
Fuel and Chemical Products from Biomass Syngas:
A Comparison of Gas Fermentation to
Thermochemical Conversion Routes
Derek W. Griffin and Michael A. Schultz
LanzaTech Inc., 725-C East Irving Park Road, Roselle, IL 60172; mike@lanzatech.com (for correspondence)
Published online 23 March 2012 in Wiley Online Library (wileyonlinelibrary.com). DOI 10.1002/ep.11613
Increasing demand for renewable feedstock-based bio-
fuels is driving the interest and rapid development of proc-
esses to produce fuels and chemicals from biomass-gener-
ated syngas. Biomass is gasified to produce syngas that can
be converted via thermochemical routes to fuels and chemi-
cals such as alcohols, olefins, and fuel grade hydrocarbons.
An alternate route to produce liquid products from syngas
is through gas fermentation, a hybrid thermochemical/
biochemical process. Biomass gasification, thermochemical
syngas
conversion
routes,
and
gas
fermentation
are
described including a comparison for converting biomass to
ethanol via the current thermochemical route versus gas
fermentation.
Ó 2012 American Institute of Chemical Engineers
Environ Prog, 31: 219–224, 2012
Keywords: biomass, gasification, biomass to liquids, ther-
mochemical biofuels, gas fermentation
INTRODUCTION
As the worldwide population and the consumption of fos-
sil fuel-derived fuels and chemicals increase, there is the ever
growing need to increase the use of renewable feedstocks as
an energy source. It is estimated that the United States has
the potential to produce more than 1 billion dry tons of bio-
mass that would not affect current food production and
could produce enough biofuels to meet more than one-third
of the transportation fuel demand [1]. Biomass is most com-
monly used for heat/power generation through direct com-
bustion or by gasification to produce synthesis gas (syngas)
for combined heat and power (CHP) applications. Alternate
uses for biomass-derived syngas include thermochemical
conversions to fuels or chemicals such as methanol, ethanol,
hydrogen, diesel, naphtha, or gasoline.
Gasification is an approach to convert all the carbon con-
tained in biomass (cellulose, hemicellulose, and lignin) into a
medium energy content gas consisting of H
2
and CO, syngas.
A main benefit to thermochemical routes compared to cur-
rent biochemical biomass technologies is that only the cellu-
lose content of biomass can be converted to product in cur-
rent biochemical routes. Gas fermentation is a hybrid ther-
mochemical/biochemical
process
where
syngas
can
be
generated through biomass gasification which is then con-
verted to fuels and chemicals by a biological microbial reac-
tion. Initial commercial projections indicate that such gas fer-
mentation processes can convert syngas to liquid products
with high carbon conversion efficiency, high yields, and at
lower costs than alternative thermochemical biomass syngas
conversion routes.
Currently, there is an increasing effort to convert biomass
to fuels and develop second generation biofuel technologies
that can more efficiently convert the energy content of bio-
mass to liquid products. This article describes biomass gasifi-
cation and current thermochemical processes to convert syn-
gas to fuels and chemicals. These biomass to liquids routes
generally have higher costs compared with conventional coal
or petcoke gasification processes due to the higher feedstock
handling costs, potential higher feedstock costs, limitations
on scale, and maturity of biomass gasification, including the
difficulty in gasifying biomass when compared with coal.
These thermochemical routes are then compared to an alter-
native syngas conversion route, gas fermentation.
BIOMASS GASIFICATION
Gasification of biomass occurs at high temperature, 600–
1,000
8C or higher, in the presence of an oxidizing agent such as
air, steam, or oxygen. The solid carbon content in the biomass
is oxidized over several steps: dehydration, pyrolysis, combus-
tion, and reduction [2] producing a gaseous mixture called syn-
thesis gas (or syngas) by a series of reactions, such as combus-
tion, partial oxidation, methanation, water-gas shift (WGS),
Boudouard reaction, among others [2–4]. Syngas contains
mostly carbon monoxide and hydrogen with varying amounts
of carbon dioxide, methane, water vapor, and low levels of
impurities such as H
2
S, NH
3
, COS, HCl, or HCN. The composi-
tion, impurity level, and amount of syngas produced are deter-
mined by the flow rate, type, and properties of the biomass and
the gasifier operating conditions including oxidizing agent
equivalence ratio, gasification pressure, and gasifier tempera-
ture profile. Most biomass feedstocks have the following prop-
erties: carbon content ranging from 40 to 60 wt % on a dry basis
[3, 5], moisture content up to 50 wt % [6], and higher heating
values ranging from 12 to 20 MJ/kg [3]. Other key biomass
properties include percentage of ash content and volatile mat-
ter.
