
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
ARTICLES 813
Cancer Risks and Mortality in Heterozygous ATM
Mutation Carriers
Deborah Thompson , Silvia Duedal , Jennifer Kirner , Lesley McGuffog ,
James Last , Anne Reiman , Philip Byrd , Malcolm Taylor , Douglas F. Easton
Background: Homozygous or compound heterozygous
mutations in the ATM gene are the principal cause of ataxia
telangiectasia (A-T). Several studies have suggested that
heterozygous carriers of ATM mutations are at increased risk
of breast cancer and perhaps of other cancers, but the precise
risk is uncertain. Methods: Cancer incidence and mortality
information for 1160 relatives of 169 UK A-T patients
(including 247 obligate carriers) was obtained through the
National Health Service Central Registry. Relative risks
(RRs) of cancer in carriers, allowing for genotype uncertainty,
were estimated with a maximum-likelihood approach that
used the EM algorithm. Maximum-likelihood estimates of
cancer risks associated with three groups of mutations were
calculated using the pedigree analysis program MENDEL.
All statistical tests were two-sided. Results: The overall rela-
tive risk of breast cancer in carriers was 2.23 (95% confi dence
interval [CI] = 1.16 to 4.28) compared with the general popu-
lation but was 4.94 (95% CI = 1.90 to 12.9) in those younger
than age 50 years. The relative risk for all cancers other than
breast cancer was 2.05 (95% CI = 1.09 to 3.84) in female
carriers and 1.23 (95% CI = 0.76 to 2.00) in male carriers.
Breast cancer was the only site for which a clear risk increase
was seen, although there was some evidence of excess risks of
colorectal cancer (RR = 2.54, 95% CI = 1.06 to 6.09) and
stomach cancer (RR = 3.39, 95% CI = 0.86 to 13.4). Carriers
of mutations predicted to encode a full-length ATM protein
had cancer risks similar to those of people carrying truncat-
ing mutations. Conclusion: These results confi rm a moderate
risk of breast cancer in A-T heterozygotes and give some
evidence of an excess risk of other cancers but provide no
support for large mutation-specifi c differences in risk. [J Natl
Cancer Inst 2005;97:813 – 22]
Ataxia telangiectasia (A-T) is a rare autosomal recessive
neurologic disorder, characterized by progressive cerebellar
degeneration and oculocutaneous telangiectasia. A-T appears to
be completely penetrant and is typically diagnosed in early
childhood, although the precise clinical phenotype varies from
patient to patient. Most cancers in A-T patients are childhood
lymphoid leukemias and lymphomas, but there is also a substan-
tial risk of epithelial tumors later in life ( 1 ) . Almost all cases
of A-T have been shown to be associated with mutations in
the ATM gene, the product of which plays a central role in the
recognition and repair of double-strand DNA breaks and in the
activation of cell cycle checkpoints ( 2 ) . Most A-T patients are
compound heterozygotes; homozygous carriers are uncommon,
except in consanguineous families or in the case of a few
population- specifi c founder mutations.
It has frequently been suggested that the blood relatives of
A-T patients (i.e., obligate or potential heterozygous ATM
mutation carriers) have an increased risk of cancer, primarily
breast cancer. Clearly, it is important to reliably establish the
cancer risks in heterozygous carriers to provide appropriate
advice to the relatives of A-T patients. However, the question
may also have wider public-health relevance. Some estimates of
the frequency of ATM mutation carriers in Western populations
are as high as 1% ( 3 , 4 ) , so that a relatively modest increase in
breast cancer risk could equate to a substantial population attrib-
utable risk.
Studies assessing the risk of breast cancer in heterozygous
ATM mutation carriers fall in two broad categories. First, several
groups have compared breast cancer incidence and/or mortality
in relatives of A-T patients with that in the general population or
in married-in family members ( 5 – 10 ) . A review of four such
studies estimated the breast cancer relative risk (RR) to be 3.9
(95% confi dence interval [CI] = 2.1 to 7.2) ( 11 ) . Subsequent
studies have found slightly more modest results, with relative
risks between 2.4 and 3.4; most studies report that relative risks
are higher among younger women ( 5 , 9 , 11 , 12 ) .
An alternative approach is to compare the frequency of ATM
mutations in breast cancer case patients with that in control
subjects. Case – control studies have almost uniformly failed to
fi nd an increased frequency of pathogenic ATM mutations in case
patients, even when restricted to early-onset cancers ( 4 , 13 – 16 ) .
A review of 10 studies showed that ATM mutations are statisti-
cally signifi cantly more frequent in breast cancer case patients
selected on the basis of a family history of breast cancer than in
unselected case patients ( 17 ) , although other studies have not
replicated this result ( 18 ) .
The fi ndings from the family studies and the case – control
studies are not necessarily incompatible, given the widths of the
confi dence intervals; the sample sizes in many studies are too
small to detect a modest increase in risk. Moreover, some studies
have suggested that certain missense ATM mutations, notably
7271T>G, may be associated with higher risks of breast cancer
( 14 , 17 , 19 – 22 ) , whereas most of the earlier population-based
studies used mutation detection techniques that are biased in
favor of detecting truncating mutations.
In addition to the potential association between ATM and
breast cancer, several studies have reported an increase in the
Affi liations of authors: Cancer Research UK Genetic Epidemiology Unit, Uni-
versity of Cambridge, Cambridge, UK (DT, SD, JK, LM, DFE); Cancer Research
UK Institute for Cancer Studies, University of Birmingham, Birmingham, UK
(JL, AR, PB, MT) .
Correspondence to: Douglas F. Easton, PhD, CR-UK Genetic Epidemiology
Unit, Cambridge University Department of Public Health and Primary Care,
Strangeways Research Laboratories, Worts Causeway, Cambridge, CB1 8RN, UK
(e-mail: douglas.easton@phpc.cam.ac.uk ).
See “ Notes ” following “ References. ”
DOI: 10.1093/jnci/dji141
Journal of the National Cancer Institute, Vol. 97, No. 11, © Oxford University
Press 2005, all rights reserved.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from

814 ARTICLES
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
overall risk of cancer in relatives of A-T patients. One review
found that the risk of non-breast cancers in carriers was almost
double that expected in the general population ( 11 ) . Several
cancer sites have been mentioned in this context, but no statisti-
cally signifi cant associations with particular cancers have been
reported to date ( 6 , 9 , 12 , 23 , 24 ) . If the risks of any other specifi c
tumor types are genuinely increased in heterozygous ATM
carriers, no study has yet had suffi cient power to demon-
strate this.
This study aimed to provide more precise estimates of the
risks of cancer in heterozygous ATM mutation carriers by exam-
ining the cancer incidence and mortality experienced by the rela-
tives of 169 A-T patients from 139 families living in the UK. This
is by far the largest group of A-T families outside the US to have
been studied to date and represents the large majority of A-T case
patients diagnosed in the UK during the last 20 years. A second
aim was to investigate potential differences in cancer risks asso-
ciated with different types of ATM mutations.
S
UBJECTS
AND
M
ETHODS
Data Collection
Families were ascertained on the basis of at least one family
member having been given a clinical diagnosis of A-T. The ma-
jority of the families (121 families) were ascertained via contact
with the A-T Society, a UK support group for people with A-T
and their families, or after referral by their pediatric neurologist
to the Cancer Research UK Institute for Cancer Studies for diag-
nosis, genetic testing, and research purposes. In addition, to avoid
biasing the cohort towards relatives of living A-T patients, a list
of all death certifi cates since 1979 that mentioned A-T was ob-
tained from the Offi ce of National Statistics, leading to the inclu-
sion of a further 18 families. Forty-four of the families were
included in a previous study ( 7 ) , but the data used here include a
larger number of relatives, 7.5 years of additional follow-up, and
information about cancer incidence and mortality.
After we sought permission to contact the parents of each
A-T patient from his or her general practitioner, the parent or
parents who had agreed to participate in the study were sent
a questionnaire requesting basic information about themselves
and their children, siblings, parents, and grandparents (i.e., the
siblings, aunts, uncles, grandparents, and great-grandparents of
the A-T patient). All parents returning questionnaires gave writ-
ten informed consent. The requested information for each rela-
tive comprised name, date and place of birth, vital status, and
date of death, where applicable, whether he or she had ever had
a cancer, and if so, the type of cancer, age at diagnosis, and place
of treatment. Dates of birth were confi rmed from national birth
registers, and birth, death, and marriage registers were used to
trace relatives in families for which the questionnaire was
incomplete. Data were also obtained in this way for families for
whom no questionnaire was available and for families ascer-
tained via death certifi cate. An attempt was made to “ fl ag ” each
of the relatives listed above through the National Health Service
Central Register (NHSCR). The NHSCR receives notifi cation
of all deaths in the UK and all cancer registrations from
cancer registries covering the UK, and the study coordinator
was informed of these events in study subjects. Individuals
were
excluded from the study if tracing was not possible.
Cancer diagnoses were included in the analysis only if they had
been confi rmed by the NHSCR, to allow valid comparison with
population-based incidence rates.
Ethical approval was obtained from the South Birmingham
Research Ethics Committee and the Birmingham and the Black
Country Health Authority. Approval for use of the NHSCR for
tracing was given by the Patient Information Advisory Group.
