162,163]
S. flexneri
2aCVD1207
Deletions in icsA, sen, set and
guaAB
No symptoms in humans when
administered in a single dose of
up to 1
10
8
CFU
Challenge only performed in
guinea pigs, provided 85%
protection
Immunogenicity in humans may not be
sufficient with just one dose
[94,144]
S. flexneri 2a SC602
Deletions in icsA gene and the
iuc locus.
Mild diarrhoea and fever in
humans at 1
10
4
CFU
3 out of 7 volunteers challenged
experienced mild diarrhoea
Provides protection or reduces symp-
toms to infection with S. flexneri 2a.
Further attenuation may be required
[136,164]
52
A.V.
Jennison,
N.K.
Verma
/
FEMS
M
ircobiolo
gy
Reviews
28
(2004)
43–58

SFL124 to serotype X by the insertion of the bacterio-
phage SfX serotype-conversion gene cluster [145]. This
approach has been utilised to insert the serotype con-
version gene cluster of bacteriophage SfV and the glu-
cosyl transferase gene of bacteriophage SfII in tandem
into the SFL124 chromosome. The resulting strain dis-
played the 3,4 group antigen and both the II and V type
antigens as detected by monovalent antiserum and
simultaneously induced a serotype-specific immune
response to both serotypes 2a and 5a in the mouse
pulmonary model (unpublished data, this lab). This
strategy could be easily applied to any newly developed
serotype Y vaccine candidates, ultimately generating a
variety of S. flexneri polyvalent vaccine strains which
could be combined into vaccine cocktails designed for
specific geographical areas.
5. Conclusions
Through in vitro and in vivo studies we are beginning
to develop a detailed picture of how S. flexneri invades
the intestinal mucosa and causes disease. Further re-
search into the hostÕs immune response will ultimately
reveal all inflammatory mediators involved in shigellosis
and clarify whether cellular immunity plays an impor-
tant role in the control of Shigella and in protection
against reinfection. Virulence factors are crucial to
S. flexneri for the development and maintenance of
disease. Undoubtedly, many more remain to be identi-
fied and characterised. Discovery of new virulence fac-
tors and an understanding of gene regulation and
conditional gene expression will be greatly assisted by
the release of the S. flexneri 2a genome sequence. A
number of the Shigella vaccine strategies mentioned
above have shown promise in animal studies and initial
human clinical trials, significantly advancing the status
of Shigella vaccine development. However, to com-
pletely protect against natural Shigella infections, the
development of vaccine cocktails and polyvalent vac-
cines must be addressed. Future vaccine research should
encompass trials of mixed vaccines designed to confer
protection against multiple serotypes.
References
[1] Wei, J., Goldberg, M.B., Burland, V., Venkatesan, M.M., Deng,
W., Fournier, G., Mayhew, G., Plunkett III, G., Rose, D.,
Darling, A., Mau, B., Perna, N.T., Payne, S.M., Runyen-
Janecky, L., Zhou, S., Schwartz, D.C. and Blattner, F.R. (2003)
Complete genomic sequence and comparative genomics of
Shigella flexneri serotype 2a strain 2457T. Infect. Immun. 71,
2775–2786.
[2] Jin, Q., Yuan, Z., Xu, J., Wang, Y., Shen, Y., Lu, W., Wang, J.,
Liu, H., Yang, J., Yang, F., Zhang, X., Zhang, J., Yang, G., Wu,
H., Qu, D., Dong, J., Sun, L., Xue, Y., Zhao, A., Gao, Y., Zhu,
J., Kan, B., Ding, K., Chen, S., Cheng, H., Yao, Z., He, B.,
Chen, R., Ma, D., Qiang, B., Wen, Y., Hou, Y. and Yu, J. (2002)
Genome sequence of Shigella flexneri 2a: insights into pathoge-
nicity through comparison with genomes of Escherichia coli K12
and O157. Nucleic Acids Res. 30, 4432–4441.
[3] Bennish, M.L. (1991) Potentially lethal complications of shigel-
losis. Rev. Infect. Dis. 13, S319–S324.
[4] Kotloff, K.L., Winickoff, J.P., Ivanoff, B., Clemens, J.D.,
Swedlow, D.L., Sansonetti, P.J., Adak, G.K. and Levine,
M.M.M. (1999) Global burden of Shigella infections: implica-
tions for vaccine development and implementation of control
strategies. Bull. World Health Organ. 77, 651.
[5] Bennish, M.L. and Wojtyniak, B.J. (1991) Mortality due to
shigellosis: community and hospital data. Rev. Infect. Dis. 13,
S245–S251.
[6] Ashkenazi, S., Levy, I., Kazaronovski, V. and Samra, Z. (2003)
Growing antimicrobial resistance of Shigella isolates. J. Anti-
microb. Chemother. 51, 427–429.
[7] Moss, J.E., Cardozo, T.J., Zychlinsky, A. and Groisman, E.A.
(1999) The SelC-associated SHI-2 pathogenicity island of
Shigella flexneri. Mol. Microbiol. 33, 74–83.
[8] Rajakumar, K., Sasakawa, C. and Adler, B. (1997) Use of a
novel approach, termed island probing, identifies the Shigella
flexneri she pathogenicity island which encodes a homolog of the
immunoglobulin A protease-like family of proteins. Infect.
Immun. 65, 4606–4614.
[9] Turner, S.A., Luck, S.N., Sakellaris, H., Rajakumar, K. and
Adler, B. (2001) Nested deletions of the SRL pathogenicity
island of Shigella flexneri 2a. J. Bacteriol. 183, 5535–5543.
[10] Walker, J.C. and Verma, N.K. (2002) Identification of a putative
pathogenicity island in Shigella flexneri using subtractive hy-
bridisation of the S. flexneri and Escherichia coli genomes.
FEMS Microbiol. Lett. 10580, 1–8.
[11] Allison, G.E. and Verma, N.K. (2000) Serotype-converting
bacteriophages and O-antigen modification in Shigella flexneri.
Trends Microbiol. 8, 17–23.
[12] Mallet, C.P., VanDeVerg, L.L., Collins, H.H. and Hale, T.L.
(1993) Evaluation of Shigella vaccine safety and efficacy in an
intranasally challenged mouse model. Vaccine 11, 190–196.
[13] Oaks, E.V., Wingfield, M.E. and Formal, S.B. (1985) Plaque
formation by virulent Shigella flexneri. Infect. Immun. 48, 124–
129.
