
Association Between Sexual Behavior
and Cervical Cancer Screening
Anthony M.A. Smith, Ph.D.,
1
Wendy Heywood, B.A. (Hons),
1
Richard Ryall, B.App.Sc. (Hons),
1
Julia M. Shelley, Ph.D.,
1,2
Marian K. Pitts, Ph.D.,
1
Juliet Richters, Ph.D.,
3
Judy M. Simpson, Ph.D.,
4
and Kent Patrick, Ph.D.
1
Abstract
Background: Not much is known about whether women who follow Pap testing recommendations report the
same pattern of sexual behavior as women who do not.
Methods: Data come from part of a larger population-based computer-assisted telephone survey of 8656 Aus-
tralians aged 16–64 years resident in Australian households with a fixed telephone line (Australian Longitudinal
Study of Health and Relationships [ALSHR]). The main outcome measure in the current study was having had a
Pap test in the past 2 years.
Results: Data on a weighted sample of 4052 women who reported sexual experience (ever had vaginal inter-
course) were analyzed. Overall, 73% of women in the sample reported having a Pap test in the past 2 years.
Variables individually associated with Pap testing behavior included age, education, occupation, cohabitation
status, residential location, tobacco and alcohol use, body mass index (BMI), lifetime and recent number of
opposite sex partners, sexually transmitted infection (STI) history, and condom reliance for contraception. In
adjusted analyses, women in their 30s, those who lived with their partner, and nonsmokers were more likely to
have had a recent Pap test. Those who drank alcohol at least weekly were more likely to have had a recent test
than irregular drinkers or nondrinkers. Women with no sexual partners in the last year were less likely to have
had a Pap test, and women who reported a previous STI diagnosis were more likely to have had a Pap test in the
past 2 years.
Conclusions: There are differences in Pap testing behavior among Australian women related to factors that may
affect their risk of developing cervical abnormalities. Younger women and regular smokers were less likely to
report a recent test. Screening programs should consider the need to focus recruitment strategies for these
women.
Introduction
P
articipation in Papanicolaou (Pap) Screening
has
been associated with a number of sociodemographic
characteristics, although not consistently.
1–12
Little research
has investigated interval between Pap tests and sexual be-
havior, however, which is surprising, given the primary cause
of cervical cancer is sexually acquired persistent human
papillomavirus (HPV).
13–16
In the United States, having had a
Pap test in the past year was more common among women
reporting one of the following: being £ 15 years at first sex,
having ‡ 10 lifetime sex partners, having a history of pelvic
inflammatory disease (PID), having a history of sexually
transmitted disease (STD), and with male sexual partners
having sex with other women in the past 12 months.
3
A Swedish study interviewed women on a cytology register,
finding that women with two or more sex partners during the
last 5 years were more likely to have had a recent Pap test,
whereas age at first sex and total number of sex partners were
not associated with screening. Not being screened was posi-
tively associated with the nonuse of oral contraceptives and
frequent condom use.
2
Having two or more sex partners was
associated with Pap testing in the last 3 years in a Spanish
study, although age at first sex was not.
17
Finally, a recent U.S.
1
Australian Research Centre in Sex, Health & Society, La Trobe University, Melbourne, Australia.
2
School of Health and Social Development, Deakin University, Melbourne, Australia.
3
School of Public Health and Community Medicine, University of New South Wales, Sydney, Australia.
4
Sydney School of Public Health, University of Sydney, Australia.
JOURNAL OF WOMEN’S HEALTH
Volume 20, Number 7, 2011
ª Mary Ann Liebert, Inc.
DOI: 10.1089/jwh.2010.2585
1091

study found women in same-sex relationships were less likely
to have had a Pap test in the last 3 years than were women in
relationships with men.
18
Given the importance of regular cervical screening and the
paucity of research on and inconsistency of findings about the
relationship between having a recent Pap test and sexual and
behavioral factors, further examination of this topic is essen-
tial. Here, we examine if women who report their last Pap test
as being no more than 2 years ago (the Australian recom-
mendation for Pap testing is every 2 years once sexually ac-
tive
19
) report the same patterns of sociodemographic and
behavioral characteristics, including sexual behavior, as do
women reporting longer time since their most recent
test. In Australia, all or part of the costs associated with Pap
testing are subsidized by the universal healthcare system,
Medicare.
20
Materials and Methods
Recruitment
The present study was a component of the Australian
Longitudinal Study of Health and Relationships (ALSHR).
