
Combustion, Explosion, and Shock Waves, Vol. 37, No. 6, pp. 655–663, 2001
Numerical Analysis of Conditions for Ignition
of Compact Metal Specimens and Foil in Oxygen
UDC 620.22;546.21
V. I. Bolobov
1
and N. A. Podlevskikh
1
Translated from Fizika Goreniya i Vzryva, Vol. 37, No. 6, pp. 46–55, November–December, 2001.
Original article submitted November 10, 2000.
The conditions for ignition of compact specimens and a foil made of iron, nickel,
copper, and stainless steel in oxygen under pressures of 0.1–70 MPa are studied with
the use of modified equations and dimensionless parameters of the heat theory of
ignition of metal particles. Calculation results are compared with experimental data.
At present time, oxygen finds various applications
in science and engineering, where it is used at high tem-
peratures and pressures. To ensure safe operation of
structural materials under these conditions, it is neces-
sary to study ignition mechanisms of metals and alloys
shaped like compact articles and to infer how the oxy-
gen pressure affects their ignition temperature T
∗
exp
. It
has been shown experimentally (see, e.g., [1–7]) that
compact specimens made of widely used materials such
as stainless steel [4], copper and copper alloys [5], nickel
and nickel alloys [6], and iron and carbon steel [7] ignite
in oxygen under conditions of natural convection only
at the melting temperature of the metal (T
melt
) or basic
component of the oxide film. The pressure of the oxi-
dizing medium (up to 70 MPa) has little effect on the
values of T
∗
exp
[4–7].
2
In the present paper, the critical parameters of ig-
nition of the above-mentioned materials are studied by
a numerical method under experimental conditions of
[4–7]; the question of whether the dimensionless param-
eters of the heat theory of ignition of metal particles [8]
may be used in the calculations is considered.
CHOOSING THE KINETIC CONSTANTS
An analysis of the data available in the literature
shows that little is known about the kinetics of pre-
ignition oxidation of metal structural materials in oxy-
1
Russian Scientific Center “Applied Chemistry,”
St. Petersburg 197198.
2
According to [3], specimens of molybdenum-containing al-
loys ignite at T < T
melt
after the oxide film is destroyed by
MoO
2
and MoO
3
vapors for T
∗
exp
= f (p
O
2
).
gen under high pressure. Therefore, as the initial data
for calculations, we chose the kinetic constants of high-
temperature oxidation of iron, stainless steel, and nickel
in decomposition products of N
2
O (0.33/0.67 O
2
/N
2
composition; p = 1.5 MPa) determined in [9] under the
conditions close to the experimental conditions of [4–
7]. (In [9], an increase in the diameter of the middle
part of a cylindrical bar was measured after its oxi-
dation and Joule heating. It was found that, for all
materials, the time evolution of the oxide-film thickness
was described by the equation of a parabola; the activa-
tion energy E and the preexponent K
0
in the Arrhenius
equation were calculated for the constant of oxidation
rate K
T
determined at T = 1415–1570 K.) For cop-
per, we used the kinetic parameters of high-temperature
(T = 823–1173 K) oxidation of the material in air deter-
mined by the gravimetric method in [10]. To estimate
the effect of discrepancies in the values of the kinetic
constants determined by different authors on calcula-
tion results, we solved the problem of ignition of iron
repeatedly with the use of the data on the kinetics of
the oxide film of the material in air [10].
To estimate the discrepancy in the kinetics of ox-
idation of metals in oxygen at different pressures and
in an O
2
–N
2
mixture, we used the following considera-
tions. For metals and alloys with oxide films possessing
protective properties (as in our case), the rate of high-
temperature oxidation is limited by the diffusion rate of
anions and cations through a layer of solid oxides and
depend little [for nickel, v
∼ K
m
p
, where m
≈ 0.17] or
does not depend (for iron, steel, and copper) [10] on
the partial pressure of a gaseous oxidizer (for the values
of p exceeding the oxide-dissociation pressure, which is
0010-5082/01/3706-0655 $25.00 c
2001
Plenum Publishing Corporation
655
656
Bolobov and Podlevskikh
TABLE 1
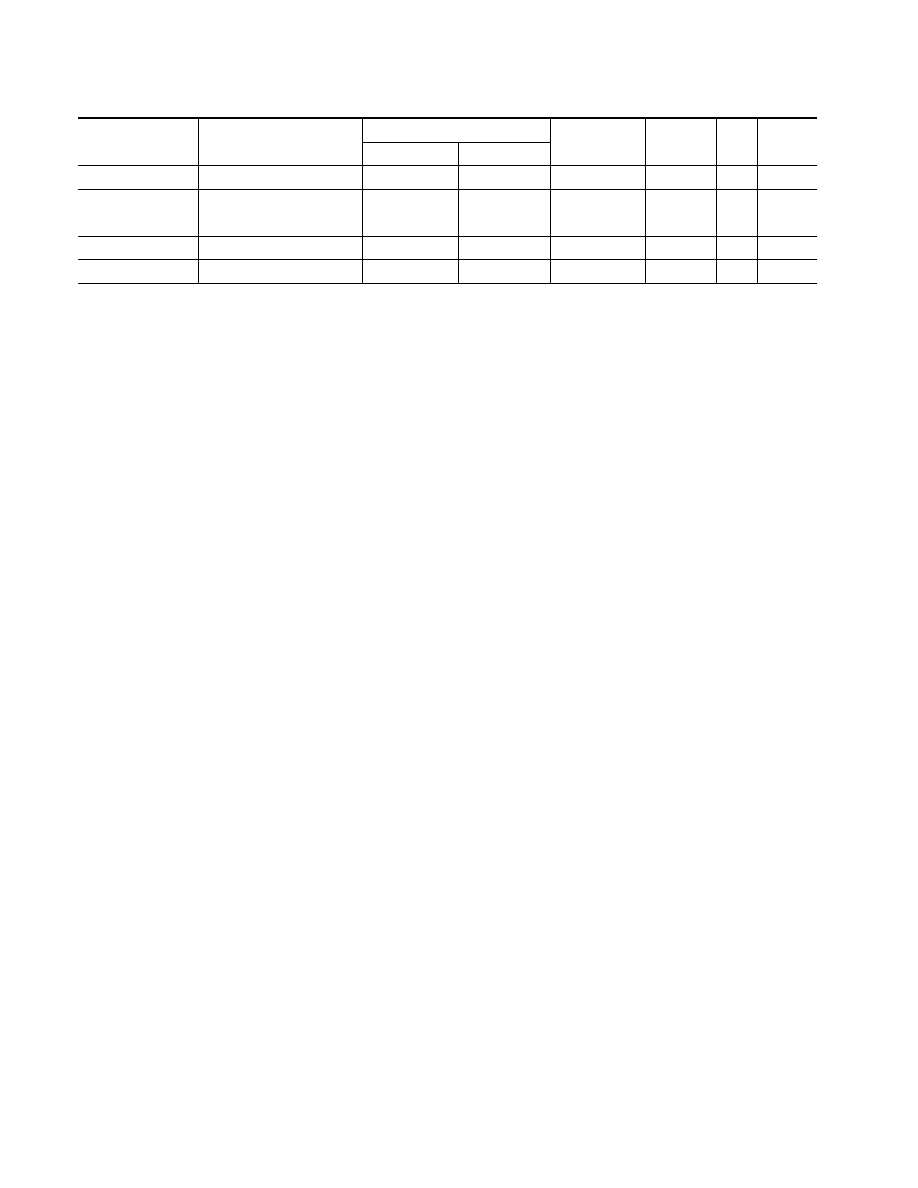
Results of X-Ray Phase and Gravimetric Tests of Specimens in Oxygen and Kinetic Constants Chosen
Material
Composition of scale
δ, µm
K
0
,
E,
m
Q,
for p
O
2
= 0.2 and 20 MPa
p
O
2
=0.2 MPa
p
O
2
=20 MPa
m
2
/sec
kJ/mole
MJ/kg
Copper
0.95Cu
2
–0.05Cu
360
370
5.9
· 10
−5
[10]
158 [10]
0
0.8
Iron
0.85FeO–0.15Fe
34
80
110
6.1
· 10
−4
[10]
170 [10]
0
3.0
9.5
· 10
−4
[9]
190 [9]
Nickel
NiO
—
—
7.8
· 10
−4
[9]
225 [9]
0.17
2.5
12Kh18N10T steel
(Cr,Fe)
2
O
3
0.86.2
0.85.0
0.8 0.14 [9]
0.8290 [9]
0.80
0.84.2
much lower than 0.1 MPa). For this reason, the oxi-
dation of these metals in air (nitrogen–oxygen mixture)
and oxygen at different pressures occurs in the same
manner, and they cannot be distinguished by kinetics
or scale structure [11].