Description of Cohort
A total of 169 A-T patients from 139 separate families were
included in the study. Three families each contained three
siblings with A-T, and 23 families each included a pair of siblings
with A-T. One pair of cousins with A-T occurred in a consan-
guineous family. The number of relatives per family for whom
information was available ranged from two to 28 (median = 17),
giving a total of 2102 blood relatives (excluding 15 stepparents
of A-T patients or of their parents). We excluded 510 relatives
with unknown dates of birth, 152 who were born prior to 1891,
and an additional 153 relatives who could not be traced by the
NHSCR. Follow-up for parents was defi ned as starting at the
birth of their fi rst child with A-T, and follow-up for maternal and
paternal grandparents began at the birth of the A-T patient’s
mother and father, respectively. Follow-up for maternal and
paternal great-grandparents started 28 years before the birth of
the A-T patient’s mother and father respectively, to approximate
the date of the relevant grandparent’s birth (28 years was the
average age of parents at the birth of a child with A-T in the
cohort). This left-truncation of the follow-up period was
performed to avoid biasing the cohort toward individuals who
had, by defi nition, still been alive at the time that the A-T patient
(or his or her parent or grandparent, respectively) was born.
Follow-up for all other relatives began at their own dates of
birth, because the A-T patient’s birth was not dependent on their
being alive at any particular point in time. One father was
excluded because his last follow-up (when he joined the armed
forces) was before the birth of his fi rst child. The cohort included
a total of 1286 relatives.
For the analysis of cancer incidence, follow-up prior to 1971
was excluded because cancer registry information was not
complete before then. Only fi rst cancers were considered, non-
melanoma skin cancers were excluded, and only cancers reported
by the cancer registry were counted. Follow-up was assumed to
cease at the earliest of July 1, 2002, the date of death, the 80th
birthday, or when the individual was last reported as being alive
and cancer-free. Of the 1286 relatives, 126 contributed no person-
years to the cancer incidence analysis because their dates of last
follow-up or death were before 1971. The proportion of relatives
excluded for any of the above reasons did not differ between
families with and without questionnaires ( P = 0.4).
According to the defi nitions above, the cohort for the cancer
incidence analysis consisted of 1160 relatives of A-T patients
from 132 families, who contributed a total of 26 220 person-years
to the analysis (median = 9 relatives per family, 27.2 years per
relative). The distribution between different types of relative is
shown in Table 1 . The number of male and female relatives was
approximately equal; 573 (49.4%) males contributed 12 664
person-years (48.3%), and 587 (50.6%) females contributed
13 557 person-years (51.7%). The median year of birth was 1942
(interquartile range [IQR] = 1924 to 1958). During the follow-up
period, there were 355 deaths, with a median age at death of
71 years (IQR = 62 to 81 years). The remaining 805 relatives
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from

Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
ARTICLES 815
were still alive when they exited the cohort, at a median age of
50 years (IQR = 39 to 63 years).
The follow-up period for mortality was defi ned in the same
way as for cancer incidence, except that follow-up commenced
on January 1, 1950. In this cohort, 644 male and 625 females
contributed a total of 41 276 person-years (median = 34.2 years
per relative, IQR = 22.0 to 43.7).
Genotyping
To identify the ATM mutations present in the families of A-T
patients, mutation screening of the ATM gene was performed at
the Cancer Research UK Institute for Cancer Studies using lym-
phoblastoid cell lines derived from blood samples of A-T patients.
In all these A-T patients, including those for whom mutations
have not yet been found, loss of ATM protein was confi rmed by
Western blotting of protein extracts from the lymphoblastoid cell
lines. Proteins were separated by sodium dodecyl sulfate
–
polyacrylamide gel electrophoresis on 6% gels and transferred
electrophoretically to nitrocellulose membranes that were incu-
bated with a monoclonal mouse
–
anti-human ATM antibody
(11G12) ( 39 ) . Formerly, screening for ATM mutations had been
carried out using restriction enzyme fi ngerprinting of PCR-
amplifi ed cDNA ( 22 ) . More recently, ATM mutations in A-T
patients were identifi ed by denaturing high-performance liquid
chromatography analysis of PCR-amplifi ed exons, followed by
sequencing. For those mutations identifi ed by exon sequencing
that potentially altered splicing of the RNA transcript, cDNA
sequencing was also performed to confi rm sequence deletion
or insertion. At least one pathogenic ATM mutation was identi-
fi ed in 118 A-T patients from 95 families (79% of the families
ascertained via the A-T Society). In eight families the A-T
patients have been shown to be homozygous for different ATM
mutations, and a further 40 families have been shown to carry
two distinct ATM mutations. No mutation has yet been identifi ed
in 12 families, and samples are not currently available for a
further 30 families. Mutations were found in both parents from
33 families, in the mother but not the father in eight families, and
in the father but not the mother in 10 families.
Subsequent to the initial data collection, A-T patients in two
families have been shown to carry mutations in the MRE11 gene
(including the consanguineous family containing a pair of cous-
ins with A-T), rather than in ATM, and so should more properly
be described as having A-T – Like Disorder (ATLD) ( 25 ) . MRE11-
associated ATLD is diffi cult to distinguish clinically from A-T,
although the characteristic telangiectasia features are absent in
ATLD patients. These families were, however, included in the
main analysis, because study entry was defi ned on the basis of a
clinical, rather than a genetic, diagnosis of A-T.
Statistical Analysis
Standardized incidence ratios (SIR) were used to compare
the cancer incidence in relatives with that expected in the
general population. Expected numbers of cancers in each indi-
vidual were based on the age, sex, and calendar-period specifi c
incidence rates given for England and Wales in Cancer in Five
Continents Volumes III to VIII ( 26 – 31 ) using the PYEARS
Table 1. Cancer incidence in 1160 relatives of A-T patients from 132 families *
N
No. eligible
Pyears
Obs
Exp
SIR
(95% CI)
All cancer incidence, excluding breast cancer
Relationship to A-T patient
Parent
280
247
5025
9
10.6
0.85
(0.39 to 1.62)
Sibling
105
90
1776
1
0.44
2.28
(0.06 to 12.7)
Half-sibling
11
8
189
0
0.05
0.00
Aunt/uncle
437
352
10 344
22
12.6
1.75
(1.10 to 2.64)
Grandparent
454
325
7054
49
39.7
1.23
(0.92 to 1.63)
Great-grandparent
802
131
1622
14
18.4
0.76
(0.41 to 1.28)
Parent’s half-sibling
13
7
210
0
0.27
0.00
Approximate carrier probability
1
280
247
5025
9
10.6
0.85
(0.39 to 1.62)
0.67
105
90
1776
1
0.44
2.28
(0.06 to 12.7)
0.5
902
685
17 587
71
52.4
1.36
(1.06 to 1.72)
0.25
815
138
1832
14
18.7
0.75
(0.41 to 1.26)
All
2102
1160
26 220
95
82.1
1.16
(0.95 to 1.41)
Breast cancer incidence (female relatives)
Relationship to A-T patient
Mother
127
2640
5
2.67
1.87
(0.61 to 4.36)
Sister
45
968
0
0.09
0.00
Half-sister
4
81
0
0.00
0.00
Aunt
174
5047
9
3.11
2.90
(1.33 to 5.50)
Grandmother
173
3950
8
6.89
1.16
(0.50 to 2.29)
Great-grandmother
62
810
1
1.70
0.59
(0.01 to 3.27)
Parent’s half-sister
2
62
0
0.01
0.00
Approximate carrier probability
1
127
2640
5
2.67
1.87
(0.61 to 4.36)
0.67
45
968
0
0.09
0.00
0.5
351
9077
17
10.0
1.70
(0.99 to 2.72)
0.25
64
872
1
1.72
0.58
(0.01 to 3.24)
All
587
13 557
23
14.5
1.59
(1.01 to 2.38)
* N = total number of relatives in the cohort, Pyears = person-years at risk, Obs = observed cancers, Exp = expected cancers, SIR = standardized incidence ratios,