21
The aim of the ALSHR was to document the natural history of
sexual and reproductive health of the Australian adult pop-
ulation. To be able to identify a statistically significant relative
risk of approximately 2.0 when exposures have a prevalence
of at least 10%–20% and outcomes have an incidence over 3
years of at least 2%–4.5%, the ALSHR surveyed 4290 men and
4366 women in all states and territories of Australia. Only
women who reported sexual experience (ever had vaginal
intercourse), the target population for regular Pap testing,
were included in the current analysis.
Survey
Women were asked a range of sociodemographic ques-
tions, including age, education, occupation, cohabitation sta-
tus, residential location, language spoken at home, country of
origin, and frequency of use of tobacco and alcohol. Because
of previously reported associations between body mass index
(BMI) and Pap testing,
1,6,7
BMI, calculated as weight in kilo-
grams divided by height in meters squared, was investigated
using self-reported weight and height. Four categories cor-
responding to current World Health Organization (WHO)
recommendations were used: underweight, BMI < 18.5 kg/m
2
;
normal weight, BMI = 18.5–24.9 kg/m
2
; overweight, BMI =
25–29.9 kg/m
2
; obese, BMI ‡ 30 kg/m
2
.
22
Potential variables of interest were sexual identity (het-
erosexual, homosexual/lesbian, bisexual), age at first vaginal
intercourse, lifetime number of opposite sex sexual partners
(any form of sexual experience), and number of opposite sex
sexual partners in the year before the interview (any form of
sexual experience). Additional variables, including history of
sexually transmitted infections (STIs) (self-reported diagnosis
of chlamydia, genital herpes, genital warts, syphilis, gonor-
rhea, PID, bacterial vaginosis, trichomoniasis, or crablice) and
current reliance on condoms for contraception, were also in-
vestigated because of associations with Pap testing in past
research.
2,3
Finally, Pap testing behavior was assessed by two ques-
tions: if the woman had ever had a Pap test or Pap smear, and,
if so, at what age she had her most recent Pap test. Using these
two questions and the respondent’s current age, we derived
a binary variable indicating whether the women had had a
Pap test in the previous 2 years. A 2-year cutoff was se-
lected as being consistent with Australian cervical screening
guidelines.
19
Procedure
Approval for this study was granted by the human research
ethics committees of La Trobe University, the University of
New South Wales, and Deakin University and was conducted
during 2004 and 2005 using computer-assisted telephone in-
terviewing. Participants were first contacted through random
digit dialing and, after having the study explained to them,
either gave their verbal consent to be interviewed or refused.
Of those contacted, 56.0% agreed to participate. Age was
the only selection criterion used in this study, with partici-
pants interviewed only if aged between 16 and 64 years.
Where two or more eligible respondents lived in a household,
one was randomly chosen to be interviewed. All interviews
were conducted in English.
Data analysis
All frequencies were weighted by household size and
rounded to the nearest integer. Chi-square tests were used to
assess bivariate associations between Pap testing in the past 2
years and demographic characteristics, age at first vaginal
intercourse, number of lifetime opposite sex partners, number
of opposite sex partners in the last year, STI history, and
condom reliance. Variables were included in the initial mul-
tivariate logistic regression model only if bivariate analysis
gave p < 0.25.
23
The least significant variables were sequen-
tially removed from the model until all remaining variables
were statistically significant at p < 0.05. Removed variables
were then checked one at a time to ensure they were not
significant in the final model. All analyses were weighted by
household size. p values reported are for design-based F sta-
tistics from the weight-adjusted chi-square tests (unadjusted)
and Wald tests (adjusted) of the overall effect of each factor.
All associations between variables are reported as relative
risk (RR) with 95% confidence intervals (CI) estimated from
log-binomial regression. Log-binomial was used instead of
logistic regression as odds ratios (ORs) overstate the RR when
the outcome of interest is common ( > 10%), as is the case for
Pap testing.
24
Analyses were conducted using Stata, version
10.1 (StataCorp, College Station, TX).
Results
Of the 4366 women who completed the survey, 4236 indi-
cated they had ever had vaginal intercourse. A further 40
women were excluded because of invalid answers to the two
Pap test questions. After these exclusions, the following ana-
lyses are based on a weighted sample of 4052 women, 2969
(73%) of whom reported having a Pap test in the previous
2 years.
Sociodemographic and lifestyle factors
Table 1 displays the percentage and number of women
from each sociodemographic background who reported
having a Pap test in the last 2 years. In bivariate analyses, age
was strongly associated with recent Pap testing. In addition,
1092
SMITH ET AL.

