
Enzymatic hydrolysis of microcrystalline cellulose in reverse micelles
Nan Chen, Jun-Bao Fan, Jin Xiang, Jie Chen, Yi Liang
⁎
State Key Laboratory of Virology, College of Life Sciences, Wuhan University, Wuhan 430072, China
Received 24 August 2005; received in revised form 12 March 2006; accepted 28 March 2006
Available online 4 April 2006
Abstract
The activities of cellulases from Trichoderma reesei entrapped in three types of reverse micelles have been investigated using microcrystalline
cellulose as the substrate. The reverse micellar systems are formed by nonionic surfactant Triton X-100, anionic surfactant Aerosol OT (AOT), and
cationic surfactant cetyltrimethyl ammonium bromide (CTAB) in organic solvent mediae, respectively. The influences of the molar ratio of water to
surfactant
ω
0
, one of characteristic parameters of reverse micelles, and other environmental conditions including pH and temperature, on the
enzymatic activity have been studied in these reverse micellar systems. The results obtained indicate that these three reverse micelles are more
effective than aqueous systems for microcrystalline cellulose hydrolysis, and cellulases show
“superactivity” in these reverse micelles compared with
that in aqueous systems under the same pH and temperature conditions. The enzymatic activity decreases with the increase of
ω
0
in both AOT and
Triton X-100 reverse micellar systems, but reaches a maximum at
ω
0
of 16.7 for CTAB reverse micelles. Temperature and pH also influence the
cellulose hydrolysis process. The structural changes of cellulases in AOT reverse micelles have been measured by intrinsic fluorescence method and a
possible explanation for the activity changes of cellulases has been proposed.
© 2006 Elsevier B.V. All rights reserved.
Keywords: Cellulase; Microcrystalline cellulose; Reverse micelle; Enzymatic hydrolysis
1. Introduction
The Trichoderma reesei cellulase complex is a true cellulase
mixture in the most rigid sense, which includes endoglucanases
(EC 3.2.1.4), exoglucanases (cellobiohydrolases, EC 3.2.1.91),
and
β-glucosidases (EC 3.2.1.21), being able to hydrolyze the β-
1,4-glucosidic linkages of cellulose, and convert crystalline,
amorphous, and chemically derived celluloses quantitatively to
glucose
. Cellulose is the most abundant renewable energy
source on earth. Complete hydrolysis of cellulose yields the
easily fermentable sugar glucose. When this product is biolo-
gically converted into other products, such as ethanol, it can
provide environmental, economic, and strategic benefits on a
large scale. Cellulose hydrolysis will be one of the most impor-
tant biotechnologies in the 21st century
. Therefore, there is
great interest in understanding the process of enzymatic
cellulose degradation and its industrial applications
Reverse micelles are at present considered as simple organic
media mimicking membranous biological systems, to perform
biocatalysis. Reverse micelles containing enzymes have been
thought of as
“micro-reactors” in which the enzymes can be
sheltered and protected from the detrimental effects of solvents
. The structure of a reverse micelle consists of an aqueous
micro-domain (water pool) surrounded by inward-facing polar
heads of the surfactants. The outward-facing hydrophobic chains
of the surfactants are soluble in the non-aqueous bulk solvent
. According to the different surfactants used in different
systems, reverse micelles can be divided into anionic, cationic,
and nonionic classes
. The water concentration in reverse
micelles is generally defined in terms of the parameter
ω
0
, the
molar ratio of water to surfactant. Owing to the water limiting
conditions in reverse micelles,
ω
0
becomes an important deter-
minant of the rates of enzymatic hydrolytic reactions. Therefore
it is of interest to study the correlation between
ω
0
and the
enzyme-catalyzed reaction rate in reverse micelles.
Biopolymers can be solubilized in the water pool of the
reverse micelles and be easily studied by various methods, since
the system is stable, homogeneous, and transparent. Continuous
efforts have been made by various workers to demonstrate that
Biochimica et Biophysica Acta 1764 (2006) 1029
–1035
http://www.elsevier.com/locate/bba
Abbreviations: AOT, Aerosol OT or sodium bis(2-ethylhexyl)sulfosuccinate
⁎ Corresponding author. Tel.: +86 27 6875 4902; fax: +86 27 6875 2560.
E-mail address:
(Y. Liang).
1570-9639/$ - see front matter © 2006 Elsevier B.V. All rights reserved.
doi:

reverse micellar water pool constitutes a unique microenviron-
ment for hydrophilic enzymes, which protects them against
denaturation by the surrounding organic solvents. The structure
of this microenvironment may affect reaction rates through a
variety of mechanisms
:
(1) Shifts of thermodynamic equilibrium in enzyme-catalyzed
condensation and hydrolysis reactions due to a control-
lable water concentration.
(2) Possibility of suppressing product inhibition.
(3) Possibility of enhanced activity and stability of enzymes.
For a few enzymes, an enhancement of the turnover number
over that found in aqueous solution has been observed. In the
case of
α-chymotrypsin, as reported first by Menger and Yamada
, and later by other groups
, this enhancement takes
place at rather low
ω
0
values. The enhancement is, however,
modest. Martinek and co-workers
and Luisi's group
have observed much larger enhancements in the case of
peroxidase and ribonuclease.
Nowadays, the key challenge for the application of cellulases
to the breakdown of cellulosic biomass into sugars for fermen-
tation to ethanol and other commodity products is to make
biomass depolymerization more rapid and less costly. Thanks to
the properties mentioned above, the use of reverse micelles as
reaction media could be an effective method for increasing the
enzymatic hydrolysis of cellulose.
The principal goal of this work is to examine comprehensively
the kinetics of enzymatic hydrolysis of microcrystalline cellulose
in reverse micelles, the subsequent fermentation to chemical
products such as ethanol, and the possibility of future application
in industry. Toward this goal, the following objectives were
achieved for the first time: (i) we compared the enzymatic activity
in reverse micelles composed of nonionic surfactant Triton X-
100, anionic surfactant Aerosol OT or sodium bis(2-ethylhexyl)
sulfosuccinate (AOT), and cationic surfactant cetyltrimethylam-
monium bromide (CTAB) with that in aqueous medium under the
same pH and temperature conditions; (ii) we investigated the
influence of
ω
0
, pH, and temperature on the enzymatic hydrolysis
and thereby obtained an optimum environment for reaction; (iii)
we studied the structural changes of cellulases in AOT reverse
micelles by intrinsic fluorescence method.