CI = confi dence interval.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from
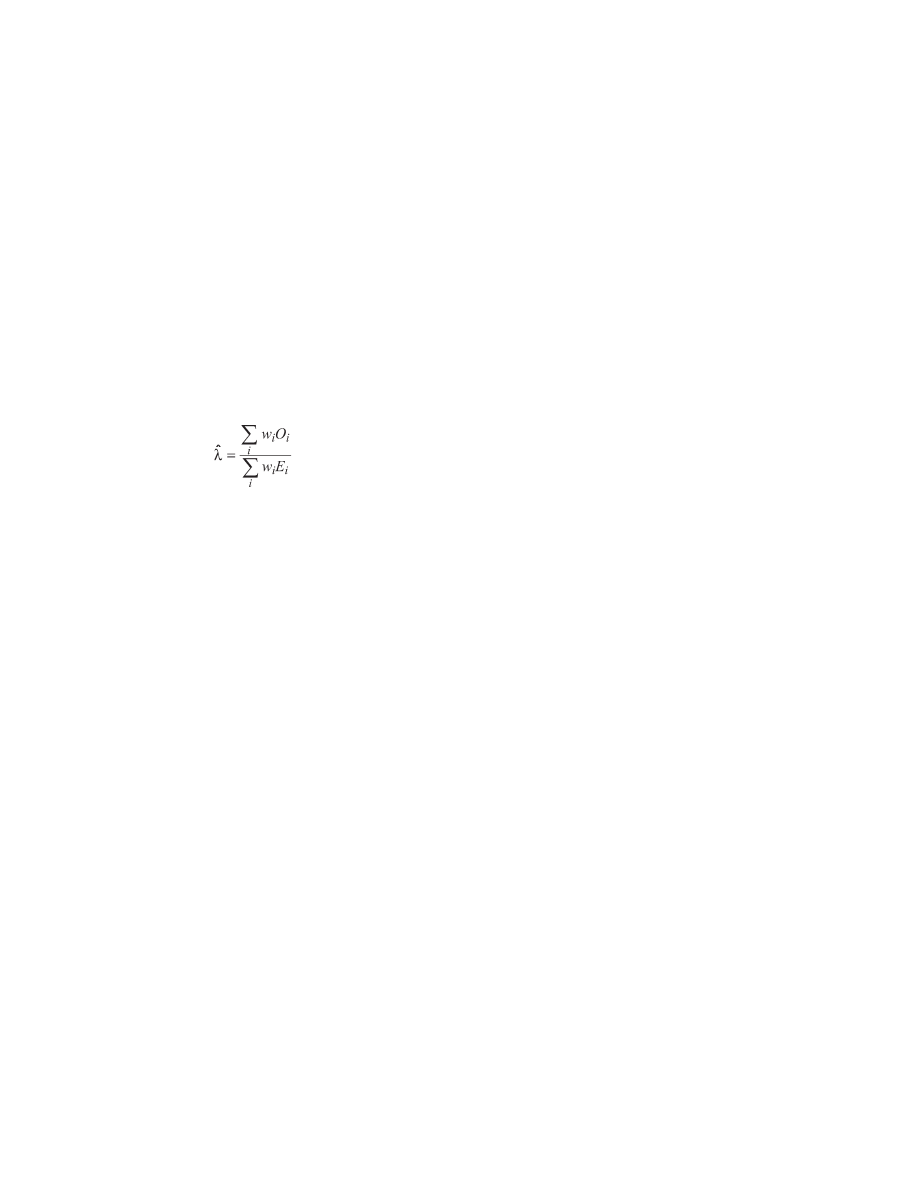
816 ARTICLES
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
program ( 32 ) . The 95% confi dence intervals (CIs) were derived
as exact confi dence limits for a Poisson mean ( 33 ) . For the mor-
tality analysis, mortality rates were taken from data provided by
the UK Offi ce of National Statistics, and standardized mortality
ratios (SMR) were computed.
The parents of the A-T patients are all obligate ATM mutation
carriers. No other relatives have been tested for mutations, so
their carrier probabilities were estimated on the basis of their
position within the pedigree, using the program MENDEL ( 34 ) ,
assuming that A-T is a fully penetrant recessive disorder, with
mutant ATM alleles segregating according to standard Mendelian
inheritance rules. The frequency of mutant alleles within the UK
population was taken to be 0.3%, equivalent to approximately
fi ve new A-T cases per year. The results were not sensitive to
small variations in this value.
These estimated carrier probabilities ( w
i
, for the i
th
individual)
were used to obtain estimates of the relative risk of cancer
associated with carrying one ATM mutation, with the observed
and expected numbers of cancers in each relative (O
i
and E
i
respectively) weighted by their estimated carrier probability; i.e.,
if the relative risk is denoted λ , then
The relative risk of cancer for the noncarriers in the cohort,
φ ,
was computed in the same way but with the O
i
and E
i
weighted
instead by the estimated probability of not carrying a mutation,
1 − w
i
. Estimates of λ and
φ were obtained using the EM algo-
rithm to iteratively update the individual carrier probability esti-
mates and the relative risks ( 35 ) . Confi dence intervals were
derived from the estimated covariance matrix for λ and
φ ( 36 ) .
For almost all individual cancer sites, there was insuffi cient in-
formation to give stable simultaneous estimates of λ and
φ . Si-
multaneously estimating λ and
φ for all sites combined gave no
evidence of an overall excess of cancer incidence, cancer mortal-
ity, or non-cancer mortality in noncarrier relatives; therefore, all
estimates of λ presented are those estimated under the constraint
that
φ = 1 (i.e., noncarrier incidence rates assumed to equal
general population rates).
Relative risks were also estimated separately for carriers who
were younger than 50 years of age and for those aged 50 years or
older. The cutpoint of 50 years was chosen to distinguish approx-
imately between pre- and postmenopausal breast cancers. For
consistency, the same cutpoint was also used for other cancers.
Cumulative risks of cancer in carriers were estimated by apply-
ing the estimated carrier relative risks (younger than 50 years of
age and 50 years or older) to the population rates for England
and Wales (1992 – 1997) ( 29 ) .
Strictly, the relative risk estimates are not maximum-
likelihood estimates because the dependence between the carrier
probabilities of relatives from the same family is ignored in the
iteration. However, the resulting estimates are consistent, whereas
a full-likelihood analysis would theoretically require adjustment
for familial aggregation of cancer, which is problematic to spec-
ify. In practice, the differences between the estimates presented
here and the hypothetical full-likelihood estimates are likely to
be negligible because there was rarely more than one cancer of
the same type per family (i.e., no family had multiple cases of
stomach or lung cancer; two families had two cases of breast
cancer, and three families had two cases of colorectal cancer).
Genotype – Phenotype Correlation
Given the large number of distinct pathologic ATM mutations
recorded in A-T patients (81 distinct mutations in this cohort), it is
impossible to evaluate risks associated with individual mutations.
Because it had been previously hypothesized that the cancer risk
might be related to the residual expression of the mutant ATM
protein ( 21 ) , we classifi ed mutations into three groups, according
to whether any ATM protein was likely to be expressed from a
mutant allele and, if so, whether the protein was likely to have
kinase activity: A) frameshift mutations and substitutions leading
to premature termination codons, resulting in no expression of the
ATM protein from that allele; B) large (exon) or small (codon)
in-frame deletions allowing some expression of a mutant ATM
protein ( 37 ) that lacks kinase activity; and C) missense mutations
allowing expression of mutant ATM with reduced kinase activity
( 37 ) . We have also included in this group the IVS40 – 1050A>G
(5672ins137) “ leaky ” splicing mutation that can express a low
level of normal ATM protein with kinase activity ( 37 – 39 ) .
The full list of observed mutations assigned to each group is
given in the Supplementary Table (available at http://jncicancer
spectrum.oupjournals.org/jnci/content/vol97/issue11 ). Western blo -
tting is routinely performed on lymphoblastoid cell lines derived
from A-T patients to check for loss of ATM protein as part of the
confi rmation of diagnosis. The presence of some ATM protein
was confi rmed in A-T cells carrying all group B and C mutations
(Supplementary Table, available at http://jncicancerspectrum.
oupjournals.org/jnci/content/vol97/issue11 ). If ATM protein is
expressed, its kinase activity can be assayed by in vitro phos-
phorylation of p53 ( 39 ) or detected with phosphospecifi c
antibodies to in vivo targets (e.g., p53ser15) ( 37 ) . The ATM
protein associated with the 7636del9 mutation (group B) has no
detectable kinase activity ( 37 ) , although the carriers of both the
7271T>G and 5672ins137 ATM mutations (group C) express
ATM protein with kinase activity ( 37 , 39 ) , as do the carriers of
the other three mutations in group C. Absence of detectable
kinase activity was examined and confi rmed in nine patients
with group B mutations (data not shown).
The pedigree analysis program MENDEL ( 34 ) was used to
obtain maximum-likelihood estimates of the cancer risks associ-
ated with the three groups of mutations, assuming that all
mutations must belong to one of these groups (even if there is
currently insuffi cient evidence to say which). An iterative
maximum-likelihood approach was necessary because of the
incomplete genotype information available. This is an extension
of the EM algorithm approach described earlier that allows for
the nonindependence of genotypes within the same family. Along
with relative risk parameters for breast cancer and all non-breast
cancers in heterozygous mutation-carrying relatives, parameters
for the relative risks of lymphoid tumors in A-T patients [C81 –
C96 inclusive, ICD revision 10 ( 40 ) ] were included in the mod-
els. A single relative risk parameter was used to model the risk of
lymphoid tumors in A-T patients with no group C mutation (i.e.,
no kinase activity), whereas the relative risk parameter for pa-
tients with at least one group C mutation (i.e., some kinase activ-
ity) was fi xed at 1.0. The inclusion of these parameters should
improve the ability of the program to correctly predict the carrier
status of untested individuals and hence give more precise rela-
tive risk estimates. In this analysis, 12 relative risk parameters
were estimated for heterozygous carriers: three breast cancer
relative risk parameters for women younger than 50 years of age
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
ARTICLES 817
Table 2. Cancer incidence in 247 parents of A-T patients from 132 families *
Cancer site
Obs
Exp
SIR
(95% CI)
Esophagus
1
0.27
3.65
(0.09 to 20.4)
Colorectal
1
1.45
0.69
(0.02 to 3.83)
Lung
3
1.89
1.59
(0.33 to 4.63)
Breast (female)
5
2.67
1.87
(0.61 to 4.36)
Prostate
1
0.55
1.83
(0.05 to 10.2)
Bladder
2
0.63
3.17
(0.38 to 11.4)
Brain
1
0.34
2.98
(0.08 to 16.6)
All sites
14
13.3
1.06
(0.58 to 1.77)
All except breast
9
10.6
0.85
(0.39 to 1.62)
*
Obs = observed cancers, Exp = expected cancers, SIR = standardized
incidence ratio, CI = confi dence interval.
