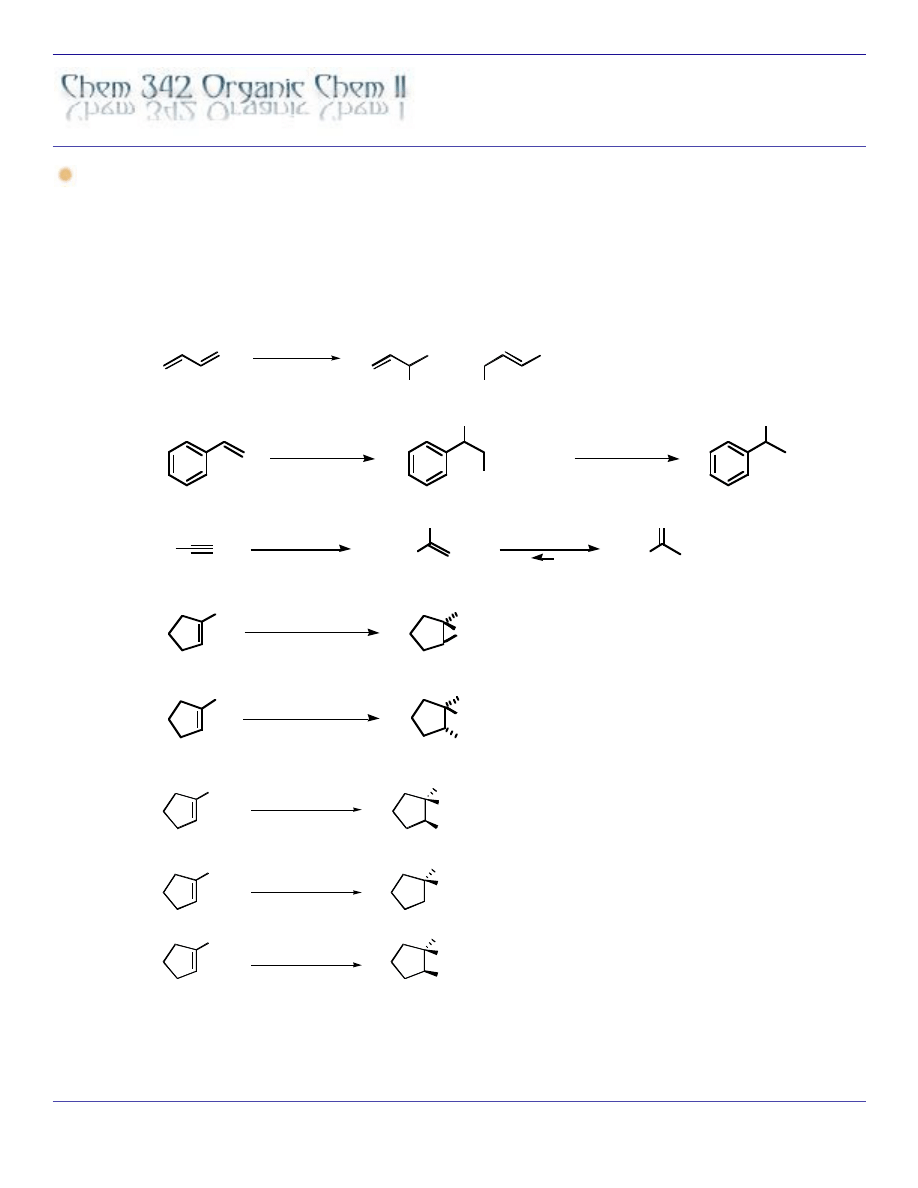
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
REACTIONS
For Final Exam
Reactions for Final Exam
Although you don’t need to know all of the mechanisms for these reactions, knowing them
makes it easier to understand the reactions and to figure out a reaction if you forget it. Consult the
lecture notes for more details on the mechanisms and issues of stereo- and regioselectivity.
ELECTROPHILIC ADDITION REACTIONS
HX
X
+
X
OCH
3
HgO
2
CCF
3
NaBH
4
OCH
3
Hg(O
2
CCF
3
)
2
CH
3
OH
R
R
OH
R
O
HgSO
4
H
3
O
+
enol
ketone
CH
3
CH
3
O
MCPBA
CH
3
CH
3
OH
Br
Br
2
H
2
O
CH
3
BH
3
H
2
O
2
, OH
-
CH
3
H
OH
CH
3
Hg(OAc)