[14] Sereny, B. (1957) Experimental keratoconjunctivitis Shigellosa.
Acta Microbiol. Acad. Sci. Hung., 4.
[15] Wassef, J.S., Keren, D.F. and Mailloux, J.L. (1989) Role of M
cells in initial antigen uptake and in ulcer formation in the rabbit
intestinal loop model of shigellosis. Infect. Immun. 57, 858–863.
[16] DuPont, H.L., Levine, M.M., Hornick, R.B. and Formal, S.B.
(1989) Inoculum size in shigellosis and implications for expected
mode of transmission. J. Infect. Dis. 159, 1126–1127.
[17] Small, P., Blankenhorn, D., Welty, D., Zinser, E. and Slonczewski,
J.L. (1994) Acid and base resistance in Escherichia coli and Shigella
flexneri: role of rpoS and growth pH. J. Bacteriol. 176, 1729–1737.
[18] Philpott, D.J., Edgeworth, J.D. and Sansonetti, P.J. (2000) The
pathogenesis of Shigella flexneri infection: lessons from in vitro
and in vivo studies. Philos. Trans. R. Soc. Lond. B. Biol. Sci.
355, 575–586.
[19] Mounier, J., Vasselon, T., Hellio, R., Lesourd, M. and Sanso-
netti, P.J. (1992) Shigella flexneri enters the human colonic Caco-
2 epithelial cells through the basolateral pole. Infect. Immun. 60,
237–248.
[20] Neutra, M.R., Pringault, E. and Kraehenbuhl, J.P. (1996)
Antigen sampling across epithelial barriers and induction of
mucosal immune responses. Annu. Rev. Immunol. 14.
[21] Sansonetti, P.J. and Phalipon, A. (1999) M cells as ports of entry
for enteroinvasive
pathogens: mechanisms
of interaction,
consequences for the disease process. Semin. Immunol. 11,
193–203.
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
53

[22] Beatty, W.L. and Sansonetti, P.J. (1997) Role of lipopolysac-
charide in signaling to subepithelial polymorphonuclear leuko-
cytes. Infect. Immun. 65, 4395–4404.
[23] Perdomo, J.J., Gounon, P. and Sansonetti, P.J. (1994) Polymor-
phonuclear leukocyte transmigration promotes invasion of colonic
epithelial monolayer by Shigella flexneri. J. Clin. Invest. 93, 633–
643.
[24] Sansonetti, P.J., Arondel, J., Cavaillon, J.M. and Huerre, M.
(1995) Role of interleukin-1 in the pathogenesis of experimental
shigellosis. J. Clin. Invest. 96, 884–892.
[25] Sansonetti, P.J., Arondel, J., Huerre, M., Harada, A. and
Matsushima, K. (1999) Interleukin-8 controls bacterial transepi-
thelial translocation at the cost of epithelial destruction in
experimental shigellosis. Infect. Immun. 67, 1471–1480.
[26] Perdomo, O.J., Cavaillon, J.M., Huerre, M., Ohayon, H.,
Gounon, P. and Sansonetti, P.J. (1994) Acute inflammation
causes epithelial invasion and mucosal destruction in experimen-
tal shigellosis. J. Exp. Med. 180, 1307–1319.
[27] Sakaguchi, T., Kohler, H., Gu, X., McCormick, B.A. and
Reinecker, H. (2002) Shigella flexneri regulates the tight
junction-associated proteins in human intestinal epithelial cells.
Cell. Microbiol. 4, 367–381.
[28] Kuwae, A., Yoshida, S., Tamano, K., Mimuro, H., Suzuki, T.
and Sasakawa, C. (2001) Shigella invasion of macrophage
requires the insertion of IpaC into the host plasma membrane.
J. Biol. Chem. 276, 32230–32239.
[29] High, N., Mounier, J., Prevost, M.C. and Sansonetti, P.J. (1992)
IpaB of Shigella flexneri causes entry into epithelial cells and
escape from the phagocytic vacuole. EMBO J. 11, 1991–1999.
[30] Chen, R., Smith, M.R., Thirumalai, K. and Zychlinsky, A.
(1996) A bacterial invasin induces macrophage apoptosis by
binding directly to ICE. EMBO J. 15, 3853–3860.
[31] Dinarello, C.A. (1998) Interleukin-1b, Interleukin-18 and the
Interleukin-1b converting enzyme. Ann. N.Y. Acad. Sci. 856, 1–
11.
[32] Zychlinsky, A., Thirumalai, K., Arondel, J., Cantey, J.R.,
Aliprantis, A.O. and Sansonetti, P.J. (1996) In vivo apoptosis
in Shigella flexneri infections. Infect. Immun. 64, 5357–5365.
[33] Nutten, A., Sansonetti, P.J., Huet, G., Bourdon-Bisiaux, C.,
Meresse, B., Colombel, J. and Desreumaux, P. (2002) Epithelial
inflammation response induced by Shigella flexneri depends on
mucin gene expression. Microbes Infect. 4, 1121–1124.
[34] Menard, R., Sansonetti, P.J. and Parsot, C. (1994) The secretion
of the Shigella flexneri Ipa invasions is activated by epithelial
cells and controlled by IpaB and IpaD. EMBO J. 13, 5293–5302.
[35] Watarai, M., Funato, S. and Sasakawa, C. (1996) Interaction of
Ipa proteins of Shigella flexneri with alpha5beta1 integrin
promotes entry of the bacteria into mammalian cells. J. Exp.
Med. 183, 991–999.
[36] Alho, A.M. and Underhill, C.B. (1989) The hyaluronate receptor
is preferentially expressed on proliferating epithelial cells. J. Cell
Biol. 108, 1557–1565.
[37] Skoudy, A., Mounier, J., Aruffo, A., Ohayon, H., Gounon, P.,
Sansonetti, P.J. and Tran Van Nhieu, G. (2000) CD44 binds to
the Shigella IpaB protein and participates in bacterial invasion of
epithelial cells. Cell. Microbiol. 2, 19–33.
[38] Tran Van Nhieu, G. and Sansonetti, P.J. (1999) Mechanism of
Shigella entry into epithelial cells. Curr. Opin. Microbiol. 2, 51–55.