(one for each mutation group), three for women older than 50
years of age, and three relative risk parameters each for male and
female non-breast cancers.
Two families segregating the 7271T>G mutation were ex-
cluded from the genotype
–
phenotype analysis, because the
identifi cation of these families had prompted the hypothesis that
the 7271T>G missense mutation (a group C mutation) was as-
sociated with a particularly elevated breast cancer risk ( 22 ) . One
further family was excluded due to uncertainty about the function
of its one identifi ed mutation. The two families carrying muta-
tions in the MRE11 gene were also excluded from this analysis
(although they had been included in the main cohort analysis).
This analysis was therefore based on 134 families, i.e., 268
mutant alleles. One hundred thirty-eight mutations have been
identifi ed (45 families have either two known mutations or two
copies of the same mutation, and 48 families have one known
mutation). Of these mutations, 86 were from group A, 34 were
from group B, 18 were from group C, and one was of uncertain
function (3403del174). The ATM mutation frequency (0.3%)
was divided among the three groups of mutations according to
these proportions. Estimating the allele frequencies as parameters
within the model gave essentially the same results.
To improve the statistical power, the analysis was repeated
with 16 additional breast cancers that were not eligible for the
main analysis, because they either occurred before 1971 or after
age 80 years, or were not confi rmed by the NHSCR. Although
including these cases might bias the overall relative risk estimate,
there is no reason to believe that they would be biased toward any
particular mutation group. Model selection was carried out using
a conventional likelihood ratio test approach. All P values are
two-sided; in the text, “ statistically signifi cant ” is used to denote
a P of <.05.
R
ESULTS
Overall Results for Cohort
After the exclusions described above, the cohort consisted of
1160 relatives of A-T patients from 132 families (26 220 person-
years). A total of 118 fi rst cancers were reported by the NHSCR,
compared with the 96.7 expected (SIR = 1.22, 95% CI = 1.02 to
1.46). Fifty-four of the cases were in men (50.3 expected), and 64
were in women (46.3 expected) (SIR = 1.07, 95% CI = 0.82 to
1.40, and SIR = 1.38, 95% CI = 1.08 to 1.77, in men and women,
respectively). The median age was 50 years.
Analysis by Type of Relative
The distribution of individuals, person-years, and cancer cases
among relatives of each type is shown in Table 1 . Over all types
of relative, the incidence of all cancers other than breast cancer
was similar to that of the general population (SIR = 1.16, 95%
CI = 0.95 to 1.41). The excess was attributable largely to excess
risks in aunts/uncles (SIR = 1.75, 95% CI = 1.10 to 2.64) and
grandparents (SIR = 1.23, 95% CI = 0.92 to 1.63). No statis -
tically signifi cant excess was observed in parents or great-
grandparents. The overall number of breast cancers in relatives
was slightly higher than expected (SIR = 1.59, 95% CI = 1.01 to
2.38, Table 1 ). Five of the 23 eligible breast cancers were in
mothers, nine in aunts, eight in grandmothers, and one in a great-
grandmother.
The 14 cancers diagnosed in parents of A-T patients are listed
in Table 2 . No cancer site showed a statistically signifi cant ex-
cess. Overall, the cancer incidence in parents was similar to that
predicted using general population rates.
Weighted Relative Risk Estimation
Consistent with previous observations, a statistically signifi cant
excess of female breast cancer in heterozygous ATM mutation car-
riers was seen (RR = 2.23, 95% CI = 1.16 to 4.28, Table 3 ) com-
pared with the general population. Excluding breast cancer, there
remained some evidence of an overall increased cancer risk to
ATM carriers compared with that of the general population (RR =
1.47, 95% CI = 1.00 to 2.16), which was slightly greater in female
carriers (RR = 2.05, 95% CI = 1.09 to 3.84) than in male carriers
(RR = 1.23, 95% CI = 0.76 to 2.00). In addition, a statistically
signifi cant excess risk was observed for colorectal cancer (RR =
2.54, 95% CI = 1.06 to 6.09), and there was some suggestion of an
excess of stomach cancer (RR = 3.39, 95% CI = 0.86 to 13.4).
Table 3. Cancer incidence in 1160 relatives of A-T patients from 132 families,
with estimated relative risks (RRs) and 95% confi dence intervals (CIs) to
heterozygous ATM carriers estimated using the EM algorithm *
Cancer site
ICD 9
Obs
Exp
RR
(95% CI)
Buccal cavity
140 – 149
2
1.78
1.59
(0.15 to 16.8)
and
pharynx
Esophagus
150
3
2.17
2.34
(0.47 to 11.6)
Stomach
151
10
4.74
3.39
(0.86 to 13.4)
Colorectal
152 – 154
20
12.1
2.54
(1.06 to 6.09)
Gallbladder
156
3
0.53
12.2
(1.26 to 118)
Pancreas
157
4
2.63
2.41
(0.34 to 17.1)
Lung
162
21
18.2
1.38
(0.64 to 2.97)
Breast (female)
174
23
14.6
2.23
(1.16 to 4.28)
Uterus
179
2
2.15
1.38
(0.09 to 22.4)
Ovary
183
3
2.67
1.90
(0.20 to 18.2)
Prostate
185
6
5.34
1.29
(0.30 to 5.48)
Bladder
188
5
5.22
1.41
(0.41 to 4.82)
Brain
191
2
1.93
0.06
(0.01 to 0.33)
Unknown
199
4
5.19
0.70
(0.10 to 4.92)
Myeloma
203
3
1.09
4.49
(0.32 to 62.2)
Other female
184
2
0.43
10.2
(0.30 to 345)
genital
All sites
95
82.1
1.47
(1.00 to 2.16)
except
breast
Male: all sites
54
50.4
1.23
(0.76 to 2.00)
Female: all sites
41
31.8
2.05
(1.09 to 3.84)
except breast
* The cancer sites shown are those for which at least two cases were observed.
In addition, there was a single observed case of each of the following cancers:
melanoma, cervix, testis, kidney, and thyroid. ICD = International Classifi cation
of Disease, Obs = observed cancers, Exp = expected cancers.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from
818 ARTICLES
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
Age Groups
The estimated relative risks for carriers younger than 50 years
of age and 50 years of age or older are summarized in Table 4 .
The overall relative risk of cancer was greater for both male and
female carriers younger than 50 years of age, with little evidence
of an excess risk for carriers aged 50 years and older (RR = 1.04,
95% CI = 0.59 to 1.83 in males; RR = 1.64, 95% CI = 0.81 to
3.30 in females, excluding breast cancer). The estimated relative
risk of breast cancer in carriers younger than 50 years of age was
close to 5 (RR = 4.94, 95% CI = 1.90 to 12.9), but there was no
statistically signifi cant risk for women 50 years of age and older.
The overall excess cancer risk in carriers younger than 50 years
of age appeared to be due to several different cancer types (for
myeloma, RR = 43.3, 95% CI = 2.70 to 694; for stomach cancer,
RR = 15.8, 95% CI = 1.63 to 153). One of the two buccal cavity
cancers was a nasopharyngeal cancer in the 6-year-old brother
of an A-T patient; this was the only juvenile cancer in a relative.
