Current Medicinal Chemistry, 2005, 12, 1395-1411
1395
Recent Developments in the Medicinal Chemistry of Cannabimimetic
Indoles, Pyrroles and Indenes
J.W. Huffman
*
and L.W. Padgett
H. L. Hunter Chemistry Laboratory, Clemson University, Clemson, South Carolina 29634-0973, USA
Abstract: During the development of new nonsteroidal anti-inflammatory agents, it was discovered that 1-
aminoalkyl-3-aroylindoles have affinity for the cannabinoid brain (CB
1
) receptor. This has led to the
development of over 100 cannabimimetic aminoalkylindoles, and the development of SAR for these compounds.
Later work demonstrated that the aminoalkyl moiety was not necessary, and could be replaced by a four- to six-
membered alkyl chain without loss of affinity. Investigation of these indoles led to the discovery of a CB
2
selective ligand, 3-(1-naphthoyl)-N-propylindole. Subsequent work has provided several additional CB
2
selective indoles. On the basis of a proposed pharmacophore for the cannabimimetic indoles, a series of pyrroles
and indenes were developed, some of which are potent cannabinoids. SAR for several series of pyrroles have
been developed. Two groups have described cannabimimetic indenes, which have been employed as rigid
models for the receptor interactions of cannabimimetic indoles with the CB
1
receptor. There is some evidence
that the indoles bind to a somewhat different site on the receptor than traditional cannabinoids, and interact
with the receptor primarily by aromatic stacking.
Keywords: Cannabinoid, aminoalkylindole, pyrrole, indene, receptor, indole.
INTRODUCTION
subsequent work from the same group confirmed that
compounds of this group bind to the cannabinoid brain
receptor, some with quite high affinity [11]. One rigid AAI,
WIN-55,212-2 (4), has particularly high affinity for the
cannabinoid receptors, and has been employed extensively in
a number of investigations into the pharmacology of this
group of compounds.
In the years following the elucidation of the structure of
∆
9
-tetrahydrocannabinol (
∆
9
-THC, 1), the principal
psychoactive constituent of marijuana (Cannabis sativa L.)
[1], a comprehensive set of structure-activity relationships
(SAR) based on the partially reduced dibenzopyran structure
of THC was developed [2-5]. Subsequently, a group at Pfizer
developed a series of very potent non-traditional
cannabinoids [6-9]. These SAR were extended to the Pfizer
compounds, which lack the dibenzopyran ring present in
traditional cannabinoids, but exhibit typical cannabinoid
pharmacology. CP-55,940 (2, DMH = 1,1-dimethylheptyl)
is representative of this group of compounds, and is almost
certainly the most well-known of these Pfizer non-traditional
cannabinoids.
H
3
C
N
O
N
O
O
H
3
C
N
N
O
O
H
3
CO
4
3
O
CH
3
CH
3
OH
H
3
C
H
3
C
OH
DMH
OH
HO
1
2
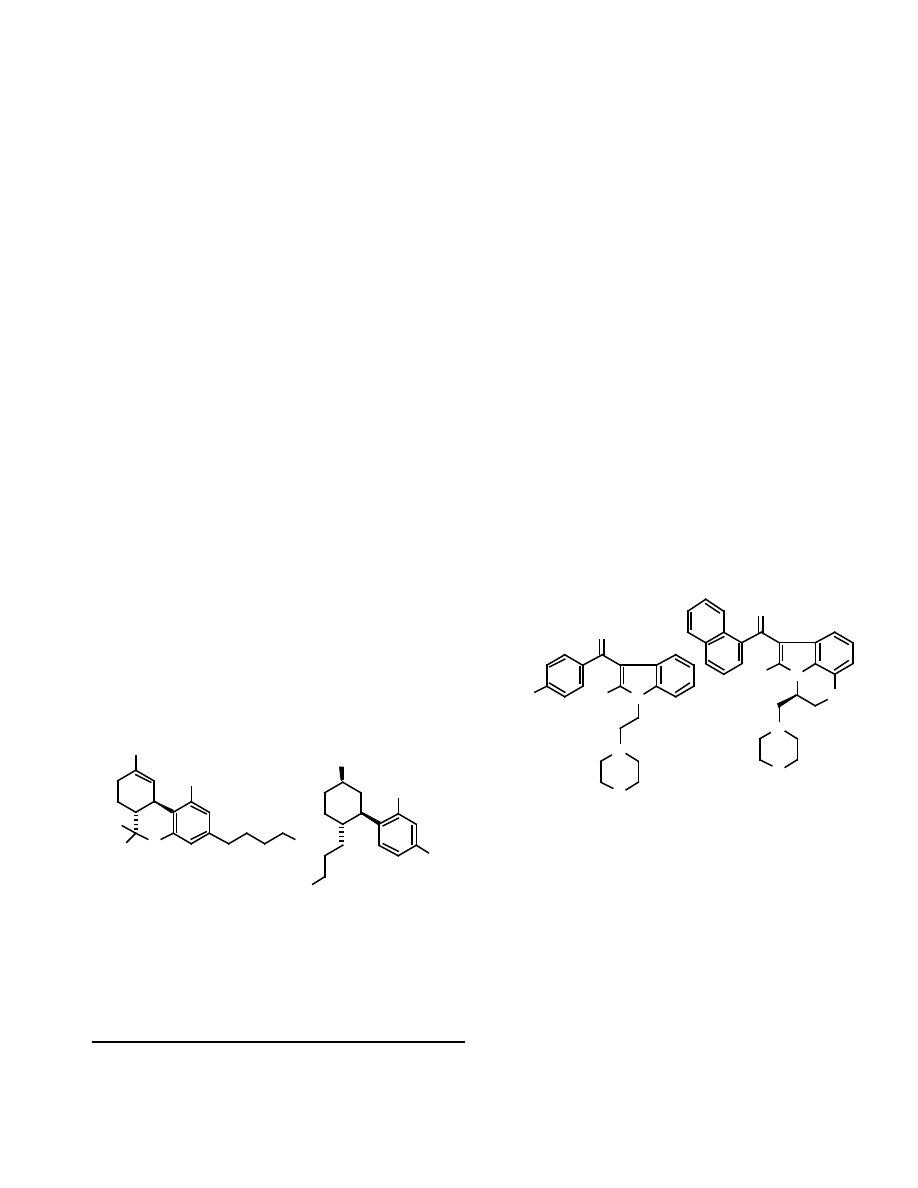
Fig. (2). Structures of pravadoline and WIN-55,212-2.
A few years later, Huffman et al. found that the
aminoalkyl portion of the molecule could be replaced by an
alkyl group to provide indole derivatives, such as JWH-007
(5), that exhibit typical cannabinoid pharmacology [12]. It
was also found that the benzene ring of the indole is not
essential for either receptor affinity or in vivo effects, and
cannabimimetic pyrrole derivatives (6, R = various alkyl
groups) were reported by the Clemson group [13]. A detailed
presentation of the SAR, of several of these cannabimimetic
indoles and pyrroles was published several years ago [14].
Several aminoalkylindenes structurally related to the
cannabimimetic AAIs have also been prepared, some of
which have high affinity for the cannabinoid receptors [15,
16].
In these compounds, the indole nitrogen is replaced
with a carbon atom to give 7 and similar compounds.
Fig. (1). Structures of
∆
9
-THC and CP 55,940.
In 1991, a group at Sterling Winthrop reported that
pravadoline (3) and related compounds inhibited the
contractions of the electrically stimulated mouse vas deferens
(MVD), are antinociceptive in vivo and inhibit adenylate
cyclase [10]. These aminoalkylindoles (AAIs) were found to
interact with a G-protein coupled receptor in the brain, and
*Address correspondence to this author at the H. L. Hunter Chemistry
Laboratory, Clemson University, Clemson, South Carolina 29634-0973,
USA; E-mail: huffman@clemson.edu
0929-8673/05 $50.00+.00
© 2005 Bentham Science Publishers Ltd.