2. Materials and methods
2.1. Materials
Cellulase mixture from Trichoderma reesei (Shanghai Boao Bio-technical Co.,
Shanghai, China, average MW
∼52 kDa) was used without further purification. Its
concentration was determined by the weight method and its filter paper activity was
determined by the anthrone method
to be 15 U/mg. Here, U is defined as 1 mg
sugar formed per min at 25.0 °C and pH 5.4. The molar concentration of cellulases
in the entire reaction volume of Triton X-100, AOT, and CTAB reverse micelles
was 0.23
μM. Microcrystalline cellulose was obtained from Shanghai Forth
Reagent factory (Shanghai, China). Its behavior, such as size, solubility, pH, and
temperature stability, is described in
. Triton X-100, AOT, and CTAB were
purchased from Amresco (Amresco Chemical Co., Solon, OH), Sigma (Sigma-
Aldrich Co, St. Louis, MO), and Shanghai Boao Bio-technical Co., respectively.
All chemicals used were made in China and of analytical grade. All reagent
solutions were prepared in 0.02 M citric acid-phosphate buffer (pH 4.7
–7.4) for
enzyme assay and all fluorescence experiments.
2.2. Reverse micellar solutions
The enzyme-containing reverse micelles were prepared by injecting a
buffered enzyme stock solution at a given pH and concentration into 250 mM
Triton X-100 in xylene/n-hexanol (1:2, v/v), 50 mM AOT in isooctane, or 50 mM
CTAB in isooctane/chloroform (1:1, v/v), to obtain different values of
ω
0
, while
being vigorously shaken at room temperature until a completely transparent
solution had been obtained (ca 10
–15 s). For example, 20 μl of 35 μM buffered
enzyme stock solution was added into 3.0 ml of AOT/isooctane solution to obtain
AOT reverse micelles at
ω
0
of 7.4.
Since the structures of biomacromolecules are very sensitive to the organic
solvent involved, the embedding of cellulases in reverse micelles was performed
using the popular
“injection method”
. That is, the aqueous solution of
cellulases was injected into the surfactant organic solvent solution.
2.3. Enzyme assays
The activities of cellulases were determined by estimating the glucose units
released from microcrystalline cellulose. The substrate concentration (3.33 mg/ml) in
the entire reaction volume of Triton X-100, AOT, and CTAB reverse micelles was
kept above the enzyme saturation level. Here, the enzyme saturation level was
determined by investigating the enzymatic hydrolysis of microcrystalline cellulose in
aqueous systems with different cellulose concentrations. The sugar formed reached a
maximum when the substrate concentration was 3.33 mg/ml, and the concentration
of cellulose was kept constant at this level in our experiments. For the experiments in
the reverse micelles, the enzyme-containing reverse micelles were incubated at 30.0,
35.0, 40.0, 45.0, 50.0, 55.0, and 60.0 °C for 5 min. The reaction was started by the
addition of cellulose, and then the reaction mixture was incubated at the same
temperature for 5 min. After centrifuging at 5000 rpm for 3 min, 1.0 ml of the upper
solution without cellulose, containing 6
–36 μl of water pool was taken and diluted by
the addition of 60
–360 μl of buffer. The mixture was vigorously shaken and allowed
to equilibrate thermally for 45 min. After the addition of 1.0 ml chloroform and
centrifugation at 8000 rpm for 5 min, an aliquot of the supernatant (10
μl) was taken
and diluted by the addition of buffer to 1.0 ml for the anthrone analysis. The anthrone
assay of reverse micelles containing only cellulases was also performed under the
same conditions in order to correct the observed background given by cellulases.
For the experiments in aqueous systems, the buffered solutions of cellulases
(pH 6.0) were incubated at 50.0 °C for 5 min. The reaction was started by the
addition of cellulose, and the reaction mixture was incubated at 50.0 °C for 5 min.
The sample was centrifuged at 5000 rpm for 3 min, and an aliquot of the
supernatant (10
μl) was taken and diluted by the addition of buffer to 1.0 ml for
the anthrone analysis.
2.4. Intrinsic fluorescence spectroscopy
Intrinsic fluorescence spectroscopic measurements on the structural changes of
cellulases in AOT reverse micelles at different water pool volumes and in aqueous
systems were carried out at 50.0 °C using a LS-55 luminescence spectrometer
(PerkinElmer Life Sciences, Shelton, CT). The excitation wavelength at 295 nm
was used for the intrinsic fluorescence measurements and the emission spectra were
recorded between 320 and 400 nm. The excitation and emission slits were both
10 nm, and the scan speed was 200 nm min
− 1
. The enzyme-containing AOT
reverse micelles were prepared as described above and the enzyme concentration in
Table 1
The behavior of the substrate, microcrystalline cellulose
Granularity
(
μm)
Solubility in water
pH
Temperature stability
15
–40
Insoluble (
b0.2%)
6.9
–7.3
Darkening of color and slow
softening only occur
at temperatures above 140 °C.
Decomposition occurs
at temperatures above 200 °C.
1030
N. Chen et al. / Biochimica et Biophysica Acta 1764 (2006) 1029
–1035
the whole system was 0.23
μM. The prepared enzyme-containing reverse micelles
were placed in a 1-cm thermostated quartz fluorescence cuvette. The fluorescence
measurements of the protein samples were carried out with optical density less than
0.3 at 295 nm in order to avoid the inner filter effects. The fluorescence of reverse
micelles containing only buffer were also measured under the same conditions in
order to correct the observed fluorescence. Each spectrum was scanned for two or
three times to acquire the final fluorescence emission spectra.