The validity of this conclusion was supported in [9]
by comparing the thicknesses and phase compositions
of oxide films on metals oxidized in an O
2
–N
2
mixture
(p = 1.5 MPa) and air (p = 0.1 MPa). In this paper,
we verified this conclusion by the gravimetric method:
specimens of iron, stainless steel, and copper were kept
for t = 3600 sec under pressure 0.2 and 20 MPa [5] at
1273 K until their ignition.
It was found that an increase in pressure by two
orders of magnitude has little effect on the rate of pre-
ignition oxidation of the materials in question and the
phase composition of the scale in oxygen at different
pressures is close to the composition of films formed on
these materials in decomposition products of N
2
O [9]
and in air [10, 11].
The experimental values of the
increment in the specimen mass m calculated for the
thickness δ with allowance for the phase composition
are listed in Table 1. The kinetic parameters of oxida-
tion of metals borrowed from [9, 10] and thermal effects
Q [12] calculated with allowance for the phase compo-
sition of the scale are also given therein.
MATHEMATICAL MODEL
OF THE PROCESS
Using the basic assumptions of the theory of ig-
nition of particles [8], we calculated the conditions for
ignition of large-scale structures. To this end, we con-
sidered the possibility of the thermal mechanism [8] of
ignition of iron, nickel, stainless steel, and copper taken
in the form of:
— compact specimens subjected to the Joule heating in
“cold” oxygen under natural convection (experimental
conditions in [4–7]);
— foil heated in vacuum in contact with oxygen of the
same temperature [7].
In the first case, we considered the thermal bal-
ance of the middle part of a nonuniformly heated metal
bar of diameter d = 3 mm and working-part length
L = 40 mm in oxygen (p = 0.1–70 MPa). In contrast
to the heat-removal conditions for particles [8] or a uni-
formly heated plate [13], which ignite in an immovable
medium at relatively low pressure and temperature, this
problem is characterized by the necessity of taking into
account the heat losses in the “cold” ends of the speci-
men, heat losses due to radiation, and dependence of the
intensity of convective heat exchange on the pressure of
the medium. In view of these factors, the analytical
solution of the problem with the use of dimensionless
parameters [8] encounters serious difficulties. In the sec-
ond case, we calculated thermograms of self-heating of
a vertically installed foil of height H = 5 mm and thick-
ness h = 0.01–0.1 mm at different initial temperatures.
It was assumed that the foil was in contact with oxygen
(p = 0.14 MPa) at the same temperature (the dimen-
sions of specimens and the pressure correspond to the
experimental conditions of [7]).
The problem was solved under the following as-
sumptions used in [14] in calculating the critical ignition
parameters of compact metal specimens in fluorine:
• heat exchange with the medium is almost quasi-
stationary;
• the radial temperature distribution can be ig-
nored (the Biot criterion Bi
≤ 0.01); for the foil, the
temperature gradients are negligible in all directions;
• the temperature profile along the working part of
the specimen is close to a parabola;
• the temperatures of the gas and specimen ends
are equal and do not vary with time; for the foil, the
temperature of the gas is equal to the temperature of
the furnace and does not vary with time;
• the radiant flux toward the specimen can be ig-
nored in view of the low temperature of the walls of the
reaction chamber in the tests of [4–7];

Numerical Analysis of Conditions for Ignition of Compact Metal Specimens and Foil
657
• the kinetic equations governing oxidation of met-
als are valid within the entire temperature range con-
sidered.
With allowance for [8], the generalized equations
governing the self-heating of the specimen and foil have
the form
h
eff
2
c
p
ρ
dT
dt
= q + q
ch.r.
− q
α
− q
ε
− q
λ
;
(1)
q
ch.r.
= Qρ
dδ
dt
= Qρ
K
0
K
m
p
δ
n
exp
−
E
RT
,
(2)
where t is the time, T is the current temperature of the
central part of the specimen, R is the universal gas con-
stant, δ is the thickness of the oxide film, ρ is the density
of the metal, c
p
is the specific heat at constant pressure,
n is the exponent characterizing the dependence of the
oxidation rate on the oxide-film thickness, h
eff
is the
radius of the cylindrical specimen or the foil thickness,
K
0
is the preexponent in the Arrhenius equation, K
p
is
the ratio of the pressure used in the calculations to the
partial pressure of oxygen to which the kinetic param-
eters of oxidation correspond, m is the experimentally
determined coefficient that takes into account the prop-
erties of the oxide film, Q is the thermal effect of the
reaction per 1 kg of oxygen times the ratio of densities of
oxide and metal, q and q
ch.r.
are the heat fluxes applied
to the specimen owing to the Joule heat and chemical
reaction of metal oxidation, respectively, per unit area
of the central part of the specimen (for the foil, q = 0),
q
λ
, q
α
, and q
ε
are the thermal fluxes due to the heat
conduction to the cold ends of the specimen, convection,
and radiation, respectively, per unit area of the central
part of the bar, q
λ
= λ
w
d(T
− T
amb
)/2a
2
(for the foil,
q
λ
= 0), q
α
= λ
0
(T
− T
amb
)Nu/l (Nu = 0.54Ra
0.25
for
natural convection for Ra > 1) [15], q
ε
= εσ
0
T
4
[for the
foil, q
ε
= εσ
0
(T
4
− T
4
amb
)]. Here T
amb
is the ambient
temperature, l is the specimen diameter or foil thick-
ness, ε is the emissivity of metals with an oxide film
(ε
≈ 0.8 [16]), σ
0
is the Stefan–Boltzmann constant, λ
0
and λ
w
are the thermal conductivities of the gas and
metal, respectively [λ
0
= f (p, (T
amb
+ T )/2)], a is the
distance from the center of the bar to the “cold” clamps
of current taps in the tests in [4–7] (a = L/2), and Nu
and Ra are the Nusselt and Rayleigh criteria, respec-
tively.