[39] Kohler, H., Rodrigues, S.P. and McCormick, B.A. (2002) S.
flexneri interactions with the basolateral membrane domain of
polarised model intestinal epithelium: role of LPS in cell invasion
and in activation of the mitogen activated protein kinase ERK.
Infect. Immun. 70, 1150–1158.
[40] Aderem, A. and Ulevitch, R.J. (2000) Toll-like receptors in the
induction of the Innate Immune Response. Science 406, 782–787.
[41] Ingalls, R.R., Monks, B.G., Savedra, R., Christ, W.J., Delude,
R.L., Medvedev, A.E., Espevik, T. and Golenbock, D.T. (1998)
CD11/CD18 and CD14 share a common lipid A signalling
pathway. J. Immunol. 161, 5413–5420.
[42] Hueck, C.J. (1998) Type III protein secretion systems in bacterial
pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62,
379–433.
[43] Menard, R., Sansonetti, P.J. and Parsot, C. (1993) Non-polar
mutagenesis of the ipa genes defines IpaB, IpaC and IpaD as
effectors of Shigella entry into epithelial cells. J. Bacteriol. 175,
5899–5906.
[44] Sasakawa, C., Kamata, K., Sakai, T., Makino, S., Yamada, M.,
Okada, N. and Yoshikawa, M. (1988) Virulence-associated
genetic regions comprising 31 kilobases of the 230-kilobase
plasmid in Shigella flexneri 2a. J. Bacteriol. 170, 2480–2484.
[45] Menard, R., Sansonetti, P.J., Parsot, C. and Vasselon, T. (1994)
Extracellular association and cytoplasmic partitioning of the
IpaB and IpaC invasins of Shigella flexneri. Cell 79, 515–525.
[46] Page, A.L., Sansonetti, P.J. and Parsot, C. (2002) Spa15 of
Shigella flexneri, a third type of chaperone in the type III
secretion pathway. Mol. Microbiol. 43, 1533–1542.
[47] Harrington, A.T., Hearn, P.D., Picking, W.L., Barker, J.R.,
Wessel, A. and Picking, W.D. (2003) Structural characterization
of the N terminus of IpaC from Shigella flexneri. Infect. Immun.
71, 1255–1264.
[48] Blocker, A., Gounon, P., Larquet, E., Niebuhr, K., Cabiaux, V.,
Parsot, C. and Sansonetti, P.J. (1999) The tripartite type III
secretion of Shigella flexneri inserts IpaB and IpaC into hosts
membranes. J. Cell Biol. 147, 683–693.
[49] Tran Van Nhieu, G., Caron, E., Hall, A. and Sansonetti, P.J.
(1999) IpaC induces actin polymerization and filopodia forma-
tion during Shigella entry into epithelial cells. EMBO J. 18,
3249–3262.
[50] Bourdet-Sicard, R., Rudiger, M., Jockusch, B.M., Gounon, P.,
Sansonetti, P.J. and Tran Van Nhieu, G. (1999) Binding of the
Shigella protein IpaA to vinculin induces F-actin depolymeriza-
tion. EMBO J. 18, 5853–5862.
[51] Tran Van Nhieu, G., Ben-ZeÕev, A. and Sansonetti, P.J. (1997)
Modulation of bacterial entry into epithelial cells by association
between vinculin and the Shigella IpaA invasin. EMBO J. 16,
2717–2729.
[52] Niebuhr, K., Giuriato, S., Pedron, T., Philpott, D.J., Gaits, F.,
Sable, J., Sheetz, M.P., Parsot, C., Sansonetti, P.J. and Payrastre,
B. (2002) Conversion of PtdIns(4,5)P
2
into PtdIns(5)P by the S.
flexneri effector IpgD reorganizes host cell morphology. EMBO
J. 21, 5069–5078.
[53] Yoshida, S., Katayama, E., Kuwae, A., Mimuro, H., Suzuki, T. and
Sasakawa, C. (2002) Shigella deliver an effector protein to trigger host
microtubule destabilization, which promotes Rac1 activity and
efficient bacterial internalization. EMBO J. 21, 2923–2935.
[54] De Geyter, C., Vogt, B., Benjelloun-Touimi, Z., Sansonetti, P.J.,
Ruysschaert, J.M. and Cabiaux, V. (1997) Purification of IpaC, a
protein involved in entry of Shigella flexneri into epithelial cells
and characterization of its interaction with lipid membranes.
FEBS Lett. 400, 149–154.
[55] Kueltzo, L.A., Osiecki, J., Barker, J., Picking, W.L., Ersoy, B.,
Picking, W.D. and Middaugh, C.R. (2003) Structure–function
analysis of Invasion Plasmid Antigen C (IpaC) from Shigella
flexneri. J. Biol. Chem. 278, 2792–2798.
[56] Mantis, N., Prevost, M.C. and Sansonetti, P.J. (1996) Analysis of
epithelial cell stress response during infection by Shigella flexneri.
Infect. Immun. 64, 2474–2482.
[57] Kane, C.D., Schuch, R., Day, W.A. and Maurelli, A.T. (2002)
MxiE regulates intracellular expression of factors secreted by the
Shigella flexneri 2a type III secretion system. Infect. Immun. 184,
4409–4419.
[58] Bernardini, M.L., Mounier, J., dÕHauteville, H., Coquis-Ron-
don, M. and Sansonetti, P.J. (1989) Identification of icsA, a
plasmid locus of Shigella flexneri that governs bacterial intra-
54
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58

and intercellular spread through interaction with F-actin. Proc.
Natl. Acad. Sci. USA. 86, 3867–3871.
[59] Lett, M., Sasakawa, C., Okada, N., Sakai, T., Makino, S.,
Yamada, M., Komatsu, K. and Yoshikawa, M. (1989) irG, a
plasmid-coded virulence gene of Shigella flexneri: identification
of the virG protein and determination of the complete coding
sequence. J. Bacteriol. 171, 353–359.
[60] Sansonetti, P.J., Arondel, J., Fontaine, A., dÕHauteville, H. and
Bernardini, M.L. (1991) OmpB (osmo-regulation) and IcsA (cell–
cell spread) mutants of Shigella flexneri; vaccine candidates and
probes to study the pathogenesis of shigellosis. Vaccine 9, 416–
422.
[61] Goldberg, M.B., Barzu, O., Parsot, C. and Sansonetti, P.J.
(1993) Unipolar localization and ATPase activity of IcsA, a
Shigella flexneri protein involved in intracellular movement. J.