Cumulative Cancer Risks
Cumulative risks of cancer were estimated by applying the
estimated relative risks for carriers to the incidence rates in the
general population. The cumulative risk of breast cancer in het-
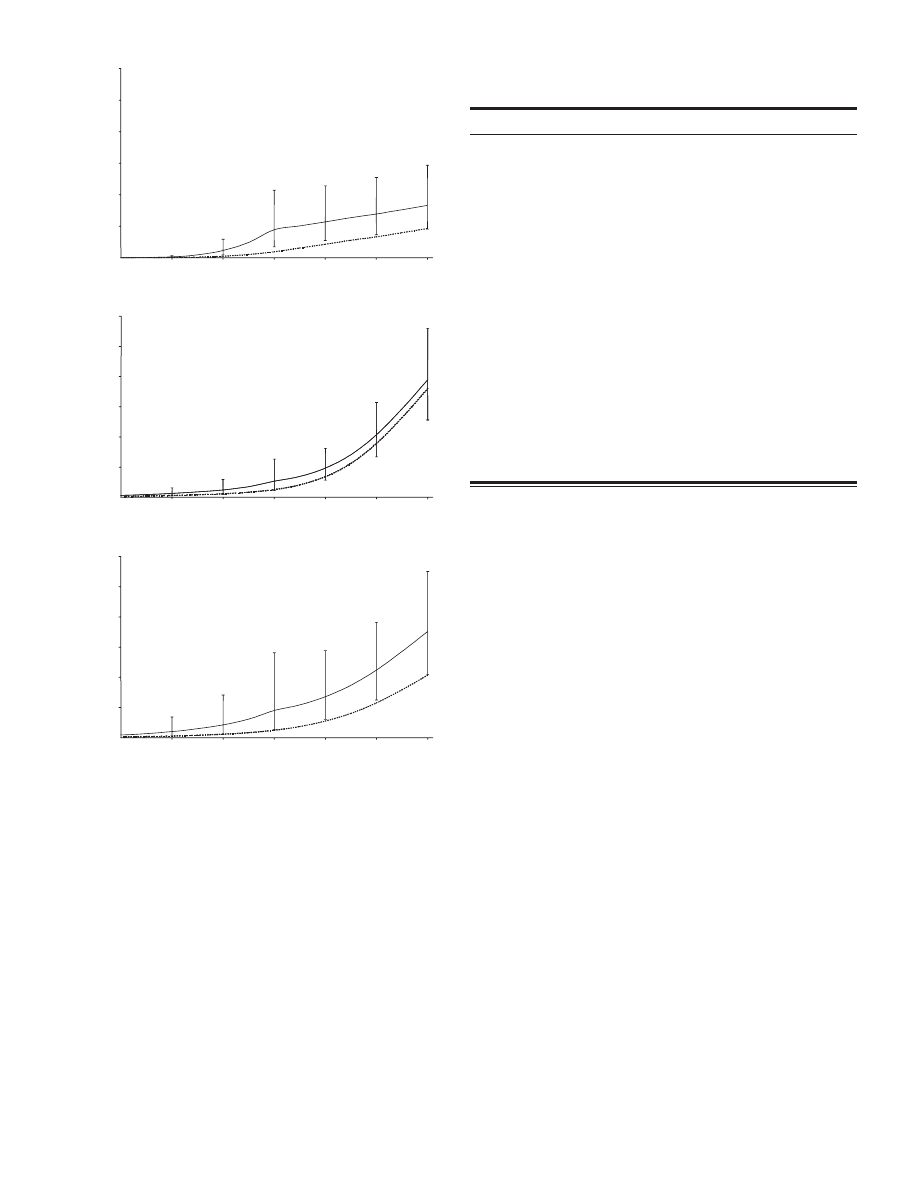
erozygous ATM mutation carriers was estimated to be 8.8% (95%
CI = 3.5% to 21.4%) by age 50 years and 16.6% (95% CI = 9.1%
to 29.3%) by age 80 years ( Fig. 1, A ). The latter risk, that ap-
proximately one woman in six will develop breast cancer, com-
pares with a risk of approximately one in 11 in the general
population of England and Wales (1992 – 1997) ( 29 ) . The esti-
mated risk of any other cancer type by age 50 years was 5.3%
(95% CI = 2.2% to 12.6%) in males and 9.0% (95% CI = 2.6% to
28.1%) in females, compared with 2.5% and 2.4%, respectively
in the general population ( 29 ) . The cumulative risk of any non-
breast cancer by age 80 years was similar in male and female
carriers (38.9%, 95% CI = 25.6% to 56.0%; and 35.1%, 95%
CI = 20.9% to 55.0%, respectively), although the risk in females
was more strongly elevated above the population risk ( Fig. 1,
B and C ).
Based on the observed case frequency over the period 1979 –
1997, we estimate the heterozygous carrier frequency to be 0.4%.
Therefore, our best estimate of the fraction of breast cancer cases
attributable to ATM mutations is 0.5% overall, rising to 1.6% for
cases diagnosed before age 50 years.
Mortality
The overall mortality rate in males was almost identical to that
expected (SMR = 1.01, 95% CI = 0.87 to 1.16). However, this
rate refl ected the combination of a modestly increased risk of
cancer deaths (SMR = 1.35, 95% CI = 1.07 to 1.70) with a slight,
statistically non-signifi cant defi cit of non-cancer deaths (SMR =
0.88, 95% CI = 0.74 to 1.05). The relative risk of non-cancer
death was similar in female relatives (SMR = 0.85, 95% CI =
0.67 to 1.09), but a higher risk of cancer deaths (SMR = 1.82,
95% CI = 1.43 to 2.32) in these relatives resulted in an overall
borderline statistically signifi cantly increased mortality rate
(SMR = 1.16, 95% CI = 0.98 to 1.37) as compared with the gen-
eral population. The mortality in fathers was close to that ex-
pected in the general population, as was the mortality in other
male relatives (data not shown). The mortality in female relatives
other than mothers was also close to that expected in the general
population. However, there were only two deaths in mothers
(a lung cancer and a pancreatic cancer), as compared with an
expected 7.90 deaths.
Seventeen deaths from breast cancer were observed in female
relatives (SMR = 2.08, 95% CI = 1.21 to 3.32). Ten of these were
included in the incidence analysis; the other seven were ineligible
because they were reported only on death certifi cates and not by
the NHSCR.
Statistically signifi cant excess cancer mortality was observed
in ATM carriers of both sexes (SMR = 1.88, 95% CI = 1.14 to
3.10 and SMR = 3.56, 95% CI = 1.83 to 6.93 for males and fe-
males, respectively), whereas non-cancer mortality was slightly,
but not statistically signifi cantly, lower than expected ( Table 5 ).
There was no evidence of excess mortality from either vascular
or respiratory disease. Statistically signifi cant excesses in mortal-
ity in ATM carriers were estimated for breast cancer (RR = 4.18,
95% CI = 1.38 to 12.7), stomach cancer (RR = 4.19, 95% CI =
1.49 to 11.8), colorectal cancer (RR = 3.19, 95% CI = 1.24 to
8.23), and lung cancer (RR = 2.36, 95% CI = 1.24 to 4.50) as
compared with the general population.
ATM carrier relative risks were also estimated separately for
deaths before or after age 50 years ( Table 6 ). The estimated cancer
Table 4. Cancer incidence, by age group, in 1160 relatives of A-T patients from 132 families, with estimated relative risks (RRs) and 95% confi dence intervals
(CIs) to heterozygous ATM carriers estimated using the EM algorithm *
Less than 50 years old
50 years or older
Site
Obs
Exp
RR
(95% CI)
Obs
Exp
RR
(95% CI)
Stomach
3
0.33
15.8
(1.63 to 153)
7
4.51
2.16
(0.40 to 11.6)
Colorectal
2
1.10
3.20
(0.55 to 18.3)
18
11.0
2.45
(0.90 to 6.69)
Gallbladder
0
0.04
0
3
0.49
13.5
(1.39 to 132)
Lung
1
1.05
0.78
(0.02 to 39.0)
20
17.2
1.42
(0.65 to 3.11)
Breast
11
4.34
4.94
(1.90 to 12.9)
12
10.1
1.14
(0.48 to 2.72)
Prostate
0
0.04
0
6
5.30
1.31
(0.31 to 5.57)
Female genital
0
0.07
0
2
0.36
12.3
(0.36 to 423)
All sites
30
15.4
3.16
(1.77 to 5.65)
88
81.2
1.20
(0.81 to 1.78)
Male: all sites
9
5.33
2.14
(0.86 to 5.30)
45
45.1
1.04
(0.59 to 1.83)
Female: all sites
10
5.78
3.81
(1.09 to 13.4)
31
26.0
1.64
(0.81 to 3.30)
except
breast
* The cancer sites shown are those for which either the overall carrier RR was statistically signifi cantly greater than 1 or for which there were 10 or more cases. In
addition, there were two or more cases in the younger age group of buccal cavity and pharynx cancer (two cases), uterus cancer (two cases), and myeloma (two cases).
Obs = observed cancers, Exp = expected cancers.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
ARTICLES 819
particularly elevated in ATM carriers below age 50 years (stom-
ach cancer, RR = 14.0, 95% CI = 3.18 to 61.9; and colorectal
cancer, RR = 11.0, 95% CI = 2.55 to 47.2).
Genotype – Phenotype Correlations
Risks of breast and non-breast cancers in relatives were esti-
mated for the three categories of ATM mutation. There were no
statistically signifi cant differences between the mutation groups
in the risks of either non-breast cancer ( P = .5) or breast cancer
( P = .8). When 16 additional breast cancer cases were included,
the risk was highest for patients with mutations expressing some
protein without kinase activity (group B) (comparing groups B
and A, RR = 2.5, 95% CI = 0.7 to 8.9) and slightly lower for those
with mutations retaining kinase activity (group C) (comparing
groups C and A, RR = 0.9, 95% CI = 0.1 to 8.9), although the
differences were not statistically signifi cant ( P = .4).
D
ISCUSSION
We have studied the cancer incidence and the mortality in
1160 blood relatives of A-T patients from 132 families and have
found evidence for an increased risk of breast cancer in heterozy-
gous ATM mutation carriers, chiefl y at young ages, accompanied
by a more moderate increase in the risk of other cancers. The
overall estimated breast cancer relative risk to heterozygous ATM
carriers was 2.23 (95% CI = 1.16 to 4.28 ) , with a relative risk
of 4.94 (95% CI = 1.90 to 12.9) in carriers younger than 50 years
of age. This is equivalent to a lifetime (until age 80 years) risk of
approximately one woman in six, as compared with one in 11 in
the general population of England and Wales.
