* Corresponding author. Tel.: 0049 3731 39 2666; fax: 0049 3731
39 3129; e-mail:heimann@orion.hrz.tu-freiberg.de
1 In this paper the cement chemistry notation will be used: C"CaO,
P"P
2
O
5
, H"H
2
O, S"SiO
2
, T"TiO
2
and Z"ZrO
2
.
Biomaterials 19 (1998) 1507 — 1511
Development of plasma-sprayed bioceramic coatings with bond
coats based on titania and zirconia
H. Kurzweg
!, R.B. Heimann!,*, T. Troczynski", M.L. Wayman#
!Department of Mineralogy, Freiberg University of Mining and Technology, Brennhausgasse 14, 09596 Freiberg, Germany
"Department of Metals and Materials Engineering, University of British Columbia, Vancouver, Canada, B.C., V6T 1Z4
#Department of Chemical and Materials Engineering, University of Alberta, Edmonton, Alberta, Canada, T6G 2G6
Abstract
Bond coats for plasma-sprayed hydroxyapatite (HAp) coatings on Ti—6Al—4V hip endoprotheses are being developed for improved
in vivo performance. Bond coat powders consisting of (i) CaO-stabilized zirconia, (ii) a eutectic composition of titania and
non-stabilized zirconia, and (iii) titania were applied by atmospheric plasma spraying (APS) to Ti—6Al—4V-coupons and 100
lm-thick
Ti—6Al—4V foils. Subsequently, a thick layer of HAp was sprayed onto the thin bond coats.
Peel tests on Ti—6Al—4V foil/bond coat/HAp top coat assemblies revealed that titania and titania/ zirconia bond coats increased the
peel adhesion strength in a statistically significant way from 22 N m
~1 (HAp without a bond coat) to'42 and 32 N m~1, respectively.
Microstructural investigations by SEM on cross-sections of coatings leached in simulated body fluid for up to 28 days led to the
conclusion that the chemically very stable bond coats act as an improved chemical barrier against in vivo release of metal ions from
the implant, as well as an improved adhesive bond by development of very thin well-adhering reaction layers, presumbly composed of
perovskite, calcium dititanate, and/or calcium zirconate.
( 1998 Elsevier Science Ltd. All rights reserved
1. Introduction
Bioactive ceramics such as calcium phosphate in general,
and hydroxyapatite (C10P3H,1 HAp) in particular, are in
widespread use as implant substitute components or as
interfacing osseoconductive layers in metallic surgical
implants. Since incomplete fixation to living bone of
uncoated cementless joints for total hip replacement
(THP) is a common problem, application of hydroxyapa-
tite coatings by plasma spray processing to the surfaces
of titanium alloy hip endoprothetic implants constitutes
the state-of-the-art procedure to induce osseointegration
by bonding osteogenesis [1—3].
The in vivo performance of such coatings depends on
a large array of factors, most notably coating thickness,
chemical composition, crystallinity, phase purity, cohe-
sive and adhesive strengths, and resorption resistance
[4]. In particular, adhesion strength of the coating to
the implant surface appears to be a property that needs
to be maximized to avoid cracking, shearing off, and
chipping of the HAp coating during emplacement of the
implant.
Some limited improvement of the adhesion strength
can be achieved by carefully controlling the plasma spray
parameters [5], or by microstructural engineering of the
spray powder through pre-spray annealing [6]. A com-
pletely different way to achieve improved adhesion is to
consider biocompatible bond coats.
The aim is to support the mechanical inter-locking be-
tween the HAp-coating and the titanium alloy substrate
by a chemical bonding. Preliminary work indicated that
the application of a dicalcium silicate (C2S) bond coat
(10—50
lm thick)/hydroxyapatite top coat (30—130 lm
thick) system to a Ti—6Al—4V substrate by atmospheric
plasma spraying (Plasmadyne 3610-D; plasma current:
700—900 A; plasma gas: argon/helium 10%) led to a sta-
tistically significant increase of the adhesion strength to
over 30 MPa [7].