The initial conditions: for t = 0, T = T
amb
[the
value of T
amb
is constant for the specimen (323 K) and
varied for the foil] and δ
0
= 10
−9
m [8].
To obtain a family of curves of the temperature of
the central part of the specimen as a function of time,
we integrated Eqs. (1) and (2) by a numerical method
for increasing values of q. For each thermogram, we de-
termined the maximum temperature of the central part
of the specimen T
max
and the final stationary tempera-
ture T
fin
at which the value of q
ch.r.
becomes negligible
because of the oxide-film growth. We note that, un-
der given heat-exchange conditions, the specimen would
have the temperature T
fin
if there were no chemical in-
teraction with the medium. It was assumed that the
temperature T
fin
corresponds to the initial temperature
T
0
of the specimen self-heated owing to the chemical
reaction at its surface. We constructed the curves T
max
and T
fin
= f (q) to determine the critical parameters of
ignition q
∗
and T
∗
0
. We assumed that the critical condi-
tions occur if a small variation in the heat flux around
q
∗
leads to a jump in the temperature T
max
, i.e., the
following condition holds:
dT
max
dq
q=q
∗
→ ∞.
(3)
For a value of q closest to q
∗
, we determined the
maximum temperature at the central part of the spec-
imen under subcritical conditions (T
∗
max
) and the max-
imum subcritical self-heating (∆T
max
= T
∗
max
− T
fin
).
The values of q = q
∗
corresponded to the ignition tem-
perature of the metal T
∗
0
= lim
q
→q
∗
T
fin
, which was taken
to be the initial temperature of self-heating that led to
ignition of the specimen.
For the foil, we integrated Eqs. (1) and (2) for in-
creasing values of T
0
(in this case, T
0
= T
amb
) to find
the minimum initial temperature of self-heating T
∗
0
that
corresponds to ignition of the metal. For T
0
≤ T
∗
0
, we
determined the maximum temperature T
∗
max
attained in
the foil without ignition and the maximum subcritical
heating of the material (∆T
max
= T
∗
max
− T
0
).
IGNITION CONDITIONS
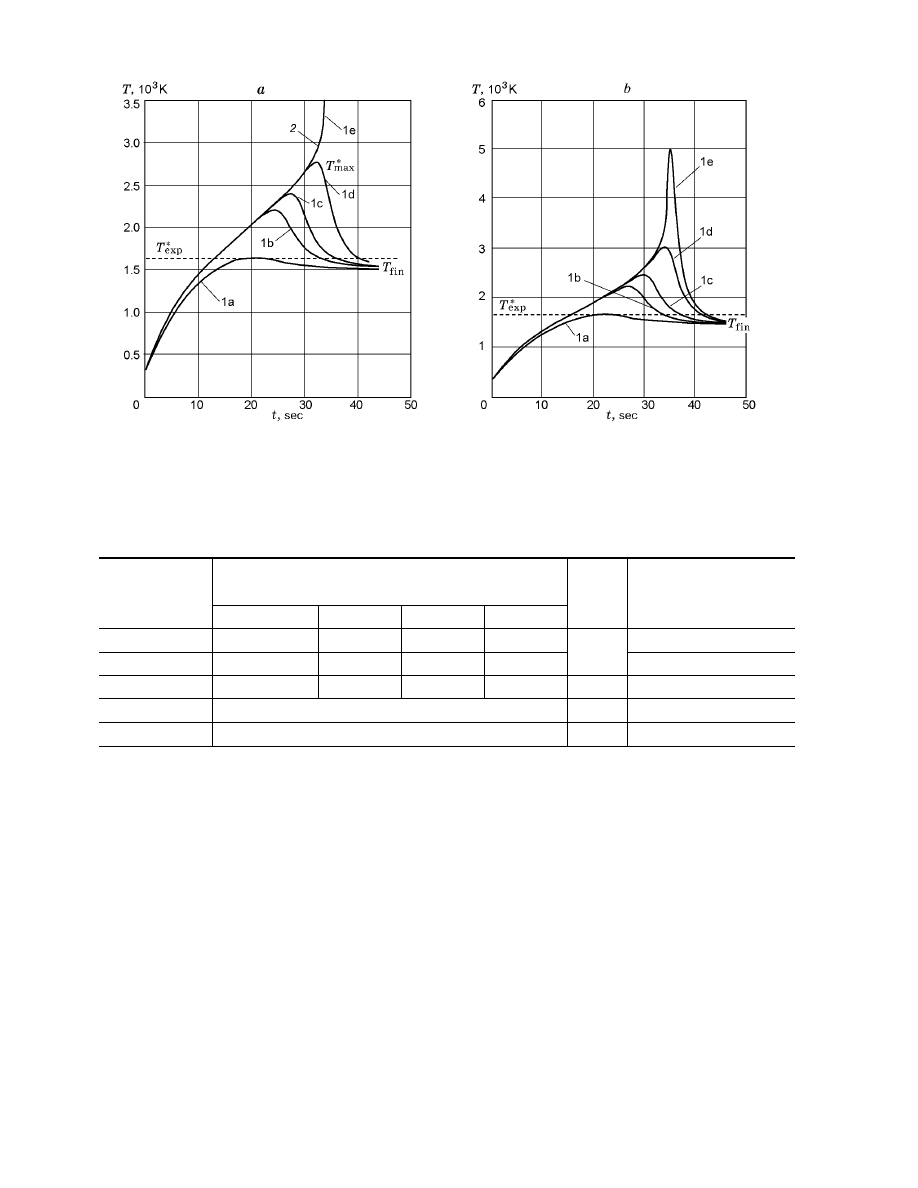
FOR COMPACT SPECIMENS
Calculations show that theoretically,
3
among the
metals considered, compact specimens made of iron (for
both kinds of oxidation kinetics [9, 10]) and stainless
steel can ignite in oxygen according to the thermal
mechanism [8]. It is noteworthy that, for iron [10], the
ignition mechanism has some distinguishing features.