Table 5. Mortality in 1269 relatives of A-T patients from 132 families,
with estimated relative risks (RRs) and 95% confi dence intervals (CIs)
to heterozygous ATM carriers estimated using the EM algorithm *
Death cause
ICD 9
Obs
Exp
RR
(95% CI)
Cancer deaths
Esophagus
150
3
2.68
1.09
(0.08 to 14.6)
Stomach
151
15
7.14
4.19
(1.49 to 11.8)
Colorectal
152 – 154
18
9.87
3.19
(1.24 to 8.23)
Pancreas
157
8
3.61
3.21
(0.89 to 11.5)
Lung
162
39
24.9
2.36
(1.24 to 4.50)
Breast (female)
174
17
8.18
4.18
(1.38 to 12.7)
Ovary
183
3
2.76
1.84
(0.19 to 17.8)
Prostate
185
2
3.09
0.93
(0.14 to 6.29)
Bladder
188
3
2.53
1.87
(0.19 to 18.0)
Brain
191
3
2.19
1.53
(0.11 to 20.7)
Unknown
199
8
4.33
2.76
(0.59 to 12.9)
Myeloma
203
2
1.03
1.51
(0.01 to 358)
Other †
4
1.68
4.00
(0.45 to 35.3)
Male: all cancer
70
51.9
1.88
(1.14 to 3.10)
sites
Female: all cancer
66
36.3
3.56
(1.83 to 6.93)
sites
Female: all cancer
49
28.1
3.21
(1.64 to 6.27)
sites except breast
Circulatory disease
119
135
0.78
(0.53 to 1.17)
Respiratory disease
43
35.2
1.63
(0.81 to 3.28)
Injury and poisoning
8
14.5
0.17
(0.04 to 0.68)
Male: all non-cancers
128
144
0.75
(0.52 to 1.08)
Female: all non-cancers
67
78.4
0.79
(0.45 to 1.38)
* Obs = observed cancers, Exp = expected cancers, ICD = International
Classification of Disease.
† The “ other ” cancers were three female genital cancers and a cancer of the
middle ear.
mortality relative risks were higher for carriers younger than 50
years of age than for carriers aged 50 years and older (RR = 3.59,
95% CI = 1.74 to 7.38; and RR = 2.23, 95% CI = 1.44 to 3.45,
respectively). Consistent with the incidence analysis, the relative
risk of breast cancer mortality was higher for carriers below age
50 years (RR = 6.08, 95% CI = 1.05 to 35.3) than for carriers
aged 50 years and older (RR = 3.45, 95% CI = 0.89 to 13.4).
Mortality from stomach cancer and colorectal cancer was also
B
0
20
30
40
50
60
70
80
age in years
% cumulative risk
C
0
10
20
30
40
50
60
20
30
40
50
60
70
80
age in years
% cumulative risk
A
0
10
20
30
40
50
60
20
30
40
50
60
70
80
age in years
% cumulative risk
10
20
30
40
50
60
Fig. 1. Cumulative risks of cancer in heterozygous ATM mutation carriers,
estimated from cancer incidence in 1160 relatives of A-T patients from 132 UK
families. A ) Estimated cumulative risks of breast cancer in female heterozygous
ATM mutation carriers. B ) Estimated cumulative risks of all cancers in male
heterozygous ATM mutation carriers. C ) Estimated cumulative risks of all
cancers other than breast cancer in female heterozygous ATM mutation carriers.
Estimated cumulative risks to carriers along with 95% confi dence intervals ( solid
lines ) and cumulative risks in the general population [England and Wales, 1992 –
1997 ( 29 ) hatched lines ] are shown, at each 10-year age point. Cumulative risks
were obtained by applying the estimated RRs to carriers below and above age 50
(estimated using the EM algorithm) to the general population rates.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from

820 ARTICLES
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
The estimated risk of any cancer in male carriers by the age of
80 years was only slightly higher than in the general population
(39% vs. 36%), whereas the risk by age of 80 years of any cancer
other than breast cancer in female carriers was considerably
higher than in the general population (35% vs. 21%).
Although there was little evidence for an overall excess risk of
cancers other than breast cancer in ATM heterozygotes, there was
some evidence for excess risks of colorectal cancer and stomach
cancer. We also observed a clear excess mortality from cancer,
with statistically signifi cant excess risks of stomach, colorectal,
and lung cancer deaths. The higher relative risks based on mor-
tality might refl ect some underreporting of cancers by the NHSCR
but could also refl ect a more aggressive behavior of cancers in
ATM carriers. Two previous studies have hinted at a possible as-
sociation between ATM and cancers of the gastrointestinal tract,
although neither association was statistically signifi cant ( 7 , 9 ) . In
contrast to our study, neither study found any evidence of a spe-
cifi c excess of colorectal cancers in relatives of A-T patients.
Some limitations of this study that may lead to biased relative
risk estimates include incomplete ascertainment of families, pos-
sible nonpaternity or de novo ATM mutations, and the possibility
that some A-T patients may not carry ATM mutations. A further
limitation is that we were able to genotype only the parents of
A-T patients and not any other relatives. Although this reduced
the power of the study and the precision of the relative risk esti-
mates, it should not result in any bias, providing that ATM muta-
tions are inherited according to Mendelian rules. The precision of
the estimates was also limited by the number of available A-T
families. Further precision should, however, be obtained through
combined analysis of our data with those from other European
studies.
We have attempted to minimize bias in this study by system-
atically following a defi ned cohort of relatives of all known A-T
patients and by basing analysis only on registered cancers and
deaths reported through national records, to allow direct compa-
rability of observed and expected rates. Nevertheless, some po-
tential biases remain. First, families in which a parent died at a
young age might be less likely to have participated in the study.
We attempted to minimize this bias by including additional fami-
lies ascertained through a mention of A-T on a death certifi cate.
That some bias remains is borne out by a marked defi cit in
mortality in mothers, with two deaths observed, compared with
nearly eight expected. This bias is refl ected in the slight defi cit
in overall mortality from nonmalignant causes and suggests that
the excess mortality from and incidence of cancer may therefore
have been underestimated.
Other events that would reduce the number of mutations in
relatives, and hence underestimate the risks, are nonpaternity and
de novo mutations. One A-T patient in the cohort is known to
carry an inherited truncating mutation alongside a de novo pater-
nal missense mutation, 8189A>C. This is generally considered to
be a very rare event in A-T. There was no evidence of incompat-
ible paternal genotypes among the genotyped parents of the A-T
patients. Although there may be instances of false paternity
among grandparents or great-grandparents, any such events would
not affect the carrier probabilities of as many family members.
Strictly speaking, the estimates are a weighted average of the
risks conferred by ATM and MRE11 mutations. The apparent
A-T cases in two of the families are in fact due to compound
heterozygous mutations in the MRE11 gene. The relative sizes of
the two genes would suggest that approximately 6% of A-T pa-
tients might in fact carry MRE11 mutations, i.e., approximately
six further families ( 25 ) . MRE11 acts in the same DNA damage
response pathway as does ATM, but mutations in the two genes
need not predispose to cancer to the same extent; there is no
evidence that homozygous Mre11 mutations are associated with
tumors in mice ( 41 ) . If MRE11 mutations conferred no excess
cancer risk, then the ATM excess cancer risk estimated in this
study could be underestimated by approximately 6%.
In addition to the excesses of breast, colorectal, and stomach
cancer noted above, a statistically signifi cant excess of cancer of
the gallbladder was also observed, but this was based on only three
cases. A high relative risk was estimated for “ other female genital
cancers, ” but this was based on just two cases and was not statisti-
cally signifi cant. Three further female genital cancers were also
reported but did not contribute to the analysis. A high relative risk
(RR = 4.5) was also estimated for myeloma, based on three cases.
It is noteworthy that these were the only lymphoid tumors seen in
relatives and that no myelomas were observed in A-T patients.
The apparent excesses at some or all of these sites could be
due to chance, given the number of cancer sites evaluated, and
larger studies will be required to determine whether these effects
are genuine. Conversely, moderate risks of other cancers in ATM
carriers cannot be ruled out. The modest overall increase in the
risk of non-breast cancer appears to be due largely to a combina-
tion of small increases at many sites; it is notable that all the rela-
tive risk estimates in Table 3 are greater than 1, with the exception
of brain cancer and cancers of unknown site.