In applications for bioceramic coatings bond coats
should also, in addition to improving adhesion strength:
(1) prevent direct contact between Ti and HAp since this
is thought to catalyse the thermal transformation of
0142-9612/98/$19.00
( 1998 Elsevier Science Ltd. All rights reserved.
PII S 0 1 4 2 - 9 6 1 2 ( 9 8 ) 0 0 0 6 7 - 2

Table 1
Plasma spray conditions
(CaO)ZrO
2
TiO
2
#
ZrO
2
TiO
2
HAp
Plasma power
(kW)
42.2
42.2
41.6
25.8
Argon : Hydrogen (l min
~1) 40 : 12
40 : 12
40 : 12
50 : 4
Carrier gas Ar
(l min
~1) 2.6
2.6
2.6
6.0
Powder feed rate (g min
~1) 18.5
23.0
20.0
23.5
Stand-off
(mm)
100
100
80
100
distance
HAp towards tri- or tetracalcium phosphate or even
non-biotolerant CaO [8, 9],
(2) reduce the release of metal ions from the substrate to
the surrounding living tissue that has been shown to
induce massive hepatic degeneration in mice [10] and
impaired development of human osteoblasts [11],
(3) reduce the thermal gradient at the substrate/coating
interface caused by the rapid quenching of the molten
particle splats that leads to deposition of amorphous
HAp with a concurrent decrease in resorption resis-
tance [6] and hence to reduced in vivo performance,
i.e. longevity of the implant,
(4) prevent a steep gradient in the coefficients of thermal
expansion between substrate and coating that pro-
motes the formation of strong tensile forces in the
coating giving rise to crack generation, chipping
and/or delamination, as well as
(5) cushion damage by cracking and delamination of the
coating initiated by cyclic micromotions of the im-
plant during movement of the patient in the initial
phase of the healing process [12].
Thus, it is highly desirable to engineer the substrate/
HAp coating interface in such a way that by application
of a suitable thin biocompatible bond coat layer the
advantages addressed above can be realized.
Suitable bond coats are being developed within the
binary system ZrO2—TiO2 [13]. The plasma spray
conditions, the in vitro resorption resistance and the
adhesion strength of those coating systems will be de-
scribed below.
2. Plasma spraying of hydroxyapatite/ bond coat systems
Various hydroxyapatite/bond coat systems were
plasma sprayed onto grit-blasted Ti—6Al—4V coupons
of dimension 50
]20]2 mm3 and onto Ti—6Al—4V foils
of dimensions 120
]16]0.1 mm3 using atmospheric
plasma spray equipment (Plasmatechnik AG, F4 torch).
Grit blasting of the coupons was performed using silicon
carbide (grain size range 0.71—1.0 mm) at an air pressure
of 500 kPa and a distance of 50 mm from the target. Grit
blasting of the foils was done using alumina grit
(600—800
lm) at an air pressure of 250 kPa and a distance
of 50 mm. After grit blasting the coupons and foils were
cleaned ultrasonically with acetic acid ethylester and
ethyl alcohol. Foils were attached to copper blocks using
a 1 : 2 mixture (by weight) of a silicone sealant (Dow
Corning 732) and copper powder (ALCAN 154, grain
size range (44
lm). The adhesive was cured at room
temperature for 12 h. The presence of copper powder in
the adhesive provides, during plasma spraying, excellent
conduction of heat away from the foil into the copper
block acting as an effective heat sink. The type of adhes-
ive used allows for an easy removal of the foil from the
copper block for post-spray sample preparation.
Bond coats of thickness 10—15
lm, deposited onto
Ti—6Al—4V substrate coupons and foils, consisted of par-
tially CaO-stabilized zirconia (DYNAZIRKON C, Hu¨ls
AG, grain size 0.45—60
lm, series 2), a mechanically
mixed powder of 73 mol% titania and 27 mol% non-
stabilized zirconia, corresponding to the eutectic com-
position (Type 9303, Carl Roth GmbH, grain sizes
0.18—26
lm, series 3), and titania (AMDRYTM 6500, Sul-
zer Metco GmbH, grain size 5—22
lm, series 4). The
plasma spray parameters used are shown in Table 1.