For stainless steel and iron [9] in oxygen at different
pressures, the calculated thermograms (Fig. 1a) are sim-
ilar to the curves of heating of metal particles [8]: in the
region of subcritical heating, the temperature reaches
the maximum T
max
and tends to the temperature T
fin
that the specimen would have if there were no chemical
interaction with the medium (curves 1a–e in Fig. 1a); in
the ignition region, the temperature curves have an in-
flection point (for a temperature close to T
∗
max
in the re-
3
Without allowance for phase transformations.
658
Bolobov and Podlevskikh
Fig. 1. Calculated thermograms of heating and ignition of cylindrical iron specimens (d = 3 mm) in oxygen
for p = 0.1 MPa, where curves 1a–1e refer to T
fin
and curve 2 refers to T
max
: (a) kinetics of [9] for q = 432
(1a), 478.1036 (1b), 478.1886 (1c), 478.1915 (1d), and 478.1916 kW/m
2
(1e); (b) kinetics of [10] for q = 385
(1a), 411.279 (1b), 411.3687 (1c), 411.3778 (1d), and 411.3780 kW/m
2
(1e).
TABLE 2
Ignition Conditions for Compact Metal Specimens in Oxygen
Calculated critical parameters
Material
for p = 0.1 and 70 MPa (in parentheses)
T
∗
exp
, K
∆T
exp
= T
∗
exp
− T
∗∗
0
, K
q
∗
, 10
3
kW/m
2
T
∗
max
, K
T
∗
0
, K
∆T
max
, K
Iron [10]
0.41 (1.29)
—
1445 (1540)
1450 (1828)
1644
234 (175)
Iron [9]
0.48 (1.4)
2672 (2894)
1525 (1606)
1147 (1288)
176 (129)
Stainless steel [9]
0.55 (1.54)
2295 (2357)
1708 (1756)
603 (631)
1683
63 (49)
Nickel [9]
Degenerated ignition conditions
≥1673
28 (22)
Copper [10]
No ignition or noticeable self-heating
1338
4 (3)
Note. T
∗∗
0
is the initial temperature of self-heating of compact specimens, for which the melting point is attained.
gion of subcritical heating) after which the temperature
increases progressively with time (curve 2 in Fig. 1a) to
infinity (if the total burnup of the specimen is ignored).
The regions of subcritical heating and ignition are sep-
arated by the critical value of the heat flux q
∗
for which
the dependence T
max
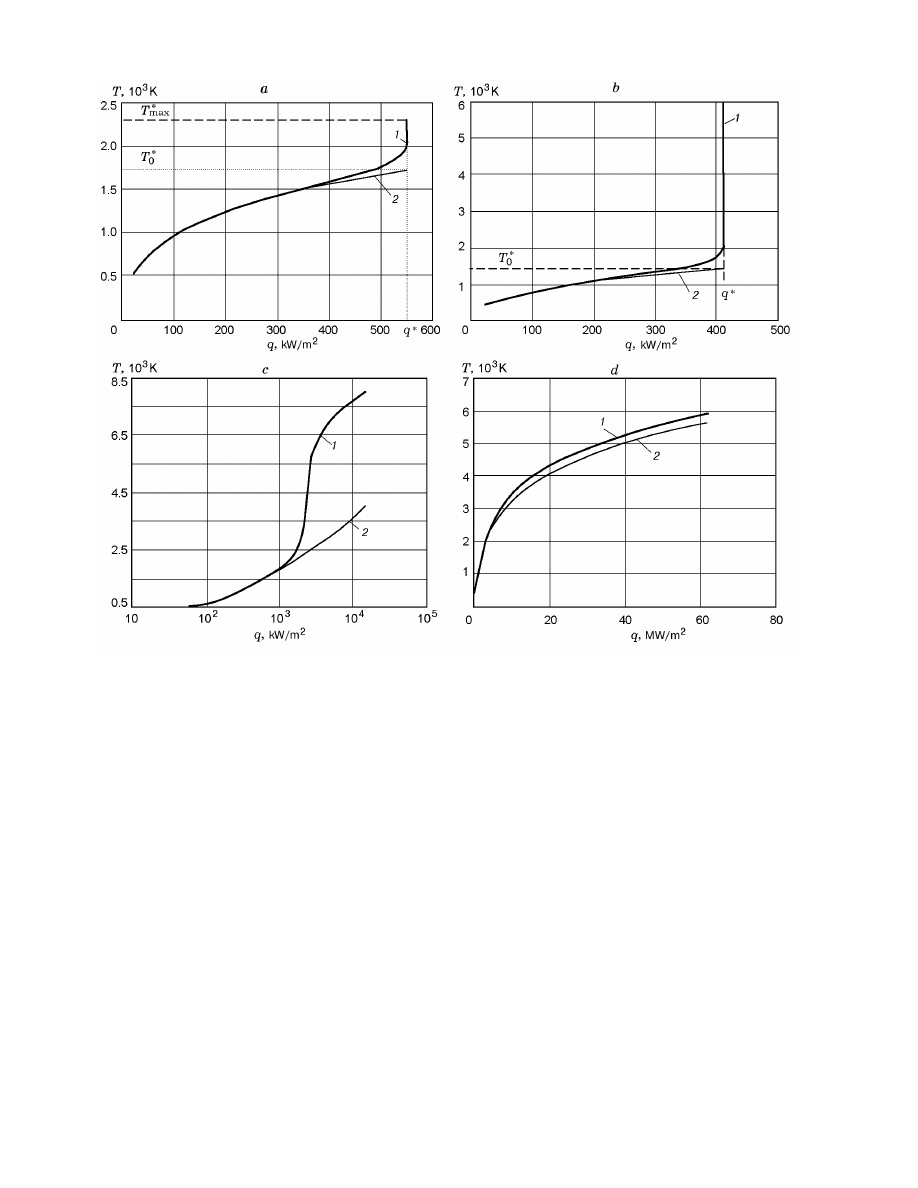
(q) (curve 1 in Fig. 2a) satisfies
condition (3). In this case, the maximum temperature
of iron [9] and stainless steel specimens under subcritical
conditions is a finite quantity (T
∗
max
in Figs. 1a and 2a).
If the kinetic parameters of oxidation of iron [10]
are used in Eq. (2), the maximum temperature jumps
from 2895 to 5000 K for a very small variation in q from
411.3778 to 411.3780 kW/m
2
(see Fig. 1b). Then, the
temperature tends to the stationary value T
fin
rather
than to infinity as in the case of iron [9]. For large val-
ues of q, the maximum is shifted toward higher tempera-
tures. Therefore, it is impossible to determine uniquely
the value of T
∗
max
for iron [10] (Fig. 2b).