The delivery and pretreatment of biomass includes har-
vesting,
collection,
transport,
preprocessing/preparation,
feedstock handling, and drying. The biomass generally has to
go through size reduction (chipping, grinding, chopping,
etc.) so it can be fed to the gasifier where the gasifier config-
uration may require more intensive feedstock preparation
such as torrefaction (or pyrolysis) so that the material can go
through densification to pellets or granules. The overall
Ó 2012 American Institute of Chemical Engineers
Environmental Progress & Sustainable Energy (Vol.31, No.2) DOI 10.1002/ep
July 2012
219

upstream process incurs a significant capital expense and
adds to the overall cost of the biomass feedstock which can
range from $16 to 70 per dry ton [3, 6].
The main classes of gasifiers are fixed or moving bed, flu-
idized bed, and entrained flow and are described in numer-
ous gasifier resources with major western technology pro-
viders illustrated by Basu [5, p. 168]. Fluidized bed gasifiers
are good candidates for biomass gasification due to their
scalability, mixing and temperature uniformity, and high car-
bon conversion and are the most commonly used gasifiers
for biomass currently [2]. Entrained-flow gasifiers are the
most common type of large-scale gasification unit for coal,
petcoke, and refinery residues but require that the feedstock
be pulverized to a small size which is difficult and costly for
most biomass sources. Entrained-flow gasifiers provide a
promising option due to several advantages, such as low tar
production and high carbon conversion, yet they are furthest
from large-scale commercialization for biomass due to the
difficulty of the required feedstock preparation [7].
Biomass syngas generally contains tars, particulates, and
contaminants and requires a certain level of cleanup based
on the downstream use of the syngas. For instance, particu-
lates and tars can clog downstream unit operations such as
gas turbines, reciprocating engines, and fuel cells, whereas
sulfur compounds can act as Fischer–Tropsch or tar-reform-
ing catalyst poisons thus necessitating a very clean syngas [8].
Gas cleanup can include tar reforming, particulate and con-
taminant removal, and acid gas (CO
2
and H
2
S) removal and
is generally performed at low temperatures due to the techni-
cal challenges of hot gas cleanup [9, 10]. Particulates are nor-
mally removed from cold or hot syngas by cyclones and fil-
ters, whereas some tars and condensables can be removed
by a quench of the hot syngas. A common tar removal
method is through catalytic reforming at temperatures up to
1200
8C producing additional syngas from cracking the tars. A
key part to syngas cleanup is the removal of H
2
S and COS
by physical sorbents such as Rectisol
Ò
or Selexol
Ò
which can
be designed for any CO
2
removal rate, up to 100% [11]. Acid
gas removal (AGR) systems contribute a significant portion of
the capital and operating costs of the gasification system due
to the high pressure and low temperature operation.
CONVENTIONAL UTILIZATION OF BIOMASS SYNGAS: HEAT, POWER, AND/OR
LIQUIDS
Biomass syngas has a number of uses including CHP
applications and conversion to liquids/chemicals through
synthesis reactions. The end-use of the syngas will dictate
the desired syngas composition and the corresponding syn-
gas conditioning required which is performed by tar reform-
ing, syngas reforming, and/or a WGS reaction. A variety of
biomass feedstocks are generally used for syngas production
including
bagasse/sugar
cane,
corn
stover/corn,
straw,
switchgrass, miscanthus, and wood with wood being the
most commonly used biomass fuel currently.
Biomass Syngas to Heat and/or Power
Biomass syngas can be used to generate heat in the form
of steam and/or also generate electricity, referred to as bio-
power. There are currently hundreds of biopower facilities
worldwide with biomass contributing 16% of the total renew-
able electricity generation in the United States [12]. Biomass
fuels can be burned in conventional combustion systems to
generate biopower, but these systems can suffer from low
thermal efficiencies [10]. CHP, or cogeneration, systems pro-
duce both heat and electricity with energy efficiencies of 45–
65% [12]. The syngas produced from biomass gasification
requires removal of tars and particulates to eliminate the
potential for clogging the moving parts of gas and steam tur-
bines. Contaminants, such as ammonia, formic acid, and
alkali vapors can damage downstream equipment and need
to be condensed and removed during gas cleanup [13].
Biomass Syngas to Liquids
Currently biomass is gasified mostly for CHP applications
but there is extensive interest and research in biomass to liq-
uid platforms. Converting syngas to liquids through synthesis
reactions requires a very clean gas with a specific gas com-
position thus requiring gas cleanup and conditioning to
remove particulates, tars, sulfur compounds, ammonia, etc.
and to adjust the syngas composition. Common chemicals
produced from syngas include methanol, ammonia, and Fi-
scher–Tropsch liquids.
Methanol is an important feedstock for producing chemi-
cals such as acetic acid and gasoline. The synthesis of metha-
nol is an exothermic gas-phase catalytic reaction operated at
high pressure, up to 300 bar, and high temperature, up to
200–400
8C [5]. The CO and H
2
present in syngas are reacted
to form methanol; the most common route to generate syn-
gas for methanol synthesis is through steam reforming of
methane. The syngas produced from biomass gasification
generally undergoes a WGS reaction to convert CO to H
2
to
produce the desired H
2
/CO ratio of
>2/1 [3]. This gas-phase
synthesis suffers from low single-pass conversion requiring
the unreacted syngas to be recycled back to the reactor add-
ing additional costs associated with the recompression and
heating of this gas stream.