2
CH
3
OH
H
2
O
NaBH
4
1)
2)
CH
3
OsO
4
CH
3
OH
NaHSO
3
OH
1)
2)
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
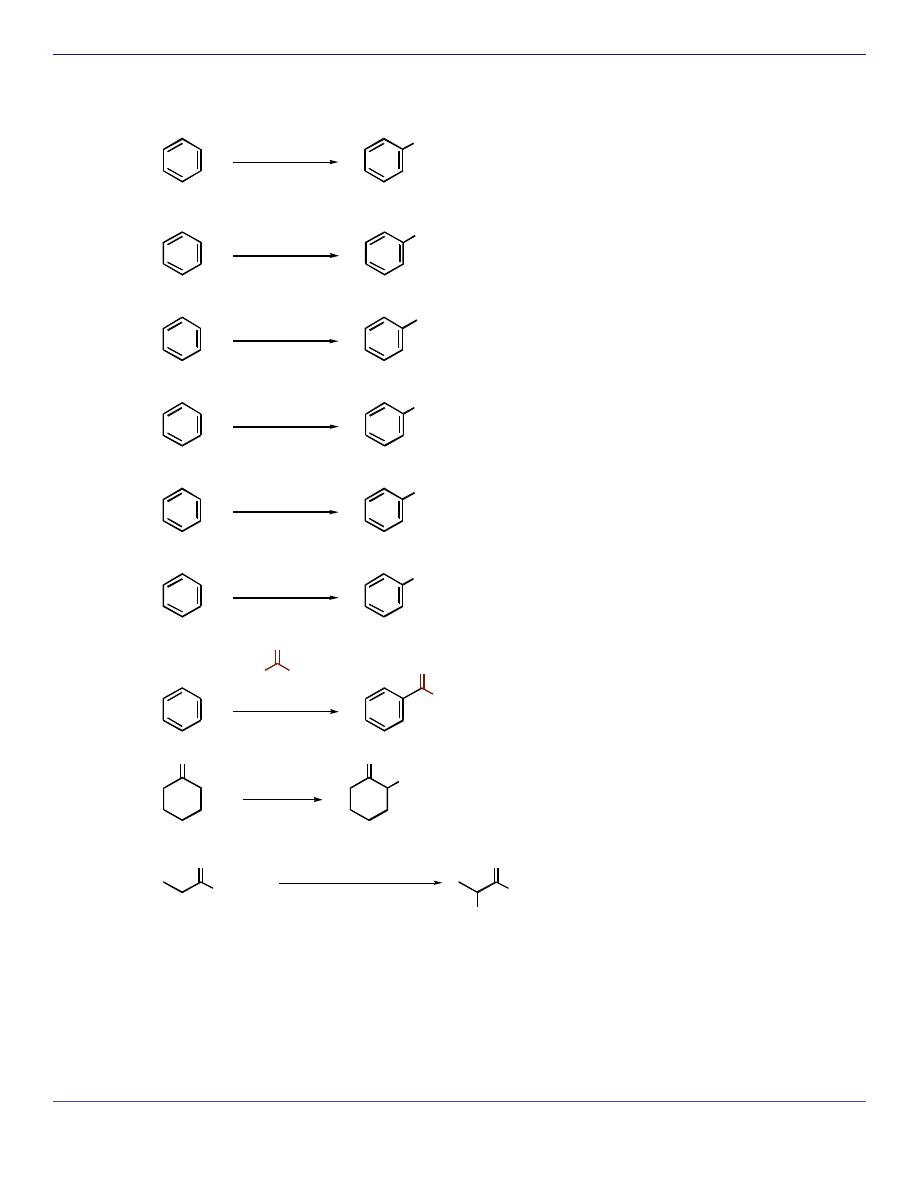
ELECTROPHILIC SUBSTITUTION REACTIONS
Br
2
FeBr
3
Br
Cl
2
FeCl
3
Cl
I
2
CuI
2
I
HNO
3
H
2
SO
4
NO
2
SO
3
H
2
SO
4
SO
3
H
R-Cl
AlCl
3
R
R
Cl
O
AlCl
3
R
O
O
Br
2
CH
3
CO
2
H
O
Br
Br
2
PBr
3
O
OH
O
OH
1)
2) H
2
O
Br
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
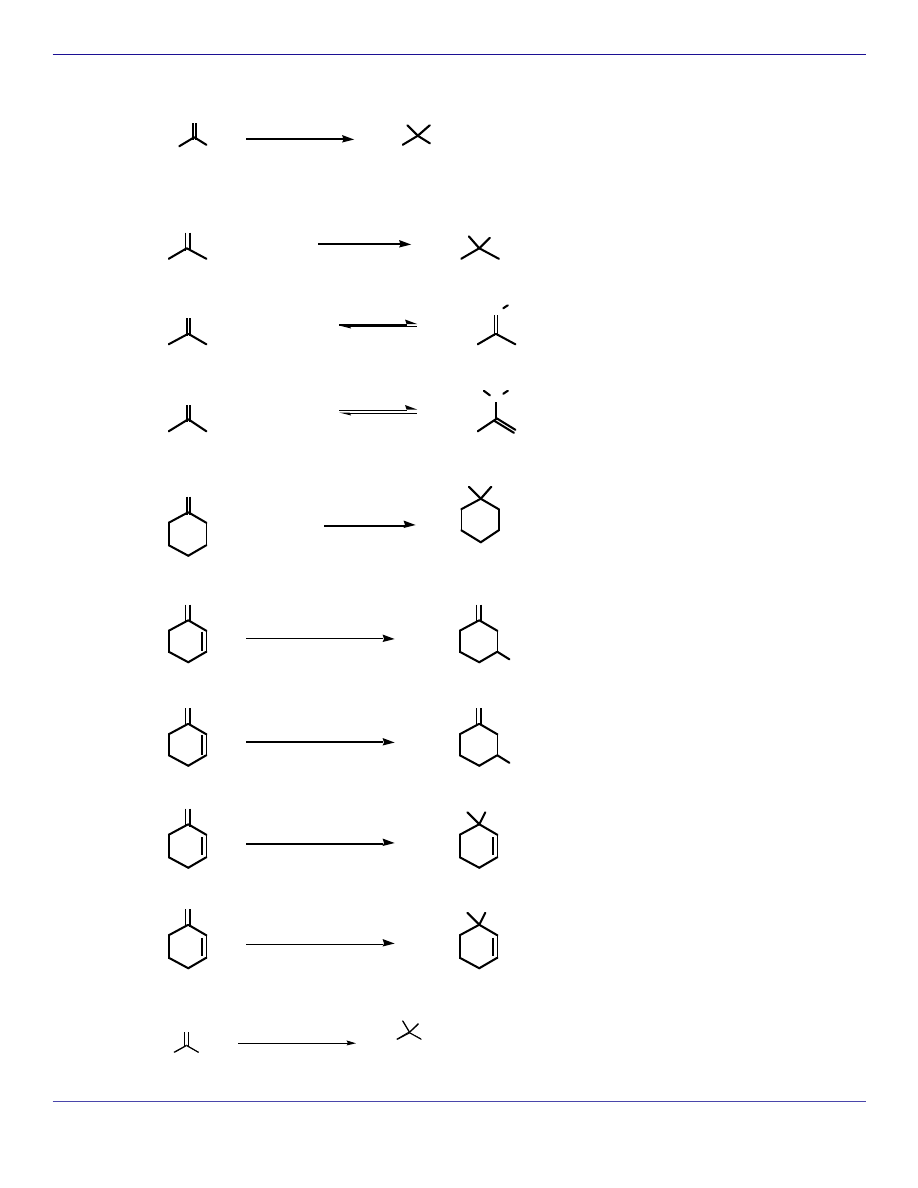
NUCLEOPHILIC ADDITION REACTIONS
Ph
H
O
Ph
H
H
O
CN
H
CN
cyanohydrin
KOH
O
HO
OH
H
2
O
hydrate
+
H
+
or
-
OH
catalyst
O
R-NH
2
H
+
N
R
H
2
O
+
+
imine
O
R
2
NH
H
+
N
R
R
H
2
O
+
+
enamine