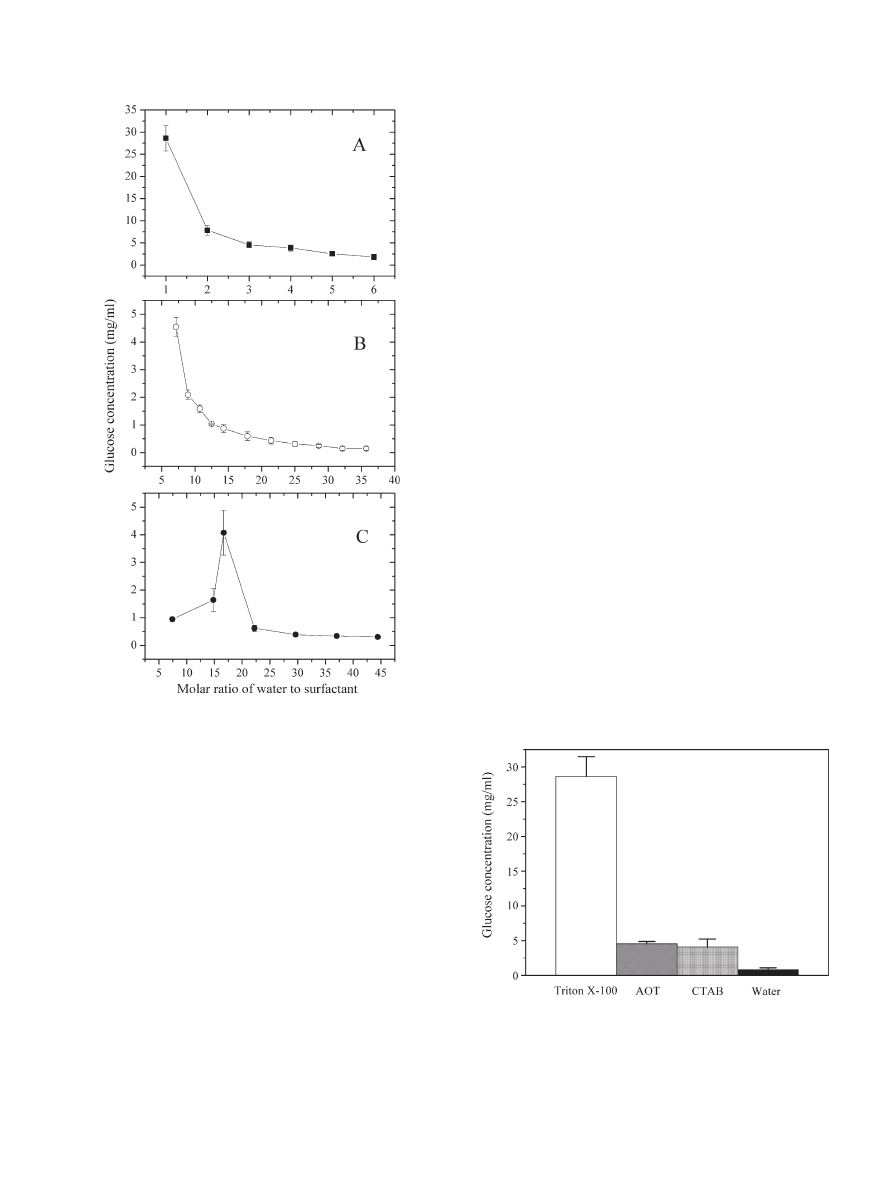
3. Results
3.1. Effect of
ω
0
on the activities of cellulases in Triton X-100,
AOT, and CTAB reverse micelles
The change of
ω
0
affects all kinetic data including the
enzymatic activity. Because the reverse micelles formed by
different surfactants and organic solvents have different contents
of buffer solution, different ranges of
ω
0
were used for Triton X-
100, AOT, and CTAB reverse micelles in the present study. The
activities of cellulases at various values of
ω
0
, represented by the
glucose concentrations after hydrolysis in the water pools of the
reverse micelles, are presented in
and compared with that in
aqueous medium at the same pH and temperature (
). As
shown in
, with increasing the value of
ω
0
, the activities of
cellulases in Triton X-100 and AOT reverse micelles decreased
gradually. Lower glucose concentrations were observed in the
bigger water pools of these two types of reverse micelles, com-
pared with those in the smaller water pools. From
, it can
also be seen that the activity of cellulases in CTAB reverse
micelles increased with increasing the value of
ω
0
from 7.4 to
16.7, reaching a maximum when
ω
0
was 16.7. With further
increasing the value of
ω
0
to 44.0, however, the activity of
cellulases declined. As shown in
, the activities of cellulases
in the smallest water pools of Triton X-100 and AOT reverse
micelles (the values of
ω
0
were 1.0 and 7.4 for Triton X-100 and
AOT reverse micelles respectively) and in the optimum water
pools of CTAB reverse micelles were much higher than those in
aqueous systems under the same pH and temperature conditions.
It should be pointed out that when
ω
0
N17.9 (or 22.2), the glucose
concentration in AOT (or CTAB) reverse micelles was lower than
that in aqueous systems. The activities of cellulases at higher
ω
0
in
were even closer to zero. A possible explanation for this
observation may be due to the deduction of the background given
by cellulases in the anthrone assay.
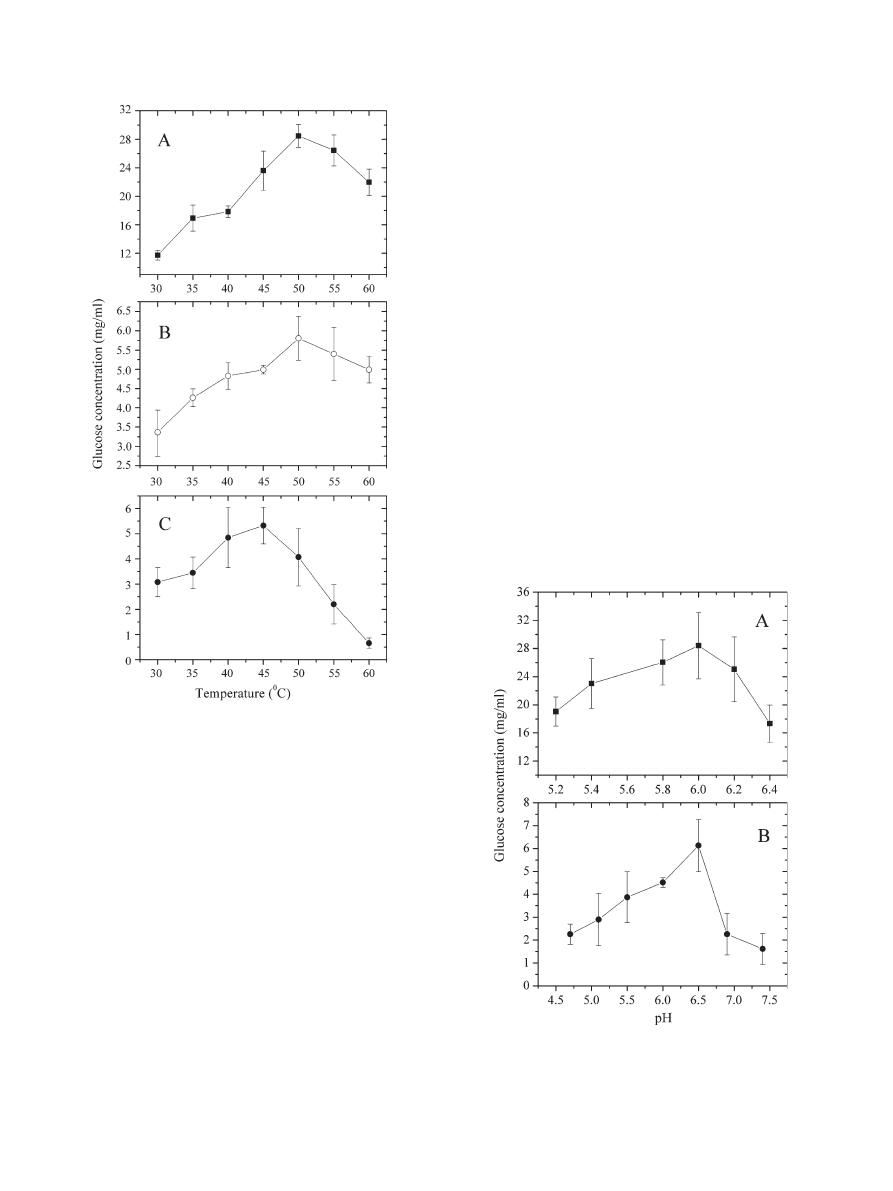
3.2. Effect of temperature on the activities of cellulases in
Triton X-100, AOT, and CTAB reverse micelles
shows the temperature dependence of the activities of
cellulases in Triton X-100, AOT, and CTAB reverse micelles at
pH 6.0 and at a specified
ω
0
(
ω
0
= 1.0 for Triton X-100 reverse
micelles, 7.4 for AOT reverse micelles, and 16.7 for CTAB reverse
Fig. 1. The activities of cellulases represented by glucose released in reverse micelles
at different molar ratios of water to surfactant. A: glucose concentration in the water
pools of Triton X-100 reverse micelles after cellulose hydrolysis (solid square). The
range of
ω
0
was 1.0
–6.0. B: glucose concentration in the water pools of AOT reverse
micelles (open circle). The range of
ω
0
was 7.4
–37.0. C: glucose concentration in the
water pools of CTAB reverse micelles (solid circle). The range of
ω
0
was 7.4
–44.4.
Experiments were performed at 50.0 °C in 0.02 M citric acid
–phosphate buffer at
pH 6.0. The data with error bars were expressed as mean ± S.D. (n = 2
–3).
Fig. 2. Comparison of the activities of cellulases represented by glucose
released in Triton X-100 reverse micelles (white bar), AOT reverse micelles
(grey bar), and CTAB reverse micelles (light grey bar) with that in aqueous
systems (black bar). All the four systems were under the same pH and