The synthesis of ammonia from syngas is another high
pressure, up to 350 bar, gas-phase catalytic reaction that con-
verts pure hydrogen and nitrogen to ammonia in the pres-
ence of iron catalysts [5]. A pure hydrogen stream can be
generated from clean syngas by converting CO to H
2
through
a WGS reaction where the remaining CO and other inert spe-
cies are removed by a pressure-swing adsorption unit [3].
The pure H
2
stream is then mixed with nitrogen to be used
in conventional ammonia synthesis, which also has low sin-
gle-pass conversion requiring recycle of the unconverted gas.
Fischer–Tropsch synthesis is a mature technology where
syngas is converted to liquid hydrocarbons over an iron cata-
lyst at high pressure, 20–300 bar, and at a temperature range
of 200–350
8C [5]. A variety of long-chained hydrocarbons can
be produced by FT synthesis where a syngas feed composi-
tion with a H
2
/CO ratio of 0.6–2.0 can produce synthetic
fuels, such as naphtha, diesel, and gasoline, in the C
5
to C
10
range [5]. The reaction conversion depends on the type of re-
actor used with as high as 80% single-pass conversion for a
slurry-phase reactor or
<40% conversion with traditional
fixed-bed reactors [11]. Numerous studies on biomass to
liquids have been performed [3, 11, 14–17] including com-
bined generation of power [18] or synthetic natural gas [19].
Most
biomass
gasification
plants
are
conceptually
designed based on a 2,000 dry ton per day feedstock
throughput. There is an upper limit on how much biomass
can be harvested and delivered to a given plant for the pro-
cess to be economical. The suggested practical upper limit
for collection of switchgrass is 4,000 dry tons per day for a
biomass to liquids, diesel and naphtha plant which would
produce 5,000 barrel/day compared to 50,000 bpd for con-
ventional coal to liquid plants [14]. A method to increase the
scale of biomass gasification applications is to co-fire biomass
with coal or petcoke with biomass consisting of up to 20%
by weight of the feed.
Biomass Syngas to Ethanol
Increasing attention is being given to alternate methods
for producing ethanol to supplant land/food-derived ethanol
that is produced by sugar fermentation, referred to as bio-
chemical ethanol. This section describes two routes to pro-
duce ethanol from syngas (1) a catalytic thermochemical
220
July 2012
Environmental Progress & Sustainable Energy (Vol.31, No.2) DOI 10.1002/ep
route using syngas derived from biomass gasification and (2)
a combined thermochemical/biochemical route, which uses a
biocatalyst to convert biomass syngas to ethanol.
Thermochemical Route to Ethanol
The thermochemical route to ethanol is similar to the pro-
cess for producing methanol described in the previous sec-
tion but uses a different catalyst. There is significant work
currently being performed to improve the performance of
gas-phase catalysts for the production of ethanol from syn-
gas; this section compiles information provided in recent
NREL reports [6, 8, 20], which describe the current and future
status of the thermochemical route to ethanol from biomass
syngas.
The reactions that produce ethanol from syngas occur at
high pressure, as high as 200 bar, with reactions above 300
8C
producing oxygenate and hydrocarbon products. The reac-
tions are exothermic providing the heat of reaction where
steam can be generated by removal of any excess heat. The
conventional catalyst is a sulfide-type mixed alcohol catalyst,
where the optimal catalyst is Rh-based with some catalysts
costing more than $2000/lb. These catalysts are characterized
by poor selectivity to ethanol due to the faster reaction rate
for MeOH and low single-pass conversion of 40–50% requir-
ing syngas recycle. The catalysts used experience a tradeoff
between catalyst lifetime and selectivity to ethanol with some
catalysts sensitive to CO
2
levels
>7%. Therefore, depending
on the catalyst used, extensive gas cleaning may be required
to remove most or all the CO
2
from the syngas (or gas
recycled from the synthesis reactor).
The reactor effluent consists of mixed alcohols (including
higher alcohols such as propanol and butanol), gaseous
byproducts such as CH
4
and CO
2
, and unconverted syngas.
This hot stream is cooled to condense out the alcohols for
separation and the unreacted syngas can be used as fuel,
recycled back to any gas reformers, or recycled back to the
alcohol synthesis reactor (after any required cleaning or com-
pression). Crude alcohols are depressurized and then dehy-
drated using a molecular sieve system where the MeOH/
water stream can be used to generate steam for gasification
or distillation. The mixed alcohol stream is sent to a distilla-
tion system to separate the methanol and ethanol from the
higher alcohols. The methanol can be used to regenerate the
mol sieve, as fuel for any reformers, or recycled to the syn-
thesis reactor to improve ethanol yields. The ethanol can be
produced at american society for testing materials (ASTM)
fuel standards,
>99.5 wt %, from this distillation configura-
tion.