Subsequently, a thick (150—180
lm and 100—120 lm, re-
spectively) layer of hydroxyapatite (AMDRY
TM 6021,
Sulzer Metco Deutschland GmbH) was sprayed onto the
bond coat using the parameters shown in Table 1. In
addition, foils were sprayed with HAp using the same
parameters but without using a bond coat (series 1) to
establish a bench mark for estimating the effect of the
various bond coats on the adhesion behaviour.
3. The in vitro resorption resistance
Investigations into the microstructure and the chem-
ical behaviour during immersion in simulated body fluid
(Hank’s Balanced Salt Solution, HBSS) will give valuable
information on the anticipated in vivo performance of
such coating systems.
5 mm slices cut from the as-sprayed coupons were
immersed in 50 ml of simulated body fluid (Hank’s Bal-
anced Salt Solution, HBSS) for 7, 14 and 28 days to study
the in vitro resorption resistance of the various coating
systems. The composition of HBSS is shown in Table 2.
The leaching experiments were performed in cylindrical
glass containers held in a constant temperature bath at
37$0.5°C. At the end of the leaching period the sample
was removed from the HBSS, cleaned with distilled
water, acetone and methanol, and dried in a dust-free
environment.
After the immersion the samples were investigated by
SEM.
It could be shown that the HAp coatings onto titanium
alloy substrates with various bond coats, display differ-
ent stability against leaching in HBSS. In particular,
1508
H. Kurzweg et al. / Biomaterials 19 (1998) 1507—1511

Table 2
Composition of Hank’s Balanced Salt Solution in 1 l deionized water
8000 mg NaCl
140 mg CaCl2
400 mg KCl
100 mg MgCl2
60 mg Na2HPO4
60 mg KH2PO4
1000 mg MgSO4
Fig. 1. SEM micrograph of a cross-section of a titania bond coat/hy-
droxyapatite top coat system.
Table 3
Calculated peel strengths (N/m) for the coating systems investigated
Series
Regression line
Peel strength
Dt
3
(a)
D
t
.,2
(N/m)
1
y"34.7#1.59x
21.9
2
y"30.3#1.68x
18.0
2.54
2.07
3
y"51.1#1.61x
31.8
2.15
2.06
4
y"65.2#1.55x
42.1
2.67
2.07
stresses introduced into the hydroxyapatite by thermally
induced transformation of the zirconia bond coat (series
2) lead to extensive scaling and concurrent leaching dur-
ing treatment in HBSS. In contrast to this, titania/zirco-
nia bond coat/ HAp top coat (series 3) and, in particular,
titania bond coat/HAp top coat ‘tandems’ (series 4) stand
up well to the leaching with little damage done to the
cohesion of the coating system. For the latter samples the
amounts of titania and zirconia measured in the HBSS
after 28 days remained below 5 ng l
~1.
Figure 1 shows a sample of series 4 leached for 28 days
in HBSS. The cross-section shows the titania bond coat
very well adhering to the titanium alloy substrate that in
some places has separated from the HAp top coat, pre-
sumably due to damage done during preparation. The
outermost leached HAp layer of approximately 30
lm
thickness has a Ca/P ratio of 1.27 and shows some
cracking perpendicular to the interface indicating mech-
anical weakening during leaching.
4. The peel adhesion test
Since the uncontrolled mode of coating/ substrate sep-
aration in the conventional tensile pull test contributes to
a large extent to the uncertainty of the test results a modi-
fied ASTM D3167-76 peel test was introduced recently
[14, 15].
A thin metal foil is attached to a massive copper block
that provides mechanical support and acts as a heat sink.