Table
1. Association Between Having a Pap Test in Previous 2 Years and Demographic, Lifestyle,
and Sexual Behavior Correlates
Total n
Yes %
Unadjusted RR (95% CI)
a
Adjusted RR (95% CI)
b
Age
c
p < 0.001
p < 0.001
16–19
176
28
0.39 (0.30-0.51)
0.36 (0.26-0.48)
20–29
633
76
0.92 (0.87-0.96)
0.92 (0.87-0.97)
30–39
943
85
-
-
40–49
1059
82
0.96 (0.93-1.00)
0.96 (0.93-0.99)
50–59
950
65
0.77 (0.73-0.81)
0.78 (0.74-0.82)
60–64
291
54
0.64 (0.58-0.71)
0.66 (0.60-0.74)
Level of education
p < 0.001
p = 0.88
Less than secondary
1113
68
-
-
Secondary/college
1927
73
1.08 (1.04-1.14)
1.01 (0.97-1.05)
Postsecondary
1012
79
1.15 (1.09-1.21)
0.99 (0.95-1.04)
Occupation
p < 0.001
p = 0.09
Professional
1393
77
-
-
Associate professional
1566
76
1.00 (0.96-1.04)
0.99 (0.95-1.03)
Tradesperson
175
70
0.92 (0.84-1.02)
0.97 (0.89-1.06)
Unskilled
844
65
0.87 (0.83-0.92)
0.96 (0.91-1.01)
Cohabitation status
c
p < 0.001
p = 0.006
Live together
2845
78
-
-
Live separately
452
68
0.90 (0.84-0.96)
0.96 (0.90-1.03)
No regular partner
756
60
0.81 (0.76-0.86)
0.90 (0.84-0.98)
Residential location
p = 0.03
p = 0.13
Metropolitan
2023
75
-
-
Regional/remote
1983
72
0.96 (0.93-1.00)
0.98 (0.95-1.01)
Language spoken at home
p = 0.34
p = 0.05
English
3904
73
-
-
Other
148
69
0.93 (0.84-1.04)
0.91 (0.81-1.02)
Country of origin
p = 0.32
p = 0.15
Australia
3188
74
-
-
Overseas
865
72
0.97 (0.93-1.01)
0.96 (0.92-1.00)
Tobacco use
c
p < 0.001
p < 0.001
None
3101
75
-
-
Less often than weekly
61
79
1.05 (0.92-1.19)
1.00 (0.90-1.11)
Weekly to daily
891
68
0.92 (0.87-0.96)
0.90 (0.86-0.95)
Alcohol use
c
p < 0.001
p = 0.006
None
986
69
-
-
Less often than weekly
1396
73
1.05 (1.00-1.10)
1.05 (1.00-1.10)
Weekly to daily
1669
76
1.10 (1.05-1.15)
1.08 (1.03-1.13)
Body mass index
p = 0.008
p = 0.26
Underweight
170
68
0.90 (0.81-1.00)
1.01 (0.92-1.11)
Normal
1941
75