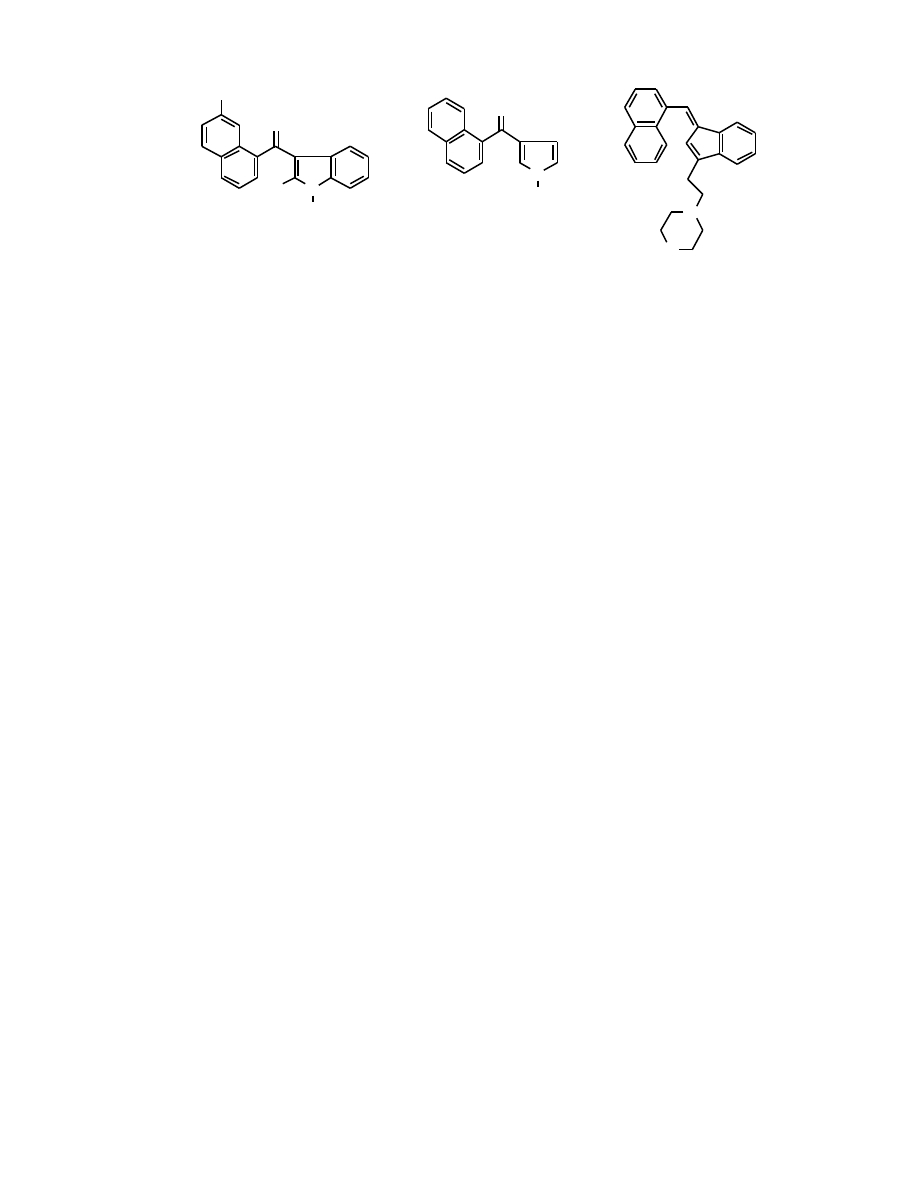
1396 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
N
O
R
H
3
C
N
O
R
N
O
5 R = n-C
5
H
11
, R' = H
8 R = n-C
3
H
7
, R' = H
9 R = n-C
3
H
7
, R' = CH
3
6
7
R'
Fig. (3). Structures of cannabimimetic indoles, pyrroles and indenes.
The chemistry and pharmacology of cannabimimetic
indoles, including the aminoalkylindoles, and the related
pyrroles and indenes were reviewed in 1999 [17]. The
present review will cover developments in this field from late
1998 through mid-2004. The similarities and differences in
the interactions of these compounds and traditional
cannabinoids with cannabinoid receptors will be discussed.
Prior to describing the medicinal chemistry and
pharmacology of these compounds, a brief introduction to
some of the more common methods employed to evaluate
the pharmacology of cannabinoids will be described.
[29]. This functional assay measures G-protein coupled
receptor activation using [
35
S]GTP
γ
S binding.
The most common in vivo protocol is a mouse model
[21], in which a battery of three or four procedures is
employed. These measure spontaneous activity (SA),
antinociception (as tail flick, TF), hypothermia (as decrease
in rectal temperature, RT) and catalepsy (as ring immobility,
RI). A variety of other procedures have been employed to
evaluate in vivo pharmacology, however the mouse model is
widely accepted, and this protocol was used for the majority
of the compounds discussed in this review that were
evaluated in vivo. An extensive review of cannabinoid
receptors and the pertinent bioassays has been published
recently [30].
PHARMACOLOGY METHODS
CANNABIMIMETIC INDOLES
A cannabinoid receptor in rat brain was first described in
1988 [18]. This G-protein coupled receptor was
subsequently cloned, and the primary structure was
determined [19]. A human cannabinoid central nervous
system receptor has been identified, which is virtually
identical (97% homology) to that from the rat [20]. It is
generally accepted that the overt physiological effects of
cannabinoids are mediated through this receptor [21, 22]. In
1993, a second human cannabinoid receptor which shows
44% identity to the brain receptor was identified and cloned
[23]. The transmembrane portion of this receptor shows 66%
identity to the central nervous system receptor. This receptor
is found primarily in the immune system [24]. The central
nervous system receptor is designated as the CB
1
receptor,
and that found principally in the immune system as the CB
2
receptor. Affinity for the CB
1
receptor measures the ability of
the substrate to displace a potent cannabinoid, usually
tritiated CP-55,940 (2), from its binding site in a membrane
preparation as described by Compton et al. [22].
Alternatively, the displacement of tritiated WIN-55,212-2 (4)
has been employed [11]. Affinity for the CB
2
receptor is
determined by the ability of a ligand to displace CP-55,940
(2) from its binding site in transfected cell lines [23, 25, 26]
or a mouse spleen membrane preparation [27]. An alternative
method for the in vitro evaluation of cannabinoid activity
employs the inhibition of electrically evoked contractions of
the isolated mouse vas deferens (MVD) [28].
Following the observation that pravadoline (3) inhibited
contractions of the electrically stimulated MVD [10], the
group at Sterling Winthrop carried out an extensive study of
the SAR of well over 100 related compounds [11, 31].
Subsequently, a series of sterically constrained AAIs was
prepared, and it was found that the most effective compound
in MVD activity was WIN-55,212-2 (4). These analogs were
also evaluated in a binding assay, in which the ability of the
ligand to displace tritiated 4 from its binding site in a rat
brain membrane preparation was measured. There was a
positive correlation between these binding data and the
MVD assay, and it was concluded that there were many
similarities both in vitro and in vivo, between the AAIs and
traditional cannabinoids. Confirmatory evidence that the
AAIs and traditional cannabinoids bind to the same receptor
was found by Kuster et al. who determined the affinities of
several AAIs for the WIN-55,212-2 binding site [32]. These
compounds were also evaluated in the standard behavioral
protocol for cannabinoids, and were found to exhibit typical
cannabinoid pharmacology [33].
The Winthrop group reported well over 100 various
cannabimimetic indoles, all of which belong to the sub-
group of aminoalkylindoles [10, 11, 31, 34]. These workers
stated that the necessary criteria for CB
1
receptor affinity
includes an aroyl group at C-3 of the indole, which, for
maximum affinity should be 1-naphthoyl or substituted 1-
naphthoyl. However, no SAR for aromatic substituents were
presented [31]. Other polycyclic aromatics at C-3 were less
effective than naphthalene. A number of substituted 3-
benzoylindoles were described, however, they had uniformly
Two functional assays are employed to determine the
efficacy of cannabinoid receptor ligands at both CB
1
and
CB
2
. One of these measures the agonist induced attenuation
of the ability of forskolin to stimulate the production of
cAMP [28]. The other assay measures the ability of a
cannabinoid receptor ligand to stimulate GTP
γ
S binding
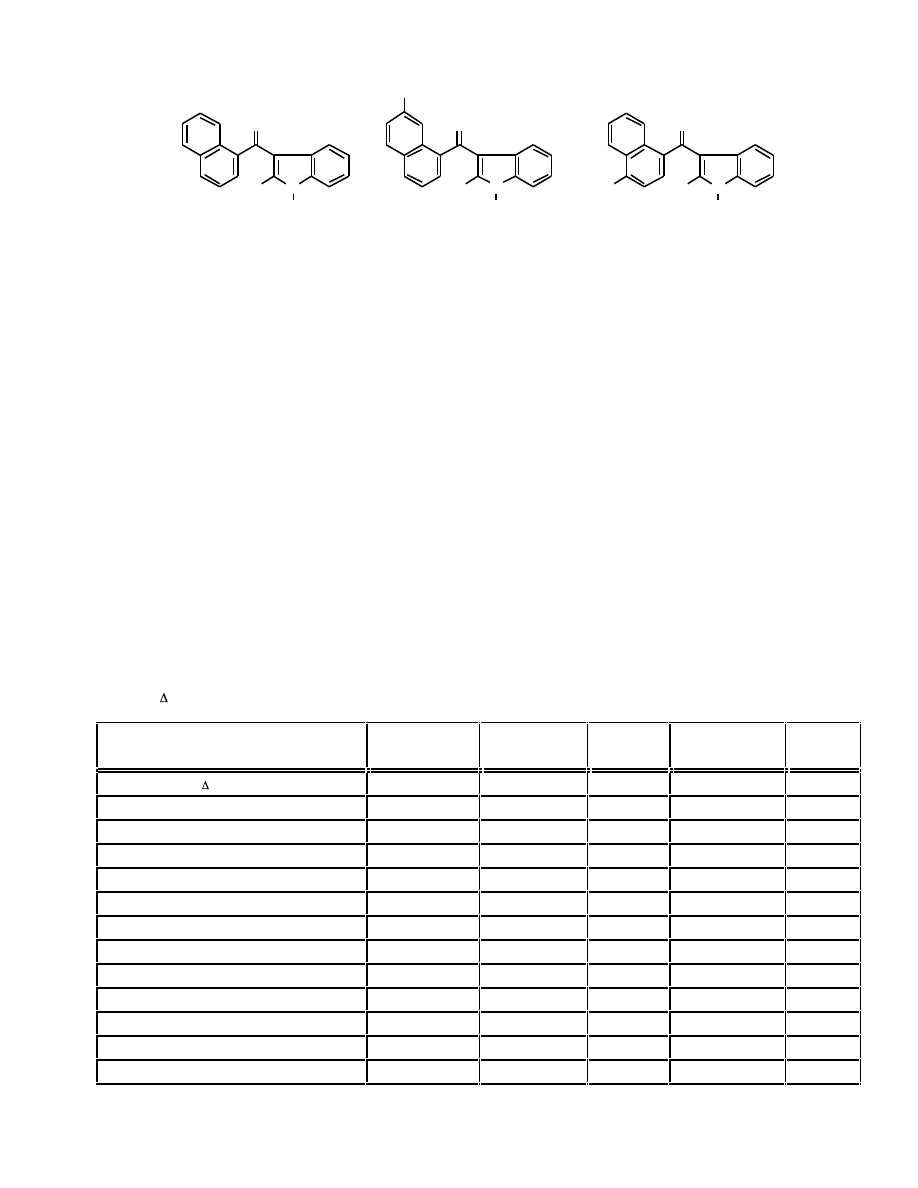
Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1397
N
R
O
R'
N
R
O
R'
CH
3
N
R
O
R'
CH
3
O
10 R' = H
13 R' = CH
3
11 R = H
14 R' = CH
3
12 R = H
15 R' = CH
3
Fig. (4). Structures of various cannabimimetic indoles. R = C
3
H
7
or C
5
H
11
.