After grit blasting a coating is deposited onto this thin
metal foil. The block, foil and coating assembly is glued
to a stiff aluminium plate, and the copper block is then
removed. The coated titanium foil/epoxy/aluminium
plate assembly was mounted on a jig (for a detailed
description cp.[14]) in an INSTRON 4200 universal test-
ing machine (Instron Corporation, Canton, MA). The
end of the foil was clamped to the upper grip of the
machine and pulled away from the coating at a constant
rate of 2.5 mm min
~1. The load and the crosshead dis-
placement were recorded digitally, and the correspond-
ing stress—strain curve calculated from the recorded data.
Different dead weights were attached to the other end of
the foil in order to make the foil conform to the mandrel
of the jig. The peeling force was averaged over 5—10 mm
of crosshead displacement, and more weight was added
as the test progressed. Since the measured force is
the sum of the peel force, the dead weight, the fric-
tional force and the plastic work per increment
peeled, from a plot of the measured force versus the
known dead weight, the peel force can be obtained
[16, 17]. Peeling off the foil from the coating causes
a crack to propagate precisely along the coating/foil
interface in a stable and controllable manner since the
sample geometry forces the crack tip to move along the
interface [18] where it encounters the local least-energy
path. While the conventional tensile pull test measures
failure stress, expressed as the ratio of applied force to
coating area in dimension: N m
~2, the novel peel test
measures the energy required to separate the coating and
the foil along a line in dimension: N m
~1.
Fig 2 shows typical results of the peel adhesion test.
Here the normalized total force (F/w) is plotted against
dead weight (D/w) from which lines the normalized peel
strengths (G/w) of a hydroxyapatite coating without
a bond coat (series 1, Fig. 2a) and a hydroxyapatite/
titania bond coat system (series 4, Fig. 2b) were
calculated. The calculated peel strengths of all samples
are shown in Table 3 together with the relevant linear
regression lines y"a#bx where x is the normalized
dead weight D/w.
Based on the comparison of the intersection with the
y-axes, (a) of series 1 (no bond coat) to series i (i"2, 3, 4),
a significance test was done [19]. The calculated
Dt3(a)D-
values are always higher than the tabulated t.,2 values
H. Kurzweg et al. / Biomaterials 19 (1998) 1507—1511
1509
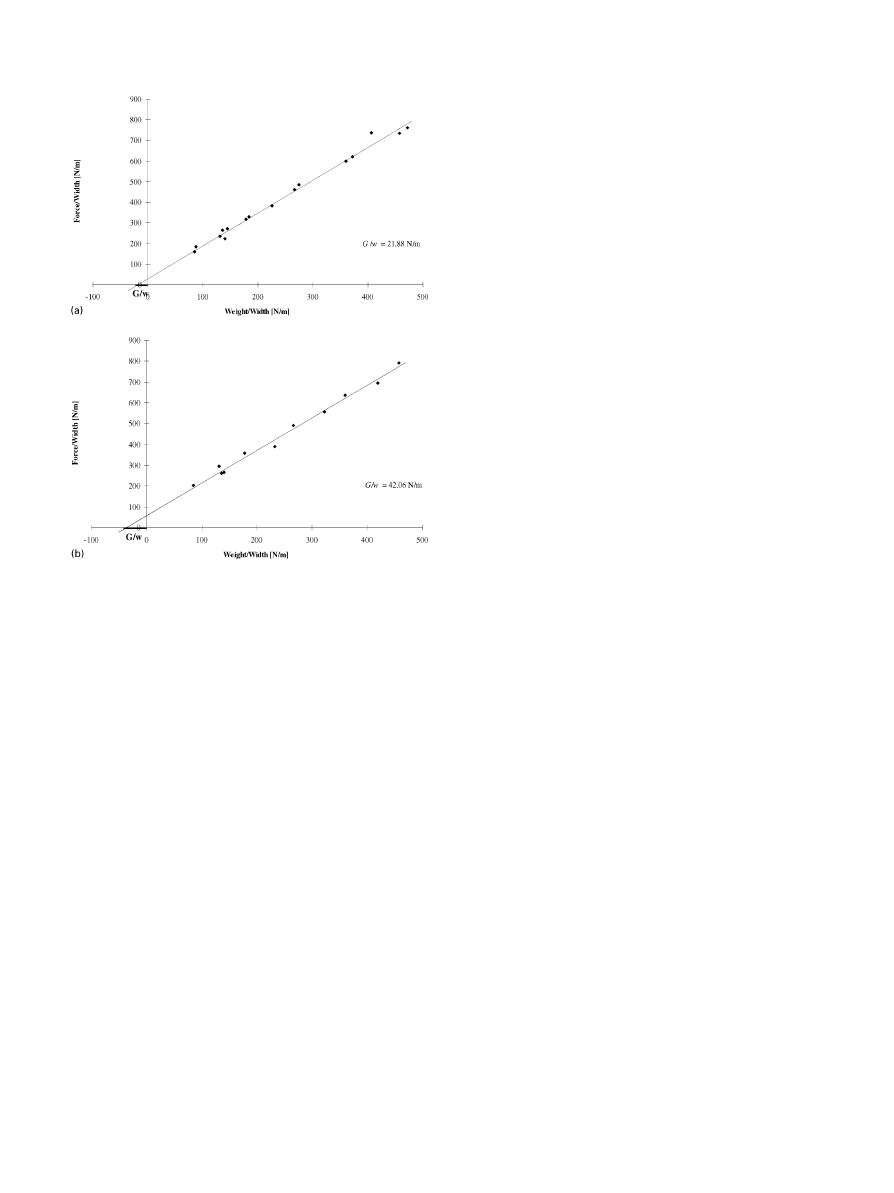
Fig. 2. Peel strength of series 1 (a) and series 4 (b).
for m degrees of freedom and a level of confidence