-
-
Overweight
1089
75
0.98 (0.94-1.02)
1.01 (0.97-1.05)
Obese
740
69
0.90 (0.86-0.95)
0.95 (0.91-1.00)
Sexual identity
p = 0.19
p = 0.31
Heterosexual
3961
73
-
-
Homosexual
36
65
0.92 (0.71-1.19)
0.97 (0.72-1.31)
Bisexual
55
62
0.84 (0.68-1.03)
0.90 (0.72-1.12)
Age at first vaginal sex
p = 0.61
p = 0.72
< 16 years
581
72
-
-
‡ 16 years
3471
73
1.00 (0.95-1.05)
1.00 (0.96-1.05)
Lifetime number of opposite sex partners
p < 0.001
p = 0.15
1
987
68
-
-
2–5
1610
72
1.06 (1.01-1.11)
1.03 (0.98-1.08)
6–10
773
78
1.13 (1.07-1.20)
1.04 (0.99-1.10)
11 +
533
79
1.14 (1.08-1.21)
1.04 (0.98-1.11)
Number of opposite sex partners in last year
c
p < 0.001
p = 0.04
0
623
60
0.78 (0.73-0.84)
0.91 (0.84-0.99)
1
3139
76
-
-
2 +
276
70
0.94 (0.87-1.02)
1.00 (0.93-1.08)
STI history
c
p < 0.001
p = 0.004
No
3342
72
-
-
Yes
710
80
1.09 (1.05-1.14)
1.06 (1.02-1.09)
(continued)
CERVICAL SCREENING AND SEXUAL BEHAVIOR
1093
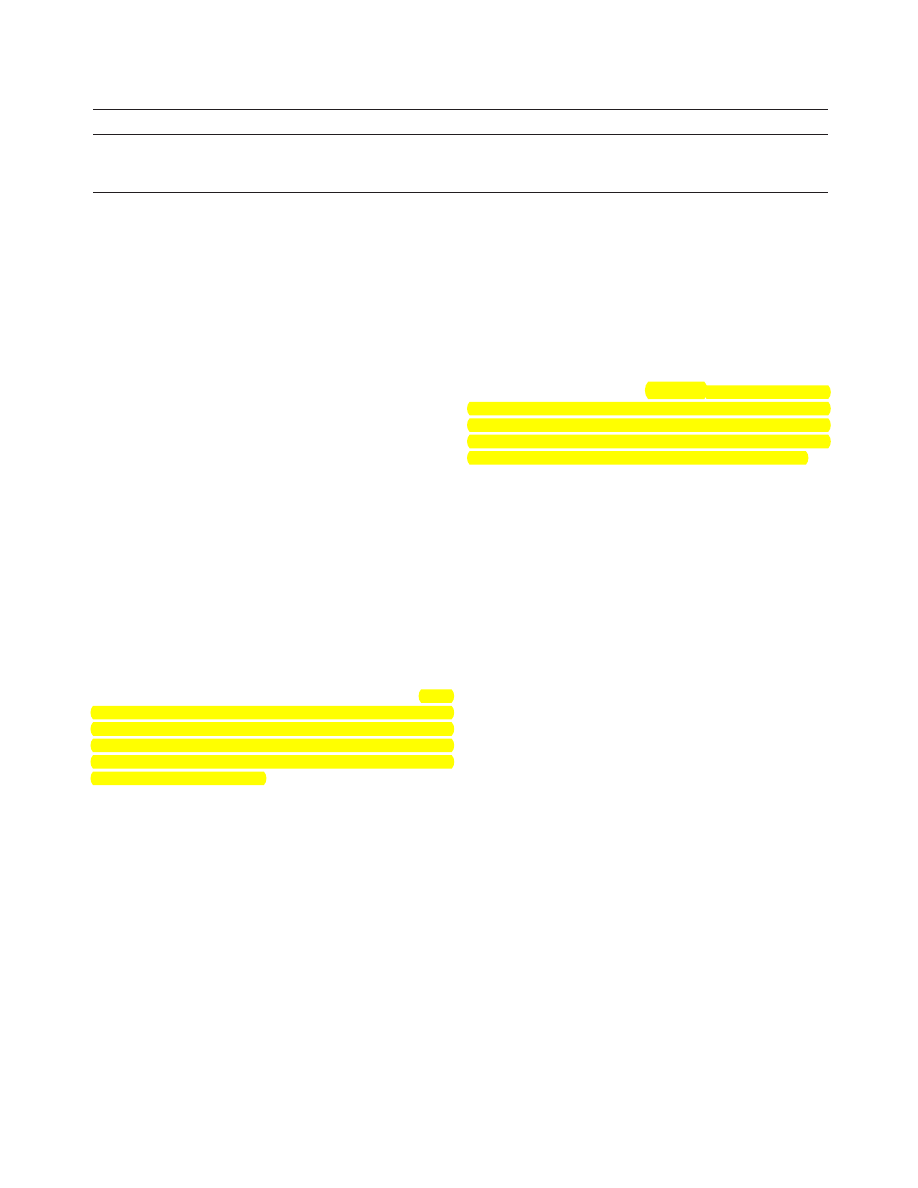
education, occupation, cohabitation status, residential loca-
tion, frequency of tobacco and alcohol use, and BMI were all
individually associated with having had a Pap test in the last
2 years.
The final multivariate model contained age, cohabitation
status, tobacco use, alcohol use, number of opposite sex
partners in the last year, and STI history. Although number of
opposite sex partners was initially removed from the model
because of nonsignificance, it became significant when added
back into the final model and was, therefore, retained. In
multivariate analysis, younger and older women were less
likely to have had a Pap test than women in their 30s. Those
without a regular partner were also less likely to have had a
recent Pap test, as were women who smoked regularly.
Conversely, women who drank alcohol regularly were more
likely to have had a Pap test than women who did not drink.
