Ž
.
Journal of Molecular Catalysis B: Enzymatic 5 1998 187–189
Highly selective transformation by plant catalysts
Hiroki Hamada
a,)
, Yuka Miyamoto
a
, Nobuyoshi Nakajima
b
, Tsutomu Furuya
a
a
Dept. of Applied Science, Okayama UniÕ. of Sci., 1-1 Ridai-cho, Okayama 700, Japan
b
Dept. of Nutritional Sci., Okayama Prefectural UniÕ., Soja, Okayama 719-11, Japan
Received 26 September 1997; accepted 29 November 1997
Abstract
This review outlines the recent progress in the biotransformation of foreign substrate by plant cultured suspension cells.
The reaction types and stereochemistry involved in the biotransformations are described. q 1998 Elsevier Science B.V. All
rights reserved.
Keywords: Biotransformation; Plant catalyst; Asymmetric reduction; Hydroxylation
1. Introduction
It is well known that plants are the source of
valuable products and some useful basic materi-
als such as cellulose, wood and rubber. In addi-
tion, secondary products such as terpenoids,
cardenolides,
coumarins,
anthraquinones,
flavonoids, glucosinolates and alkaloids are also
produced by plants and are used as drugs,
Ž
.
flavours, pigments food ingredient and agro-
chemicals. Hitherto, some secondary metabo-
lites from plant cultured suspension cells have
w
x
been produced 1,2 . However the formation and
accumulation of several secondary metabolites