For nickel, the calculated thermograms are similar
to the thermograms of iron [10] (Fig. 1b), whereas the
dependence of the maximum temperature on heat flux
is described by an S-shaped curve (Fig. 2c) typical of
the degenerated ignition conditions. According to the
calculations, copper specimens do not ignite and do not
experience noticeable self-heating up to a temperature
of 6000 K, which is the maximum within the temper-
Numerical Analysis of Conditions for Ignition of Compact Metal Specimens and Foil
659
Fig. 2. Temperatures T
max
(curves 1) and T
fin
(curves 2) versus heat flux for steel (iron [9]) (a), iron [10] (b),
nickel (c), and copper (d).
ature range considered (Fig. 2d). The fact that nickel
and copper do not ignite by the thermal mechanism [8]
can be explained by intense conductive heat removal
from the specimens owing to the high conductivities λ
w
of these materials [at 1273 K, 80 and 336 W/(m
· K),
respectively] compared to iron and steel [30 and 29
W/(m
· K), respectively].
The parameters q
∗
, T
∗
max
, T
∗
0
, and ∆T
max
= T
∗
max
−T
fin
of materials that can ignite according to the ther-
mal mechanism [8] are listed in Table 2 for p
O
2
= 0.1
and 70 MPa.
A comparison of the data given in Table 2 with
the kinetic constants of oxidation used in calculations
(see Table 1) shows that the parameters of the metals
T
∗
max
, T
∗
0
, and ∆T
max
correlate with the corresponding
values of the activation energy of the oxidation reaction
E: as E increases, the maximum temperature T
∗
max
to
which a compact specimen from a given metal could
be heated without ignition and the maximum subcriti-
cal self-heating of the specimen ∆T
max
decrease rapidly,
whereas the ignition temperature T
∗
0
increases.
As for the effect of pressure, an analysis of the
components of heat removal in the period prior to ig-
nition q
out
shows that heat is removed from the cen-
tral part of iron and steel specimens mainly by ra-
diation and heat conduction (e.g., for iron, q
out
=
0.57q
ε
− 0.37q
λ
− 0.06q
α
) at low pressures (0.1 MPa)
and by convection (q
out
= 0.23q
ε
− 0.13q
λ
− 0.64q
α
) at
high pressures (70 MPa). Since, by assumption, the ox-
idation rate weakly depends on the oxygen pressure and
q
ch.r.
= const(p
O
2
), an increase in pressure and, hence,
in q
out
causes a monotonic increase in q
∗
, T
∗
max
, T
∗
0
, and
∆T
max
.
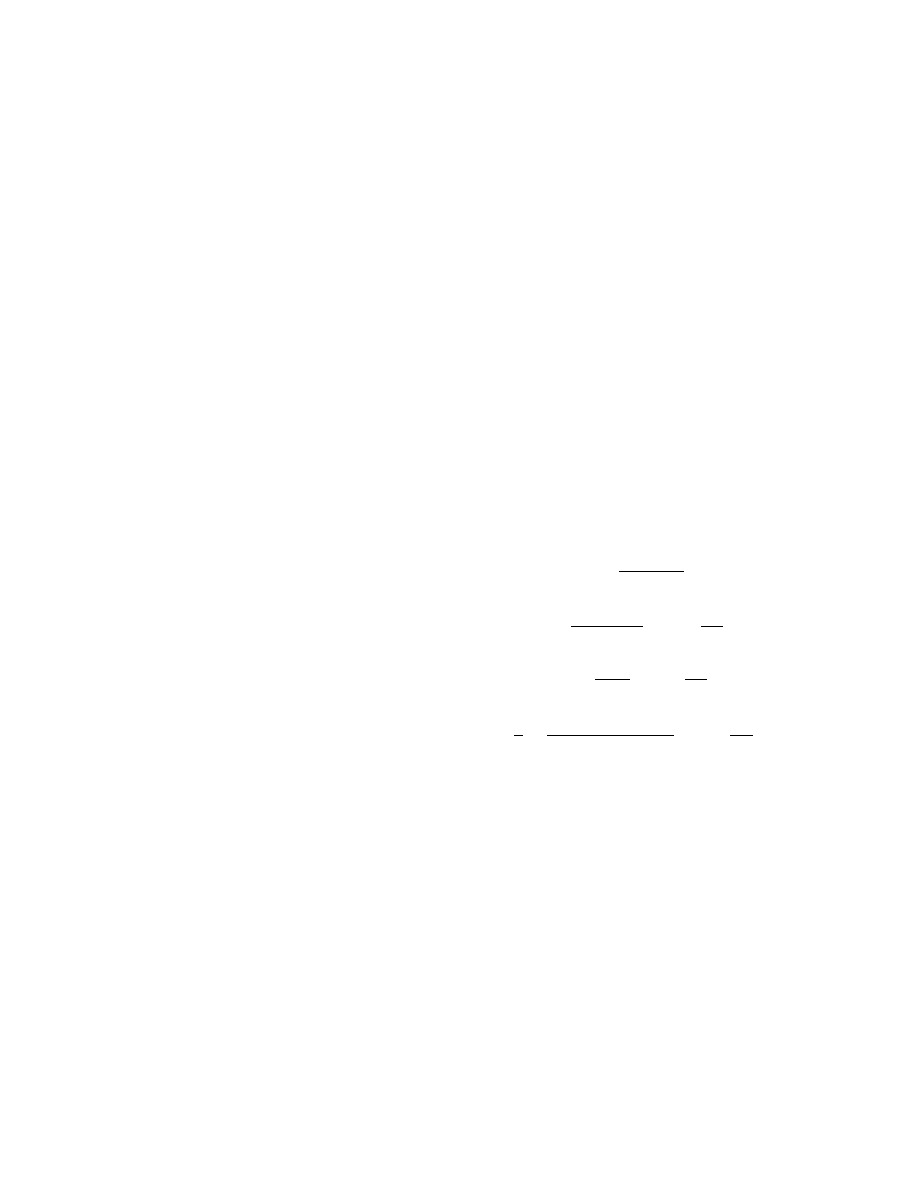
660
Bolobov and Podlevskikh
COMPARISON
WITH EXPERIMENTAL RESULTS
The experimental values of the ignition tempera-
tures of materials in oxygen are listed in Table 2 (T
∗
exp
and dashed curve in Fig. 1). For iron, T
∗
exp
is equal to
the temperature at which a liquid eutectic FeO–γFe is
formed at the metal–oxide interface [7]. For stainless
steel and nickel, the value of T
∗
exp
is close to the melt-
ing point of the metal [4, 6]. For copper, T
∗
exp
corre-
sponds to the melting point of the eutectic Cu
2
O–αCu
[5]. The last column in Table 2 gives the calculated val-
ues of subcritical self-heating of specimens (∆T
exp
) to
the temperature T
max
that corresponds to the ignition
temperature T
∗
exp
observed in experiments.
One can see from Table 2 that, for all the ma-
terials considered, the calculated maximum tempera-
tures of subcritical heating T
∗
max
are higher than the
temperature T
∗
exp
= T
melt
, at which the metal or ox-
ide film melts. As a result, the oxide film is broken
and the specimens ignite. Depending on the material,
the calculated values of self-heating of specimens to
their experimentally determined ignition temperature
(∆T
exp
= T
∗
exp
− T
∗∗
0
) are different: several hundreds of
degrees for iron and several tens of degrees for steel
and nickel, whereas self-heating for copper is practi-
cally absent. (For all the materials, the value of ∆T
exp
decreases as the pressure increases, which can be ex-
plained by the fact that the period of induction and,
hence, the thickness of the oxide film increase by the
beginning of self-heating.)