Bacteriol. 175, 2189–2196.
[62] Steinhauer, J., Agha, R., Pham, T., Varga, A.W. and Goldberg,
M.B. (1999) The unipolar Shigella surface protein IcsA is
targeted directly to the bacterial old pole: IcsP cleavage of IcsA
occurs over the entire bacterial surface. Mol. Microbiol. 32, 367–
377.
[63] Charles, M., Perez, M., Kobil, J.H. and Goldberg, M.B. (2001)
Polar targeting of Shigella virulence factor IcsA in Enterobac-
teriacae and Vibrio. Proc. Natl. Acad. Sci. USA 98, 9871–9876.
[64] Sandlin, R.C., Goldberg, M.B. and Maurelli, A.T. (1996) Effect
of O side-chain length and composition on the virulence of
Shigella flexneri 2a. Mol. Microbiol. 22, 63–73.
[65] Sandlin, R.C., Lampel, K.A., Keasler, S.P., Goldberg, M.B.,
Stolzer, A.L. and Maurelli, A.T. (1995) Avirulence of rough
mutants of Shigella flexneri: requirement of O antigen for correct
unipolar localisation of IcsA in the bacterial outer membrane.
Infect. Immun. 63, 229–237.
[66] Van Den Bosch, L., Manning, P.A. and Morona, R. (1997)
Regulation of O-antigen chain length is required for Shigella
flexneri virulence. Mol. Microbiol. 23, 765–775.
[67] Robbins, J.R., Monack, D.M., McCallum, S.J., Vegas, A.,
Pham, E., Goldberg, M.B. and Theriot, J.A. (2001) The making
of a gradient: IcsA (VirG) polarity in Shigella flexneri. Mol.
Microbiol. 41, 861–872.
[68] Morona, R., Daniels, C. and Van Den Bosch, L. (2003) Genetic
modulation of Shigella flexneri 2a lipopolysaccharide O antigen
modal chain length reveals that it has been optimized for
virulence. Microbiology 149, 925–939.
[69] Purdy, G.E., Hong, M. and Panyne, S. (2002) Shigella flexneri
DegP facilitates IcsA surface expression and is required for
efficient intercellular spread. Infect. Immun. 70, 6355–6364.
[70] Suzuki, T., Miki, H., Takenawa, T. and Sasakawa, C. (1998)
Neural Wiskott–Aldrich syndrome protein is implicated in the
actin based motility of Shigella flexneri. EMBO J. 17, 2767–2776.
[71] Goldberg, M.B. (1997) Shigella actin-based motility in the
absence of vinculin. Cell Motil. Cytoskeleton. 37, 44–53.
[72] Suzuki, T., Saga, S. and Sasakawa, C. (1996) Functional analysis
of Shigella VirG domains essential for interaction with vinculin
and actin-based motility. J. Biol. Chem. 271.
[73] Egile, C., Loisel, T.P., Laurent, V., Li, R., Pantaloni, D.,
Sansonetti, P.J. and Carlier, M.F. (1999) Activation of the
CDC42 effector N-WASP by the Shigella flexneri IcsA protein
promotes actin nucleation by Arp2/3 complex and bacterial
actin-based motility. J. Cell Biol. 146, 1319–1332.
[74] Suzuki, T., Mimuro, H., Suetsugu, S., Miki, H., Takenawa, T.
and Sasakawa, C. (2002) Neural Wiskott–Aldrich syndrome
protein (N-WASP) is the specific ligand for Shigella VirG among
the WASP family and determines the host cell type allowing
actin-based spreading. Cell. Microbiol. 4, 223–233.
[75] Monack, D.M. and Theriot, J.A. (2001) Actin-based motility is
sufficient for bacterial membrane protrusion formation and host
cell uptake. Cell. Microbiol. 2, 633–647.
[76] Rathman, M., de Lanerolle, P., Ohayon, H., Gounon, P. and
Sansonetti, P.J. (2000) Myosin light chain kinase plays an
essential role in S. flexneri dissemination. J. Cell Sci. 113, 3375–
3386.
[77] Sansonetti, P.J., Mounier, J., Prevost, M.C. and Mege, R.M.
(1994) Cadherin expression is required for the spread of Shigella
flexneri between epithelial cells. Cell 76, 829–839.
[78] Page, A.L., Ohayon, H., Sansonetti, P.J. and Parsot, C. (1999)
The secreted IpaB and IpaC invasins and their cytoplasmic
chaperone IpgC are required for intercellular dissemination of
Shigella flexneri. Cell. Microbiol. 1, 183–193.
[79] Suzuki, T., Murai, T., Fukuda, T., Tobe, T., Yoshikawa, M. and
Sasakawa, C. (1994) Identification and characterization of a
chromosomal virulence gene, vacJ, required for intercellular
spreading of Shigella flexneri. Mol. Microbiol. 11, 31–41.
[80] Raqib, R., Lindberg, A.A., Wretlind, B., Bardhan, P.K.,
Andersson, U. and Andersson, J. (1995) Persistence of local
cytokine production in shigellosis in acute and convalescent
stages. Infect. Immun. 63, 289–296.
[81] Hathaway, L.J., Griffin, G.E., Sansonetti, P.J. and Edgeworth,
J.D. (2002) Human monocytes kill Shigella flexneri but then die
by apoptosis associated with suppression of proinflammatory
cytokine production. Infect. Immun. 70, 3833–3842.
[82] Zychlinsky, A., Prevost, M.C. and Sansonetti, P.J. (1992)
Shigella flexneri induces apoptosis in infected macrophages.
Nature 358, 167–169.
[83] Biet, F., Locht, C. and Kremer, L. (2002) Immunoregulatory
functions of interleukin 18 and its role in defense against
bacterial pathogens. J. Mol. Med. 80, 147–162.
[84] Way, S.S., Borczuk, A.C., Dominitz, R. and Goldberg, M.B.
(1998) An essential role for gamma interferon in innate
resistance to Shigella flexneri infection. Infect. Immun. 66,
1342–1348.
[85] Mandic-Mulec, I., Weiss, J. and Zychlinsky, A. (1997) Shigella
flexneri is trapped in polymorphonuclear leukocyte vacuoles and
efficiently killed. Infect. Immun. 65, 110–115.