Thermochemical/Biochemical Route to Ethanol Via
Gas Fermentation
There are currently three companies pursuing commerci-
alization of syngas fermentation to produce fuel/chemicals:
Coskata, IneosBio, and LanzaTech; the LanzaTech process is
described here with many similarities to the technologies of
other companies. The gas fermentation process consists of
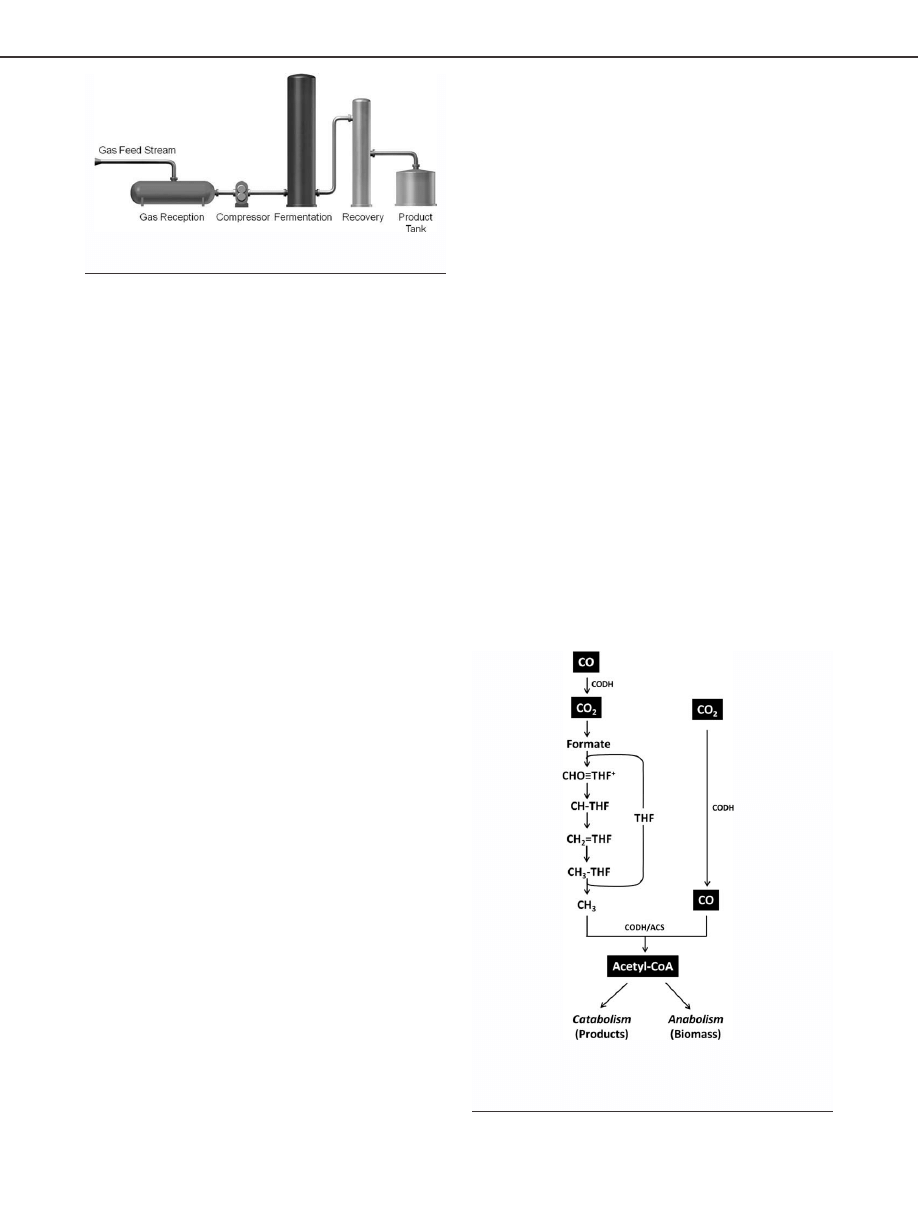
three main sections as shown in Figure 1: gas conditioning,
anaerobic gas fermentation, and product recovery.
In the gas conditioning section, the biomass gasification
syngas is cooled to fermentation temperature, using heat re-
covery to generate steam for process heat or to generate
export power. The LanzaTech microbe (biocatalyst) is toler-
ant to a number of impurities, including sulfur compounds,
so extensive cleanup or AGR is not required. Vega et al. have
demonstrated that anaerobic bacteria can convert CO to
products in the presence of significant amounts of H
2
S or
COS [21]. Following gas cleanup, there may be additional gas
compression and deoxygenation required depending on
the operating conditions of the gasifier and properties of
the syngas.