Table 6. Mortality, by age group, in 1269 relatives of A-T patients from 132 families, with estimated relative risks (RRs) and 95% confi dence intervals (CIs) to
heterozygous ATM carriers estimated using the EM algorithm *
Less than 50 years old
50 years or older
Death cause
Obs
Exp
RR
(95% CI)
Obs
Exp
RR
(95% CI)
Cancer deaths
Stomach
4
0.58
14.0
(3.18 to 61.9)
11
6.55
2.94
(0.75 to 11.5)
Colorectal
5
0.87
11.0
(2.55 to 47.2)
13
8.99
2.23
(0.67 to 7.46)
Pancreas
0
0.27
8
3.34
3.65
(1.01 to 13.2)
Lung
2
1.60
2.16
(0.37 to 12.5)
37
23.3
2.38
(1.19 to 4.76)
Breast
5
1.89
6.08
(1.05 to 35.3)
12
6.30
3.45
(0.89 to 13.4)
Male: all sites
8
4.65
2.55
(0.98 to 6.62)
62
47.3
1.75
(0.99 to 3.08)
Female: all sites except breast
9
3.98
4.45
(1.06 to 18.6)
40
24.1
2.92
(1.44 to 5.91)
Male: non-cancer deaths
17
22.3
0.61
(0.27 to 1.39)
111
122
0.79
(0.53 to 1.18)
Female: non-cancer deaths
10
12.6
0.78
(0.23 to 2.68)
57
65.7
0.79
(0.42 to 1.48)
* Obs = observed cancers, Exp = expected cancers.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from

Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
ARTICLES 821
Our study is comparable in design to two recent European
studies, one based in France ( 42 ) and the other in the Nordic
countries ( 9 ) . The Nordic study obtained cancer incidence data
for 1218 relatives of A-T patients from 50 families via record
linkage to national cancer registries. Its authors estimated the
breast cancer relative risk for ATM carriers to be 2.4 (95% CI =
1.3 to 4.1), which is very similar to our estimate. Breast cancer
was the only individual cancer with a statistically signifi cant ex-
cess; apart from breast cancer, they observed only 15% more
cancers than expected in relatives of A-T patients.
The French study was based on 1423 relatives of A-T patients
from 34 families. ATM genotyping was performed on over a
quarter of the relatives, but not all cancer cases had been formally
confi rmed. The relative risk of breast cancer, weighted by prior
carrier probability (RR = 2.43, 95% CI = 1.32 to 4.09) was also
very similar to our estimate (RR = 2.23, 95% CI = 1.16 to 4.28).
In the French study, the relative risk was higher for women below
age 45 years, but no excess was seen in women above this age
(RR = 6.32, 95% CI =1.94 to 15.2, and RR = 0.68, 95% CI = 0.08
to 2.46, respectively). There was no evidence of an increased risk
of cancers other than breast cancers in carriers in this study ( 42 ) .
The results presented here are generally in line with the French
and Nordic studies. Our study has the advantage of being based
on a far larger number of families, and, although the number of
eligible relatives in our cohort was slightly smaller, the exclusion
of cousins and great-aunts/uncles meant that the cohort had a
higher density of mutation carriers. Previous studies have either
presented separate relative risks for each type of relative, often
with large confi dence intervals as a consequence of the small
numbers of cases in each group, or have pooled all relatives into
a single group, without taking into account their different carrier
probabilities. In contrast, our use of the EM algorithm to obtain
maximum-likelihood estimates of the carrier relative risks, based
on weighting the information from all relatives, made more effi -
cient use of the data. In common with the Nordic study (but not
the French study), we considered only cancer cases that had been
formally confi rmed. Neither of the other European studies con-
sidered both cancer incidence and mortality.
We found no evidence for any difference in risk of breast or
other cancer according to the type of ATM mutation. If anything,
the trend was toward a lower breast cancer risk for the group C
mutations, in contrast with previous reports that showed that
missense mutations, in particular 7271T>G, are associated with
a markedly increased risk of breast cancer ( 19 – 22 ) . Our esti-
mates were necessarily imprecise, because group C mutations
were the least frequent in this set; after the exclusion of the two
hypothesis-generating 7271T>G families ( 22 ) , there was only
one breast cancer in a family branch known to carry a group C
mutation. Furthermore, because the 5762ins137 mutation ac-
counted for 14 out of 18 of the known group C family branches,
the results may not be generalizable to all ATM mutations retain-
ing kinase activity.
The mutation categories were devised in the context of A-T
patients with two germline mutations in trans , whereas the analy-
sis of cancer risks was restricted to heterozygous carriers, in whom
these particular differences between mutations may be less impor-
tant. For a single mutation in the presence of a wild-type allele,
alternative mechanisms may become relevant to the disease pro-
cess, potentially including haploinsuffi ciency (group A), domi-
nant-negative effects (groups B and C), or some gain in function
(groups B and C). For example, lymphoblastoid cell lines with a
heterozygous missense mutation have been shown to have higher
ATM mRNA expression than do cell lines with a truncating muta-
tion and to have poorer cell survival following irradiation ( 43 ) .
A recent study of 34 French A-T families found no difference
between the breast cancer risks associated with heterozygous
truncating and missense/in-frame deletion ATM mutations but
identifi ed three groups of truncating mutations with particularly
high breast cancer relative risks, each relating to a known binding
domain ( 42 ) . However, we observed no breast cancers in the
seven family branches with mutations that truncate the ATM
protein in these domains.
It is important to note that our results do not exclude the pos-
sibility of more substantial heterogeneity at the mutation level.
Despite all these uncertainties, the results do appear to confi rm
that a substantial risk of breast cancer is conferred by mutations
that eliminate the ATM protein and that the risk is not restricted
to a subset of missense mutations.
The breast cancer risk we have estimated would be suffi cient
to classify an ATM carrier as “ moderate risk, ” according to
recent guidelines of the National Institute for Clinical Excellence
(2004). These guidelines suggest that annual mammography
beginning at age 40 years may be appropriate in this risk group.
However, given the role of ATM in radiation-induced DNA
repair, it is not clear whether mammographic screening would be
benefi cial in ATM carriers. Recent studies have suggested that
magnetic resonance imaging may be a sensitive screening tool in
women at high risk of breast cancer, such as BRCA1 and BRCA2
carriers ( 44 ) , and it may provide an alternative management
approach for ATM carriers. Further research would be needed to
evaluate the appropriateness of any specifi c screening for gastric,
colorectal, or other cancers.
In conclusion, this study has confi rmed an approximately
twofold-increased risk of breast cancer in female carriers of ATM
mutations, with a higher relative risk for those younger than 50
years. We also identifi ed increased risk of colorectal cancer and a
possible increased risk of stomach cancer. Combined analyses
with similar cohorts and further follow-up will be required to
provide reliable risk estimates for other cancer sites and to inves-
tigate mutation-specifi c effects.
R
EFERENCES
(1) Morrell D, Cromartie E, Swift M. Mortality and cancer incidence in 263
patients with ataxia-telangiectasia. J Natl Cancer Inst 1986 ; 77 : 89 – 92.
(2) Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al.
A single ataxia telangiectasia gene with a product similar to PI-3 kinase.
Science 1995 ; 268 : 1749 – 53.
(3)
Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH,
Bishop DT. The incidence and gene frequency of ataxia-telangiectasia in the
United States. Am J Hum Genet 1986 ; 39 : 573 – 83.
(4) FitzGerald MG, Bean JM, Hegde SR, Unsal H, MacDonald DJ, Harkin DP,
et al. Heterozygous ATM mutations do not contribute to early onset of breast
cancer. Nat Genet 1997 ; 15 : 307 – 10.
(5) Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in
families with ataxia-telangiectasia. N Engl J Med 1987 ; 316 : 1289 – 94.
(6) Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161
families affected by ataxia-telangiectasia. N Engl J Med 1991 ; 325 : 1831 – 6.
(7) Inskip HM, Kinlen LJ, Taylor AM, Woods CG, Arlett CF. Risk of breast can-
cer and other cancers in heterozygotes for ataxia-telangiectasia. Br J Cancer
1999 ; 79 : 1304 – 7.
(8) Janin N, Andrieu N, Ossian K, Lauge A, Croquette MF, Griscelli C, et al.
Breast cancer risk in ataxia telangiectasia (AT) heterozygotes: haplotype
study in French AT families. Br J Cancer 1999 ; 80 : 1042 – 5.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from

822 ARTICLES
Journal of the National Cancer Institute, Vol. 97, No. 11, June 1, 2005
(9)
Olsen JH, Hahnemann JM, Borresen-Dale AL, Brondum-Nielsen K,
Hammarstrom L, Kleinerman R, et al. Cancer in patients with ataxia-
telangiectasia and in their relatives in the nordic countries. J Natl Cancer
Inst 2001 ; 93 : 121 – 7.
(10) Su Y, Swift M. Mortality rates among carriers of ataxia-telangiectasia
mutant alleles. Ann Intern Med 2000 ; 133 : 770 – 8.
(11)
Easton DF. Cancer risks in A-T heterozygotes.
Int J Radiat Biol
1994 ; 66 : S177 – 82.
(12) Geoffroy-Perez B, Janin N, Ossian K, Lauge A, Croquette MF, Griscelli C,
et al. Cancer risk in heterozygotes for ataxia-telangiectasia. Int J Cancer
2001 ; 93 : 288 – 93.
(13) Bebb DG, Yu Z, Chen J, Telatar M, Gelmon K, Phillips N, et al. Absence of
mutations in the ATM gene in forty-seven cases of sporadic breast cancer.
Br J Cancer 1999 ; 80 : 1979 – 81.