[86] Weinrauch, Y., Drujan, D., Shapiro, S.D., Weiss, J. and
Zychlinsky, A. (2002) Neutrophil elastase targets virulence
factors of enterobacteria. Nature 417, 91–94.
[87] Zhang, J., Jin, L., Champion, G., Seydel, K.B. and Stanley, S.L.
(2001) Shigella infection in a SCID mouse–human intestinal
xenograft model: role for neutrophils in containing bacterial
dissemination in human intestine. Infect. Immun. 69, 3240–3247.
[88] Gomez, H.F., Ochoa, T.J., Carlin, L.G. and Cleary, T.G. (2003)
Human lactoferrin impairs virulence of Shigella flexneri. J.
Infect. Dis. 187, 87–95.
[89] Salzman, N.H., Ghosh, D., Huttner, K.M., Paterson, Y. and
Bevins, C.L. (2003) Protection against enteric salmonellosis in
transgenic mice expressing a human intestinal defensin. Nature
422, 522–526.
[90] Islam, D. and Christensson, B. (2000) Disease dependant changes
in T-cell populations in patients with shigellosis. APMIS 108, 251–
260.
[91] Islam, D., Bardhan, P.K., Lindberg, A.A. and Christensson, B.
(1995) Shigella infection Induces cellular activation of T and B
cells and distinct species-related changes in peripheral blood
lymphocyte subsets during the course of the disease. Infect.
Immun. 63, 2941–2949.
[92] Islam, D., Wretlind, B., Lindberg, A.A. and Christensson, B.
(1996) Changes in the peripheral blood T-cell receptor V b
repertoire in vivo and in vitro during shigellosis. Infect. Immun.
64, 1391–1399.
[93] Zwillich, S.H., Duby, A.D. and Lipsky, P.E. (1989) T-lymphocyte
clones responsive to Shigella flexneri. J. Clin. Microbiol. 27, 417–421.
[94] Kotloff, K.L., Noriega, F.R., Samandari, T., Sztein, M.B.,
Losonsky, G.A., Nataro, J.P., Picking, W.D., Barry, E.M. and
Levine, M.M. (2000) Shigella flexneri 2a strain CVD 1207, with
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
55

specific deletions in virG, sen, set and guaBA, is highly attenuated
in humans. Infect. Immun. 68, 1034–1039.
[95] van de Verg, L.L., Mallet, C.P., Collins, H.H., Larsen, T.,
Hammack, C. and Hale, T.L. (1995) Antibody and cytokine
responses in a mouse pulmonary model of Shigella flexneri
serotype 2a infection. Infect. Immun. 63, 1947–1954.
[96] Nelson, M.R., Shanson, D.C., Hawkins, D.A. and Gazzard,
B.G. (1992) Salmonella, Campylobacter and Shigella in HIV-
seropositive patients. AIDS 6, 1495–1498.
[97] Way, S.S., Borczuk, A.C. and Goldberg, M.B. (1999) Thymic
independance of adaptive immunity to the intracellular pathogen
Shigella flexneri serotype 2a. Infect. Immun. 67, 3970–3979.
[98] DuPont, H.L., Hornick, R.B., Synder, M.J., Libonati, J.P.,
Formal, S.B. and Gangarosa, E.J. (1972) Immunity in shigellosis.
II. Protection induced by oral live vaccine or primary infection.
J. Infect. Dis. 125, 12–16.
[99] Formal, S.B., Oaks, E.V., Olsen, R.E., Wingfield, M.E., Snoy,
P.J. and Cogan, J.P. (1991) Effect of prior infection with virulent
Shigella flexneri 2a on the resistance of monkeys to subsequent
infection with Shigella sonnei. J. Infect. Dis. 164, 533–537.
[100] Mel, D.M., Bogoljub, L.A., Nikolic, B.D. and Radovanic, M.L.
(1968) Studies on vaccination against bacillary dysentery. 4. Oral
immunization with live monotypic and combined vaccines. Bull.
World Health Organ. 39, 375–380.
[101] Van De Verg, L.L., Bendiuk, N.O., Kotloff, K., Marsh, M.M.,
Ruckert, J.L., Puryear, J.L., Taylor, D.N. and Hartman, A.B.
(1996) Cross-reactivity of Shigella flexneri serotype 2a O antigen
antibodies following immunization or infection. Vaccine 14,
1062–1068.
[102] Raqib, R., Qadri, F., Sarker, P., Mia, S.M., Sansonetti, P.J.,
Albert, M.J. and Andersson, J. (2002) Delayed and reduced
adaptive humoral immune responses in children with shigellosis
compared with in adults. Scand. J. Immunol. 55, 414–423.
[103] Phalipon, A., Cardona, A., Kraehenbuhl, J.P., Edelman, L.,
Sansonetti, P.J. and Corthesy, B. (2002) Secretory component: a
new role in secretory IgA-mediated immune exclusion in vivo.
Immunity 17, 107–115.
[104] Iijima, H., Takahashi, I. and Kiyono, H. (2001) Mucosal
immune network in the gut for the control of infectious diseases.
Rev. Med. Virol. 11, 117–133.
[105] Cohen, D., Block, C., Green, M.S., Lowell, G.H. and Ofek, I.
(1989) Immunoglobulin M, A and G antibody response to
lipopolysaccharide O antigen in symptomatic and asymptomatic
Shigella infections. J. Clin. Microbiol. 27, 162–167.
[106] Islam, D., Veress, B., Bardhan, P.K., Lindberg, A.A. and
Christensson, B. (1997) Quantitative assessment of IgG and IgA
subclass producing cells in rectal mucosa during shigellosis. J.
Clin. Pathol. 50, 513–520.
[107] Oberhelman, R.A., Kopecko, D.J., Salazar-Lindo, E., Gotuzzo,
E., Buysse, J.M., Venkatesan, M.M., Fernandez-Prada, C.,
Guzman, M. and Leon-Barua, R. (1991) Prospective study of
systemic and mucosal immune responses in dysenteric patients to
specific Shigella invasion plasmid antigens and lipopolysaccha-
rides. Infect. Immun. 59, 2341–2350.
[108] Rasolofo-Razanamparany, V., Cassel-Beraud, A.M., Roux, J.,
Sansonetti, P.J. and Phalipon, A. (2001) Predominance of
serotype-specific mucosal antibody response in Shigella flexneri
-infected humans living in an area of endemicity. Infect. Immun.
69, 5230–5234.