The gas fermentation process is a biological process that
consumes CO and H
2
to produce chemicals such as ethanol,
2,3-butanediol [22], and others. The biocatalyst is an aceto-
genic organism in the Clostridium family of anaerobic bacte-
ria and is classified as World Health Organization (WHO) risk
group 1, similar to baker’s yeast. The microbes use the Wood
Ljungdahl pathway to produce biomass and ethanol; a high
level scheme of this pathway is shown in Figure 2 with
details on the microbial pathway that produces chemicals
from CO and H
2
described by Ko
¨pke et al. [23] and available
online at Advanced Biofuels USA [24]. Other products such
as acetate, acetone, isopropanol, and butanol can be pro-
duced by alternate biosynthetic pathways present in various
microbe strains.
The LanzaTech process has been shown to be flexible to
the H
2
/CO ratio in the input gas, such that it can convert a
range of gases such as CO-rich industrial off-gases (such as
steel mill off-gas [25]) and H
2
-rich biomass syngas. The
Figure 1. Syngas fermentation process overview.
Figure 2. Simplified scheme of Wood Ljungdahl pathway.
CODH, carbon monoxide dehydrogenase; ACS, acetyl-CoA
synthase complex; and THF, tetrahydrofolate.
Environmental Progress & Sustainable Energy (Vol.31, No.2) DOI 10.1002/ep
July 2012
221

microbes convert CO
1 H
2
to ethanol by the following
reactions:
6CO
þ 3H
2
O
! C
2
H
5
OH
þ 4CO
2
6H
2
þ 2CO
2
! C
2
H
5
OH
þ 3H
2
O
The CO acts as the only carbon source, H
2
acts as a
source of energy. If there is a lack of H
2
, the microbes can
perform an internal WGS reaction to convert CO to H
2
, yet
this reduces the amount of CO that can be converted to
products. LanzaTech has developed a strain of Clostridia bac-
teria that has shown improved efficiency for producing etha-
nol over its parent strain [26].
After gas conditioning, the gas is fed to the bioreactor
along with fresh media that consists mostly of water with a
specific mixture of nutrients, salts, and metals. The main
challenge associated with the bioreactor system is the issue
of the low solubility of CO and H
2
in water. To overcome
this, LanzaTech has developed a unique bioreactor design
using novel gas introduction methods to maximize the gas to
liquid mass transfer and enhance the gas dissolution. Key fer-
mentation process parameters include nutrient levels, pH,
temperature, and pressure with common operating condi-
tions in the range of pH 4–6, 35–42
8C, and 0–5 bar.
The vent gas from the bioreactors contains all inert com-
ponents such as N
2
, CH
4
, and CO
2
that were present in the
feed stream, as well as unconverted carbon monoxide and
hydrogen, plus additional carbon dioxide generated by the
fermentation. The gas is scrubbed to remove any additional
ethanol and water and then cleaned before venting to
remove any harmful contaminants, such as H
2
S, to meet
emission standards. If the vent gas contains sufficient heating
value, it can be used as a fuel gas to supplement the process
heat balance. Carbon capture can also be used at this stage
to mitigate carbon emissions.
The fermentation broth is sent to the product recovery sec-
tion to concentrate the ethanol to fuel grade at
>99.5 wt %.
The microbes are separated from the liquid mixture and sent
to an anaerobic digestion system, generating biogas, which
can also be used as a fuel gas. LanzaTech is also working
with a partner to develop a novel system for recovery of 2,3-
butanediol from the fermentation broth. Results indicate that it
is possible to recover both the ethanol and the 2,3-butanediol,
with both products meeting purification specification, at a util-
ity consumption less than recovery of ethanol alone by distil-
lation. Although additional capital is required, the recovery of
the higher value 2,3-butanediol leads to a payback of less
than 1 yr for the incremental capital.
COMPARISON OF ROUTES
The previous sections have briefly described the alterna-
tive routes for converting biomass-derived syngas to heat,
power, fuel, or chemicals. Each process consists of a biomass
gasification train to generate syngas, but the choice and oper-
ation of the gasifier affect the syngas composition and prop-
erties. Generating heat or power does not require a specific
syngas composition but higher thermal value gas will result
in increased net energy export from the process. Chemical
synthesis of fuels or chemicals requires a specific H
2
/CO ratio
in the gas; if this desired composition cannot be achieved in
the gasifier itself, gas conditioning by reforming or WGS is
used to adjust the syngas composition. Most chemical synthe-
sis routes are gas-phase, high-temperature, and high-pressure
reactions requiring expensive catalysts that suffer from low
single-pass conversion. Therefore, the unreacted syngas
requires recompression and heating for recycle back to the
reactor adding additional costs to the synthesis step. Each of
these cases also requires extensive gas cleanup to remove
H
2
S, COS, and/or CO
2
.