low affinity for the receptor. At C-2 of the indole, a group
larger than methyl destroyed activity, and a hydrogen at C-2
was slightly superior to a methyl group. This group stated
that an aminoethyl group appended to the indole nitrogen
was optimum for cannabinoid activity, and that a cyclic
amine, such as morpholine, piperidine or thiomporpholine
was necessary as part of the aminoethyl group. Subsequent
to outlining these SAR, the Winthrop group employed
CoMFA to develop a pharmacophore for the aminoalkyl
subgroup of the cannabimimetic indoles [35]. These authors
concluded that it was probable that these indole analogues
and classical cannabinoids may partly overlap in their
interactions with the CB
1
receptor, however no specific areas
of commonality were suggested.
the aminoalkyl group is not necessary for cannabimimetic
activity, but that an alkyl substituent of four to six carbon
atoms on nitrogen is necessary [12, 14, 36]. CB
1
receptor
affinity and in vivo potency are maximized with an N-pentyl
substituent. For useful CB
2
receptor selectivity, high affinity
for this receptor with minimum affinity for the CB
1
receptor
is essential. Both JWH-015 and another CB
2
selective
indole, JWH-046, 1-propyl-2-methyl-3-(7-methyl-1-
naphthoyl)indole (9), have a propyl substituent on nitrogen,
and in the effort to develop CB
2
selective compounds,
emphasis was placed on preparing N-propyl indoles. For
developing SAR at the CB
1
receptor N-pentyl substituents
were employed. In agreement with the Winthrop data, a 2-
methyl group slightly attenuates activity relative to an
unsubstituted 2-position, and larger substituents lead to
inactivity [36]. Various 3-(1-naphthoyl) substituents were
investigated and it was found that a 7-methyl substituent has
little effect on activity, while a 4-methoxy group enhances
affinity for the CB
1
receptor [36]. Larger 4-alkoxyl groups
effectively render the compound inactive. Receptor affinities
and in vivo potencies of several N-propyl- and N-pentyl-3-(1-
naphthoyl)indoles (10), 3-(7-methyl-1-naphthoyl)indoles
In 1996, Showalter et al. reported that JWH-015, 1-
propyl-2-methyl-3-(1-naphthoyl)indole (8) has selective
affinity for the CB
2
receptor [25]. This observation, plus
efforts to establish SAR for the cannabimimetic indoles led
Huffman et al. to prepare a number of indole derivatives,
some of which are very potent cannabinoids [12, 14, 36].
The principal difference between the SAR described by this
group and that of the Winthrop workers is the finding that
Table 1.
Receptor Affinities (CB
1
and CB
2
) and In Vivo Potency for Cannabimimetic Indoles 10–15, WIN-55,212-2 (4) and
9
-THC (1)
Compound
K
i
(nM)
(CB
1
)
K
i
(nM)
(CB
2
)
ED
50
SA
µ mol/kg
a
TF
RT
9
-THC (1)
41±2
b
36±10
b
0.92
2.7
2.5
WIN-55212-2 (4)
1.9±0.1
b
0.3±0.2
b
0.25
c
0.82
c
23.0
c
8 (JWH-015)
383±72
b
14±5
b
18.7
84.7
99.1
10, R = n-Propyl (JWH-072)
1050±55
c
170±54
e
NT
NT
NT
10, R = n-Pentyl (JWH-018)
9.5±4.5
b
2.9±2.6
b
0.44
~0.09
1.7
13, R = n-Pentyl (JWH-007)
9.5±4.5
d
2.9±2.6
e
0.70
0.25
4.3
11, R = n-Propyl (JWH-076)
214±11
f
106±46
f
NT
NT
NT
14, R = n-Propyl (JWH-046)
343±38
e
16±5
e
No Max
No Max
No Max
14, R = n-Pentyl (JWH-048)
11±1
e
0.5±0.1
e
<2.7
e
<2.7
e
<2.7
e
12, R = n-Propyl (JWH-079)
63±3
e
32±6
e
5.5
f
10
f
12.3
f
15, R = n-Propyl (JWH-094)
476±67
e
97±3
e
NT
NT
NT
12, R = n-Pentyl (JWH-081)
1.2±0.1
e
12±2
e
0.15
f
0.22
f
0.17
f
15, R = n-Pentyl (JWH-098)
4.5±0.1
e
1.9±0.3
e
NT
NT
NT
a
Refs. 12, 14 and 36.
b
Ref. 25.
c
Ref. 33.
d
Ref. 14.
e
Ref. 37.
f
Huffman et al. unpublished work.
1398 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
N
O
R
R'
H
3
CO
N
O
R
C
2
H
5
16 R = C
3
H
7
, R' = C
4
H
9
17 R = C
4
H
9
, R' = C
5
H
11
18 R = C
5
H
11
, R' = C
6
H
13
19 R = C
3
H
7
to C
7
H
15
Fig. (5). Structures of 2-alkyl cannabimimetic indoles.
(11), 3-(4-methoxy-1-naphthoyl)indoles (12) and the
corresponding 2-methylindoles (13, 14, 15) are presented in
Table 1. Data for (
∆
9
-THC, 1) and WIN-55,212-2 are also
included in Table 1.
nM. None of the other compounds in this series have K
i
<
200 nM. With the exception of indole 16, the weak affinities
of these 2-alkylindoles is in accord with the generalization
that an alkyl group larger than methyl at the indole C-2
position leads to a loss of affinity for the CB
1
receptor [10,
11].
The CB
1
and CB
2
receptor affinities for 34
cannabimimetic indoles 10–15 were reported by Aung et al.
in 2000 [37]. The receptor affinities and some in vivo
pharmacology for these compounds had been presented
previously [17, 36, 38], and the data for those compounds in
which R = C
3
H
7
and R = C
5
H
11
are included in Table 1.
The N-alkyl substituents were varied from methyl through n-
heptyl, and in accordance with earlier work, it was found that
a nitrogen substituent of four to six carbon atoms provided
maximum affinity for the CB
1
receptor [12, 14. 17]. In
general, indoles with an n-propyl group appended to
nitrogen have little affinity for the CB
1
receptor, but JWH-
079, 1-propyl-3-(4-methoxy-1-naphthoyl)indole (12, R =
C
3
H
7
, Table 1) is an exception. This compound has
moderate affinity for the CB
1
receptor with K
i
= 63.0 ± 3.0
nM. None of the indole derivatives with N-methyl or N-
ethyl groups have appreciable affinity for the CB
1
receptor
[37].
The CB
2
receptor affinities for cannabimimetic indoles
10-15 follow the same general trend as their CB
1
receptor
affinities [37]. Those compounds with N-methyl or N-ethyl
substituents have little affinity for the CB
2
receptor, and the
CB
2
affinities tend to increase with increasing alkyl chain
length up to n-hexyl, then decrease by several orders of
magnitude with the n-heptyl nitrogen substituent. However,
within any series of indoles 10-15, there is much less
variation in the CB
2
receptor affinities for those compounds
with n-propyl to n-hexyl nitrogen substituents. For instance,
in compounds 15, the CB
1
receptor affinities range from 476
± 67 nM for JWH-094 (15, R = (C
3
H
7
) to 4.5 ± 0.1 nM for
JWH-098 (15, R = C
5
H
11
). In contrast, the CB
2
affinities for
the same series vary from 1.9 ± 0.3 nM for the pentyl
analog, JWH-098, to 97 ± 2.7 nM for the propyl compound,
JWH-094. While many of these compounds have some
selectivity for the CB
2
receptor, only two, JWH-015 (8) and
JWH-046 (9) have the combination of low affinity for the
CB
1
receptor and high affinity for the CB
2
receptor that is
necessary for a useful CB
2
selective ligand [37].