For this reason, iron is a
unique material (among the materials studied in [4–7])
for which the maxima of subcritical heating on exper-
imental thermograms are pronounced.
Judging from
small values of ∆T
exp
, compact specimens of copper,
nickel, and steel ignite by the Friedman–Macek mecha-
nism [17], whereas the ignition of iron specimens can be
considered as a transient mechanism between that de-
scribed in [17] and the Khaikin–Bloshenko–Merzhanov
thermal mechanism [8].
CONDITIONS FOR IGNITION OF FOIL
Calculations show that, owing to small heat re-
moval q
out
, all the considered metals in the form of foil
can ignite in oxygen at a temperature lower than the
temperature of any phase transformations in the metal
or oxide film which can lead to the failure of the latter
(T
∗
0
< T
melt
). The calculated values of T
∗
0
and ∆T
max
for foil ignited in oxygen (p = 0.14 MPa) and experi-
mental values of T
∗
exp
are listed in Table 3. (For iron, the
value of T
∗
exp
is taken from [7]; the data for steel, nickel,
and copper were obtained by the method proposed in [7]
and were not published previously.)
As in the case of compact specimens, the ignition
temperature of foil T
∗
0
increases with the activation en-
ergy of pre-ignition oxidation of the metal. It is minimal
for copper (E = 158 kJ/mole) and maximal for stainless
steel (E = 290 kJ/mole).
Good correlation between the calculated and ex-
perimental values of T
∗
0
supports the conclusion that
the foil specimens from these materials ignite in oxygen
according to the thermal mechanism [8]. The validity
of the assumptions used in the calculations is also con-
firmed.
ANALYSIS OF CRITICAL
PARAMETERS OF IGNITION
We consider the question whether the critical pa-
rameters of ignition of metal particles (χ and Ω
∗
) [8]
can be used to determine the temperature parameters
of ignition (T
∗
max
, T
∗
0
, and ∆T
max
) of large-scale metal
bodies and bodies under different conditions of heat ex-
change with the ambient medium.
We calculated the parameters introduced in [8],
which can be written as
γ
≡
c
p
RT
2
h
eff
2EQδ
∗
0
;
χ
≡
QρEk
0
K
m
p
T
2
αRδ
∗
0
exp
−
E
RT
;
θ
≡
∆T E
RT
2
;
β
≡
T R
E
;
Ω
≡
χ
γ
=
2Q
2
(E/R)
2
ρK
0
K
m
p
T
4
αc
p
h
eff
exp
−
E
RT
,
(4)
where α = ∂q
out
/∂T is the overall heat-transfer coeffi-
cient and δ
∗
0
is the thickness of the oxide film at the mo-
ment the critical self-heating begins [δ
∗
0
= δ
0
= 10
−9
m
for foil and
≈10
−5
m (according to calculations) for the
specimens].
Table 4 lists the dimensionless parameters calcu-
lated at T = T
∗
0
for compact specimens (metals ignited
by the thermal mechanism [8]) and foil (given in ascend-
ing order of the values of E of the materials studied).
The values of α at T = T
∗
0
used in the calculations of χ
and Ω
∗
are also given in Table 4.
In accordance with [8], the criterion that describes
the critical conditions for ignition of metal particles is
chosen depending on the parameter γ: χ
cr
= 1/e for
γ
1 and Ω
∞
cr
= 1.57 for γ
1 (for a parabolic law of
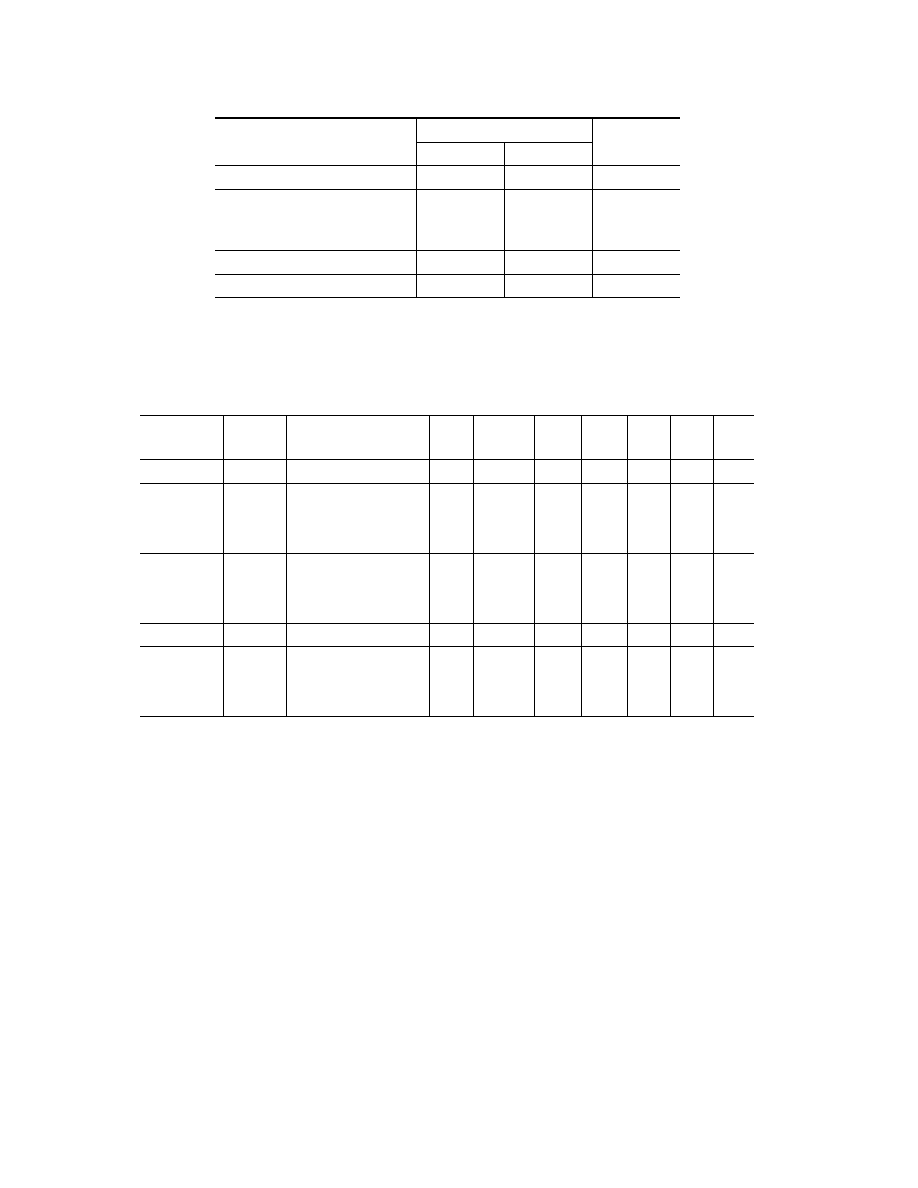
Numerical Analysis of Conditions for Ignition of Compact Metal Specimens and Foil
661
TABLE 3
Values of T
∗
0
and ∆T
max
for Foil Ignited in Oxygen
Material and thickness of the foil
T
∗
0
, K
∆T
max
, K,
calculations
experiment
calculations
Copper, h = 0.01 mm
1133
1153
543
1182 [10]
532 [10]
Iron, h = 0.5 mm
1233
∗
1273 [9]
547 [9]
Nickel, h = 0.05 mm
1393
1543
520
Stainless steel, h = 0.01 mm
1396
1483
218
Note. The asterisk indicates the value for iron containing 0.22% C (St. 3 steel).