O
ROH
H
+
RO
OR
+ 2
an acetal
1,4-addition
O
CH
3
NH
2
O
NHCH
3
1,4-addition
O
O
Ph
1) Ph
2
CuLi
2) H
3
O
+
1,2-addition
O
1) PhMgBr
2) H
3
O
+
HO
Ph
1,2-addition
O
1) PhLi
2) H
3
O
+
HO Ph
R
Cl
O
R
Ph
1) PhMgBr
2) H
3
O
+
HO Ph
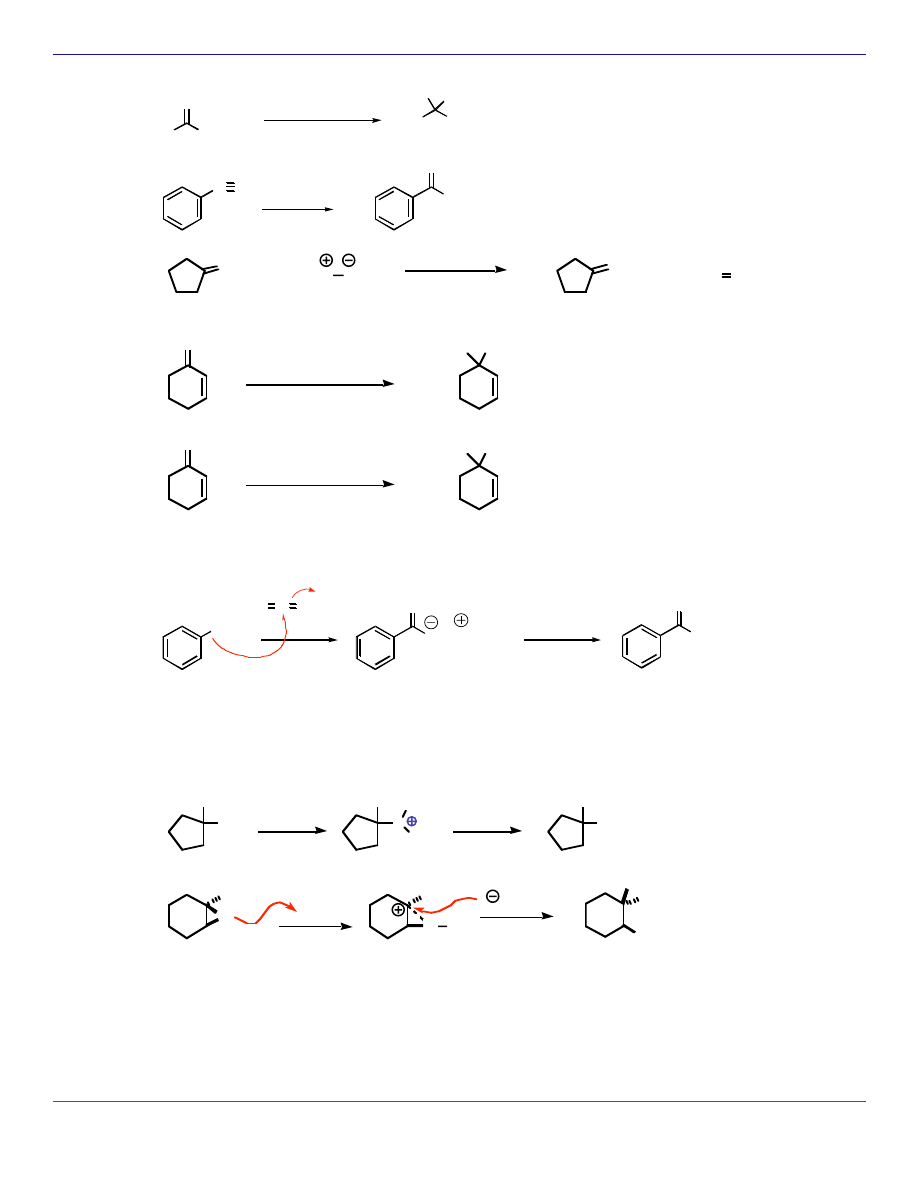
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
R
OCH
3
O
R
Ph
1) PhMgBr
2) H
3
O
+
HO Ph
C N
CH
3
1) CH
3
MgBr
2) H
2
O
O
O
+
Ph
3
P CH
2
phosphonium
ylide
CH
2
+
Ph
3
P O
1,2-addition
O
1) NaBH
4
2) H
3
O
+
HO
H
1,2-addition
O
1) LiAlH
4
2) H
3
O
+
HO H
The addition of hydrides to carbonyls is also considered reductions and more examples are listed
under reduction reactions.