temperature conditions, and the values of
ω
0
were 1.0, 7.4, and 16.7 for Triton
X-100, AOT, and CTAB reverse micelles, respectively. Experiments were
performed at 50.0 °C in 0.02 M citric acid
–phosphate buffer at pH 6.0. The
data with error bars were expressed as mean ± S.D. (n = 2
–3).
1031
N. Chen et al. / Biochimica et Biophysica Acta 1764 (2006) 1029
–1035
micelles). The temperature range was 30.0
–60.0 °C. As shown in
, the activities of cellulases in Triton X-100 and AOT (or
CTAB) reverse micelles increased with the increase of the tem-
peratures from 30.0 to 50.0 °C (or 45.0 °C). With a further increase
of the temperature to 60.0 °C, however, the activities of cellulases
declined. In control experiments, the optimum temperature for the
same enzymatic reaction in aqueous systems was 50.0 °C (figure
not shown), similar to that measured in these reverse micelles.
3.3. pH dependence of the activities of cellulases in Triton
X-100 and CTAB reverse micelles
shows the pH-dependence of the activities of cellulases
in Triton X-100 (
ω
0
= 1.0) and CTAB (
ω
0
= 16.7) reverse mi-
celles at 50.0 °C. The pH ranges were 5.2
–6.4 and 4.7–7.4 for
Triton X-100 and CTAB reverse micelles, respectively. As
shown in
, the activity of cellulases in Triton X-100 reverse
micelles increased with the increase of the pH values from 5.2 to
6.0, reaching a maximum at pH 6.0. With further increasing the
pH to 6.4, however, the activity of cellulases declined. From
, it can also be seen that the optimum pH value for this
enzymatic reaction in CTAB reverse micelles was 6.5, which is
closed to the physiological pH value. However, the optimum pH
value for the same enzymatic reaction in aqueous systems
(pH 5.4, figure not shown) was more acidic than those measured
in both reverse micelles.
The influences of
ω
0
, temperature, and pH on cellulose
hydrolysis were studied as described above, and the optimum
conditions for Triton X-100, AOT, and CATB reverse micelles
were thus obtained. When
ω
0
= 1.0 and 7.4, temperature = 50.0 °C,
and pH = 6.0, the enzymatic hydrolysis of cellulose reached a
maximum in Triton X-100 and AOT reverse micelles, respec-
tively. When
ω
0
= 16.7, temperature = 45.0 °C, and pH = 6.5, the
enzymatic hydrolysis of cellulose reached a maximum in CTAB
reverse micelles. The activity of cellulases in Triton X-100 reverse
micelles under such optimum conditions was 35-fold larger than
that in aqueous systems. Similarly, the activities of cellulases in
AOT and CTAB reverse micelles under such optimum conditions
were 5-fold and 7-fold larger than that in aqueous systems,
respectively.
Fig. 3. Effect of temperature on the activities of cellulases represented by glucose
released in Triton X-100, AOT, and CTAB reverse micelles. A: glucose
concentration in Triton X-100 reverse micelles after hydrolysis (solid square). B:
glucose concentration in AOT reverse micelles after hydrolysis (open circle). C:
glucose concentration in CTAB reverse micelles after hydrolysis (solid circle).
The temperature range was 30.0
–60.0 °C. The values of ω
0
for Triton X-100,
AOT, and CTAB reverse micelles were 1.0, 7.4, and 16.7, respectively.
Experiments were performed in 0.02 M citric acid
–phosphate buffer at pH 6.0.
The data with error bars were expressed as mean ± S.D. (n = 2
–3).