Sexual behavior and Pap testing
In bivariate analysis, sexual identity, age at first sex, and
current reliance on condoms were not associated with having
a Pap test in the past 2 years, whereas lifetime number of sex
partners, number of sex partners in the past 12 months, and
STI history were associated with Pap testing (Table 1). After
taking into account significant sociodemographic and sexual
behavior variables, women reporting no sexual partners in the
previous year were less likely to have had a recent Pap test,
and those reporting at least one diagnosed STI were more
likely to have had a Pap test.
Finally, we expected there would be a strong relationship
between number of lifetime sexual partners and number of
sexual partners in the last year. We, therefore, calculated ad-
justed RRs for lifetime number of partners with ( p = 0.15) and
without adjusting for number of partners in the last year
( p = 0.11). Overall, this made little difference to the signifi-
cance of the factors, the adjusted RRs, and CIs (data not
shown). For consistency with other excluded variables, life-
time number of partners was adjusted for all variables sig-
nificant in the final model, including number of sexual
partners in the last year.
Discussion
Age, cohabitation status, tobacco and alcohol use, number
of opposite sex partners in the last year, and a history of STIs
were all associated with whether a women had a Pap test in
the previous 2 years. Compared with women aged 30–39
years, all other age groups were less likely to have had a Pap
test. Lower screening rates among younger women are not
unique to Australia; in England in 2005, < 50% of women
aged 20–24 who were eligible for screening had had a Pap test
in the last 5 years.
25
Furthermore, research has demonstrated
a fall in screening among younger women in developed
countries in recent years.
26,27
Women > age 50 also received
less frequent tests or had stopped screening early. This was
found despite current guidelines in Australia recommending
women should not cease screening until the age of 70 and only
if they have had two normal tests within the last 5 years.
19
Women with no regular heterosexual partner were less
likely to have been screened in the last 2 years than those who
lived with their partner. Interestingly, women who drank
regularly were more likely to have had a Pap test, whereas
those who smoked regularly were less likely to have had a
Pap test in the last 2 years. Lower levels of Pap testing among
smokers is of some concern, given that smoking increases the
risk of progression of cervical abnormalities by causing ad-
ditional damage to cells already damaged by HPV.
15
Al-
though there has been concern expressed about access to Pap
testing services in rural and regional areas of Australia,
12
we
did not find any differences in likelihood of women having
had a recent test based on their residential location after ad-
justing for confounding factors, nor did we find differences
related to socioeconomic status (education and occupation)
or sexual identity. This likely reflects universal access to
healthcare in Australia.
20
Number of recent sexual partners was associated with Pap
testing over and above the influence of sociodemographic
factors. Women who had not been sexually active in the last
year with a male partner reported lower levels of Pap testing
than women with only one partner, suggesting women who
were not currently sexually active may not be being tested
regularly. Furthermore, there was not a strong association be-
tween number of sexual partners in the last year and lifetime
number of sexual partners. More research is needed to clarify
the relationship between Pap testing and number of sexual
partners, given that risk of acquiring HPV and cervical ab-
normalities increases through having more sexual partners.
15
Consistent with previous research,
3
women with a history
of STIs were more likely to have had a Pap test in the previous
2 years. It was important to investigate STI history, as it has
been suggested that STIs, such as Chlamydia infection, lead to
cervical inflammation, which could potentially facilitate a
concurrent HPV infection to become persistent, increasing the
chance of cervical cancer.
15
It is possible, however, that being
diagnosed with an STI was a result of coming for a Pap test
rather than being a characteristic of women more likely to
present for Pap testing.
Table
1. (Continued)
Total n
Yes %
Unadjusted RR (95% CI)
a
Adjusted RR (95% CI)
b
Condom reliance for contraception
p = 0.11
d
p = 0.63
No
3533
73
-
-
Yes
520
77
1.08 (1.04-1.14)
0.99 (0.95-1.04)
a
Unadjusted relative risk (RR) and 95% confidence intervals (CI) for having a Pap test in the previous 2 years vs. not having a Pap test.
b
Adjusted RR and 95% CI for having a Pap test in the previous 2 years vs. not having a Pap test. Adjusted for age group, cohabitation
status, frequency of tobacco and alcohol use, number of opposite sex partners in the last year, and history of sexually transmitted infections.
c
Variable significant in final model.
d
Discrepancies between p values and CIs arise because p values are from chi-square tests and RR and their 95% CIs are from log-binomial
regression.