[109] Schultsz, C., Qadri, F., Hossain, S.A., Ahmed, F. and Ciznar, I.
(1992) Shigella-specific IgA in saliva of children with bacillary
dysentery. FEMS Microbiol. Immunol. 4, 65–72.
[110] Clemens, J.D., Stanton, B., Stoll, B., Shahid, N.S., Banu, H. and
Chowdhury, A.K. (1986) Breastfeeding as a determinant of
severity in shigellosis: evidence for protection throughout the
first three years of life in Bangladesh children. Am. J. Epidemiol.
123, 710–720.
[111] Phalipon, A., Kaufman, M., Michetti, P., Cavaillon, J.M.,
Huerre, M., Sansonetti, P.J. and Kraehenbuhl, J.P. (1995)
Monoclonal immunoglobulin A antibody directed against sero-
type-specific epitope of Shigella flexneri lipopolysaccharide
protects against murine experimental shigellosis. J. Exp. Med.
182, 769–778.
[112] Oaks, E.V., Hale, T.L. and Formal, S.B. (1986) Serum immune
response to Shigella protein antigens in Rhesus monkeys and
humans infected with Shigella species. Infect. Immun. 53, 57–
63.
[113] van de Verg, L.L., Herrington, D.A., Boslego, J., Lindberg, A.A.
and Levine, M.M. (1992) Age-specific prevalence of serum
antibodies to the invasion plasmid and lipopolysaccharide
antigens of Shigella species in Chilean and North American
populations. J. Infect. Dis. 166, 158–161.
[114] Way, S.S., Borczuk, A.C. and Goldberg, M.B. (1999) Adaptive
immune response to Shigella flexneri 2a cydC in immunocom-
petent mice and mice lacking immunoglobulin A. Infect. Immun.
67, 2001–2004.
[115] Cohen, D., Green, M.S., Block, C., Slepon, R. and Ofek, I.
(1991) Prospective study of the association between serum
antibodies to lipopolysaccharide O-antigen and the attack rate
of shigellosis. J. Clin. Microbiol. 29, 386–389.
[116] Lowell, G.H., MacDermott, R.P., Summers, P.L., Reeder, A.A.,
Bertovich, M.J. and Formal, S.B. (1980) Antibody-dependant
cell-mediated antibacterial activity: K lymphocytes, monocytes
and granulocytes are effective against Shigella. J. Immunol. 125.
[117] Reed, W.P. and Albright, E.L. (1974) Serum factors responsible
for killing of Shigella. Immunology 26, 205–215.
[118] Barnes, L.A., Cooper, M.L., Jerome, E.A., Durant, R.C. and
Smith, A.B. (1951) Field trial of Shigella flexneri III vaccine. VI.
Mouse protective studies. J. Immunol. 66, 515–525.
[119] Bennett, I.L., Gordon, R.S. and Barnes, L.A. (1949) A field trial
of Shigella flexneri III vaccine. II. Serum agglutination studies.
J. Infect. Dis. 85, 180–194.
[120] Cooper, M.L., Tepper, J. and Keller, H.M. (1948) Studies in
dysentery vaccination. IV. Primary vaccination of children with
monovalent vaccines of Shigella. J. Immunol. 60, 189–203.
[121] Higgins, A., Floyd, T. and Kader, M. (1955) Studies in
shigellosis. III. A controlled evaluation of monovalent Shigella
vaccine in a highly endemic environment. Am. J. Trop. Med.
Hyg. 4, 281–288.
[122] Fries, L.F., Montemarano, A.D., Mallet, C.P., Taylor, D.N.,
Hale, T.L. and Lowell, G.H. (2001) Safety and immunogenicity
of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine
administered intranasally to healthy adults. Infect. Immun. 69,
4545–4553.
[123] Ashkenazi, S., Passwell, J.H., Harlev, E., Miron, D., Dagan, R.,
Farzan, N., Ramon, R., Majadly, F., Bryla, D.A., Karpas, A.B.,
Robbins, J.R. and Schneerson, R. (1999) Safety and immuno-
genicity of Shigella sonnei and Shigella flexneri 2a O-specific
polysaccharide conjugates in children. J. Infect. Dis. 179, 1565–
1568.
[124] Cohen, D., Ashkenazi, S., Green, M.S., Lerman, Y., Slepon, R.,
Robin, G., Orr, N., Taylor, D.N., Sadoff, J.C., Chu, C.,
Shiloach, J., Schneerson, R. and Robbins, J.R. (1996) Safety
and immunogenicity of investigational Shigella conjugate vac-
cines in Israeli volunteers. Infect. Immun. 64, 4074–4077.
[125] Passwell, J.H., Harlev, E., Ashkenazi, S., Chu, C., Miron, D.,
Ramon, R., Farzan, N., Shiloach, J., Bryla, D.A., Majadly, F.,
Roberson, R., Robbins, J.R. and Schneerson, R. (2001) Safety
and immunogenicity of improved Shigella O-specific polysac-
charide-protein conjugate vaccines in adults in Israel. Infect.
Immun. 69, 1351–1357.
[126] Taylor, D.N., Trofa, A.C., Sadoff, J.C., Chu, C., Bryla, D.A.,
Shiloach, J., Cohen, D., Ashkenazi, S., Lerman, Y. and Egan, W.
(1993) Synthesis, characterization and clinical evaluation of
56
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58

conjugate vaccines composed of the O-specific polysaccharides of
Shigella dysenteriae type 1, Shigella flexneri type 2a and Shigella
sonnei (Plesiomonas shigelloides) bound to bacterial toxoids.
Infect. Immun. 61, 3678–3687.
[127] Turbyfill, K.R., Hartman, A.B. and Oaks, E.V. (2000) Isolation
and characterization of a Shigella flexneri invasin complex
subunit vaccine. Infect. Immun. 68, 6624–6632.
[128] Levenson, V.I., Chernokhvostova, E.V., Lyubinskaya, M.M.,
Salamatova, S.A., Dzhikidze, E. and Stasilevitch, Z.K. (1988)
Parental immunization with Shigella ribosomal vaccine elicits
local IgA response and primes for mucosal memory. Int. Arch.
Allergy Immunol. 87, 25–31.
[129] Levenson, V.I. and Egorova, T.P. (1990) Polysaccharide nature
of O-antigen in protective ribosomal preparations from Shigella:
experimental evidence and implications from the ribosomal
vaccine concept. Res. Microbiol. 141, 707–720.