O
O
MgBr
OH
O
H
3
O
+
MgBr
O C O
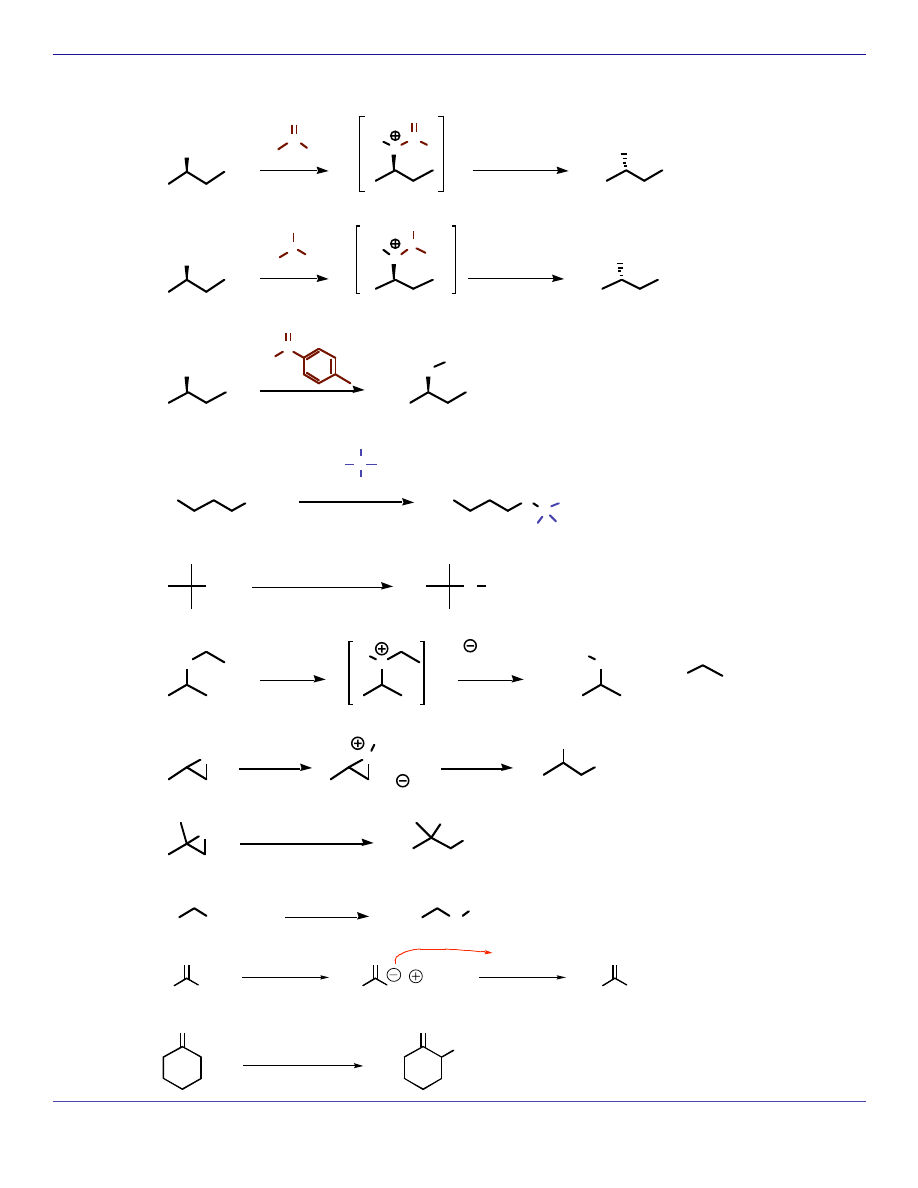
SUBSTITUTION REACTIONS (S
N
1)
CH
3
OH
HCl
CH
3
O
H
H
-H
2
O
Cl
CH
3
CH
3
O
HCl
CH
3
O H
Cl
OH
CH
3
Cl
(regioselective like S
N
1 but stereoselective like S
N
2) (also opens with water to make trans diol)
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
SUBSTITUTION REACTIONS (S
N
2)
S
O
Cl
Cl
OH
H
S
O
O
Cl
Cl
P
Br
Br
Br
OH
H
P
Br
O
Br
Br
S
O
Cl
OH
O
Tos
Tos-Cl
pyridine
Br
OH
Si Cl
CH
3
H
3
C
CH
3
Br
O
Si
CH
3
CH
3
H
3
C
pyridine
trimethylsilyl
chloride (TMS-Cl)
OH
1) NaH
2) CH
3
I
O CH
3
O
HI
O
H
I
O
H
I
+
O
HCl
O
H
Cl
Cl
OH
O
1) PhMgBr
2) H
2
O
Ph
OH
Ph
SH
CH
3
I
Ph
S
CH
3
R
OH
O
NaOH
R
O
O
Na
CH
3
I
R
O
CH
3
O
O
1) LDA
2) CH
3
I
O
CH
3
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
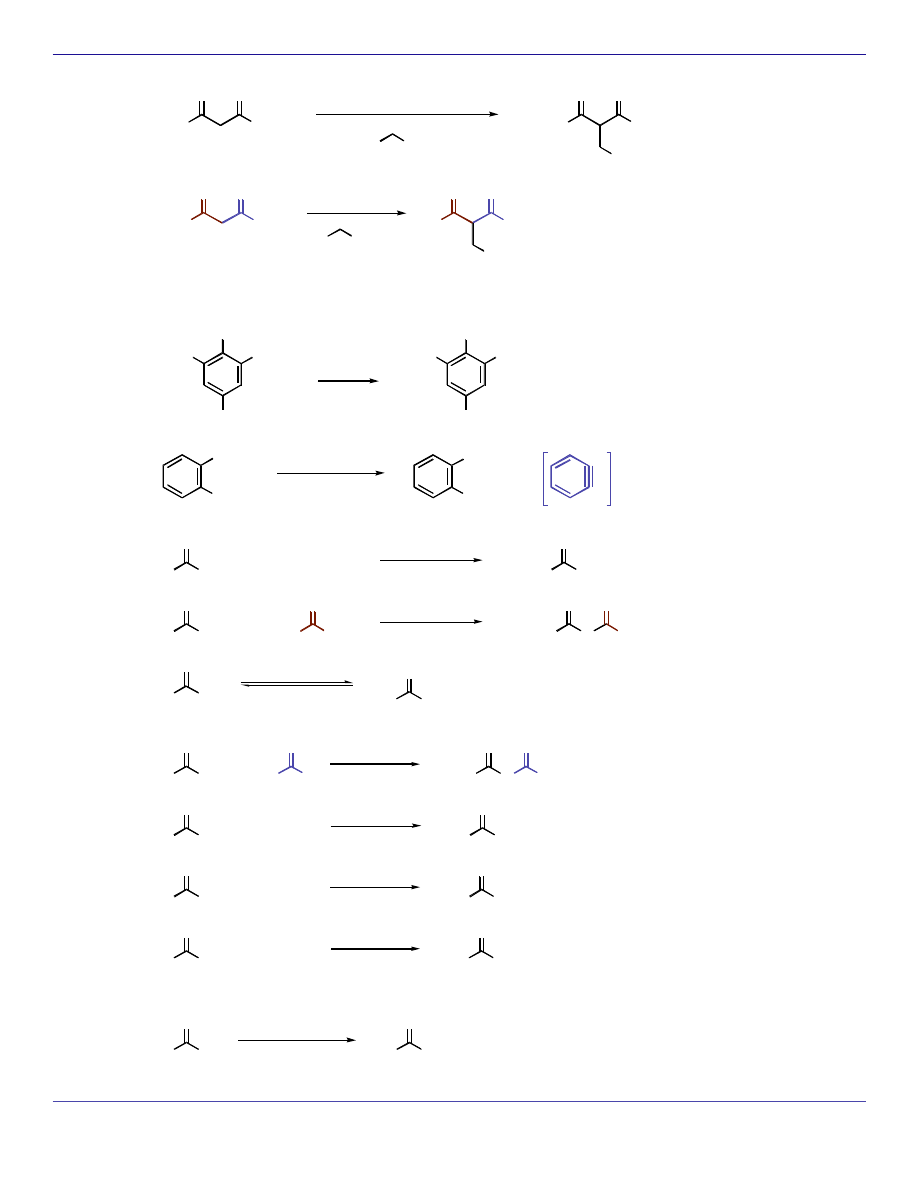
OEt
O
EtO
O
NaOEt, EtOH
R
Br
OEt
O
EtO
O
R
OEt
O
H
3
C
O
NaOEt
EtOH
R
Br