Gas fermentation of syngas uses CO as the main source of
carbon to produce fuel or chemicals with high conversion of
CO in the fermentation process. The LanzaTech gas fermen-
tation process can operate with a wide range of H
2
/CO gas
compositions eliminating the need for any syngas condition-
ing. A gasifier configuration that maximizes CO production is
optimal,
whereas
the
thermochemical
routes
generally
require a high H
2
content and use syngas conditioning to
produce a specific H
2
/CO ratio. The gas fermentation process
can also tolerate a high level of impurities where only the
vent gas from the fermentation requires scrubbing, which is
less energy intensive and less extensive than the AGR
required for the other cases described. The LanzaTech pro-
cess can convert nearly all the carbon in the CO to fuels or
chemicals compared with the low single-pass conversion
achieved in the chemical syntheses routes. In comparison
with the thermochemical route to ethanol described earlier,
syngas fermentation produces ethanol at high selectivity
resulting in lower separation costs compared with the prod-
uct stream of mixed alcohols generated catalytically. The gas
fermentation process is operated at low temperature and low
pressure so the capital and operating costs associated with
fermentation step are considerably lower than those associ-
ated with traditional chemical synthesis routes.
The two alternatives discussed for producing ethanol from
biomass are presented as case-studies in Table 1 to compare
the various routes in terms of fuel yield, carbon capture,
energy efficiency, etc. An NREL report from Dutta et al. [6]
provides specific operating data for the thermochemical
route, Case 1, including biomass properties, gasifier opera-
tion, syngas composition, syngas yield, plantwide energy effi-
ciency on a lower heating value (LHV) basis, and total car-
bon emissions. The feedstock, gasifier front-end, and syngas
properties for the thermochemical route, Case 1, are repli-
cated for the gas fermentation study, Case 2. The results for
the gas fermentation case are based on an internal LanzaTech
Table 1. Comparison of routes to produce ethanol from biomass syngas: (1) thermochemical route [6] and (2) biochemical/
thermochemical route (gas fermentation) via LanzaTech process.
Case 1: Thermochemical
to ethanol
Case 2: Gas fermentation
to ethanol
Feedstock
Woody biomass
Woody biomass
Feedstock flow rate (dry US ton per day)
2,205
2,205
Fuel production (MM gal/yr)
64.7
82.1
Carbon conversion to fuel efficiency (%)
40.7
51.6
Fuel yield (gal/dry US ton)
83.8
117.6
Overall plant energy efficiency,
LHV% (energy in feedstock
converted to final product)
45
57
CO
2
emissions (lb CO
2
/gal)
30.2
24.8
222
July 2012
Environmental Progress & Sustainable Energy (Vol.31, No.2) DOI 10.1002/ep

process model; the model assumptions are not specifically
detailed here, but the potential improvements in carbon cap-
ture and energy efficiency have been described throughout
the manuscript and are enumerated in Table 1.
The report by Dutta et al. assumes high conversion of the
carbon in the biomass feedstock to syngas by a combination
of gasification and tar reforming; a dry syngas yield of 2,050
Nm
3
dry syngas/dry ton of biomass feed is reported [6]. This
value is higher than many current biomass gasifier syngas
yields, which results in a higher fuel yield per ton of biomass
here than what is reported commercially. Thus, the data in
Table 1 is shown as a comparative example of the two routes
and is not a specific claim on the specific fuel yield or overall
plant energy efficiency of either route.
Case 1, thermochemical route to ethanol production from
mixed alcohols [6]: woody biomass feedstock is gasified in
a steam-blown indirectly heated gasifier based on run data
from the Battelle Columbus Laboratory gasifier. Following
the gasifier is a tar reformer and a physical solvent sulfur
removal system. The outlet of the tar reformer has a dry
composition containing 39% CO and a H
2
/CO ratio of
1.25; the design for the catalytic reactor inlet is for a H
2
/
CO ratio of 1.5 after the unreacted reactor off-gas is
recycled and combined with the tar reformer outlet.
Case 2, gas fermentation for ethanol production: same
operating data as Case 1 in terms of biomass feed flow
rate, gasifier and tar reformer operation, and syngas prop-
erties. There is no AGR and the fermentation input gas is
assumed to be the same as the gas produced by the tar re-
former, 39% CO and a H
2
/CO ratio of 1.25.
The data in Table 1 demonstrates the potential advantages
of gas fermentation over the thermochemical conversion of
syngas to fuel or chemicals. In this example, nearly all the
carbon in CO is selectively converted to ethanol allowing for
higher fuel yield and energy efficiency for the fermentation
process. Carbon dioxide is produced in the chemical synthe-
sis route and as a byproduct of gas fermentation; yet the
thermochemical route uses gas conditioning, such as WGS,
to further produce H
2
generating additional CO
2
. Overall, the
combined
biochemical/thermochemical
route
results
in
higher fuel yield per biomass feed, carbon conversion to
fuel, energy efficiency, and lower carbon emissions com-
pared with the thermochemical route to ethanol.
CONCLUSIONS
This article has presented an overview of biomass gasifi-
cation and thermochemical routes for converting syngas to
heat/power, fuels, and chemicals. Another route, a hybrid
thermochemical/biochemical process, was described as an al-
ternative way to convert biomass-derived syngas to valuable
liquid products. This low-temperature, low-pressure gas fer-
mentation route benefits from tolerance to several impurities
and the ability to use a flexible H
2
/CO ratio feed gas elimi-
nating the need for extensive gas cleanup or conditioning.