TABLE 4
Dimensionless Parameters of Critical Conditions for Ignition of Foil
and Compact Metal Specimens in Oxygen
Material
E,
Shape of the specimen
p
O
2
,
α,
β
θ
melt
γ
χ
Ω
∗
kJ/mole
MPa
kW/m
2
Copper [19]
158
Foil
0.14
0.30
0.060
8.0
114
284
2.49
Foil
0.14
0.34
0.058
7.8
1018
2441
2.40
Iron [19]
170
Cylinder
0.1
0.79
0.071
14.1
0.59
2.2
3.67
Cylinder
70
1.56
0.075
15.7
0.61
2.1
3.39
Foil
0.14
0.42
0.056
7.7
1054
2445
2.32
Iron [18]
190
Cylinder
0.1
0.90
0.067
11.3
0.60
2.1
3.56
Cylinder
70
1.66
0.071
11.3
0.61
2.0
3.35
Nickel [18]
225
Foil
0.14
0.55
0.052
7.21
190
419
2.21
Stainless
Foil
0.14
0.60
0.040
3.9
130
250
1.93
steel [18]
290
Cylinder
0.1
1.11
0.049
7.0
0.60
1.9
3.10
Cylinder
70
1.82
0.050
7.1
0.62
1.8
2.94
oxidation). The calculated values of γ given in Table 4
lie within the interval 10
−1
–10
3
, and the critical condi-
tions for ignition in our case should be determined from
the condition Ω
∗
= const. At the same time, one can
see that this parameter does not remain constant: it in-
creases in the transition from foil to compact specimens
and decreases with an increase in the activation energy
of the oxidation reaction of the metal for specimens of
one type. An analysis of the data given in Table 4 shows
that, for all the metal foils, the parameter Ω
∗
is a func-
tion of the product βθ:
Ω
∗
= 1.68 + 1.60βθ.
(5)
The limiting value Ω
∗
= 1.68 (for βθ = 0) is close to the
value Ω
∞
cr
= 1.57, which determines the critical condi-
tions for ignition of metal particles [8].
The form of dependence (5) can be explained
by the fact that self-heating of foil is more intense
(θ
melt
= 3.9–8.0 in Table 4) than that of a particle
(θ
melt
= 3.3 [8]) and, hence, the product βθ is larger
(0.157–0.48); therefore, it cannot be ignored as was done
in [8] in determining Ω
∞
cr
for particles.
One can see
from Table 4 that, as the activation energy increases,
the quantities β and θ
melt
decrease for specimens of one
type; therefore, for metals with high activation energy,
for example, steel, we have βθ
→ 0, and Ω
∗
→ Ω
∞
cr
[8].
(It is noteworthy that, for compact specimens, the quan-
tity Ω
∗
is also a linear function of the product βθ, and
for different oxygen pressures, the corresponding lines
pass through approximately the same point with the
coordinates Ω
∗
= 2.75 and βθ = 0.)

662
Bolobov and Podlevskikh
We found that, for Ω/Ω
∗
< 0.7, the dependence
of the dimensionless temperature θ of foil and compact
specimens on Ω/Ω
∗
can be approximated by a linear
function with a slightly different proportionality coeffi-
cient compared to [8]:
θ = 2.2(Ω/Ω
∗
).
(6)
PRACTICAL USE OF THE
CALCULATION DATA IN ENGINEERING
To answer the question whether the averaged value
of Ω
∗
can be used in determining the critical tempera-
ture parameters by Eq. (4), we calculated the ignition
temperatures T
∗
0
of specimens and foil from the mate-
rials studied for the their arithmetical mean (see Ta-
ble 4) value of Ω
∗
(Ω
∗
= 2.85). Moreover, using Eq. (6)
with Ω
∗
= 2.85, we determined the temperature T
∗∗
0
corresponding to the onset of self-heating of compact
specimens for which the melting point T
melt
is attained.
The latter was recorded in [4–7] as the ignition temper-
ature T
∗
exp
. By analogy with [8], we solved the following
transcendental equation for T
∗∗
0
:
∆T
exp
= T
melt
− T
∗∗
0
= 1.54
Q
2
(E/R) ρ K
0
K
m
p
(T
∗∗
0
)
2
αc
p
h
eff
× exp
−
E
RT
∗∗
0
.
(7)
The calculations show that, for all the materials
ignited by the thermal mechanism and for all types of
specimens and oxygen pressures, the values of T
∗
0
cal-
culated by formula (4) for Ω
∗
= 2.85 differ from those
obtained by integrating Eqs. (1) and (2) by no more
than 40 K (or 3%). The difference between the values
of T
∗∗
0
calculated by formula (7) and those computed
numerically does not exceed this error either.
Since this error is acceptable, this method of deter-
mining the conditions for ignition of specimens can be
used in engineering practice to estimate the conditions
of safe exploitation of metal structures contacting cor-
rosive media. For this purpose, the following algorithm
is proposed:
1) Using the data available in the literature, de-
termine the kinetic parameters (K
0
, E, and n) of high-
temperature oxidation of a structural material in a given
medium and the thermal effect of the reaction;
2) For quasi-stationary conditions of heat ex-
change, calculate the dependence of the overall heat-
transfer coefficient of the most heated zone of the struc-
ture on temperature;
3) Using Eq. (4) with Ω
∗
= 2.85, determine the
ignition temperature T
∗
0
of the structure from the given
material by the substitution method for corresponding
values of α = f (T ) [in this case, T
∗
0
is the minimum
temperature of the surface (chemical interaction with
the medium is not taken into account) at which self-
heating owing to the metal-oxidation reaction leads to
ignition of the structure];
4) Using the existing data on the phase composition
of the products of interaction of the material with the
corrosive medium and their physicochemical properties,
determine the minimum temperature at which the pro-
tective film on the material can be destroyed (usually,
as a result of melting of the component or metal at the
temperature T
melt
);
5) For the chosen value of T
melt
, use Eq. (7) to
calculate the temperature T
∗∗
0
corresponding to the on-
set of critical self-heating of the metal structure, which
results in the destruction of the protective film and ig-
nition of the metal;
6) Compare the determined values of T
∗
0
and T
∗∗
0
with expected temperature regimes of operation of the
structure to infer whether the operation conditions are
safe.