The1-alkyl-2-methyl-3-(4-methoxy-1-naphthoyl) indoles
(15, R = C
3
H
7
, JWH-094, R = C
4
H
9
, JWH-096 and R =
C
5
H
11
, JWH-098) were prepared by base catalyzed
alkylation of 2-methyl-3-(4-methoxy-1-naphthoyl) indole
(15, R = H). As a side reaction, alkylation of the 2-methyl
group occurred to give indoles 16-18. Unexpectedly, 1-
propyl-2-butylindole, JWH-093 (16) had rather high affinity
for the CB
1
receptor with K
i
= 40.7 ± 2.8 nM [37]. Neither
the 1-butyl-2-pentyl- (17, JWH-095) nor the 1-pentyl-2-
hexylindole (18, JWH-097) had significant affinity for the
CB
1
receptor. A series of 2-ethylindoles (19, R = C
3
H
7
through R = C
7
H
15
) was prepared [38]. In this series only
JWH-116, the n-pentyl compound (19, R = C
5
H
11
), has
significant affinity for the CB
1
receptor with K
i
= 52 ± 4.9
The rationale for replacing the N-aminoalkyl substituent
characteristic of the Winthrop cannabimimetic indoles with
an N-alkyl group was based upon molecular modeling
studies in which it was suggested that the naphthoyl
carbonyl would correspond to the phenolic hydroxyl of
traditional cannabinoids [12, 14]. The 7- and 8-positions of
the naphthalene were overlaid upon C-9 and C-10 of THC,
in which alignment the indole nitrogen corresponds to the
first carbon atom of the cannabinoid side chain (C-1'). For
the purpose of modeling, the side chain of THC was
O
CH
3
OH
H
3
C
H
3
C
CH
3
H
3
C
N
O
N(CH
3
)
2
O
32
33
a
b
c
d
e
f
a
b c
d
e
f
Fig. (6). Suggested alignment of traditional cannabinoids and AAIs [Refs. 12, 14].
Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1399
truncated by one carbon atom, and the morpholine was
modeled as a dimethylamino group (Fig. 6). The conclusion
was reached that if this alignment was correct, the
aminoalkyl group was not essential for cannabinoid activity
and could be replaced by other substituents. This alignment
was used to design the cannabimimetic indoles prepared by
the Clemson group [12, 14, 36, 37].
supports both the alignment suggested in Figure 6 and the
hydrogen bonding hypothesis. However, there is a body of
evidence suggesting that the cannabimimetic indoles
probably interact with the CB
1
receptor primarily by
aromatic stacking interactions [16, 43].
To test the hypothesis that hydrogen bonding
interactions involving the indole carbonyl group are not
important in the interaction of cannabimimetic indoles with
the CB
1
receptor, a series of 3-indolyl-1-naphthylmethanes
(21-26) was prepared and their affinities for the CB
1
receptor
were determined [44]. The CB
1
receptor affinities of these
compounds and the corresponding naphthoylindoles are
summarized in Table 2.
However, experiments using mutant CB
1
receptors led to
the suggestion that cannabimimetic indoles and traditional
cannabinoids, such as
∆
9
-THC (1) bind to different, but
partially overlapping sites on the receptor [39, 40]. These
experiments, combined with molecular modeling studies,
led to a hypothesis that a hydrogen bonding interaction of a
lysine on the third transmembrane domain of the CB
1
receptor is important in the binding of traditional
cannabinoids such as
∆
9
-THC (1), but not cannabimimetic
indoles [16, 41]. In order to test the hypothetical alignment
of traditional cannabinoids and cannabimimetic indoles that
was employed in the design of the indoles synthesized by
the Huffman group, a hybrid cannabinoid (20, JWH-161)
that combined structural features of both traditional
cannabinoids and cannabimimetic indoles was synthesized
[42]. This hybrid cannabinoid has high affinity for the CB
1
receptor, with K
i
= 19 ± 3 nM. The compound is potent in
vivo with ED
50
= 2.7, 6.2 and 3.0 µ mole/kg for spontaneous
activity (SA), tail flick (TF) and rectal temperature (RT),
respectively. It is slightly less potent than
∆
9
-THC (1 Table
1) in spontaneous activity and tail flick, but approximately
equivalent to THC in the rectal temperature measure of
hypothermia.
The CB
1
receptor affinities of indoles 21–23 (JWH-175,
JWH-184 and JWH-185), which are unsubstituted at the C-2
position of the indole, are essentially identical with K
i
= 17–
23 nM. These affinities are somewhat less than those of the
corresponding naphthoylindoles, JWH-018 (10, R =
C
5
H
11
), JWH-122 (27) and JWH-081 (12, R = C
5
H
11
),
which have K
i
= 9 ± 5, 0.69 ± 0.05 and 1.2 ± 0.1 nM,
respectively. Although the presence of a methyl or methoxy
group at the 4-position of the naphthoyl group causes a
significant increase in affinity for JWH-122 and JWH-127, in
the case of indolylnaphthylmethanes 21–23, there is little
effect on CB
1
receptor affinity associated with substitution at
C-4. In contrast to the naphthoylindole series in which a
methyl group at C-2 of the indole causes only slight
attenuation of CB
1
receptor affinity, indolylnaphthylmethanes
24–26 (JWH-196, JWH-194 and JWH-197, respectively)
have considerably reduced affinity with K
i
= 151–323 nM.
O
CH
3
OH
H
3
C
H
3
C
N
C
5
H
11
20
A structural characteristic of the Winthrop
cannabimimetic indoles is the presence of an aminoalkyl
group appended to the indole nitrogen [10, 31]. This
aminoalkyl group could conceivably interact with the CB
1
receptor by hydrogen bonding as suggested by Xie et al.
[45]. To explore this possibility, aminoalkylindoles 29
(JWH-195), 30 (JWH-192) and 31 (JWH-199), which lack
the carbonyl oxygen, and the corresponding naphthoyl
analogs, JWH-200 (32), JWH-193 (33) and JWH-198 (34),
were prepared and their affinities for the CB
1
receptor were
determined. The CB
1
receptor affinities for
aminoalkylindoles 29–34 are included in Table 2.
Fig. (7). Structure of JWH-161.
The alignment of
∆
9
-THC and cannabimimetic indoles
depicted in Fig. (6) includes the hypothesis that the indole
carbonyl interacts with the CB
1
receptor by hydrogen
bonding and was supported by the receptor affinities and in
vivo potencies of the cannabimimetic indoles, both those
with aminoalkyl groups and those with alkyl side chains.
Also, the high affinity and in vivo potency of JWH-161 (20)
Although there is little variation in CB
1
receptor affinity
as a function of substitution at C-4 of the naphthalene moiety
in indoles 21–23, there is considerable variation in the
affinities of aminoalkylindoles 29–31. The unsubstituted
analog, JWH-195 (29), has modest affinity for the CB
1
N
C
5
H
11
R
R'
N
H
3
C
O
C
5
H
11
R
21 R, R' = H
22 R = CH
3
, R' = H
23 R = OCH
3
, R' = H
24 R = H, R' = CH
3
25 R = CH
3
, R' = CH
3
26 R = OCH
3
, R' = CH
3
27 R = H
28 R = CH
3
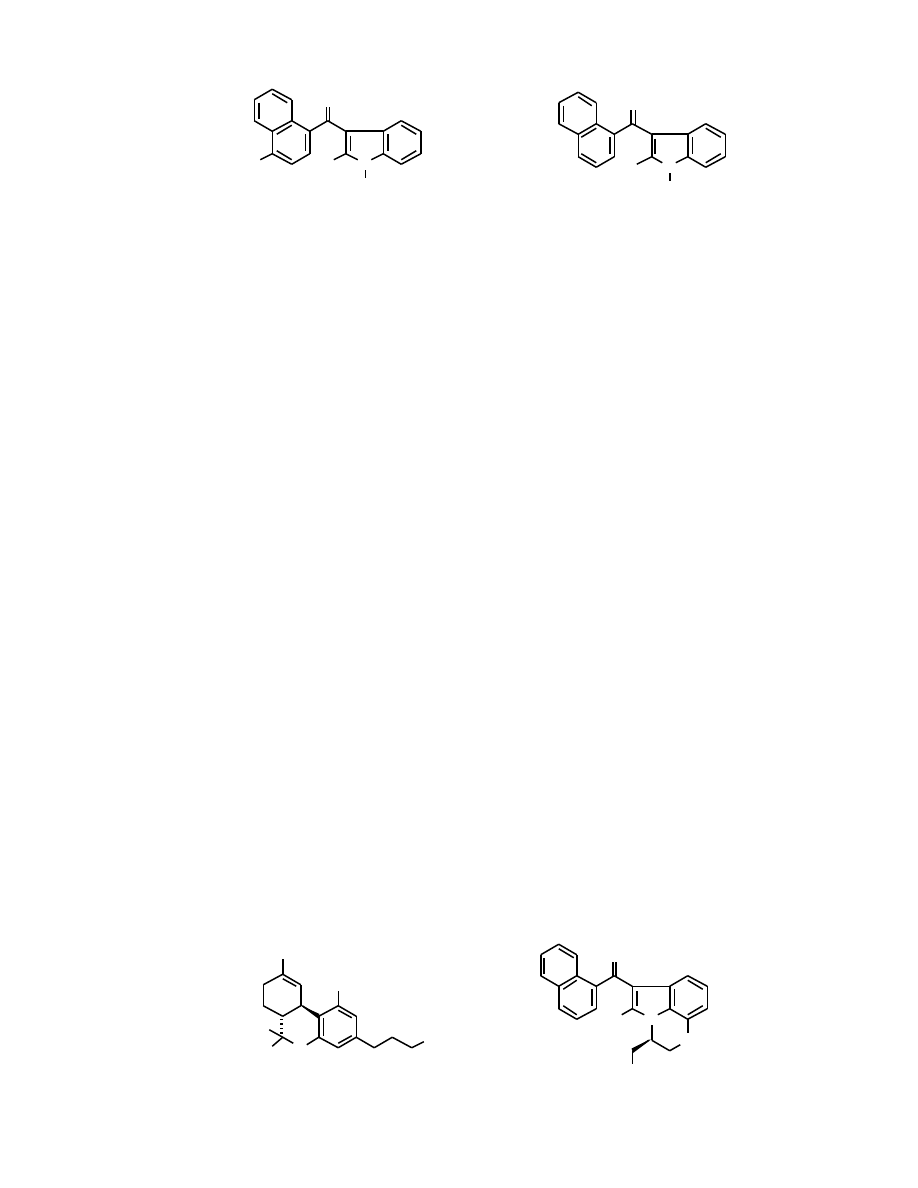
Fig. (8). Structures of indolylnaphthylmethanes and 3-(4-methyl-1-naphthoyl)indoles.