STI, sexually transmitted infection.
1094
SMITH ET AL.
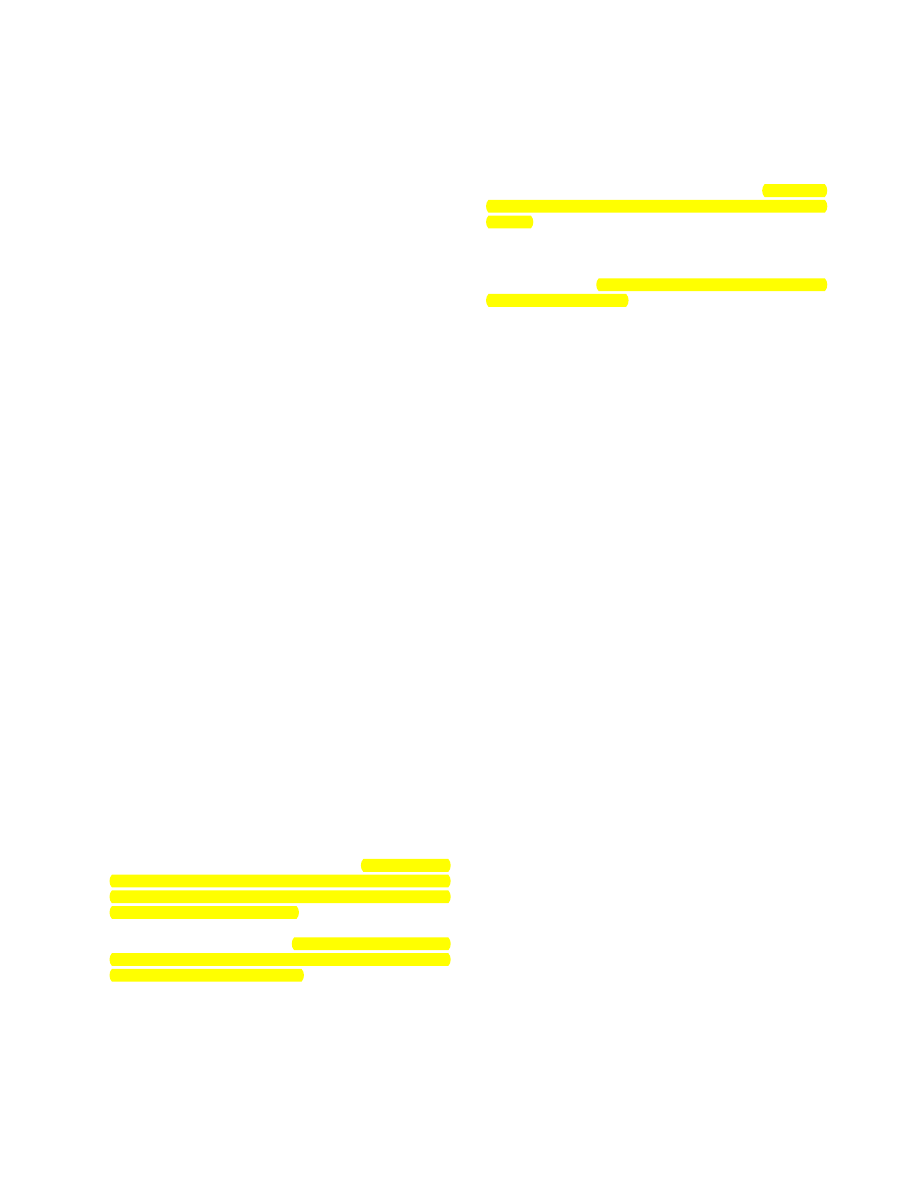
Finally, age at first sex was investigated, as it has been
linked to increased risk of acquiring HPV, either through being
an indicator for future high-risk sexual behavior or because of
greater vulnerability during adolescence.
15,28
Our findings are
consistent with past studies that have found no association
between age at first sex and Pap testing behavior.
2,17
The present study has a number of strengths and weak-
nesses. Strengths of the study were its large sample size and
wide age range. Our sample included women from nearly all
age groups that are recommended to receive Pap testing in
Australia. Limitations include the reliance on self-report and
possible social desirability bias. Recent systematic reviews
and meta-analyses of studies investigating the accuracy of
self-reports of Pap testing compared to medical records have
found women overreport their participation in screening over
a given time frame.