CONCLUSIONS
1. With the use of the mathematical model pro-
posed, the critical conditions for ignition of compact
specimens and foil from iron (for two oxidation kinet-
ics), 12Kh18N10T stainless steel, and technical nickel
and copper in oxygen at p = 0.1–70 MPa under natural
convection have been calculated.
2. It has been found that compact copper spec-
imens do not ignite at temperatures up to 6000 K,
whereas the critical conditions for ignition of nickel are
degenerate.
Theoretically, specimens from iron and
stainless steel can ignite in oxygen according to the
mechanism proposed in [8] at the maximum tempera-
tures of subcritical self-heating (T
∗
max
= 2295–2894 K),
which are much higher than the melting points of the
metals or oxide films (T
melt
= 1644–1683 K) at which
specimens ignite in tests.
3. All the considered materials in the form of foils
have been shown to be able to ignite in oxygen accord-
ing to the thermal mechanism [8] at temperatures T
∗
0
lower than the temperature at which the oxide film is
destroyed. The calculated values of T
∗
0
increase with
the activation energy of pre-ignition oxidation and sat-
isfactorily correlate with experimental data.

Numerical Analysis of Conditions for Ignition of Compact Metal Specimens and Foil
663
4. It has been shown that the parameter Ω
∗
de-
termining the critical conditions for ignition of metal
particles [8] is not a constant in the case of ignition
of compact specimens and foil (Ω
∗
= 1.93–3.67) and
increase with the activation energy of the oxidation re-
action of the materials.
5. An algorithm of calculating the ignition temper-
atures of metal structures with the use of the averaged
value of Ω
∗
has been proposed.
REFERENCES
1. L. E. Dean and W. R. Thompson, “Ignition character-
istics of metals and alloys,” J. Amer. Rocket Soc., 31,
No. 7, 78–85 (1961).
2. B. A. Ivanov and A. P. Nikonov, “Ignition and com-
bustion of compact metals in oxygen,” in: Problems in
Engineering Workplace Hygiene (collected scientific pa-
pers) [in Russian], No. 63, Metallurgiya, Moscow (1970),
pp. 55–63.
3. Yu. V. Frolov, A. N. Pivkina, and V. V. Gusel’nikov,
“Ignition and combustion of high-temperature alloys in
oxygen,” Fiz. Goreniya Vzryva, 24, No. 4, 19–21 (1988).
4. V. I. Bolobov, A. Yu. Berezin, P. F. Drozhzhin,
and A. S. Shteinberg, “Ignition of compact stainless-
steel specimens in high-pressure oxygen,” Fiz. Goreniya
Vzryva, 27, No. 3, 3–7 (1991).
5. V. I. Bolobov and A. Yu. Berezin, “Conditions for ig-
nition of copper and copper alloys in oxygen,” Fiz.
Goreniya Vzryva, 34, No. 2, 47–50 (1998).
6. V. I. Bolobov, “Effect of pressure on the ignition tem-
perature of compact samples of nickel alloys in oxygen,”
Fiz. Goreniya Vzryva, 35, No. 2, 54–58 (1999).
7. V. I. Bolobov,“Conditions for ignition of iron and carbon
steel in oxygen,” Fiz. Goreniya Vzryva, 37, No. 3, 52–57
(2001).
8. B. I. Khaikin, V. N. Bloshenko, and A. G. Merzhanov,
“Ignition of metal particles,” Fiz. Goreniya Vzryva, 6,
No. 4, 474–488 (1970).
9. V. I. Bolobov and K. M. Makarov, “High-temperature
oxidation of some metals in the presence of atomic oxy-
gen,” Zashchita Metallov, 28, No. 6, 1007–1010 (1992).
10. O. Kubashewski and B. E. Hopkins, Oxidation of Metals
and Alloys, Butterworths, London (1965).
11. J. B´
enard (ed.),
Oxydation des Metaux,
Vol. 1,
Gauthier-Villars, Paris (1962).
12. A. P. Zefirov, Thermodynamic Properties of Inorganic
Substances: Handbook [in Russian], Atomizdat, Moscow
(1965).
13. S. S. Rybanin, N. I. Peregudov, T. V. Rybanina, and
S. L. Sobolev, “Critical conditions and period of induc-
tion for combustion of a metal plate in an immobile ox-
idizing atmosphere,” Fiz. Goreniya Vzryva, 22, No. 3,
3–10 (1986).
14. V. I. Bolobov, P. F. Drozhzhin, and V. G. Nechaeva,
“High-temperature oxidation and ignition of some
metallic materials in fluorine,” Fiz. Goreniya Vzryva,
34, No. 4, 34–42 (1998).
15. S. S. Kutateladze, Fundamentals of the Heat Transfer
Theory [in Russian], Nauka, Novosibirsk (1970).
16. K. P. Yakovlev (ed.), Physicochemical Handbook [in Rus-
sian], ´
Energiya, Moscow (1962).
17. R. Friedman and A. Macek, “Ignition and combustion
of aluminium particles in hot ambient gases,” Combust.
Flame, 6, 9 (1962).
Wyszukiwarka
Podobne podstrony:
Preliminary Analysis of the Botany, Zoology, and Mineralogy of the Voynich Manuscript
Babi Yar Analysis of Yevtushenko's Writing Style and Meani
Analysis of Roosevelt's New?al and the Great?pression
Analysis of residual styrene monomer and other VOC in expand
A Cost Analysis of Typical Computer Viruses and Defenses
To Kill a Mockingbird Analysis of Boo Radley, Atticus, and doc
Al Suhaibani And Kryzanowski An Exploratory Analysis Of The Order Book, And Order Flow And Execution
Johnson Summary brief shades of embeddedness Tie strengh and obligations in direct selling parties
2002 Intestinal Effects of Mannanoligosaccharides, Transgalactooligosaccharides, Lactose and Lactulo
J Leigh Globalization Reflections of Babylon Intercultural Communication and Globalization in the
Interaction of fraternal birth order and handedness in the
Death and return of the author criticism and subjectivity in barthes foucault and derrida
Alan L Mittleman A Short History of Jewish Ethics Conduct and Character in the Context of Covenant
DANN Changing patterns of violence at Qustul and Ballana in the post Meroitic period Part One The Hu
integration and radiality measuring the extent of an individuals connectedness and reachability in a
THE USE OF THERAVADA BUDDHIST practises and perspectives in mindfulnes B CT
Vatican lists conditions for ties with China Conflict Resolutions and World Security Solutions wor
Parametric Analysis of the Ignition Conditions of Composite Polymeric Materials in Gas Flows
A Philosophy for all an analysis of the Tao
więcej podobnych podstron