1400 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
Table 2. CB
1
Receptor Affinities (mean ± SEM) of Cannabimimetic Indoles 10, 12, 15, and 21–34 [44]
Compound
K
i
(nM)
1-Pentyl-1H-indol-3-yl-(1-naphthyl)methane (21, JWH-175)
22 ± 2
1-Pentyl-1H-indol-3-yl-(4-methyl-1-naphthyl)methane (22, JWH-184)
23 ± 6
1-Pentyl-1H-indol-3-yl-(4-methoxy-1-naphthyl)methane (23, JWH-185)
17 ± 3
2-Methyl-1-pentyl-1H-indol-3-yl-(1-naphthyl)methane (24, JWH-196)
151 ± 18
2-Methyl-1-pentyl-1H-indol-3-yl-(4-methyl-1-naphthyl)methane (25, JWH-194)
127 ± 19
2-Methyl-1-pentyl-1H-indol-3-yl-(4-methoxy-1-naphthyl)methane (26, JWH-197)
323 ± 28
1-Pentyl-3-(1-naphthoyl)indole (10, R = C
5
H
11
, JWH-018)
9 ± 5
a
1-Pentyl-3-(4-methyl-1-naphthoyl)indole (27, JWH-122)
0.69 ± 0.05
1-Pentyl-3-(4-methoxy-1-naphthoyl)indole (12, R = C
5
H
11
, JWH-081)
1.2 ± 0.1
b
2-Methyl-1-pentyl-3-(1-naphthoyl)indole (13, R = C
5
H
11
, JWH-007)
9.5 ± 4.5
a
2-Methyl-1-pentyl-3-(4-methyl-1-naphthoyl)indole (28, JWH-149)
5.0 ± 2.1
2-Methyl-1-pentyl-3-(4-methoxy-1-naphthoyl)indole (15, R = C
5
H
11
, JWH-098)
4.5 ± 0.1
b
1-[2-(4-Morpholino)ethyl]-1H-indol-3-yl-1-naphthylmethane (29, JWH-195)
113 ± 28
1-[2-(4-Morpholino)ethyl]-1H-indol-3-yl-(4-methyl-1-naphthyl)methane (30, JWH-192)
41 ± 13
1-[2-(4-Morpholino)ethyl]-1H-indol-3-yl-(4-methoxy-1-naphthyl)methane (31, JWH-199)
20 ± 2
1-[2-(4-Morpholino)ethyl]-3-(1-naphthoyl)indole (32, JWH-200)
42 ± 5
1-[2-(4-Morpholino)ethyl]-3-(4-methyl-1-naphthoyl)indole (33, JWH-193)
6 ± 1
1-[2-(4-Morpholino)ethyl]-3-(4-methoxy-1-naphthoyl)indole (34, JWH-198)
10 ± 2
a
Ref. 14.
b
Ref. 37.
receptor with K
i
= 113 ± 28 nM. The 4-methylnaphthyl
compound, JWH-192 (30) has significantly greater affinity,
K
i
= 41 ± 13 nM and the 4-methoxy analog has still greater
affinity with K
i
= 20 ± 2 nM. Naphthoyl aminoalkylindoles
32–34 had been reported previously by the Winthrop group
who observed the same trend in relative CB
1
receptor
affinities, with the 4-methyl-1-naphthoyl- (33) and 4-
methoxy-1-naphthoylindoles (34) having greater affinity than
the unsubstituted analog ( 32) [31].
interact with the receptor by hydrogen bonding. To explore
this possibility, E-naphthylideneindene 35 (JWH-176) was
prepared and was found to have high affinity for the CB
1
receptor with K
i
= 26 ± 4 nM [44].
H
C
5
H
11
35
N
R'
N
O
X
29 X = H
2
, R' = H
30 X = H
2
, R' = CH
3
31 X = H
2
, R' = OCH
3
32 X = O, R' = H
33 X = O, R' = CH
3
34 X = O, R' = OCH
3
Fig. (10). Structures of JWH-176.
The high CB
1
receptor affinities of indoles 21–23, 30, 31
and indene 35 strongly support the hypothesis that
cannabimimetic indoles and related CB
1
receptor ligands
interact with the receptor primarily by aromatic stacking [16,
43]. In particular, the high affinity of indene 35, a
hydrocarbon, for the CB
1
receptor provides compelling
evidence against hydrogen bonding interactions playing a
major role in the binding of these ligands [44].
Fig. (9). Structures of aminoalkylindoles.
E-Naphthylideneindene 7 has good affinity for the CB
1
receptor with K
i
= 2.72 ± 0.22 nM, and modeling studies
support the hypothesis that 7 and other cannabimimetic
indenes interact with the receptor by aromatic stacking
interactions [16]. However, there is at least a formal
possibility that the morpholino nitrogen or oxygen may
Indoles 21–23, 30 and 31, which are unsubstituted at the
C-2 position, have significant affinity for the CB
1
receptor,
however 2-methylindoles, 24–26, have little affinity, in

Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1401
contrast to the 3-(1-naphthoyl)indole series in which the 2-
methylindoles have only slightly less affinity for the CB
1
receptor than the unsubstituted compounds. There appeared
to be no a priori explanation for the poor receptor affinities
of indoles 24–26, in comparison to the significant affinities
of indoles 21–23, 30 and 31. To obtain insight into the
origin of these differences in receptor affinity, molecular
modeling and receptor docking studies of indoles JWH-081
(12, R' = C
5
H
11
), its 2-methyl congener, JWH-098 (15, R'
= C
5
H
11
) and the corresponding pair of naphthylmethanes
JWH-185 (23) and JWH-197 (26) were carried out [44].
rather than hydrogen bonding interactions, are the primary
interactions for cannabimimetic indoles at CB
1
[41].
Cannabimimetic indoles 12, R' = C
5
H
11
and 15, R' =
C
5
H
11
, 23 and 26 are structurally related to WIN-55,212-2
(4), and it was hypothesized that the TMH 3-4-5-6 region of
CB
1
receptor would also be the binding region for these
ligands. Indoles 12, R' = C
5
H
11
and 15, R' = C
5
H
11
were
docked in this region of the receptor in their lowest energy s-
trans conformations, and indoles 23 and 26 were docked in
this same region using the global minimum energy
conformer of each.
3-(1-Naphthoyl)indoles have been shown to exist in two
principal conformations, either s-cis or s-trans. In the s-cis
conformation, which predominates when the C-2 substituent
is methyl, the carbonyl oxygen is near C-2 with the
naphthalene ring stacked over C-4. In the s-trans
conformation, which predominates when the C-2 substituent
is hydrogen, the carbonyl oxygen is near C-4 of the indole,
and the naphthalene ring is near C-2 [16]. Consistent with
these earlier findings JWH-081 (12, R' = C
5
H
11
), with a
hydrogen at C-2, has an s-trans conformation as the global
minimum energy conformer. In JWH-098 (15, R' = C
5
H
11
),
calculations revealed that the lowest energy conformer is an
s-cis conformer. The lowest energy s-trans conformation of
JWH-098 was found to be 1.22 kcal/mol higher in energy
than the global minimum s-cis conformer [44].
A hydrophobic binding pocket comprised of a valine, an
isoleucine and a phenylalanine on helix-3, plus leucine and
an isoleucine on helix-6 were identified, which permitted
simultaneous interaction of the indole and naphthalene rings
with the aromatic residues in the TMH 3-4-5-6 region of the
CB
1
receptor active state (R*). When the alkyl chain on
nitrogen was docked in this pocket, JWH-081 (12, R' =
C
5
H
11
) and JWH-098 (15, R' = C
5
H
11
) found aromatic
stacking interactions with two tryptophan residues on helix-
5 of the CB
1
receptor. In this docking position, the C-2
methyl group of JWH-098 would cause no loss of affinity,
since the C-2 methyl group occupies an open space in the
receptor binding pocket.