Fig. 4. pH dependence of the activities of cellulases represented by glucose
concentration in Triton X-100 reverse micelles at 50.0 °C (A), and CTAB reverse
micelles at 45.0 °C (B). For Triton X-100 reverse micelles, the pH range was 5.2
–
6.4 and the value of
ω
0
was 1.0. For CTAB reverse micelles, the pH range was
4.7
–7.4 and the value of ω
0
was 16.7. The data with error bars were expressed as
mean ± S.D. (n = 2
–3).
1032
N. Chen et al. / Biochimica et Biophysica Acta 1764 (2006) 1029
–1035
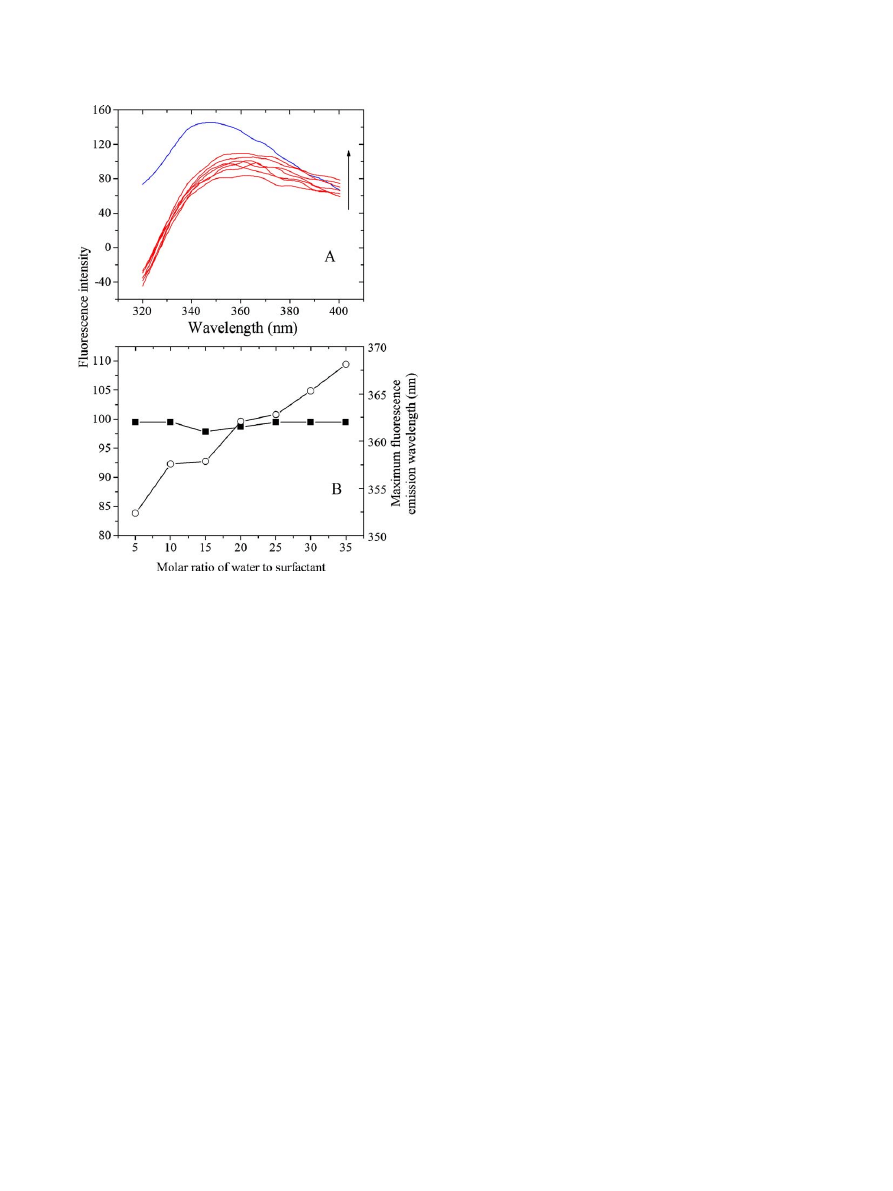
3.4. Conformational changes of cellulases with the increase of
ω
0
in AOT reverse micelles
Tryptophan is a very useful intrinsic probe since its emission
fluorescence spectra reflect the molecular environment of the
side chain; disruption of the native structure leads to changes in
the exposure of the tryptophan side chains to solvent that can
readily be monitored by recording the protein fluorescence
emission spectra
. As shown in
, a regular increase
in the intrinsic fluorescence intensity of tryptophan residues of
cellulases together with a remarkable blue shift of fluorescence
emission maximum from 362 to 348 nm took place upon
increasing the molar ratio of water to surfactant from 5 to the
infinite (the bulk aqueous systems) at 50.0 °C, suggesting that a
more rigid conformation for cellulases was induced in AOT
reverse micelles than that in aqueous systems.
4. Discussion
Studies during the past two decades have revealed that the
technological utility of enzymes in low water content systems
cannot only enhance the catalytic efficiency greatly, but also
lead enzymes to catalyze reactions which are impossible in
water
. It has been established firmly that many enzymes
can work in reverse micelles, such as lipase
, cutinase
, dihydrofolate reductase
, and lysozyme
. How-
ever, information on the activities of cellulases in reverse
micelles, which is necessary for a thorough understanding of the
mechanism for enzymatic hydrolysis of cellulose in low water
content systems, is eagerly awaited.
The results obtained herein indicated that Triton X-100, AOT,
and CTAB reverse micelles were more effective than aqueous
systems for cellulose hydrolysis under the same pH and
temperature conditions. That is, cellulases showed
“superactiv-
ity
” in these three types of reverse micelles. The “superactivity”
of other enzymes, such as
α-chymotrypsin
, ribonuclease
, and peroxidase
, in reverse micelles has been reported.
Luisi et al. have suggested that this enhancement is associated
with the artificial environments and electric fields of the enzyme,
and perhaps with the particular state of water in reverse micelles
. García-Carmona et al. have explained their observations by
a greater reactivity of the structured water in the micelle and/or
by the relatively high rigidity of the enzyme molecule caused by
the surfactant layer
. The present fluorescence study
demonstrated that a rigid conformation for cellulases induced
by reverse micelles might result in the superactivity of cellulases.
The quite high rigid surfactant shells of reverse micelles
surround and confine the molecules of the solubilized cellulases,
thereby functioning as a buffer (or absorber) of excessive
fluctuations that may destroy the catalytic conformation in the
aqueous systems.