a"0.05. These values are also included in Table 3. Thus,
there is a significant difference between the peel strengths.
It can be shown that series 2 (CaO-stabilized zirconia)
has a significantly lower peel adhesion strength than the
HAp coating without a bond coat. On the other hand,
the results for series 3 (titania/zirconia bond coat) and
series 4 (titania) show that those bond coats improve the
peel adhesion strength in a statistically significant way. In
particular, a titania bond coat leads to a peel strength
twice the value of a HAp coat without a bond coat. The
reason for this behaviour may be seen in the development
of a thin reaction zone at the immediate interface be-
tween bond coat and HAp top coat consisting of calcium
titanates and/or zirconates [20]. Calcium titanates have
been reported to occur at the interface of a titanium
substrate terminated by a thin layer of native oxide and
a HAp coating by de Groot et al. [1] (perovskite, CT)
and Ji et al. [9] (calcium dititanate, CT2).
5. Summary
Bond coats for plasma-sprayed hydroxyapatite (HAp)
coatings on titanium alloy hip endoprotheses are being
developed for improved in vivo performances.
The application of various bond coats based on titania
and zirconia to the Ti—6Al—4V substrate influences the
adhesion of the entire coating ‘tandem’ (bond coat#
HAp top coat). Titania/zirconia (73/27 mol%) and
titania bond coats increase the peel strength by 50% and
100%, respectively.
It could be shown that the HAp coatings, APS-
sprayed onto Ti—6Al—4V substrates with various bond
coats, display different stability against leaching in
simulated body fluid (HBSS).
Titania/zirconia bond coat/HAp top coat and, in par-
ticular, titania bond coat/HAp top coat ‘tandems’ stand
up well to the leaching with little damage done to the
cohesion of the coating system.
Acknowledgements
The support of the first author (HK) by the Studienstif-
tung des deutschen Volkes (Education Foundation of the
German People) during a four-months student visit to
the Department of Metals and Materials Engineering,
University of British Columbia, Vancouver, B.C., Cana-
da is gratefully acknowledged. One of the authors
(R.B.H.) is indebted to the German Federal Ministry of
Education, Research, Science and Technology (BMBF)
for sponsoring a four-months sabbatical at the Depart-
ment of Chemical and Materials Engineering, University
of Alberta, Edmonton, Alberta, Canada within the aus-
pices of the German—Canadian Agreement on Scientific
and Technological Cooperation, during which part of
this work was carried out. Thanks are also due to Dr.