29,30
Furthermore, our study says nothing
about intervals between Pap tests, only time since last Pap
test, nor does the study comment on Pap testing and same sex
partners. Finally, although the response rate may be relatively
low (56%), this should not influence findings about Pap test-
ing behavior. Similar sampling procedures have been shown
to produce representative samples in previous studies.
31
It is
possibly, however, that compliant individuals are more likely
to both complete surveys and receive healthcare screening,
such as Pap testing.
This study demonstrates that certain groups of Australian
women are vulnerable to not receiving Pap tests within the
recommended time frame, every 2 years once sexually active.
Furthermore, it demonstrates that sexual behaviors have an
impact on whether a Pap test had been conducted in the past 2
years beyond previously reported sociodemographic factors.
Screening programs should consider the need to focus re-
cruitment strategies for these women, particularly younger
women, older women, and regular smokers.
Acknowledgments
The study was funded by the National Health and Medical
Research Council through grants 234409 and 487304. We
thank Jason Ferris for assistance with data management and
the Hunter Valley Research Foundation for data collection.
Disclosure Statement
No completing financial interests exist.
References
1. Blackwell DL, Martinez ME, Gentleman JF. Women’s com-
pliance with public health guidelines for mammograms and
Pap tests in Canada and the United States: An analysis of
data from the Joint Canada/United States Survey of Health.
Womens Health Issues 2008;18:85–99.
2. Eaker S, Adami HO, Sparen P. Reasons women do not at-
tend screening for cervical cancer: A population-based study
in Sweden. Prev Med 2001;32:482–491.
3. Hewitt M, Devesa S, Breen N. Papanicolaou test use among
reproductive-age women at high risk for cervical cancer:
Analyses of the 1995 National Survey of Family Growth. Am
J Public Health 2002;92:666–669.
4. Hewitt M, Devesa SS, Breen N. Cervical cancer screening
among U.S. women: Analyses of the 2000 National Health
Interview Survey. Prev Med 2004;39:270–278.
5. Luengo Matos S, Munoz van den Eynde A. Use of Pap
smear for cervical cancer screening and factors related with
its use in Spain. Aten Primaria 2004;33:229–234.
6. Maruthur NM, Bolen SD, Brancati FL, Clark JM. The asso-
ciation of obesity and cervical cancer screening: A systematic
review and meta-analysis. Obesity 2008;17:375–381.
7. Nelson W, Moser RP, Gaffey A, Waldron W. Adherence
to cervical cancer screening guidelines for US women aged
25–64: Data from the 2005 Health Information National
Trends Survey (HINTS). J Womens Health 2009;18:1759–
1768.
8. Ortiz AP, Hebl S, Serrano R, Fernandez ME, Suarez E,
Tortolero-Luna G. Factors associated with cervical cancer
screening in Puerto Rico. Prev Chronic Dis 2010;7:A58.
9. Peterson NB, Murff HJ, Cui Y, Hargreaves M, Fowke JH.
Papanicolaou testing among women in the Southern United
States. J Womens Health 2008;17:939–946.
10. Selvin E, Brett KM. Breast and cervical cancer screen-
ing: Sociodemographic predictors among white, black,
and Hispanic women. Am J Public Health 2003;93:
618–623.
11. Siahpush M, Singh GK. Sociodemographic predictors of Pap
test receipt, currency and knowledge among Australian
women. Prev Med 2002;35:362–368.
12. Wain G, Morrell S, Taylor R, Mamoon H, Bodkin N. Var-
iation in cervical cancer screening by region, socio-economic,
migrant and indigenous status in women in New South
Wales. Aust NZ J Obstet Gynaecol 2001;41:320–325.
13. Bosch FX, Lorincz A, Munoz N, Meijer C, Shah KV. The
causal relation between human papillomavirus and cervical
cancer. J Clin Pathol 2002;55:244–264.
14. Franco EL, Rohan TE, Villa LL. Epidemiologic evidence and
human papillomavirus infection as a necessary cause of
cervical cancer. J Natl Cancer Inst 1999;91:506–511.
15. Trottier H, Franco EL. The epidemiology of genital human
papillomavirus infection. Vaccine 2006;24:4–15.
16. Walboomers JMM, Jacobs MV, Manos MM, et al. Human
papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol 1999;189:12–19.
17. de Sanjose S, Corte´s X, Me´ndez C, et al. Age at sexual ini-
tiation and number of sexual partners in the female Spanish
population: Results from the AFRODITA survey. Eur J Ob-
stet Gynecol Reprod Biol 2008;140:234–240.