The microbes used in the gas fermentation process can con-
vert nearly all the carbon in CO to fuels or chemicals at high
selectivity compared with the conventional chemical synthe-
ses routes resulting in higher overall fuel and thermal effi-
ciency. Because of higher carbon to fuel conversion effi-
ciency, the gas fermentation route produces less carbon
emissions making it an overall ‘‘greener’’ process with lower
carbon capture requirements.
One current limitation to all routes described is the com-
mercialization of large-scale biomass gasifiers, which are cur-
rently restricted to
1,000–2,000 dry ton per day feed based
on feedstock collection limitations and biomass gasifier de-
velopment. The economics of the biomass gasification proj-
ects described in this article will improve greatly at larger
scales. As discussed earlier, entrained-flow gasifiers are pref-
erential for large-scale coal and petcoke gasification due to
the high carbon conversion and low tar formation. This gasif-
ier configuration would be ideal for producing syngas for the
gas fermentation route because oxygen blown entrained-flow
gasifiers generally produce syngas with a high CO content,
40–60%. However, because of the current limitations and cost
implications associated with the biomass feedstock prepara-
tion required for entrained-flow gasifiers, these gasifier types
are furthest from large-scale commercialization for biomass.
In the ongoing effort to produce renewable fuels and chemi-
cals, commercial biomass gasification development will con-
tinue along with improving the efficiency of thermochemical
conversion routes of syngas and advancing the hybrid bio-
chemical/thermochemical gas fermentation route.
LITERATURE CITED
1. Perlack, R.D., Wright, L.L., Turhollow, A.F., Graham,
R.L., Stokes, B.J., & Erbach, D.C. (2005). Biomass as
feedstock for bioenergy and bioproducts industry: The
technical feasibility of a billion-ton annual supply, Tech-
nical Report, DOE/GO-102995–2135; ORNL/TM-2005/66,
Oak Ridge, TN: US Department of Energy; US Depart-
ment of Agriculture.
2. Swanson, R.M., Satrio, J.A., Brown, R.C., Platon, A., &
Hsu, D.D. (2010). Techno-economic analysis of biofuels
production based on gasification, Technical Report,
NREL/TP-6A20–46587, Golden, CO: National Renewable
Energy Laboratory.
3. Ciferno, J.P., & Marano, J.J. (2002). Benchmarking bio-
mass gasification technologies for fuels, chemicals and
hydrogen production, Washington, DC: US Department
of Energy, NETL Gasification Cost and Performance
Study. Available at: www.netl.doe.gov, accessed July
2011.
4. Kumar, A., Jones, D.D., & Hanna, M.A. (2009). Thermo-
chemical biomass gasification: A review of the current
status of the technology, Energies, 2, 556–581.
5. Basu, P. (2010). Biomass gasification and pyrolysis: Prac-
tical design and theory, New York, NY: Elsevier Inc.
6. Dutta, A., Talmadge, M., Hensley, J., Worley, M., Dudg-
eon, D., Barton, D., Groenendijk, P., Ferrari, D., Stears,
B., Searcy, E.M., Wright, C.T., & Hess, J.R. (2011). Pro-
cess design and economics for conversion of lignocellu-
losic biomass to ethanol, Technical Report, NREL/TP-
5100–51400, Golden, CO: National Renewable Energy
Laboratory.
7. van der Drift, H., Boerrigter, H., Coda, B., Cieplik, M.K.,
& Hemmes, K. (2004). Entrained flow gasification of bio-
mass: Ash behavior, feeding issues, and system analyses,
ECN-C-04–039, Petten, the Netherlands: Energy Research
Center of the Netherlands.
8. Phillips, S., Aden, A., Jechura, J., & Dayton, D. (2007).
Thermochemical ethanol via indirect gasification and
mixed alcohol synthesis of lignocellulosic biomass, Tech-
nical Report, NREL/TP-510–41168, Golden, CO: National
Renewable Energy Laboratory.
9. Energy and Environmental Analysis, Inc., & Eastern
Research Group, Inc. (2007). Biomass combined heat
and power catalog of technologies. Prepared for U.S.
Environmental Protection Agency, Combined Heat and
Power Partnership. Washington, DC.
10. Easterly, J., & Kasarabada, A. (2008). Advanced bio-
power technology assessment. Prepared for the Massa-
chusetts Division of Energy Resources & Massachusetts
Department of Conservation & Recreation. Overland
Park, KS: Black & Veatch.
11. Kreutz, T.G., Larson, E.D., Lui, G., & Williams, R.H.
(2008). Fischer–Tropsch fuels from coal and biomass. In
Environmental Progress & Sustainable Energy (Vol.31, No.2) DOI 10.1002/ep
July 2012
223

25th Annual International Pittsburgh Coal Conference,
Pittsburgh, PA.