The activities of cellulases in Triton X-100 and AOT reverse
micelles reached a maximum at quite low
ω
0
, which is similar to
those of other enzymes
. For several enzymes, optimal
activity is observed in the range of
ω
0
from 8 to 13. For
example, the maximal activity of
α-chymotrypsin is found at ω
0
of 7
, for lysozyme at 13
, and for lipase at around 10
. The low
ω
0
probably causes the decrease in the
conformational flexibility of the proteins in reverse micelles.
The results of the pH study presented herein were only to
determine the apparent optimal pH value for cellulose
hydrolysis in reverse micelles. Because of the micro heteroge-
neity of reverse micelles, common expressions like pH must be
carefully redefined. For instance, pH in reverse micelles is a
controversial phenomenon. It has been clearly shown that the
acidity of a compound strongly depends on its location in a
reverse micelle because an acidity gradient is formed from the
interface to the micellar center
. Upon increasing the
ω
0
value in a reverse micellar medium, the pH of solutes in the
water pool begins to resemble that in bulk water. So far, there
still has been no accepted definition or measurement of pH in
reverse micelles.
Another important factor for enzymatic activities in reverse
micelles is the temperature. The stability of reverse micelles
themselves is temperature-sensitive. Therefore, when assaying
enzyme activity in reverse micelles at different temperatures,
these effects should be considered. A suitable high temperature
leads to the percolation and changes of the electrical conductivity
Fig. 5. Conformational changes of cellulases in AOT reverse micelles with
increasing
ω
0
values. A: intrinsic fluorescence emission spectra of cellulases under
different
ω
0
conditions (red lines), compared with that in aqueous systems (blue
line). The arrow represented the value of
ω
0
for AOT reverse micelles increased
gradually from 5.0 (the bottom red line) to 35.0 (the top red line). B: effects of
ω
0
on
the intrinsic fluorescence intensity (open circle) and emission maximum (solid
square) of cellulases. The excitation wavelength was 295 nm. Experiments were
performed at 50.0 °C in 0.02 M citric acid
–phosphate buffer at pH 6.0. (For
interpretation of the references to colour in this figure legend the reader is refred to
the web version of this article.)
1033
N. Chen et al. / Biochimica et Biophysica Acta 1764 (2006) 1029
–1035

of reverse micelles
, which is advantageous to the hydrolysis
of cellulose and the diffusion of the product. The activities of
cellulases in Triton X-100, AOT, and CTAB reverse micelles
increased with the increase of the temperatures at first, reaching a
maximum at a specified temperature, which is the most critical
point according to enzyme stability and subsequent activity in
reverse micelles as well as the stability of reverse micelles.
Since the surfactant is one of the components of reverse
micelles, the influence of surfactant on the activities of
cellulases cannot be neglected. Different kinds of surfactants
have different effects on the stability and activity of enzymes.
When the net charge on the enzyme molecule is opposite to that
on the surfactant layer, the enzyme molecule interacts with the
surfactant layer remarkably. The crystal structures of cellobio-
hydrolase and endoglucanase from Trichoderma reesei have
shown that the net charge on the surfaces of these cellulases is
positive
, while the net charge is negative on the AOT
composed layer and positive on the CTAB surfactant layer. So
there may be strong electrostatic attractions between the
opposite charges of the proteins and AOT, and strong
electrostatic repulsive forces between the same charges of the
proteins and CTAB, and the surfactant
–enzyme interactions and
the electrostatic repulsive forces may partially inhibit the
activities of cellulases in both reverse micelles. This could be an
explanation why the cellulose hydrolysis efficiencies in AOT
and CTAB reverse micelles were lower than that in Triton X-
100 reverse micelles.
It remains unclear whether the substrate, microcrystalline
cellulose, can be entrapped in these reverse micelles during the
enzymatic reaction. According to the induced fit model
, the
molecules of cellulases can be entrapped in the water pools of
these reverse micelles, surrounded by surfactant layers. In the
enzyme-containing reverse micelles, there is some structured
water used for enzymatic hydrolysis of the microcrystalline
cellulose, and cellulases maintain a rigid conformation.
Microcrystalline cellulose is typically an insoluble powder
having a mean size range of about 15 to 40
μm (
), much
larger than the size of a reverse micelle (with a diameter of
hundreds of angstroms). Therefore, the substrate could not be
entrapped in these reverse micelles during the enzymatic
reaction. In the present study, we assumed that these reverse
micelles could carry cellulases in their water pools and
“force”
them to
“dock to” as well as remain seated on cellulose through
diffusion. When the reaction was started, these reverse micelles
could
“force” the sugar formed “dock into” their water pools
through hydrogen bond and diffusion. Therefore, glucose
concentration in reverse micelles we measured is really from
the water pools of the reverse micelles.
It remains unclear whether the insoluble microcrystalline
cellulose affects the stability of the reverse micelles. In other
words, when the insoluble microcrystalline cellulose was mixed
with the enzyme-containing reverse micelles, does it disturb the
structure of the reverse micelle? Pileni has given a case in his
review
that if the solute is located in the hydrocarbon phase
or in the external interface, there may be no perturbation of the
characteristic radius of the reverse micelles. According to the
assumption that cellulases may
“dock to” and remain seated on
the microcrystalline cellulose, and the fact that cellulases show
“superactivity” in these three types of reverse micelles compared
with that in aqueous systems, we conclude that the insoluble
microcrystalline cellulose does not disturb seriously the
structure of the reverse micelles.
The frequency of diffusion-controlled collision of discrete
micelles is readily calculated by Smoluchowski equation to be
10
9
–10
11
M
− 1
s
− 1
, and the values for the exchange frequency of
guest molecules in the water pools of the reverse micelles have
also been reported in the range of 10
6
and 10
8
M
− 1
s
− 1
. This
means that only one in every thousand collisions leads to an
exchange and that reverse micelles communicate with each other
on a micro-to millisecond timescale. Such high collision
frequency and exchange rate may be one of the reasons that
lead to
“superactivity” of cellulases in reverse micelles. Since all
cellulases are dependent on diffusion to
“dock to” as well as
remain seated on the non-diffusing substrate, all parameters
affecting diffusion (temperature, surrounding solution, etc.) are
very important. An environment which forces the enzymes to be
seated on cellulose for a longer time is most likely to increase the
chance for more hydrolysis instead of loosening from the
cellulose chain. This environment should, on the other hand, also
increase the risk for product inhibition, although the concentra-
tion of cellobiose/glucose needed for inhibition is rather high.