18. Buchmueller T, Carpenter CS. Disparities in health insurance
coverage, access, and outcomes for individuals in same-sex
versus different-sex relationships, 2000–2007. Am J Public
Health 2010;100:489–495.
19. Australian Government Department of Health and Ageing.
National Cervical Screening Program, 2009 Available at www
.cancerscreening.gov.au/internet/screening/publishing
.nsf/Content/NCSP-Policies-1 Accessed September 6,
2010.
20. Biggs A. Medicare. Background brief. Parliamentary Li-
brary, Parliament of Australia, 2003. Available at aph.gov
.au/library/intguide/SP/medicare.htm Accessed February
16, 2011.
21. Smith AMA, Pitts MK, Shelley JM, Richters J, Ferris J. The
Australian Longitudinal Study of Health and Relationships.
BMC Public Health 2007;7:139.
22. World Health Organization. Global database on body mass
index, 2006 Available at apps.who.int/bmi/index.jsp Ac-
cessed August 19, 2009.
23. Hosmer DW, Lemeshow S. Applied logistic regression. New
York: John Wiley, 1989.
CERVICAL SCREENING AND SEXUAL BEHAVIOR
1095

24. McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative
risk in cohort studies and clinical trials of common out-
comes. Am J Epidemiol 2003;157:940–943.
25. Lancuck L, Patnick J, Vessey M. A cohort effect in cervical
screening coverage? J Med Screen 2008;15:27–29.
26. Australian Institute of Health and Welfare. Cervical screen-
ing in Australia 2006–2007. Canberra: AIHW, 2009.
27. Lancucki L, Fender M, Koukari A, et al. A fall-off in cervical
screening coverage of younger women in developed coun-
tries. J Med Screen 2010;17:91–96.
28. Kahn JA, Rosenthal SL, Succop PA, Ho GYF, Burk RD.
Mediators of the association between age of first sexual in-
tercourse and subsequent human papillomavirus infection.
Pediatrics 2002;109:e5.
29. Howard M, Agarwal G, Lytwyn A. Accuracy of self-reports
of Pap and mammography screening compared to medical
record: A meta-analysis. Cancer Causes Control 2009;
20:1–13.
30. Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of
self-reported cancer-screening histories: A meta-analysis.
Cancer Epidemiol Biomarkers Prev 2008;17:748–757.
31. Smith AMA, Rissel CE, Richters J, Grulich AE, Visser RO.
Sex in Australia: The rationale and methods of the Austra-
lian Study of Health and Relationships. Aust N Z J Public
Health 2003;27:106–117.
Address correspondence to:
Anthony M.A. Smith, Ph.D.
Australian Research Centre in Sex, Health & Society
La Trobe University
215 Franklin Street
Melbourne, Victoria 3000
Australia
E-mail: anthony.smith@latrobe.edu.au
1096
SMITH ET AL.

Copyright of Journal of Women's Health (15409996) is the property of Mary Ann Liebert, Inc. and its content
may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express
written permission. However, users may print, download, or email articles for individual use.
Wyszukiwarka
Podobne podstrony:
Sexual behavior and the non construction of sexual identity Implications for the analysis of men who
Association between cancer screening behavior and family history among japanese women
Associations Between Symptoms of Borderline Personality Disorder, Externalizing Disorders,and Suicid
Forma, Ewa i inni Association between the c 229C T polymorphism of the topoisomerase IIb binding pr
Positive Urgency Predicts Illegal Drug Use and Risky Sexual Behavior
Childhood Maltreatment and Difficulties in Emotion Regulation Associations with Sexual and Relation
The Relationship of ACE to Adult Medical Disease, Psychiatric Disorders, and Sexual Behavior Implic
Posttraumatic Stress Symptomps Mediate the Relation Between Childhood Sexual Abuse and NSSI
Treaty of Peace Between The Allied and AssociatedPowers and Hungary And Protocol and Declaration, Si
MOU between Ralia Odinga and Muslims
Lumiste Betweenness plane geometry and its relationship with convex linear and projective plane geo
A Comparison between Genetic Algorithms and Evolutionary Programming based on Cutting Stock Problem
student sheet activity 3 e28093 object behavior and paths
“Knowing” Lolita Sexual Deviance and Normality in Nabokov’s Lolita
Bechara, Damasio Investment behaviour and the negative side of emotion
What are differences between being a ciwilian and military(1)
MOU between Ralia Odinga and Muslims
Resnick, Mike Between the Sunlight and Thunder
więcej podobnych podstron