Walter Kunert, Freiberger Nichteisen-Metall GmbH,
Freiberg, Germany who kindly granted access to the
plasma spray equipment and to Dr. N. Dorin Ruse and
Ms. Edith Breslauer, Faculty of Dentistry, University of
British Columbia for providing access to the Instron
universal testing machine.
References
[1] de Groot K, Geesink RTG, Klein CPAT, Serekian P. J Biomed
Mater Res 1987;21:1375.
[2] Klein CPAT, Patka P, van der Lubbe HBM, Wolke JGC, de
Groot K. J Biomed Mater Res 1991;25:53.
[3] Caulier H, van der Waerden JPCM, Wolke JGC, Kalk W, Naert
I, Jansen JA. In: P. Vincenzini Techna Srl, editors. Materials in
Clinical Application 1995:477.
[4] Heimann RB, Vu TA, Wayman ML. Europ J Mineral 1997;
9:597.
[5] Yang CY, Wang BC, Chang E, Wu JD. J Mater Sci: Mater Med
1995;6:249.
[6] Heimann RB, Vu TA. J Thermal Spray Technol 1997;6:145.
[7] Vu TA, Heimann RB. Second Interim Report SMWK Project
d 7541.82-0390/414, 15 February 1996.
[8] Weng J, Liu X, Zhang X, Ji X. J Mater Sci Lett 1994;13:159.
[9] Ji H, Ponton CB Marquis PM. J Mater Sci: Mater Med
1992;3:283.
[10] Pereira ML, Abreu AM, Sousa JP, Carvalho GS. J Mater Sci
Mater Med 1995;6:523.
1510
H. Kurzweg et al. / Biomaterials 19 (1998) 1507—1511

[11] Tomas H, Carvalho GS, FerNandes MH, Freire AP, Abrantes
LM. J Mater Sci: Mater Med 1996;7:291.
[12] S
+balle K. Acta Orthop Scand 1993;255(64):58.
[13] Kurzweg H. PhD thesis, Freiberg University of Mining and
Technology, Germany, in progress.
[14] Sexsmith M, Troczynski T. J Thermal Spray Technol 1996;3(4):
404.
[15] Sexsmith M, Troczynski T. J Thermal Spray Technol 1996;
5:196.
[16] Sexsmith M, Troczynski T, Breslauer E. J Adhes Sci Technol,
in press.
[17] Breslauer E, Troczynski T. J Adhes Sci Technol, to be published.
[18] Crocombe A, Adams R. J Adhes 1981;12:127.
[19] Storm R. Wahrscheinlichkeitsrechnung, mathematische Statistik
und statistische Qualita¨tskontrolle. Fachbuchverlag 1995:253—6.
[20] Figueiredo MO, Correia DOS, Santos A. In: Meriani S, Pal-
monari C, editors. Zirconia ’88. Advances in Zirconia Science and
Technology, London, New York, Elsevier, 1989:81.
H. Kurzweg et al. / Biomaterials 19 (1998) 1507—1511
1511
Wyszukiwarka
Podobne podstrony:
Microstructure and mechanical properties of plasma sprayed H
Activity of plasma sprayed yttria stabilized zirconia reinfo
Development of Carbon Nanotubes and Polymer Composites Therefrom
Development of BBM turbine
Development of financial markets in poland 1999
Development of a highthroughput yeast based assay for detection of metabolically activated genotoxin
01 [ABSTRACT] Development of poplar coppices in Central and Eastern Europe
43 597 609 Comparison of Thermal Fatique Behaviour of Plasma Nitriding
Development of vertical bulb turbine
Development of organic agriculture in Poland, Technologie
Aristoteles # Guthrie (The Development of Aristotle's Theology 1) BB
Development of wind turbine control algorithms for industrial use
An experimental study on the development of a b type Stirling engine
DEVELOPMENT OF FACTORING MARKET IN TURKEY
Progressive development of sonographic features
Advanced Methods for Development of Wind turbine models for control designe
Development of Communist Theory
Development of Plot Plan
więcej podobnych podstron