It has been reported that the refolding of denatured protein in
reverse micelles is reminiscent of intracellular chaperone-aided
protein folding
. As enzyme molecules entrapped in the
water pool interact with reverse micelles, various amphiphilic
sequences may dock onto reverse micelles in a structurally
ordered and thus stable form. Such ordered and stable structure
may be another reason that leads to
“superactivity” of cellulases
in reverse micelles.
In summary, Triton X-100, AOT, and CTAB reverse micelles
are more effective than aqueous systems for cellulose hydroly-
sis, and cellulases show
“superactivity” in these three types of
reverse micelles compared with that in aqueous systems under
the same pH and temperature conditions. Information obtained
from the present study can enhance our understanding of the
mechanism for enzymatic hydrolysis of cellulose in low water
content systems, and is helpful to the application of cellulases to
the breakdown of cellulosic biomass into sugars for fermentation
to ethanol and other commodity products. The cost of detergents
and the problems with the presence of detergents in biological
material aimed for fermentation should also be considered. So
the extension of the study of enzymatic hydrolysis of cellulose to
other reverse micelles, which are more effective for cellulose
hydrolysis and less consumable and toxic to the environment,
should lead to future applications of this new technology in
industry.
Acknowledgements
We thank Prof. Allen P. Minton and Dr. Peter McPhie
(National Institute of Diabetes and Digestive and Kidney
Diseases, National Institutes of Health) for their critical reading
of the manuscript. This work was supported by National Natural
Science Foundation of China (Grants 30370309 and 90408012),
1034
N. Chen et al. / Biochimica et Biophysica Acta 1764 (2006) 1029
–1035

Project 863 (Grant 2004AA404260) from the Ministry of
Science and Technology of China, and Program for New
Century Excellent Talents in University (Grant NCET-04-0670)
from the Ministry of Education of China.
References
[1] E.A. Bayer, H. Chanzy, R. Lamed, Y. Shoham, Cellulose, cellulases and
cellusomes, Curr. Opin. Struct. Biol. 8 (1998) 548
–557.
[2] M.E. Himmel, M.F. Ruth, C.E. Wyman, Cellulase for commodity products
from cellulosic biomass, Curr. Opin. Biotechnol. 10 (1999) 358
–364.
[3] M. Schülein, Protein engineering of cellulases, Biochim. Biophys. Acta
1543 (2000) 239
–252.
[4] M.K. Baht, Cellulases and related enzymes in biotechnology, Biotechnol.
Adv. 18 (2000) 355
–383.
[5] J. Xiang, Y. Liang, N. Chen, Studies on the binding of sodium dodecyl
sulfate to cellulase by isothermal titration calorimetry and fluorescence
titration, Acta Chimi. Sin. 61 (2003) 1949
–1954.
[6] C. Divne, J. Ståhlberg, T. Reinikainen, L. Ruohonen, G. Pettersson, J.K.C.
Knowles, T.T. Teeri, T.A. Jones, The three-dimensional crystal structure of
the catalytic core of cellobiohydrolase I from Trichoderma reesei, Science
265 (1994) 524
–528.
[7] M. Sandgren, A. Shaw, T.H. Ropp, S. Wu, R. Bott, A.D. Cameron, J.
Ståhlberg, C. Mitchinson, T.A. Jones, The X-ray crysal structure of the
Trichoderma reesei family 12 endoglucanase 3, cel12A, at 1.9 Å resolution,
J. Mol. Biol. 308 (2001) 295
–310.
[8] L.R. Lynd, C.E. Wyman, T.U. Gerngross, Biocommodity engineering,
Biotechnol. Prog. 15 (1999) 777
–793.
[9] N.E. Levinger, Chemistry. Water in confinement, Science 298 (2000)
1722
–1723.
[10] P.L. Luisi, M. Giomini, M.P. Pileni, B.H. Robinson, Reverse micelles as
hosts for proteins and small molecules, Biochim. Biophys. Acta 947
(1988) 209
–246.
[11] K. Martinek, A.V. Levashov, Y.L. Khmelnitsky, N.L. Klyachko, I.V.
Berezin, Colloidal solution of water in organic solvents: a microheter-
ogeneous medium for enzymatic reactions, Science 218 (1982) 889
–891.
[12] B. Orlich, R. Schomacker, Enzyme catalysis in reverse micelles, Adv.
Biochem. Eng. Biotechnol. 75 (2002) 185
–208.
[13] K. Martinek, A.V. Levashov, N. Klyachko, Y.L. Khmelnitski, I.V. Berezin,
Micellar enzymology, Eur. J. Biochem. 155 (1986) 453
–468.
[14] K. Martinek, N.L. Klyachko, A.V. Kabanov, Y.L. Khmelnitsky, A.V.
Levashov, The second E.C. Slater lecture. Micellar enzymology: its
relation to membranology, Biochim. Biophys. Acta 981 (1989) 161
–172.
[15] A. Sanchez-Ferrer, F. Garcia-Carmona, Biocatalysis in reverse self-
assembling structures: reverse micelles and reverse vesicles, Enzyme
Microb. Technol. 16 (1994) 409
–415.
[16] F.M. Menger, K. Yamada, Enzyme catalysis in water pools, J. Am. Chem.
Soc. 101 (1979) 6731
–6734.
[17] S. Barbaric, P.L. Luisi, Micellar solubilization of biopolymers in organic
solvents. 5. Activity and conformation of
α-chymotrypsin in isooctane-
AOT reverse micelles, J. Am. Chem. Soc. 103 (1981) 4239
–4244.
[18] R. Wolf, P.L. Luisi, Micellar solubilization of enzymes in hydrocarbon
solvents, enzymatic activity and spectroscopic properties of ribonuclease
in n-octane, Biochem. Biophys. Res. Commun. 89 (1979) 209
–217.
[19] D.M. Updegraff, Semimicro determination of cellulose in biological
materials, Anal. Biochem. 32 (1969) 420
–424.
[20] P.L. Luisi, B. Steinmann-Hofmann, Activity and conformation of enzymes
in reverse micellar solutions, Methods Enzymol. 136 (1987) 188
–216.
[21] Y. Liang, F. Du, S. Sanglier, B.R. Zhou, Y. Xia, A. Van Dorsselaer, C.