[130] Formal, S.B., LaBrec, E., Palmer, A. and Falkow, S. (1965)
Protection of monkeys against experimental shigellosis with
attenuated vaccines. J. Bacteriol. 90, 63–68.
[131] Chakrabarti, M.K., Bhattacharya, J., Bhattacharya, M.K., Nair,
G.B., Bhattacharya, S.K. and Mahalanabis, D. (1999) Killed
oral Shigella vaccine made from Shigella flexneri 2a protects
against challenge in the rabbit model of shigellosis. Acta
Paediatr. 88, 161–165.
[132] Meitert, T., Pencu, E., Ciudin, L. and Tonciu, M. (1984) Vaccine
strain Sh. flexneri T
32
-ISTRATI. Studies in animals and volun-
teers. Antidysentery immunoprophylaxis and immunotherapy by
live vaccine VADIZEN (Sh. flexneri T
32
-ISTRATI). Arch.
Roum. Pathol. Exp. Microbiol. 43, 251–278.
[133] Karnell, A., Li, A., Zhao, C.R., Karlsson, K., Nguyen, B.M. and
Lindberg, A.A. (1995) Safety and immunogenicity study of the
auxotrophic Shigella flexneri 2a vaccine SFL1070 with a deleted
aroD gene in adult Swedish volunteers. Vaccine 13, 88–99.
[134] Kotloff, K.L., Noriega, F.R., Losonsky, G.A., Sztein, M.B.,
Wasserman, S.S., Nataro, J.P. and Levine, M.M. (1996) Safety,
immunogenicity, and transmissibility in humans of CVD1203, a
live oral Shigella flexneri 2a vaccine candidate attenuated by
deletions in aroA and virG. Infect. Immun. 64, 4542–4548.
[135] Li, A., Pal, T., Forsum, U. and Lindberg, A.A. (1992) Safety and
immunogenicity of the live oral auxotrophic Shigella flexneri
SFL124 in volunteers. Vaccine 10, 395–404.
[136] Coster, T.S., Hoge, C.W., van de Verg, L.L., Hartman, A.B.,
Oaks, E.V., Venkatesan, M.M., Cohen, D., Robin, G., Fontaine-
Thompson, A., Sansonetti, P.J. and Hale, T.L. (1999) Vaccina-
tion against shigellosis with attenuated Shigella flexneri 2a strain
SC602. Infect. Immun. 67, 3437–3443.
[137] Falkow, S., Schneider, H., Baron, L.S. and Formal, S.B. (1963)
Virulence of Escherichia–Shigella genetic hybrids for the guinea
pig. J. Bacteriol. 86, 1251–1258.
[138] Levine, M.M., Woodward, D., Formal, S.B., Gemski, P., DuPont,
H.L., Hornick, R.B. and Snyder, M. (1977) Studies with a new
generation of oral attenuated Shigella vaccine: Escherichia coli bearing
surface antigens of Shigella flexneri. J. Infect. Dis. 136, 577–582.
[139] Kotloff, K.L., Herrington, D.A., Hale, T.L., Newland, J.W., van
de Verg, L.L., Cogan, J.P., Snoy, P.J., Sadoff, J.C., Formal, S.B.
and Levine, M.M. (1992) Safety, immunogenicity and efficacy in
monkeys and humans of invasive Escherichia coli K-12 vaccine
candidates expressing Shigella flexneri 2a somatic antigen. Infect.
Immun. 60, 2218–2224.
[140] Kotloff, K.L., Losonsky, G.A., Nataro, J.P., Wasserman, S.S.,
Hale, T.L., Taylor, D.N., Newland, J.W., Sadoff, J.C., Formal,
S.B. and Levine, M.M. (1995) Evaluation of the safety, immu-
nogenicity and efficacy in healthy adults of four doses of live oral
hybrid Escherichia coli–Shigella flexneri 2a vaccine strain EcSf2a-
2. Vaccine 13, 495–502.
[141] Rui, X., Xu, Y., Wan, H., Su, G. and Huang, C. (1996)
Construction of a stable and non-resistant bivalent vaccine
candidate strain against Shigella flexneri 2a and Shigella sonnei.
Chin. J. Biotechnol. 12, 89–97.
[142] Klee, S.R., Tzscgaschel, B.D., Singh, M., Falt, I., Lindberg,
A.A., Timmis, K.N. and Guzman, C.A. (1997) Construction and
characterization of genetically-marked bivalent anti-Shigella
dysenteriae and anti-Shigella flexneri Y live vaccine candidates.
Microb. Pathogenesis 22, 363–376.
[143] Carlin, N.I.A. and Lindberg, A.A. (1987) Monoclonal antibodies
specific for Shigella flexneri lipopolysaccharides: clones binding
to type IV, V and VI antigens, group 3,4 antigen and an epitope
common to all Shigella flexneri and Shigella dysenteriae type 1
strains. Infect. Immun. 55, 1412–1420.
[144] Noriega, F.R., Liao, F.M., Maneval, D.R., Ren, S., Formal, S.B.
and Levine, M.M. (1999) Strategy for cross-protection among
Shigella flexneri serotypes. Infect. Immun. 67, 782–788.
[145] Guan, S. and Verma, N.K. (1998) Serotype conversion of a
Shigella flexneri candidate vaccine strain via a novel site specific
chromosome-integration system. FEMS Microbiol. Lett. 166, 79–
87.
[146] DuPont, H.L., Hornick, R.B., Snyder, M., Libonati, J.P.,
Formal, S.B. and Gangarosa, E.J. (1972) Immunity in shigellosis.
I. Response of man to attenuated strains of Shigella. J. Infect.
Dis. 125, 5–11.
[147] Mills, J.A., Venkatesan, M.M., Baron, L.S. and Buysse, J.M.
(1992) Spontaneous insertion of an IS1-like element into the virF
gene is responsible for avirulence in opaque colonial variants of
Shigella flexneri 2a. Infect. Immun. 60, 175–182.
[148] Levine, M.M., Gangarosa, E.J., Werner, M. and Morris, G.K.
(1974) Shigellosis in custodial institutions. III. Prospective
clinical and bacteriologic surveillance of children vaccinated
with oral attenuated Shigella vaccines. J. Pediatr. 84, 803–806.