OEt
O
H
3
C
O
R
SUBSTITUTION REACTIONS (Nucleophilic Aromatic and Nucleophilic Acyl)
Cl
NaOH
25 °C
O
2
N
NO
2
NO
2
OH
O
2
N
NO
2
NO
2
NaOH
340 °C
2500 psi
Cl
OH
H
H
benzyne
intermediate
R
OH
O
+
SOCl
2
R
Cl
O
+
SO
2
+
H
Cl
R
OH
O
+
R
O
+
HO
H
R
HO
O
Heat
R
O
O
R
OH
O
HA
R'OH
R
OR'
O
R
Cl
O
+
R
O
+
Na
Cl
R
NaO
O
R
O
O
R
Cl
O
+
H
2
O
R
OH
O
+
H
Cl
R
Cl
O
+
R'OH
R
OR'
O
+
Pyridine-H
Cl
pyridine
R
Cl
O
+
NH
3
R
NH
2
O
+
NH
4
Cl
2
R
Cl
O
R
Ph
O
1)
Ph
2
CuLi
2) H
3
O
+
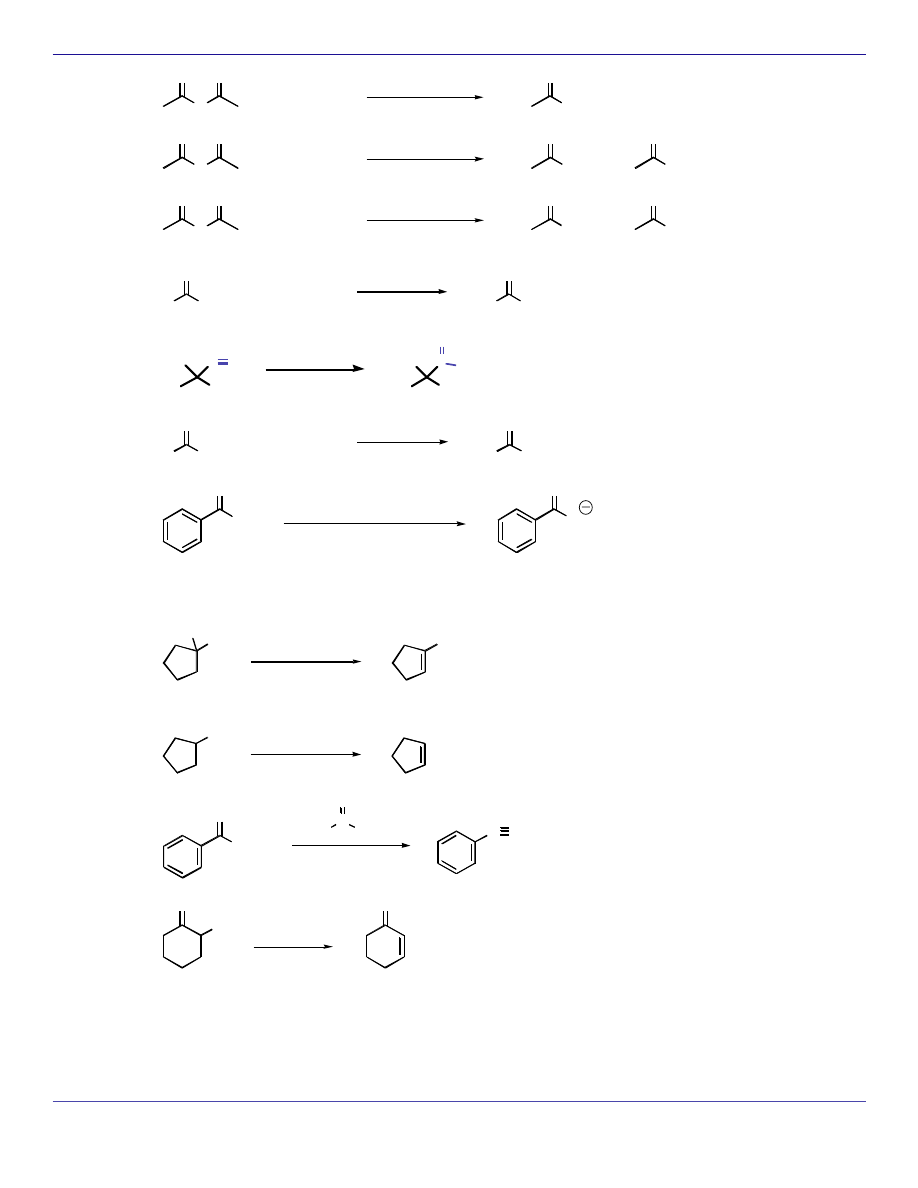
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
+
H
2
O
O
O
O
OH
O
2
+
ROH
O
O
O
OR
O
+
OH
O
+
NH
3
O
O
O
NH
2
O
+
OH
O
+
R
OCH
3
O
+
H
2
O
R
ONa
O
NaOH (cat)
CH
3
OH
Ph
H
H
O
C N
H
3
O
+
Ph
H
H
O
C
O
OH
R
NH
2
O
+
H
2
O
R
OH
O
HA (cat)
+
NH
4
A
CH
3
O
I
2
, NaOH
O
O
+
CHI
3
iodoform
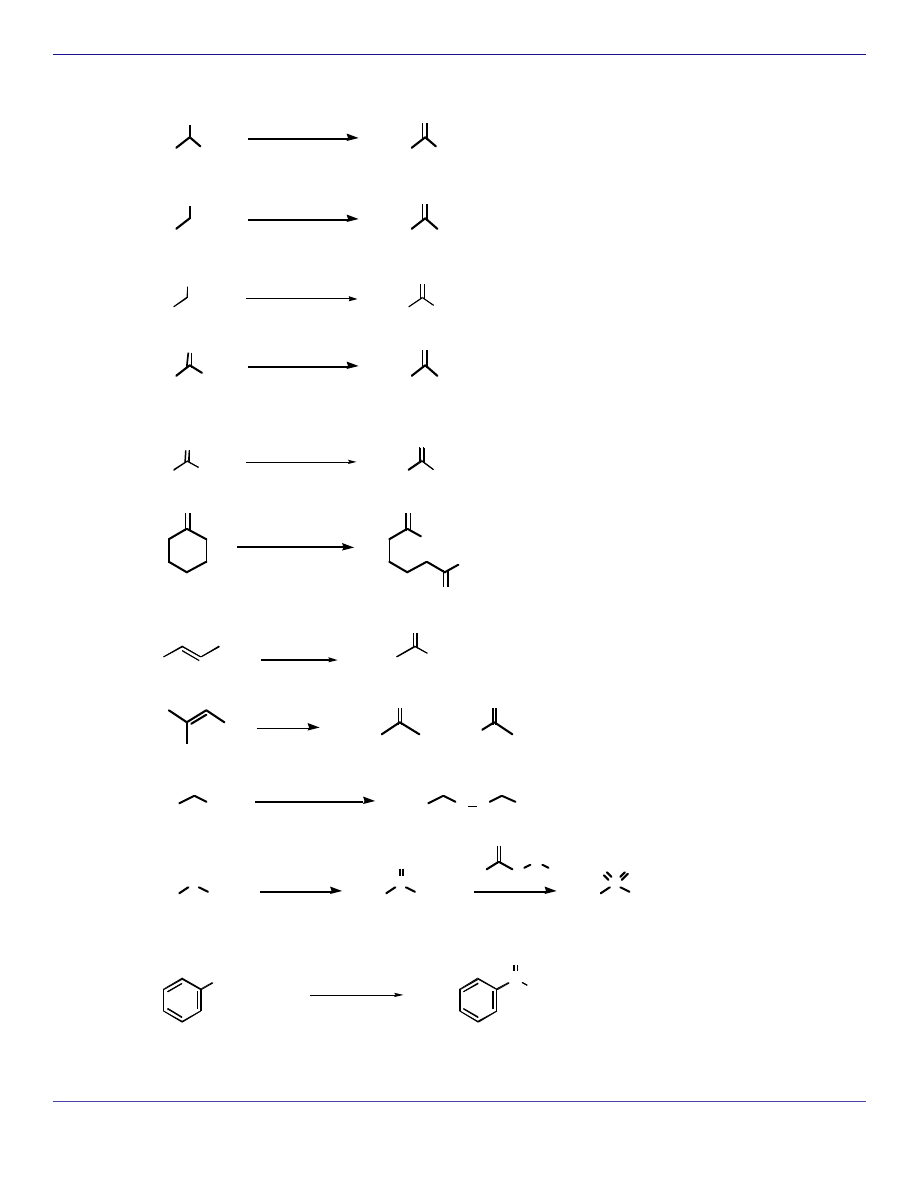
ELIMINATION REACTIONS
OH
H
3
C
CH
3
H
2
SO
4
E1
OH