12. U.S. Department of Energy, Biomass Program, Energy Ef-
ficiency and Renewable Energy. (2010). Biopower tech-
nical strategy workshop; summary report, Denver, CO.
13. Jin, H., Larson, E.D., & Celik, F.E. (2009). Performance
and cost analysis of future, commercially mature gasifica-
tion-based electric power generation from switchgrass,
Biofuels Bioproducts & Biorefining, 3, 142–173.
14. Tarka, T.J. (2009). Affordable, low-carbon diesel fuel
from domestic coal and biomass, Technical Report,
DOE/NETL-2009/1349, Washington DC: U.S. Department
of Energy, National Energy Technology Laboratory.
15. Boerrigter, H. (2006). Economy of biomass-to-liquids
(BTL) plants: An engineering assessment, ECN-C-06-019,
Petten, the Netherlands: Energy Research Center of the
Netherlands.
16. Wright, M.M., Duagaard, D.E., Satrio, J.A., & Brown, R.C.
(2010). Techno-economic analysis of biomass fast pyrol-
ysis to transportation fuels, Fuel, 89, S2–S10.
17. Swanson, R.M., Platon, A., Satrio, J.A., & Brown, R.C.
(2010). Techno-economic analysis of biomass-to-liquids
production based on gasification, Fuel, 89, S11–S19.
18. Timjensen, M.J.A., Faaij, A.P.C., Hamelinck, C.N., & Har-
develd, M.R.M. (2002). Exploration of the possibilities for
production of Fischer–Tropsch liquids and power via bio-
mass gasification, Biomass and Bioenergy, 23, 129–152.
19. Boerrigter, H., & Zwart, R.W.R. (2004). High efficiency
co-production of Fischer–Tropsch (FT) transportation
fuels and substitute natural gas (SNG) from biomass,
ECN-C-04-001, Petten, the Netherlands: Energy Research
Center of the Netherlands.
20. Ruth, M. (2005). Gridley ethanol demonstration project
utilizing biomass gasification technology: Pilot plant gas-
ifier and syngas conversion testing, Technical Report,
NREL/SR-510–37581, Golden, CO: National Renewable
Energy Laboratory.
21. Vega, J.L., Klasson, K.T., Kimmel, D.E., Clausen, E.C., &
Gaddy, J.L. (1990). Sulfur gas tolerance and toxicity of
CO-utilizing and methanogenic bacteria, Applied Bio-
chemistry & Biotechnology, 24/25, 329–340.
22. Ko
¨pke, M., Mihalcea, C., Liew, F., Tizard, J.H., Ali, M.S.,
Conolly, J.J., Al-Sinawi, B., & Simpson, S.D. (2011). 2,3-
Butanediol production by acetogenic bacteria, an alter-
native route to chemical synthesis, using industrial waste
gas, Applied & Environmental Microbiology, 77, 5467–
5475.
23. Ko
¨pke, M., Mihalcea, C., Bromley, J.C., & Simpson, S.D.
(2011). Fermentative production of ethanol from carbon
monoxide, Current Opinion in Biotechnology, 22, 320–
325.
24. Advanced Biofuels USA. (2011). Syngas fermentation:
The third pathway for cellulosic ethanol. Available at:
advancedbiofuelsusa.info, accessed July 2011.
25. Simpson, S.D., Collet, C., Forster, R.L.S., Cockrem,
M.C.M., Oakley, S.D., & Ko
¨pke, M. (2010). Carbon cap-
ture in fermentation, US Patent 0,323,417 A1.
26. Simpson, S.D., Forster, R.L.S., Tran, P., Rowe, M.J., &
Warner, I.L. (2010). Novel bacteria and methods of use
thereof, US Patent 0,311,104 A1.
224
July 2012
Environmental Progress & Sustainable Energy (Vol.31, No.2) DOI 10.1002/ep
Wyszukiwarka
Podobne podstrony:
Aspects of the development of casting and froging techniques from the copper age of Eastern Central
The Comparisons of Charles Manson to Transcendental Philosoph
Economics of Producing Fuel Pellets From Biomass
Production of Energy from Biomass Residues 020bm 496 1993
Gade, Lisa, Lynge, Rindel Roman Theatre Acoustics; Comparison of acoustic measurement and simulatio
Overview of bacterial expression systems for heterologous protein production from molecular and bioc
Energy and CO2 analysis of poplar and maize crops for biomass production in Italy Włochy 2016
Bridgewater Renewable fuels and chemicals by thermal processing of biomass
Estimation of Dietary Pb and Cd Intake from Pb and Cd in blood and urine
4 Fuel and Lubrication System
Comparison of Human Language and Animal Communication
Physical and chemical character Nieznany
Comparison of the Russians and Bosnians
Milk and Milk Products
[32] Synergism among flavonoids in inhibiting platelet aggregation and H2O2 production
więcej podobnych podstron