[149] Mel, D.M., Terzin, A.L. and Vuksic, L. (1965) Studies on
vaccination against bacillary dysentery. 3. Effective oral immu-
nization against Shigella flexneri 2a in a field trial. Bull. World
Health Organ. 32, 647–655.
[150] Venkatesan, M.M., Fernandez-Prada, C., Buysse, J.M., Formal,
S.B. and Hale, T.L. (1991) Virulence phenotype and genetic
characteristics of the T
32
-ISTRATI Shigella flexneri 2a vaccine
strain. Vaccine 9, 358–363.
[151] Sansonetti, P.J. and Arondel, J. (1989) Construction and
evaluation of a double mutant of S. flexneri as a candidate for
oral vaccination against shigellosis. Vaccine 7, 443–450.
[152] Dentchev, V., Marinova, S., Vassilev, T., Bratoyeva, M. and
Linde, K. (1990) Live Shigella flexneri 2a and Shigella sonnei I
vaccine candidate strains with two attenuating markers. II.
Preliminary results of vaccination of adult volunteers and
children aged 2–17 years. Vaccine 8, 30–34.
[153] Linde, K., Dentchev, V., Bondarenko, V., Marinova, S.,
Randhagen, B., Bratoyeva, M., Tsvetanov, Y. and Romanova,
Y. (1990) Live Shigella flexneri and Shigella sonnei I vaccine
candidate strains with two attenuating markers. I. Construction
of vaccine candidate strains with retained invasiveness but
reduced intracellular multiplication. Vaccine 8, 25–29.
[154] Linde, K., Randhagen, B., Beer, J., Dentchev, V., Marinova, S.,
Vassilev, T. and Bratoyeva, M. (1993) Shigella flexneri 2a and
sonnei I vaccine with two attenuating markers: construction,
tolerability and immunogenicity in 143 children aged 3–17 years.
Vaccine 11, 197–199.
[155] Ahmed, Z.U., Sarker, M.R. and Sack, D.A. (1990) Protection of
adult rabbits and monkeys from lethal shigellosis by oral
immunization with a thymine-requiring and temperature-sensi-
tive mutant of Shigella flexneri Y. Vaccine 8, 153–158.
[156] Ashraf, M.M., Giri, D.K., Batra, H.V., Khandekar, P., Ahmed,
Z.U. and Talwar, G.P. (1991) Potentials of Shigella flexneri Y
strain TSF21 as a candidate vaccine against shigellosis: safety,
immunogenicity and protective efficacy in Bonnet monkeys.
FEMS Microbiol. Immunol. 3, 165–170.
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
57

[157] Karnell, A., Sweiha, H. and Lindberg, A.A. (1992) Auxotrophic
live oral Shigella flexneri vaccine protects against challenge with
S. flexneri of different serotypes. Vaccine 10, 167–174.
[158] Karnell, A., Stocker, B.A., Katakura, S., Reinholt, F.P. and
Lindberg, A.A. (1992) Live oral auxotrophic Shigella flexneri
SFL124 vaccine with a deleted aroD gene: characterisation and
monkey protection studies. Vaccine 10, 389–394.
[159] Li, A., Cam, P.D., Islam, D., Minh, N.B., Huan, P.T., Rong,
Z.C., Karlsson, K., Lindberg, A.A. and Lindberg, G. (1994)
Immune response in vietnamese children after a single dose of the
auxotrophic, live Shigella flexneri Y vaccine strain SFL124. J.
Infect. 28, 11–23.
[160] Li, A., Karnell, A., Huan, P.T., Cam, P.D., Minh, N.B., Tram,
L.N., Quy, N.P., Trach, D.D., Karlsson, K. and Lindberg, G.
(1993) Safety and immunogenicity of the live oral auxotrophic
Shigella flexneri SFL124 in adult vietnamese volunteers. Vaccine
11, 180–189.
[161] Karnell, A., Cam, P.D., Verma, N.K. and Lindberg, A.A. (1993)
AroD deletion attenuates Shigella flexneri strain 2457T and
makes it a safe and efficacious oral vaccine in monkeys. Vaccine
11, 830–836.
[162] Noregia, F., Wang, J., Losonsky, G., Maneval, D., Hone, D. and
Levine, M. (1994) Construction and characterization of attenu-
ated delta aroA delta virG Shigella flexneri 2a strain CVD 1203, a
prototype live oral vaccine. Infect. Immun. 62, 5168–5172.
[163] Noriega, F.R., Losonsky, G.A., Wang, J.Y., Formal, S.B. and
Levine, M.M. (1996) Further characterisation of aroA virG
Shigella flexneri 2a strain CVD 1203 as a mucosal Shigella
vaccine and as a live-vector vaccine for delivering antigens of
enterotoxigenic Escherichia coli. Infect. Immun. 64, 23–27.
[164] Barzu, S., Fontaine, A., Sansonetti, P.J. and Phalipon, A. (1996)
Induction of a local anti-IpaC antibody response in mice by use
of a Shigella flexneri 2a vaccine candidate: implications for use of
IpaC as a protein carrier. Infect. Immun. 64, 1190–1196.
58
A.V. Jennison, N.K. Verma / FEMS Mircobiology Reviews 28 (2004) 43–58
Document Outline
- Shigella flexneri infection: pathogenesis and vaccine development
Wyszukiwarka
Podobne podstrony:
The pathogenesis of Sh flexneri infection lessons from in vitro and in vivo studies
Enteric infections, diarrhea and their impact on function and development
Inequality of Opportunity and Economic Development
Gender and Child Development
j2ee and xml development XMGP6H76DF2YXOG3KZCTWRLGLFG6YRCTQFHK6JY
2011 6 NOV Companion Animal Medicine Evolving Infectious, Toxicological, and Pa
Group and team development
Balancing Disappointment and Enthusiasm Developments in EU Balkans relations during 2003
Inequality of Opportunity and Economic Development
2011 6 NOV Companion Animal Medicine Evolving Infectious, Toxicological, and Pa
The growth and economic development, Magdalena Cupryjak 91506
Describe the role of the dental nurse in minimising the risk of cross infection during and after the
Terrorism And Development Using Social and Economic Development to Prevent a Resurgence of Terroris
Rick Strassman Subjective effects of DMT and the development of the Hallucinogen Rating Scale
Aluminum toxicokinetics regarding infant diet and vaccination(1)
Scientific and Technological Developments
więcej podobnych podstron