POBr
3
pyridine
E2
NH
2
O
Cl
S
Cl
O
C N
O
Br
pyridine
O
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
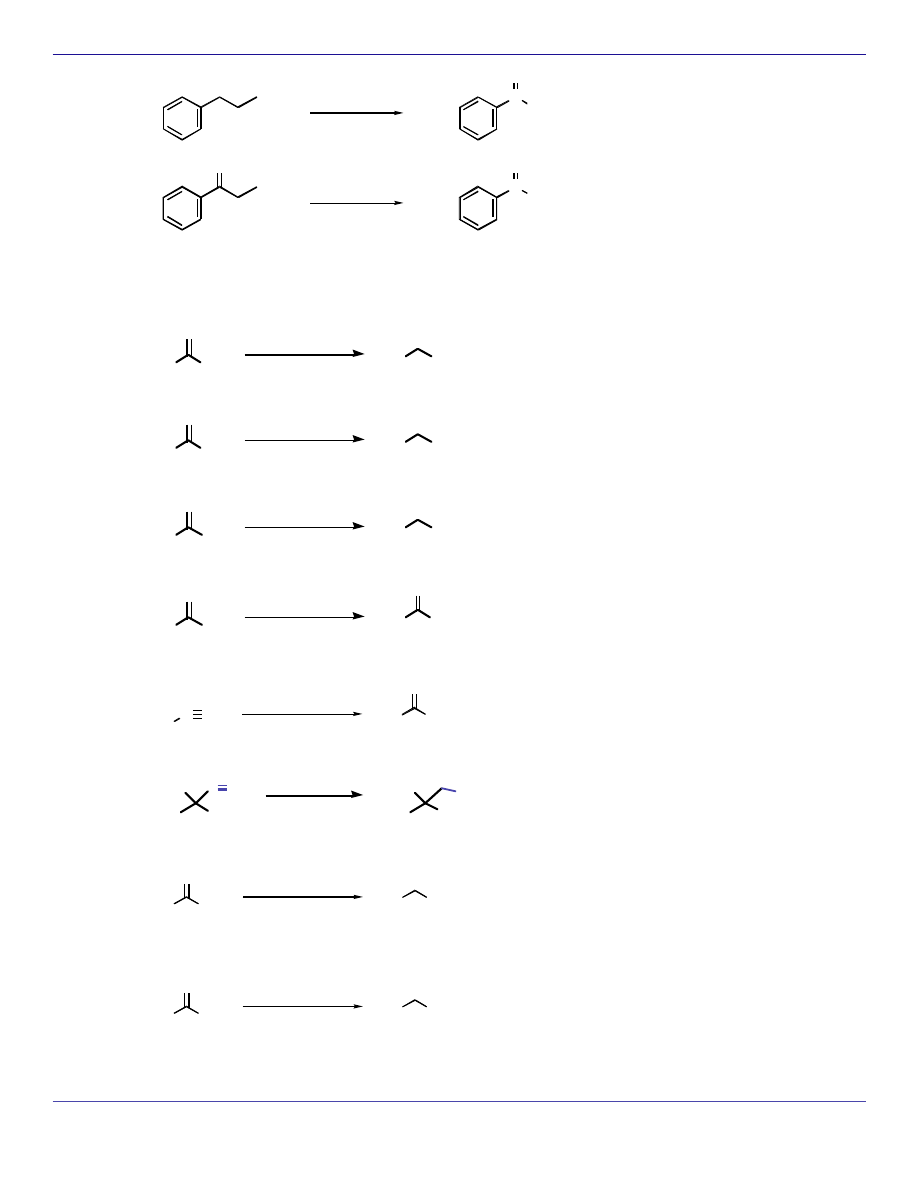
OXIDATION REACTIONS
R
R
OH
CrO
3
H
3
O
+
R
R
O
R
OH
CrO
3
H
3
O
+
R
OH
O
PCC
R
OH
R
H
O
R
O
CrO
3
H
3
O
+
R
OH
O
H
R
O
R
OH
O
H
Ag
2
O
NH
4
OH
O
KMnO
4
O
OH
O
OH
OH
O
KMnO
4
2
O
H
O
1) O
3
2) Zn, AcOH
+
Ph
SH
Ph
S
Ph
S
I
2
Ph
S
CH
3
H
2
O
2
Ph
S
CH
3
O
Ph
S
CH
3
O
O
H
3
C
O
O
H
O
a sulfoxide
a sulfone
KMnO
4
CH
3
C
O
OH
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
KMnO
4
C
O
OH
KMnO
4
C
O
OH
O
REDUCTION REACTIONS
R
OH
R
H
O
1) NaBH
4
2) H
3
O
+
R
OH
R
H
O
1) LiAlH
4
2) H
3
O
+
R
OH
R
OCH
3
O
1) LiAlH
4
2) H
3
O
+
R
H
R
OCH
3
O
1) DIBAH
2) H
3
O
+
O
R
H
R
C
1) DIBAH
2) H
3
O
+
O
N
1) LiAlH
4
2) H
3
O
+
Ph
H
H
O
C N
Ph
H
H
O
NH
2
R
OH
R
OH
O
1) LiAlH
4
2) H
3
O
+
R
OH
R
OH
O
1) BH
3
2) H
3
O
+
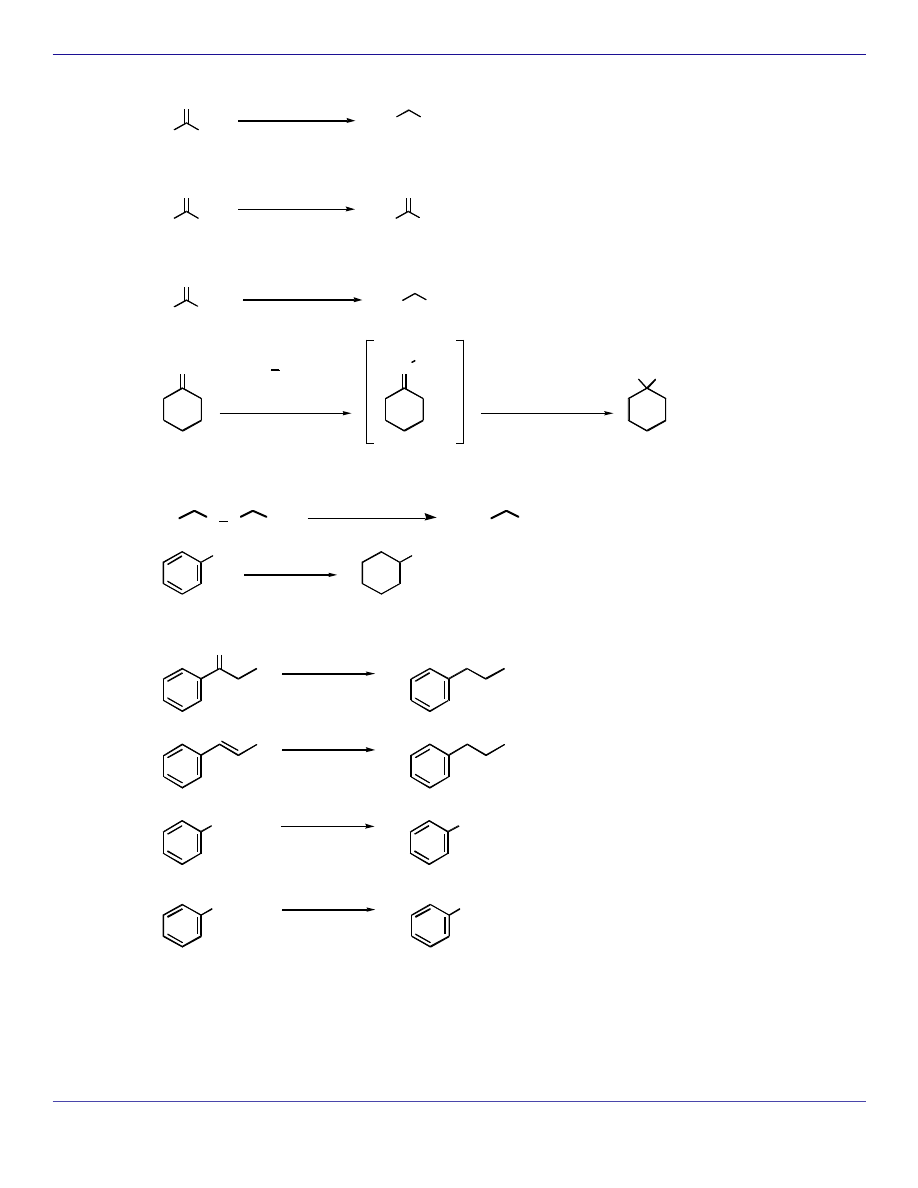