The global minimum energy conformers of JWH-185
(23) and JWH-197 (26) were docked in the same general
region of the CB
1
receptor. However, because these analogs
have conformations which orient the naphthalene and indole
rings in a very different arrangement than in 3-aroylindoles,
the orientation of the ligands in the binding pocket differs
from that of JWH-081 and JWH-098. Naphthylindoles 23
and 26, retain the stacking interactions with the tryptophan
residues on helix-5, and have an additional stacking
interaction with a phenylalanine on helix-3, which involves
the hydrogen at C-2 of the indole. While the 2-methyl
analog JWH-197 (26), can engage in aromatic stacking
interactions with the tryptophan residues on helix-5 of the
CB
1
receptor, no aromatic stacking interaction is possible
with phenylalanine on helix-3, because indole 27 lacks the
hydrogen at C-2. The nearly 20-fold drop in affinity between
JWH-185 and JWH-197 is consistent with the loss of an
aromatic stacking interaction [41].
1-Pentyl-1H-indol-3-yl-(4-methoxy-1-naphthyl)methane
(23, JWH-185, K
i
= 17 ± 3 nM) and 2-methyl-1-pentyl-1H-
indol-3-yl-(4-methoxy-1-naphthyl)methane (26, JWH-197,
K
i
= 323 ± 48 nM) are analogs of JWH-081 (12, R' =
C
5
H
11
) and JWH-098 (15, R' = C
5
H
11
), respectively, in
which the carbonyl bridge has been replaced by a methylene
group. This replacement changes the hybridization of the
bridging carbon from sp
2
in the carbonyl group, to sp
3
in the
methylene group. This changes the relative orientation of the
naphthalene and indole rings, compared to that in JWH-081,
JWH-098 and WIN-55,212-2 (4). For JWH-185 and JWH-
197, the global minimum energy conformers have the
methylene C-H bonds staggered, with respect to the indole
ring. In this conformer, the naphthalene ring is oriented
perpendicular to the plane of the indole nucleus.
1-Pentyl-1H-indol-3-yl-(4-methoxy-1-naphthyl)methane
(23, JWH-185) has attenuated CB
1
receptor
affinity (K
i
= 17
± 3 nM), relative to 1-pentyl-3-(4-methoxy-1-
naphthoyl)indole (12, R' = C
5
H
11
, JWH-081, K
i
= 1.2 ±
0.03 nM). Also in the 3-(1-naphthoyl)indole series (12, R' =
C
5
H
11
and 15, R' = C
5
H
11
), the substitution of a methyl
group at C-2 results in only a slight decrease in CB
1
receptor affinity (JWH 081, 12, R' = C
5
H
11
, K
i
= 1.2 ± 0.03
nM; JWH 098, 15, R' = C
5
H
11
, K
i
= 4.5 ± 0.1 nM).
However, substitution at C-2 in the naphthylmethane series
(23 and 26) results in a more profound 19-fold affinity loss
(JWH 185, 23, K
i
= 17 ± 3 nM; JWH 197, 26, K
i
= 323 ±
48 nM). In order to probe the origin of these affinity changes,
each of these compounds was docked in a model of the active
state (R*) of the CB
1
receptor.
The significant affinities of indoles 21-23, 30, 31 and
indene 35, none of which can interact with the CB
1
receptor
by hydrogen bonding, strongly support the hypothesis that
cannabimimetic indoles interact with the receptor primarily
by aromatic stacking interactions. The molecular modeling
and receptor docking studies agree with this conclusion, and
provide an explanation for the observation that 2-
methylindole analogues JWH-196 (24), JWH-194 (25) and
JWH-197 (26) have greatly attenuated affinities for the CB
1
receptor.
The Huffman group has very recently described the
synthesis, CB
1
and CB
2
receptor affinities for 47 indole
derivatives [46]. Their goal was the development of
structure-activity relationships for cannabimimetic indoles at
both receptors, and if possible, to obtain new selective
ligands for the CB
2
receptor. These compounds, in which
the substituents on the naphthalene moiety are varied, have
either N-propyl or N-pentyl substituents. A number of these
compounds are listed in Table 1, and includes those indoles
Cannabimimetic indoles are highly aromatic ligands, and
CB
1
receptor mutation studies in which a lysine on
transmembrane helix 3 is replaced with alanine indicate that
this lysine is not an interaction site for WIN-55,212-2 (4)
[39]. On this basis it was suggested that aromatic stacking
1402 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
with an unsubstituted naphthalene system (8, JWH-015, 10,
JWH-072 and JWH-018, 13, JWH-007), those with a 7-
methylnaphthoyl group (11 JWH-076, 14, JWH-046 and
JWH-048) and those with a 4-methoxynaphthoyl substituent
(12, JWH-079 and JWH-081, 15, JWH-094 and JWH-098).
Other cannabimimetic indoles summarized in Table 3
include those with 4-alkyl-1-naphthoyl substituents (36 and
37, R'' = CH
3
, C
2
H
5
, C
3
H
7
, C
4
H
9
), 7-ethyl-1-naphthoyl
substituents (38 and 39), plus 2-methoxy-1-naphthoyl
analogs (40 and 41), 3-(6-methoxy-1-naphthoyl)indoles (42
and 43), 7-methoxy-1-naphthoyl compounds (44 and 45),
and 4-ethoxy compounds (46 and 47). In all cases R = C
3
H
7
or C
5
H
11
.
With few exceptions, the CB
1
receptor affinities for those
indoles listed in Table 3 that have a 2-methyl substituent are
less than those of the corresponding unsubstituted analog. A
notable exception to this generalization is 1-propyl-3-(4-
methyl-1-naphthoyl)indole (JWH-120, 36, R = C
3
H
7
, R
′′
=
CH
3
) and 2-methyl-1-propyl-3-(4-methyl-1-naphthoyl)indole
(JWH-148, 36, R = C
3
H
7
, R
′′
= CH
3
), which have K
i
=
1054 ± 31 nM and K
i
= 123 ± 8 nM, respectively. While
Table 3. Receptor Affinities (CB
1
and CB
2
) for Cannabimimetic Indoles 36–47 [46]
Compound
K
i
(nM) (CB
1
)
K
i
(nM) (CB
2
)
36, R = n-Propyl, R
′′
= Methyl (JWH-120)
1054 ± 31
6.1 ± 0.7
37, R = n-Propyl, R
′′
= Methyl (JWH-148)
123 ± 8
14 ± 1.0
36, R = n-Pentyl, R
′′
= Methyl (JWH-122)
0.69 ± 0.5
a
1.2 ± 1.2
37, R = n-Pentyl, R
′′
= Methyl (JWH-149)
5.0 ± 2.1
a
0.73 ± 0.03
36, R = n-Propyl, R
′′
= Ethyl (JWH-212)
33 ± 0.9
10 ± 1.2
37, R = n-Propyl, R
′′
= Ethyl (JWH-211)
70 ± 0.8
12 ± 0.8
36, R = n-Pentyl, R
′′
= Ethyl (JWH-210)
0.46 ± 0.03
0.69 ± 0.01
37, R = n-Pentyl, R
′′
= Ethyl (JWH-213)
1.5 ± 0.2
0.42 ± 0.05
36, R = n-Propyl, R
′′
= n-Propyl (JWH-180)
26 ± 2
9.6 ± 2.0
37, R = n-Propyl, R
′′
= n-Propyl (JWH-189)
52 ± 2
12 ± 0.8
36, R = n-Pentyl, R
′′
= n-Propyl (JWH-182)
0.65 ± 0.03
1.1 ± 0.1
37, R = n-Pentyl, R
′′
= n-Propyl (JWH-181)
1.3 ± 0.1
0.62 ± 0.04
36, R = n-Propyl, R
′′
= n-Butyl (JWH-239)
342 ± 20
52 ± 6
37, R = n-Propyl, R
′′
= n-Butyl (JWH-241)
147 ± 20
49 ± 7
36, R = n-Pentyl, R
′′
= n-Butyl (JWH-240)
14 ± 1
7.2 ± 1.3
37, R = n-Pentyl, R
′′
= n-Butyl (JWH-242)
42 ± 9
6.5 ± 0.3
38, R = n-Propyl (JWH-235)
338 ± 34
123 ± 34
39, R = n-Propyl (JWH-236)
1351 ± 204
240 ± 63
38, R = n-Pentyl (JWH-234)
8.4 ± 1.8
3.8 ± 0.6
39, R = n-Pentyl (JWH-262)
28 ± 3
5.6 ± 0.7
40, R = n-Propyl (JWH-265)
3788 ± 323
80 ± 13
41, R = n-Propyl (JWH-266)
>10,000
455 ± 55
40, R = n-Pentyl (JWH-267)
381 ± 16
7.2 ± 0.14
41, R = n-Pentyl (JWH-268)
1379 ± 193
40 ± 0.6
42, R = n-Propyl (JWH-163)
2358 ± 215
138 ± 12
43, R = n-Propyl (JWH-151)
>10,000
30 ± 1.1
42, R = n-Pentyl (JWH-166)
44 ± 10
1.9 ± 0.08
43, R = n-Pentyl (JWH-153)
250 ± 24
11 ± 0.5
44, R = n-Propyl (JWH-165)
204 ± 26
71 ± 8
45, R = n-Propyl (JWH-160)
1568 ± 201
441 ± 110
44, R = n-Pentyl (JWH-164)
6.6 ± 0.7
6.9 ± 0.2
45, R = n-Pentyl (JWH-159)
45 ± 1
10.4 ± 1.4
46, R = n-Propyl (JWH-259)
220 ± 29
74 ± 7
47, R = n-Propyl (JWH-261)
767 ± 105
221 ± 14
46, R = n-Pentyl (JWH-258)
4.6 ± 0.6
10.5 ± 1.3
47, R = n-Pentyl (JWH-260)
29 ± 0.4
25 ± 1.9
a
Ref. 44.
Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1403
JWH-120 has very little affinity for the CB
1
receptor, it has
excellent affinity for the CB
2
receptor (K
i
= 6.1 ± 0.7 nM).
This compound is highly selective for the CB
2
receptor with
greater than 170-fold selectivity. In the series of indoles with
a 4-alkyl substituent (36 and 37, R'' = CH
3
, C
2
H
5
, C
3
H
7
,
C
4
H
9
), the CB
1
receptor affinities are uniformly very high
when the nitrogen substituent is pentyl. The greatest CB
1
receptor affinity in this group is JWH-210 (1-pentyl-3-(4-
ethyl-1-naphthoyl)indole, (36, R = C
5
H
11
, R
′′
= C
2
H
5
)
with K
i
= 0.46 ± 0.03 nM. The 4-propyl (JWH-182, 36, R
= C
5
H
11
, R
′′
= C
3
H
7
) and 4-methyl (JWH-122, 36, R =
C
5
H
11
, R
′′
= CH
3
) analogs have virtually the same, very
high affinity for the CB
1
receptor with K
i
= 0.65 ± 0.03 nM
and K
i
= 0.69 ± 0.5 nM, respectively. The N-pentyl
compounds with small alkyl groups at C-4 and an indole 2-
methyl group have CB
1
receptor affinities from 1.3 to 1.5
nM. With the exception of the 4-methyl compounds (JWH-
120 and JWH-148), the N-propyl compounds in this group
have relatively high CB
1
receptor affinities with K
i
= 26–70
nM. The N-pentyl-3-(4-butyl-1-naphthoyl)indoles (36,
JWH-240 and 37, JWH-242, R = C
5
H
11
,
R
′′
= C
4
H
9
) have
somewhat less affinity for the CB
1
receptor than the
congeners with smaller alkyl substituents. The N-propyl
analogs (JWH-239, 36 and JWH-241, 37, R = C
3
H
7
,
R
′′
=
C
4
H
9
), both have very modest affinity for the CB
1
receptor
with K
i
= 342 ± 20 nM (36) and K
i
= 147 ± 20 nM (37).
methoxy group in other positions of the naphthoyl moiety
[37]. In order to gain insight into the effect of methoxy
groups at other positions, 1-propyl and 1-pentyl-3-(2-
methoxy-1-naphthoyl) (40–41), 3-(6-methoxy-1-naphthoyl)
(42–43) and 3-(7-methoxy-1-naphthoyl)indoles (44–45) were
synthesized and their CB
1
and
CB
2
receptor affinities were
determined [46]. The CB
1
and
CB
2
receptor affinities for
indoles 40–45 are summarized in Table 3.
None of the 3-(2-methoxy-1-naphthoyl)indoles (40–41,
JWH-265 to JWH-268) have appreciable affinity for the CB
1
receptor, with K
i
> 380 nM. However, JWH-267 (40, R =
C
5
H
11
) and JWH-268 (41, R = C
5
H
11
) have high affinity for
the CB
2
receptor (K
i
= 7.2 ± 0.14 nM and K
i
= 40 ± 0.6
nM, respectively). 1-Pentyl-3-(2-methoxy-1-
naphthoyl)indole, JWH-267, is a very highly selective
ligand for the CB
2
receptor, with greater than 50-fold
selectivity over the CB
1
receptor.
Only one of the 3-(6-methoxy-1-naphthoyl)indoles (42-
43) has significant affinity for the CB
1
receptor. For 1-pentyl-
3-(6-methoxy-1-naphthoyl)indole (JWH-166, 42, R =
C
5
H
11
) K
i
= 44 ± 10 nM. The other three compounds in
this series, JWH-163 (42, R = C
3
H
7
), JWH-151 (43, R =
C
3
H
7
) and JWH-153 (43, R = C
5
H
11
) have K
i
> 250 nM.
However, all of the compounds in this series have from
modest to very high affinity for the CB
2
receptor, and JWH-
151 is a highly selective ligand for the receptor, with K
i
=
30 ± 1.1 nM at CB
2
with K
i
> 10,000 nM at the CB
1
receptor. In contrast to the 3-(2-methoxy-1-naphthoyl)- and
3-(6-methoxy-1-naphthoyl)indoles, which in general have at
best, very modest affinity for the CB
1
receptor, the 1-pentyl-
3-(7-methoxy-1-naphthoyl)indoles both have high affinity. 1-
Pentyl-3-(7-methoxy-1-naphthoyl)indole (JWH-164, 44, R =
C
5
H
11
), has K
i
= 6.6 ± 0.7 nM, while the 2-methyl
congener (JWH-159, 45, R = C
5
H
11
), has K
i
= 45 ± 1 nM.
The N-propyl compounds, JWH-165 and JWH-160 (44 and
45, R = C
3
H
7
) have little affinity for the CB
1
receptor with
K
i
> 200 nM.
The CB
1
receptor affinities for 7-ethyl analogs 38 and 39
(Table 3) are similar to those of the corresponding 7-methyl
compounds (11 and 14, Table 1). However, in contrast to 1-
propyl-2-methyl-3-(7-methyl-1-naphthoyl)indole (JWH-046,
14, R = C
3
H
7
), which has high affinity for the CB
2
receptor
(K
i
= 16 ± 5 nM) and modest affinity for the CB
1
receptor
(K
i
= 343 ± 38 nM), the corresponding 7-ethyl analog
(JWH-239, 39, R = C
3
H
7
) has little affinity for either
receptor.
It had been observed previously that a 4-methoxy-1-
naphthoyl substituent enhances CB
1
receptor affinity, but
virtually nothing was known concerning the effect of a
N
O
R
R'
R''
N
O
R
R'
C
2
H
5
N
OCH
3
O
R'
R
N
O
R
R'
H
3
CO
N
O
R
R'
OCH
3
N
O
R
R'
C
2
H
5
O
36 R' = H
37 R' = CH
3
R'' = CH
3
, C
2
H
5
, C
3
H
7
or C
4
H
9
38 R' = H
39 R' = CH
3
40 R' = H
41 R' = CH
3
42 R' = H
43 R' = CH
3
44 R' = H
45 R' = CH
3
46 R' = H
47 R' = CH
3
Fig. (11). Structures of cannabimimetic indoles with substituted naphthoyl systems. In all cases R = C
3
H
7
or C
5
H
11
.
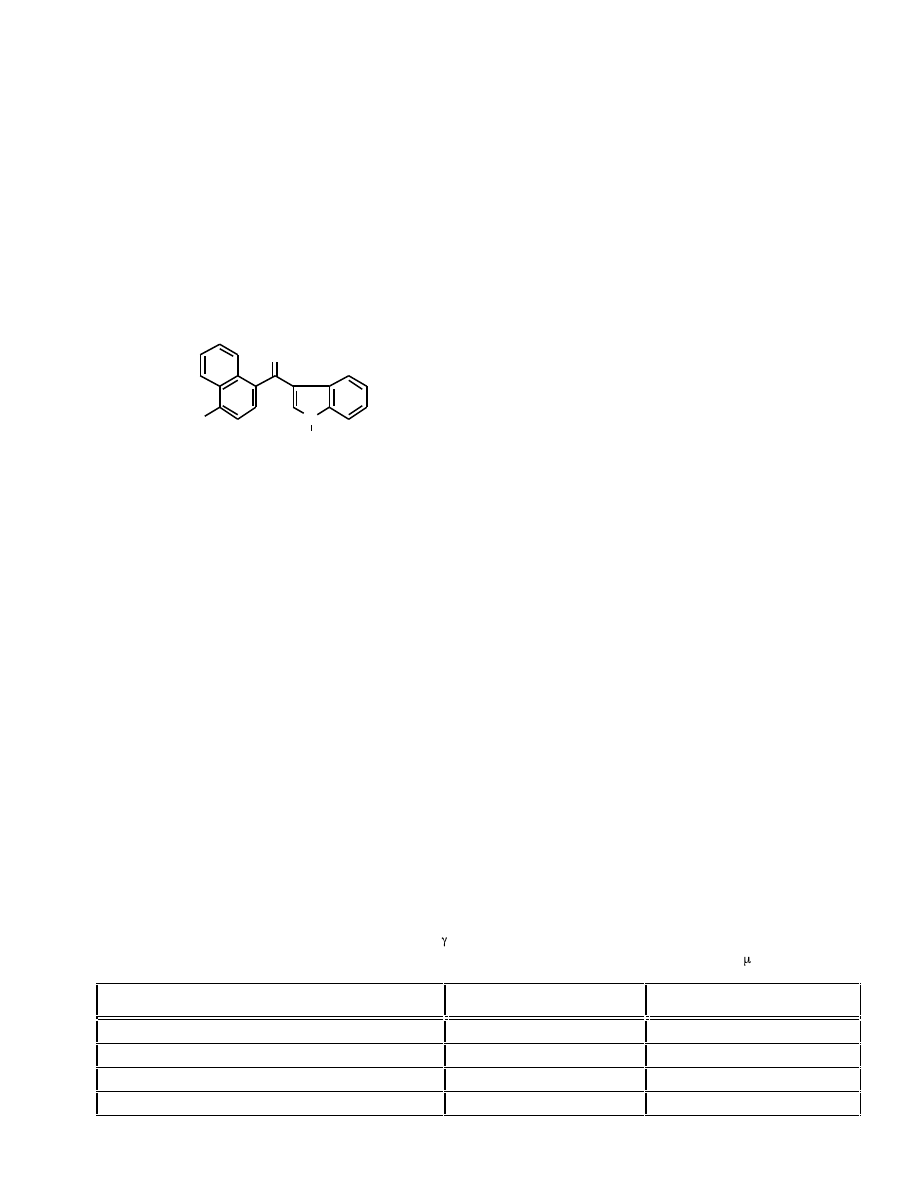
1404 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
In the course of preparing a series of N-alkyl-3-(4-
methoxy-1-naphthoyl)indoles (12, R = C
3
H
7
to C
7
H
15
), a
side reaction occurred which led to the production of the
corresponding 3-(4-alkoxy-1-naphthoyl)-N-alkylindoles (48)
via an unusual SNAr reaction [47]. The compounds in this
series have uniformly poor affinity for the CB
1
receptor with
K
i
> 200 nM. Although indoles 48 with 4-alkoxy
substituents of four or more atoms have little affinity for the
receptor, N-pentyl cannabimimetic indoles 36 and 37 with
alkyl chains of one to four carbon atoms have uniformally
high affinity for the CB
1
receptor. In order to probe the effect
of a 4-alkoxy substituent larger than methoxy, a series of 3-
(4-ethoxy-1-napthoyl)indoles (46 and 47) was prepared and
the CB
1
and CB
2
receptor affinities were determined (Table
3).