does not normally occur in the plant cultured
suspension cells and it has proven difficult to
harness this potential to industrial processes.
To overcome these problems we have studied
the biotransformation of foreign substrates by
)
Corresponding author. E-mail: hamada@das.ous.ac.jp
plant cultured suspension cells. These cells have
the ability to specifically convert cheap and
plentiful substrates into more useful com-
pounds. More recently, many studies have fo-
cused on the ability of plant cultured suspension
cells to transform foreign substrates. This paper
summarizes the selectivity in the biotransforma-
tion of foreign substrate by plant cultured sus-
pension cells.
[ ]
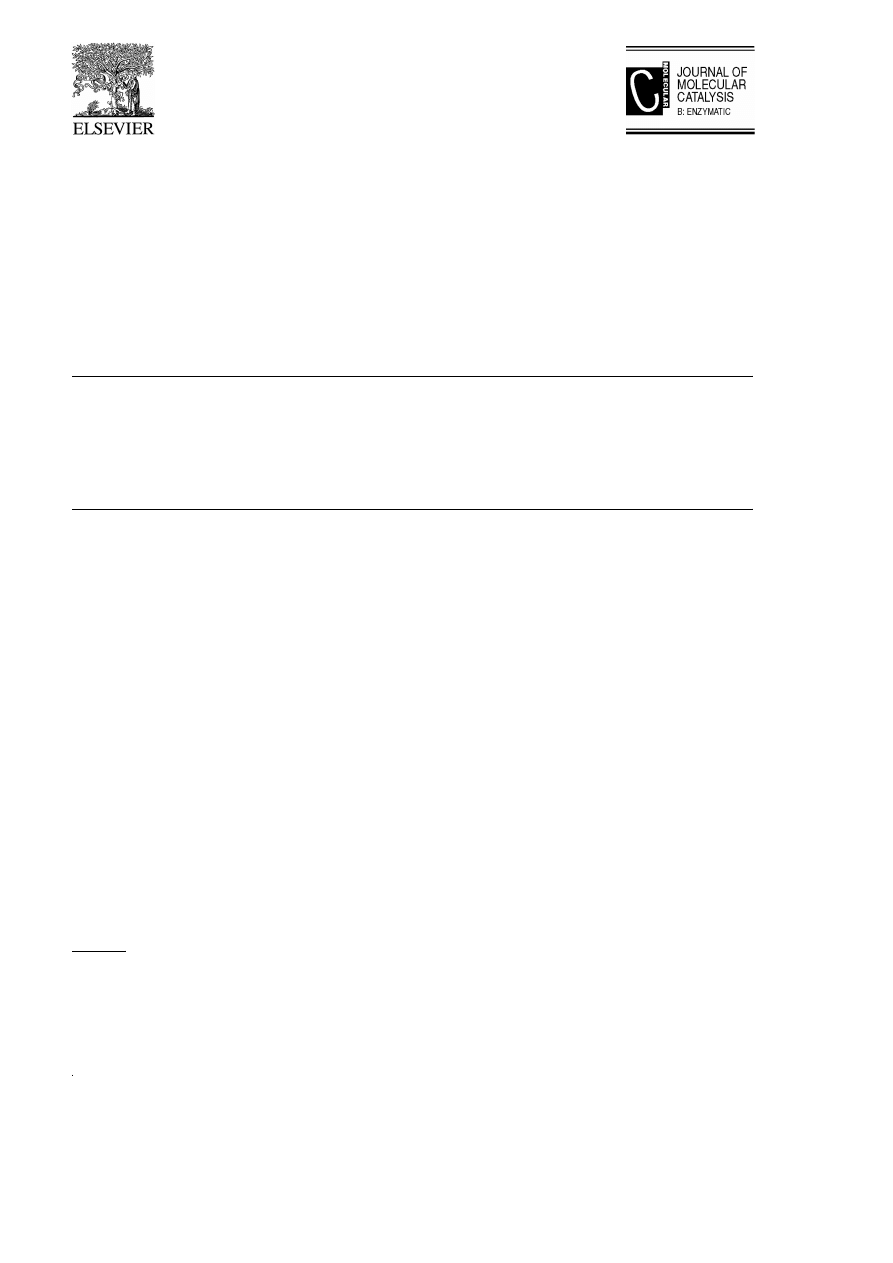
2. Biotransformation of b-keto ester 3
In the biotransformation of ethyl 2-methyl-
3-oxobutanoate by the cultured suspension cells
of Marchantia polymorpha and Glycine max,
ethyl 2-methyl-3-oxobutanoate was reduced di-
astereo- and enantio-selectively to the corre-
Ž .
sponding anti- and syn- S -hydroxyester by the
cultured suspension cells of M. polymorpha and
Ž
.
G. max, respectively Fig. 1 .
1381-1177r98r$19.00 q 1998 Elsevier Science B.V. All rights reserved.
Ž
.
PII: S 1 3 8 1 - 1 1 7 7 9 8 0 0 0 3 2 - 0
(
)
H. Hamada et al.r Journal of Molecular Catalysis B: Enzymatic 5 1998 187–189
188