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
R
Cl
O
R
OH
1) LiAlH
4
2) H
3
O
+
R
Cl
O
R
H
O
1) LiAlH(O
t
-Bu)
3
2) H
3
O
+
R
NH
2
R
NH
2
O
1) LiAlH
4
2) H
2
O
O
H
2
N NH
2
KOH
N
NH
2
a hydrazone
H
H
Ph
SH
Ph
S
Ph
S
Zn, H
3
O
+
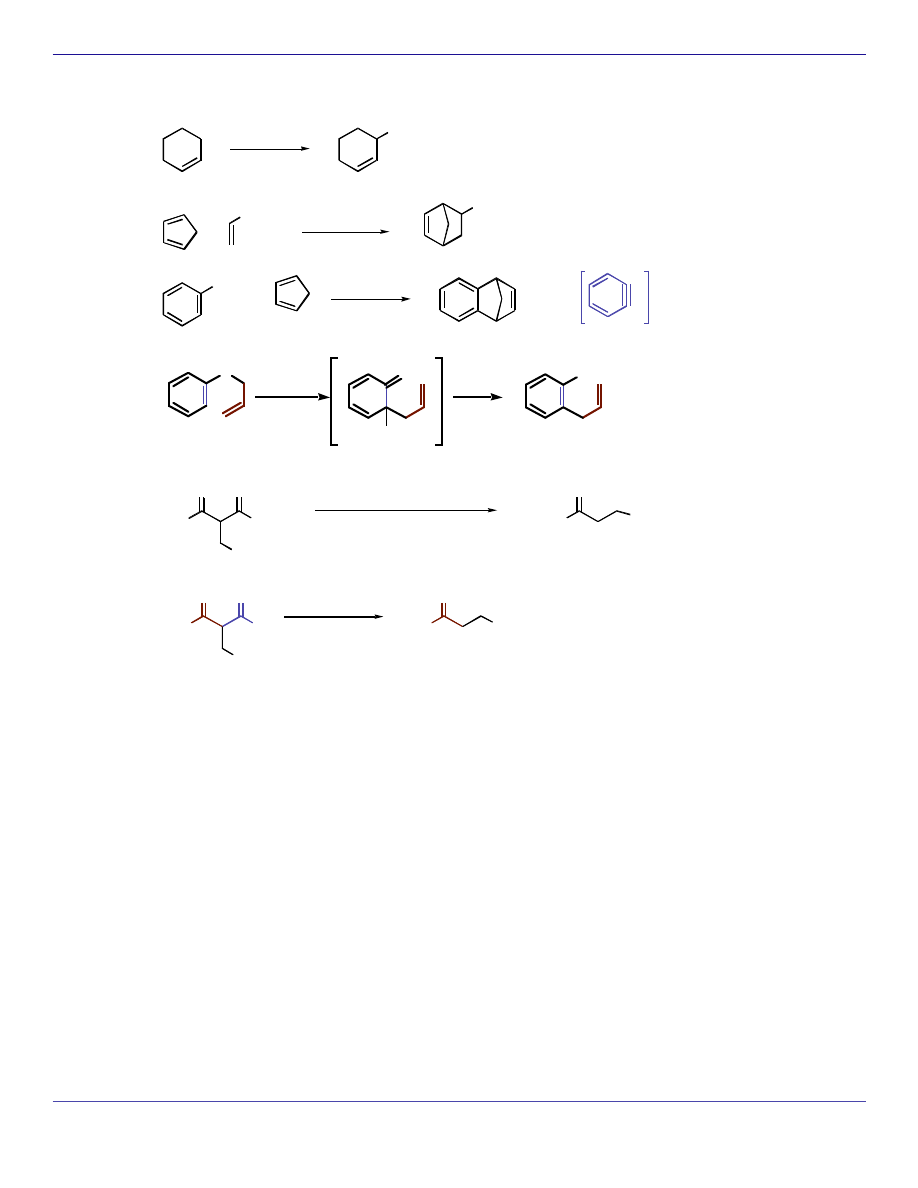
Pt, 2000 psi
CH
3
or
Rh/C, 15 psi
CH
3
H
2
Pd/C
O
H
2
Pd/C
H
2
NO
2
Pd/C
NH
2
SnCl
2
NO
2
NH
2
H
3
O
+
© Gregory R Cook
page
Chem Exam Reactions
North Dakota State University
OTHER REACTIONS
NBS
peroxides
Br
also benzylic
bromination
EWG
+
EWG
KNH
2
Cl
+
benzyne
intermediate
O
O
H
OH
heat
H
3
O
+
, Heat
HO
O
OEt
O
EtO
O
R
R
+ CO
2
H
3
O
+
, Heat
OEt
O
H
3
C
O
R
H
3
C
O
R
+ CO
2
Wyszukiwarka
Podobne podstrony:
Organic Chemistry 342 Reactions
Polymer supported catalysis in synthetic organic chemistry
Dannenberg et al 2015 European Journal of Organic Chemistry
Suggested Problems Part 2, Chemia, Chemia organiczna, Organic chemistry - lecture with exam question
Suggested problems for Chapter 22, Chemia, Chemia organiczna, Organic chemistry - lecture with exam
Chapter 19 Suggested Problems, Chemia, Chemia organiczna, Organic chemistry - lecture with exam ques
Chapter 21 Suggested Problems, Chemia, Chemia organiczna, Organic chemistry - lecture with exam ques
Industrial Organic Chemistry by Klaus Weissermel and Hans Jurgen Arpe
Suggested Problems for Chapter 18, Chemia, Chemia organiczna, Organic chemistry - lecture with exam
Hua et al 2009 European Journal of Organic Chemistry
The Organic Chemistry of Drug Synthesis VOLUME 1 DANIEL LEDNICER
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER
optimization in organic chemistry
The Organic Chemistry of Drug Synthesis VOLUME 3 DANIEL LEDNICER
Industrial Organic Chemistry by Klaus Weissermel and Hans Jurgen Arpe
więcej podobnych podstron