Maechling, M.C. Kilhoffer, J. Haiech, Unfolding of rabbit muscle creatine
kinase induced by acid. A study using electrospray ionization mass
spectrometry, isothermal titration calorimetry and fluorescence spectros-
copy, J. Biol. Chem. 278 (2003) 30098
–30105.
[22] B.R. Zhou, Y. Liang, F. Du, Z. Zhou, J. Chen, Mixed macromolecular
crowding accelerates the oxidative refolding of reduced, denatured
lysozyme: Implications for protein folding in intracellular environments,
J. Biol. Chem. 279 (2004) 55109
–55116.
[23] H. Stamatis, A. Xenakis, F.N. Kolisis, Bioorganic reactions in microemul-
sions: the case of lipases, Biotechnol. Adv. 17 (1999) 293
–318.
[24] C.M.L. Carvalho, J.M.S. Cabral, Reverse micelles as reaction media for
lipases, Biochimie 82 (2000) 1063
–1085.
[25] S. Avramiotis, C.T. Cazianis, A. Xenakis, Interfacial properties of lecithin
microemulsions in the presence of lipase. A membrane spin-probe study,
Langmuir 15 (1999) 2375
–2379.
[26] P.K. Das, A. Chaudhuri, On the origin of unchanged lipase activity profile
in cationic reverse micelles, Langmuir 16 (2000) 76
–80.
[27] C.M.L. Carvalho, M.R. Aires-Barros, J.M.S. Cabral, Cutinase: from
molecular level to bioprocess development, Biotechnol. Bioeng. 66 (1999)
17
–34.
[28] E.P. Melo, R.P. Baptista, J.M.S. Cabral, Improving cutinase stability in
aqueous solution and in reverse micelles by media engineering, J. Mol.
Catal., B Enzym. 22 (2003) 299
–306.
[29] H. Zhu, Y.X. Fan, N. Shi, J.M. Zhou, Activity and conformation changes
of Chinese hamster dihydrofolate reductase in reverse micelles, Arch.
Biochem. Biophys. 368 (1999) 61
–66.
[30] C. Grandi, R.E. Smith, P.L. Luisi, Micellar solubilization of
biopolymers in organic solvents. Activity and conformation of
lysozyme in isooctane reverse micelles, J. Biol. Chem. 256 (1981)
837
–843.
[31] F. García-Carmona, R. Bru, A. Sánchez-Ferrer, Expression of enzyme
activity, in: A. Gornez-Puyou (Ed.), Biomolecules in Organic Solvents,
CRC Press, Boca Raton, Florida, 1992, pp. 163
–188.
[32] D. Han, J.S. Rhee, Characteristics of lipase-catalyzed hydrolysis of olive
oil in AOT-isooctane reversed micelles, Biotechnol. Bioeng. 28 (1986)
1250
–1255.
[33] E. Bardez, E. Monnier, B. Valeur, Absorption and fluorescence
probing of the interface of aerosol OT reversed micelles and micro-
emulsions, J. Colloid Interface Sci. 112 (1986) 200
–207.
[34] O.A. El Seoud, A.M. Chinelatto, Acid-base indicator equilibria in aerosol-
OT reversed micelles in heptane. The use of buffers, J. Colloid Interface
Sci. 95 (1983) 163
–171.
[35] M. Almgren, R. Jóhannsson, J.C. Eriksson, Polydispersity of AOT
droplets measured by time-resolved fluorescence quenching, J. Phys.
Chem. 97 (1993) 8590
–8594.
[36] M.P. Pileni, Water in oil colloidal droplets used as microreactors, Adv.
Colloid Interface Sci. 46 (1993) 139
–163.
[37] S.S. Atik, J.K. Thomas, Transport of photoproduced ions in water in oil
microemulsions: movement of ions from one water pool to another, J. Am.
Chem. Soc. 103 (1981) 3543
–3550.
[38] A.A. Vinogradov, E.V. Kudryashova, A.V. Levashov, W.M.A.M. van
Dongen, Solubilization and refolding of inclusion body proteins in reverse
micelles, Anal. Biochem. 320 (2003) 234
–238.
[39] M. Sakono, Y. Kawashima, H. Ichinose, T. Maruyama, N. Kamiya, M.
Goto, Direct refolding of inclusion bodies using reversed micelles,
Biotechnol. Prog. 20 (2004) 1783
–1787.
[40] W. Parker, P.S. Song, Protein structures in SDS micelle
–protein
complexes, Biophys. J. 61 (1992) 1435
–1439.
1035
N. Chen et al. / Biochimica et Biophysica Acta 1764 (2006) 1029
–1035
Document Outline
- Enzymatic hydrolysis of microcrystalline cellulose in reverse micelles
- Introduction
- Materials and methods
- Results
- Effect of ω0 on the activities of cellulases in Triton X-100, AOT, and CTAB reverse micelles
- Effect of temperature on the activities of cellulases in Triton X-100, AOT, and CTAB reverse mi.....
- pH dependence of the activities of cellulases in Triton X-100 and CTAB reverse micelles
- Conformational changes of cellulases with the increase of ω0 in AOT reverse micelles
- Discussion
- Acknowledgements
- References
Wyszukiwarka
Podobne podstrony:
Dance, Shield Modelling of sound ®elds in enclosed spaces with absorbent room surfaces
Proteomics of drug resistance in C glabrata
Microstructures and stability of retained austenite in TRIP steels
MMA Research Articles, Risk of cervical injuries in mixed martial arts
Development of financial markets in poland 1999
Antigone Analysis of Greek Ideals in the Play
Analysis of Police Corruption In Depth Analysis of the Pro
Low Temperature Differential Stirling Engines(Lots Of Good References In The End)Bushendorf
01 [ABSTRACT] Development of poplar coppices in Central and Eastern Europe
13 161 172 Investigation of Soldiering Reaction in Magnesium High Pressure Die Casting Dies
Mankiewicz Boczek, J i inni Bacteria homologus to Aeromonas capable of microcystin degradation (201
feminism and formation of ethnic identity in greek culture
86 1225 1236 Machinability of Martensitic Steels in Milling and the Role of Hardness
Formation of heartwood substances in the stemwood of Robinia
54 767 780 Numerical Models and Their Validity in the Prediction of Heat Checking in Die
Causes and control of filamentous growth in aerobic granular sludge sequencing batch reactors
Development of organic agriculture in Poland, Technologie
więcej podobnych podstron