Fig. 1. Biotransformation of ethyl 2-methyl-3-oxobutanoate.
Ž
.
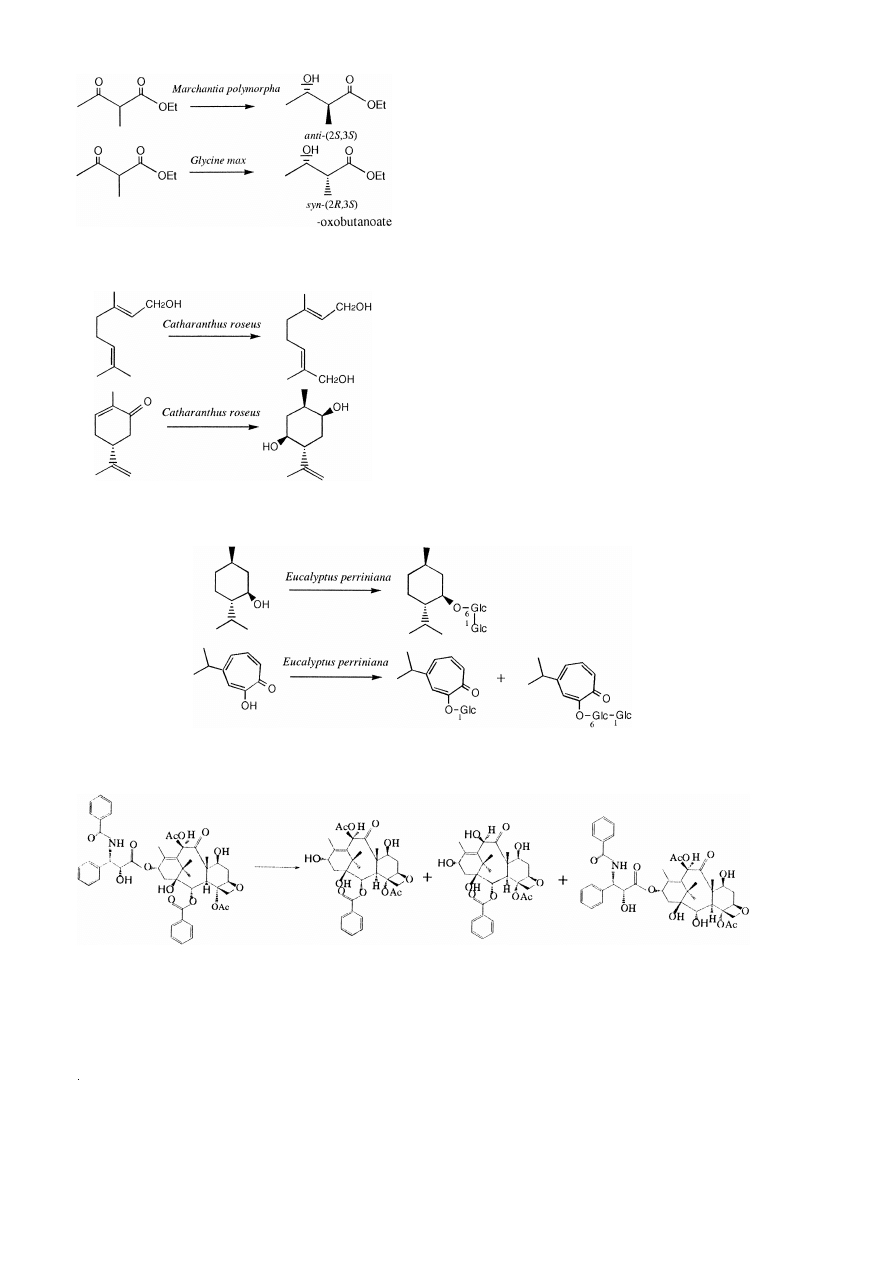
Fig. 2. Biotransformation of geraniol and y -carvone.
3. Biotransformation of terpenoids
We studied the biotransformation of carvones
and geraniol by the cultured suspension cells of
Catharanthus roseus and we found that they
Ž
.
Ž
.
hydroxylate allylic positions of
y
-,
q
-
carvones and geraniol and reduce double bonds
w x
and ketones 4 . The main product of geraniol is
10-hydroxygeraniol and the main products of
Ž
.
Ž
.
y
- and q -carvones are 5b-hydroxyneodi-
hydrocarveol and 5a-hydroxycarvone, respec-
Ž
.
tively Fig. 2 . From the study of biotransforma-
tion of monoterpenoids by the cultured suspen-
Ž
.
sion cells of C. roseus periwinkle it was found
that the cultured suspension cells of C. roseus
have the ability to hydroxylate regioselectively
at 10-position of geraniol and C-4 and C-5
positions of carvones.
Furthermore we investigated the biotrans-
Ž
formation of menthols and b-thujaplicin hino-
.
kitiol by the cultured suspension cells of Euca-
Ž
. w
x
lyptus perriniana eucalyptus
5–7 . In the bio-
Ž
.
Fig. 3. Biotransformation of y -menthol and b-thujaplicin.
Fig. 4. Biotransformation of taxol.

(
)
H. Hamada et al.r Journal of Molecular Catalysis B: Enzymatic 5 1998 187–189
189
transformation of menthols the main products of
Ž
.
Ž
.
Ž
.