(14) Buchholz TA, Weil MM, Ashorn CL, Strom EA, Sigurdson A, Bondy M,
et al. A Ser49Cys variant in the ataxia telangiectasia, mutated, gene that is
more common in patients with breast carcinoma compared with population
controls. Cancer 2004 ; 100 : 1345 – 51.
(15) Izatt L, Greenman J, Hodgson S, Ellis D, Watts S, Scott G, et al. Identifi ca-
tion of germline missense mutations and rare allelic variants in the ATM
gene in early-onset breast cancer. Genes Chromosomes Cancer 1999 ; 26 :
286 – 94.
(16) Shafman TD, Levitz S, Nixon AJ, Gibans LA, Nichols KE, Bell DW, et al.
Prevalence of germline truncating mutations in ATM in women with a
second breast cancer after radiation therapy for a contralateral tumor. Genes
Chromosomes Cancer 2000 ; 27 : 124 – 9.
(17) Thorstenson YR, Roxas A, Kroiss R, Jenkins MA, Yu KM, Bachrich T,
et al. Contributions of ATM mutations to familial breast and ovarian cancer.
Cancer Res 2003 ; 63 : 3325 – 33.
(18) Chen J, Birkholtz GG, Lindblom P, Rubio C, Lindblom A. The role of
ataxia-telangiectasia heterozygotes in familial breast cancer. Cancer Res
1998 ; 58 : 1376 – 9.
(19) Chenevix-Trench G, Spurdle AB, Gatei M, Kelly H, Marsh A, Chen X, et al.
Dominant negative ATM mutations in breast cancer families. J Natl Cancer
Inst 2002 ; 94 : 205 – 15.
(20) Dork T, Bendix R, Bremer M, Rades D, Klopper K, Nicke M, et al. Spec-
trum of ATM gene mutations in a hospital-based series of unselected breast
cancer patients. Cancer Res 2001 ; 61 : 7608 – 15.
(21) Gatti RA, Tward A, Concannon P. Cancer risk in ATM heterozygotes: a
model of phenotypic and mechanistic differences between missense and
truncating mutations. Mol Genet Metab 1999 ; 68 : 419 – 23.
(22) Stankovic T, Kidd AM, Sutcliffe A, McGuire GM, Robinson P, Weber P,
et al. ATM mutations and phenotypes in ataxia-telangiectasia families in
the British Isles: expression of mutant ATM and the risk of leukemia, lym-
phoma, and breast cancer. Am J Hum Genet 1998 ; 62 : 334 – 45.
(23)
Bernstein JL, Bernstein L, Thompson WD, Lynch CF, Malone KE,
Teitelbaum SL, et al. ATM variants 7271T>G and IVS10 – 6T>G among women
with unilateral and bilateral breast cancer. Br J Cancer 2003 ; 89 : 1513 – 6.
(24) Spurdle AB, Hopper JL, Chen X, McCredie MR, Giles GG, Newman B,
et al. No evidence for association of ataxia-telangiectasia mutated gene
T2119C and C3161G amino acid substitution variants with risk of breast
cancer. Breast Cancer Res 2002 ; 4 : R15 .
(25) Stewart GS, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NG,
et al. The DNA double-strand break repair gene hMRE11 is mutated in indi-
viduals with an ataxia-telangiectasia-like disorder. Cell 1999 ; 99 : 577 – 87.
(26) Muir C, Waterhouse J, Mack T, Powell J, et al. Cancer incidence in fi ve
continents. Vol V. Lyon (France): IARC; 1987 .
(27) Parkin DM, Muir CS, Whelan SL, Gao YT, et al. Cancer incidence in fi ve
continents. Vol VI. Lyon (France): IARC; 1992 .
(28) Parkin DM, Whelan SL, Ferlay J, Raymond L, et al. Cancer incidence in
fi ve continents. Vol VII. Lyon (France): IARC; 1997 .
(29) Parkin DM, Whelan SL, Ferlay J, Teppo L, et al. Cancer incidence in fi ve
continents. Vol VIII. Lyon (France): IARC; 2002 .
(30) Waterhouse JAH, Muir C, Correa P, Powell J. Cancer incidence in fi ve
continents. Vol III. Lyon (France): IARC; 1976 .
(31) Waterhouse JAH, Muir C, Shanmugaratnam K, Powell J. Cancer incidence
in fi ve continents. Vol IV. Lyon (France): IARC; 1982 .
(32) Coleman MP, Hermon C, Douglas A. Person-years (PYRS) — a fortran pro-
gram for cohort study analysis. IARC internal report 89/006. Lyon (France):
IARC; 1989 .
(33) Breslow NE, Day NE. Statistical methods in cancer research. Vol II — the
design and analysis of cohort studies. Lyon (France): IARC; 1987 .
(34) Lange K, Weeks D, Boehnke M. Programs for pedigree analysis: MENDEL,
FISHER, and dGENE. Genet Epidemiol 1988 ; 5 : 471 – 2.
(35) Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incom-
plete data via the EM algorithm (with discussion). J Roy Stat Soc Series B
1977 ; 39 : 1 – 38.
(36) Louis T. Finding the observed information matrix when using the EM
algorithm. J Roy Stat Soc Series B 1982 ; 44 : 226 – 33.
(37) Stewart GS, Last JI, Stankovic T, Haites N, Kidd AM, Byrd PJ, et al.
Residual ataxia telangiectasia mutated protein function in cells from ataxia
telangiectasia patients, with 5762ins137 and 7271T – >G mutations, showing
a less severe phenotype. J Biol Chem 2001 ; 276 : 30133 – 41.
(38) McConville CM, Stankovic T, Byrd PJ, McGuire GM, Yao QY, Lennox GG,
et al. Mutations associated with variant phenotypes in ataxia-telangiectasia.
Am J Hum Genet 1996 ; 59 : 320 – 30.
(39) Sutton IJ, Last JI, Ritchie SJ, Harrington HJ, Byrd PJ, Taylor AM. Adult-
onset ataxia telangiectasia due to ATM 5762ins137 mutation homozygosity.
Ann Neurol 2004 ; 55 : 891 – 5.
(40) World Health Organization. International statistical classifi cation of diseas-
es and related health problems, 1989 revision. Vol I. Geneva (Switzerland):
World Health Organization; 1992 .
(41) Theunissen JW, Kaplan MI, Hunt PA, Williams BR, Ferguson DO, Alt FW,
et al. Checkpoint failure and chromosomal instability without lymphoma-
genesis in Mre11(ATLD1/ATLD1) mice. Mol Cell 2003 ; 12 : 1511 – 23.
(42) Cavaciuti E, Lauge A, Janin N, Ossian K, Hall J, Stoppa-Lyonnet D, et al.
Cancer risk according to type and location of ATM mutation in ataxia-
telangiectasia families. Genes Chromosomes Cancer 2005 ; 42 : 1 – 9.
(43) Fernet M, Moullan N, Lauge A, Stoppa-Lyonnet D, Hall J. Cellular respons-
es to ionising radiation of AT heterozygotes: differences between missense
and truncating mutation carriers. Br J Cancer 2004 ; 90 : 866 – 73.
(44)
Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM,
Obdeijn IM, et al. Effi cacy of MRI and mammography for breast-cancer
screening in women with a familial or genetic predisposition. N Engl J Med
2004 ; 351 : 427 – 37.
(45) National Institute of Clinical Excellence Guidelines, CG0143 Familial
Breast Cancer. Available at: http://www.nice.org.uk/CG014NICEguideline .
[Last accessed: April 28, 2005 .]
N
OTES
We thank the A-T patients and their families for their willingness to participate
in this research. This study was supported by grants from Cancer Research UK
and the A-T Society. DFE is a Principal Research Fellow of Cancer Research UK.
We also thank staff at the Offi ce of National Statistics for its help.
Manuscript received December 24, 2004; revised March 29, 2005; accepted
April 12, 2005.
at Pomorska Akademia Medyczna on October 17, 2011
jnci.oxfordjournals.org
Downloaded from
Wyszukiwarka
Podobne podstrony:
A Surgical Safety Checklist to Reduce Morbidity and Mortality in a Global Population
Cancer Risk According to Type and Location of ATM Mutation in Ataxia Telangiectasia Families
A nonsense mutation (E1978X) in the ATM gene is associated with breast cancer
Population Based Estimates of Breast Cancer Risks Associated With ATM Gene Variants c 7271T4G and c
Morbidity and mortality due to cervical cancer in Poland
Variants in the ATM gene and breast cancer susceptibility
[41]Hormesis and synergy pathways and mechanisms of quercetin in cancer prevention and management
European transnational ecological deprivation index and index and participation in beast cancer scre
Caffeine effect on mortality and oviposition in successive of Aedes aegypti
What do British women know about cervical cancer symptoms and the risks
Tea polyphenols and their role in cancer prevention and chemotherapy
Variants in the ATM gene associated with a reduced risk of contralateral breast cancer
Degradable Polymers and Plastics in Landfill Sites
Estimation of Dietary Pb and Cd Intake from Pb and Cd in blood and urine
Aftershock Protect Yourself and Profit in the Next Global Financial Meltdown
General Government Expenditure and Revenue in 2005 tcm90 41888
A Guide to the Law and Courts in the Empire
więcej podobnych podstron