Ž
.
y
- and
q
-menthols are
y
- and
q
-
menthol 3-O-b-
D
-gentiobiosides, respectively. In
the case of b-thujaplicin, the cultured cells of
E. perriniana glycosylate the hydroxyl group of
b-thujaplicin. From these results it was found
that eucalyptus cultured suspension cells per-
form regioselective glycosylation, terpenoids,
Ž
.
Ž
.
Ž
e.g., y -, q -menthols and b-thujaplicin see
.
Fig. 3 .
4. Biotransformation of taxol
Taxol, a highly functionalized diterpenoid
Ž
secondary product derived from yew
taxus
.
species which is now recognizing as the best
anticancer drug against human breast cancer.
More recently we studied biotransformation of
taxol by E. perriniana cell suspension cultures.
As shown in Fig. 4, taxol was converted to
baccatin III, 10-deacetylbaccatin III and 2-de-
w x
benzoyltaxol 8 . From this result, it is found
that E. perriniana cultured suspension cells hy-
drolyze the ester group at C-13 and then the
acetyl group at C-10 of the produced baccatin
III. On the other hand, the cells regioselectively
hydrolyze the benzoyl group at C-2 of taxol.
The reaction types and stereoselectivity in
biotransformation depends on the functional
group in the foreign substrates. Therefore, bio-
transformation by plant cultured suspension cells
Ž
.
plant catalysts can be considered as an impor-
tant tool for commercial andror large scale
production of secondary products and food in-
gredients.
References
w x
Ž
.
1 T. Furuya, Yakugaku Zasshi 108 1988 675.
w x
Ž
.
2 T. Furuya, in: T. Uozumi, T. Kodama Eds. , The Production
of Useful Compounds by Plant Cell Cultures, Maruzen Ad-
vanced Technology, Tokyo, 1993, p. 1.
w x
3 K. Nakamura, H. Miyoshi, T. Sugiyama, H. Hamada, Phyto-
Ž
.
chemistry 40 1995 1419.
w x
4 H. Hamada, H. Yasumune, Y. Fuchukami, T. Hirata, I. Sattler,
Ž
.
H.J. Williams, A.I. Scott, Phytochemistry 44 1997 615.
w x
5 T. Furuya, Y. Orihara, H. Miyatake, J. Chem. Soc., Perkin
Ž
.
Trans. 1 1989 1711.
w x
Ž
.
6 Y. Orihara, H. Miyatake, T. Yuruya, Phytochemistry 30 1991
1843.
w x
7 T. Furuya, Y. Asada, Y. Matsuura, S. Mizobata, H. Hamada,
Ž
.
Phytochemistry 46 1997 1355.
w x
8 H. Hamada, K. Sanada, T. Furuya, S. Kawabe, M. Jaziri, Nat.
Ž
.
Product Lett. 9 1996 47.
Wyszukiwarka
Podobne podstrony:
Efficient VLSI architectures for the biorthogonal wavelet transform by filter bank and lifting sc
[42]Oxidative breakage of cellular DNA by plant polyphenols A putative mechanism for anticancer prop
Efficient VLSI architectures for the biorthogonal wavelet transform by filter bank and lifting sc
Kruczkowska, Joanna Openness and Light in the Dialogue between the North and the South Selected Poe
Kruczkowska, Joanna Kings and Poets Self Irony in Selected Poems by George Seferis and Derek Mahon
Highly selective synthesis of menthols from citral in a one step process
Alchemy The Art of Transformation by Paracelsus
Efficient VLSI architectures for the biorthogonal wavelet transform by filter bank and lifting sc
Zabezpieczenia przekaźnikowe transformatorów SN nN by Gabcio
fitopatologia, Microarrays are one of the new emerging methods in plant virology currently being dev
Poetry by Dane Rudhyar Selection One
Phase transfer catalysis a new Nieznany
USŁUGI, World exports of commercial services by region and selected economy, 1994-04
[60]Selective degradation of oxidatively modified protein substrates by the proteasome
Practical Analysis Techniques of Polymer Fillers by Fourier Transform Infrared Spectroscopy (FTIR)
Catalysis by metal nanoparticles
Techniques to extract bioactive compounds from food by products of plant origin
więcej podobnych podstron