the efficacy of these compounds, their ability to stimulate
GTP
γ
S binding was determined. The results of these
determinations are summarized in Table 4. The stimulation
is normalized to that produced by 3
µ
M CP-55,940 (2), a
maximally effective concentration of this standard
cannabinoid agonist. In addition to JWH-120, JWH-151 and
JWH-267, the [
35
S]GTP
γ
S binding for JWH-015, 1-propyl-
2-methyl-3-(1-naphthoyl)indole (8), the lead compound for
the search for CB
2
selective cannabimimetic indoles, was
determined, and the data are included in Table 4.
As indicated in Table 4, all four of these compounds are
potent in the [
35
S]GTP
γ
S assay with EC
50
values from 5.1
± 1.0 nM for JWH-120 (36, R = C
3
H
7
, R'' = CH
3
) to 17.7
± 1.0 nM for JWH-015 (8). One of these CB
2
receptor
ligands, 1-propyl-2-methyl-3-(6-methoxy-1-
naphthoyl)indole, JWH-151 (43, R = C
3
H
7
) is highly
efficacious with an E
max
of 108.5 ± 13.0% relative to CP-
55,940. The other three cannabimimetic indoles, 1-propyl-2-
methyl-3-(1-naphthoyl)indole, JWH-015 (8), 1-propyl-3-(4-
methyl-1-naphthoyl)indole, JWH-120 (36, R = C
3
H
7
, R'' =
CH
3
) and 1-pentyl-3-(2-methoxy-1-naphthoyl)indole, JWH-
267 (40, R = C
5
H
11
), are partial agonists relative to CP-
55,940 with E
max
values from 65.7 ± 6.4% (JWH-015) to
78.1 ± 10.7% (JWH-120).
N
O
R
RO
48 R = n-propyl to n-hept yl
Fig. (12). Structure of 4-alkoxy-1-naphthoylindoles.
Indoles 46 and 47 have weaker affinities for the CB
1
receptor than the corresponding methoxy analogs (12 and 15,
Table 1). However they follow the usual trend in that the N-
propyl indoles (JWH-259, 46, R = C
3
H
7
and JWH-261, 47,
R = C
3
H
7
) have significantly less affinity for the receptor
than the N-pentyl compounds. Neither N-propyl analog has a
CB
1
receptor affinity better than 220 nM (JWH-259). 1-
Pentyl-3-(4-ethoxy-1-naphthoyl)indole (JWH-258, 46, R =
C
5
H
11
) has very high affinity for the CB
1
receptor with K
i
=
4.6 ± 0.6 nM, however, this is somewhat less than that for
the 4-methoxy analog (JWH-081, 12, R = C
5
H
11
, K
i
= 1.2
± 0.1 nM). The 2-methyl compound (JWH-260, 47, R =
C
5
H
11
) has K
i
= 29 ± 0.4 nM which is considerably less
than that of the 2-methyl-1-pentyl-3-(4-methoxy-1-
naphthoyl)indole (JWH-098, 15, R = C
5
H
11
) with K
i
= 4.5
± 0.1 nM.
The 1-pentyl indoles provide several structural criteria for
recognition at the CB
1
receptor. As noted previously, CB
1
receptor affinity is reduced slightly by the presence of a
methyl group at the 2-position of the indole. With the
exception of the 1-pentyl-3-(2-methoxy-1-naphthoyl)indoles
(JWH-267, 40, R = C
5
H
11
), JWH-268, (41, R = C
5
H
11
)
and 1-pentyl-2-methyl-3-(6-methoxy-1-naphthoyl)indole
(JWH-153, 43, R = C
5
H
11
), all of the compounds in this
series have K
i
< 45 nM, indicative of high affinity for the
receptor. The addition of a methyl (JWH-122, 36, R =
C
5
H
11
, R
′′
= CH
3
, JWH-149, 37, R = C
5
H
11
, R
′′
= CH
3
),
ethyl (JWH-210, 36, R = C
5
H
11
, R
′′
= C
2
H
5
, JWH-213,
37, R = C
5
H
11
, R
′′
= C
2
H
5
) or propyl (JWH-182, 36, R =
C
5
H
11
, R
′′
= C
3
H
7
, JWH-181, 37, R = C
5
H
11
, R
′′
=
C
3
H
7
) group at C-4 of the naphthalene leads to a
considerable increase in CB
1
receptor affinity, however, a
butyl group at C-4 (JWH-240, 36, R = C
5
H
11
, R
′′
= C
4
H
9
,
JWH-242, 37, R = C
5
H
11
, R
′′
= C
4
H
9
) results in a slight
decrease in affinity (Table 3). Neither a 7-methyl-1-naphthoyl
(JWH-048, 14, R = C
5
H
11
, Table 1) nor a 7-ethyl-1-
naphthoyl (JWH-234, JWH-262, 39, R = C
5
H
11
, Table 3)
substituent has a significant effect on affinity for the CB
1
receptor
A particularly significant result of this study of
cannabimimetic indoles is the discovery of three new highly
selective ligands for the CB
2
receptor [44]. These
compounds are 1-propyl-3-(4-methyl-1-naphthoyl)indole,
JWH-120 (36, R = C
3
H
7
, R'' = CH
3
), which is 173-fold
selective, 1-pentyl-3-(2-methoxy-1-naphthoyl)indole, JWH-
267 (40, R = C
5
H
11
), 53-fold selective, and 1-propyl-2-
methyl-3-(6-methoxy-1-naphthoyl)indole, JWH-151 (43, R
= C
3
H
7
), which is >333 fold selective. In order to evaluate
In the N-pentyl series, a 2-methoxy-1-naphthoyl
substituent (JWH-267, 40, R = C
5
H
11
, JWH-268, 41, R =
Table 4.
EC
50
and E
max
Values (mean ± SEM) for GTP S Binding of CB
2
for Selective Ligands. Assays were carried out in
Human CB
2
Receptor-Expressing CHO Cells. E
max
Values are Reported as Per Cent Relative to 3 M CP-55,940 (2)
Compound
EC
50
(nM)
E
max
(% CP-55940)
8, (JWH-015)
17.7 ± 1.0
65.7 ± 6.4
36, R = C
3
H
7
, R'' = CH
3
(JWH-120)
5.1 ± 1.6
78.1 ± 10.7
40, R = C
5
H
11
(JWH-267)
4.9 ± 0.8
67.3 ± 2.9
43, R = C
3
H
7
(JWH-151)
12.0 ± 2.9
108.5 ± 13.0

Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1405
C
5
H
11
, Table 3) effectively destroys affinity for the CB
1
receptor, while a 4-methoxy group (JWH-081, 12, R =
C
5
H
11
, JWH-098, 15, R = C
5
H
11
, Table 1) slightly
increases affinity relative to the unsubstitued analogs.
Replacing the 4-methoxy group with a 4-ethoxy (JWH-258,
46, R = C
5
H
11
, JWH-260, 47, R = C
5
H
11
, Table 3)
diminishes CB
1
affinity somewhat. A 6-methoxy-1-
naphthoyl substituent decreases affinity for the CB
1
receptor
in the compound unsubstitued at C-2 of the indole nucleus
(JWH-166, 42, R = C
5
H
11
, Table 3); while the 2-methyl
analog (JWH-153, 43, R = C
5
H
11
,) has little affinity. In
contrast, the 7-methoxy analogs (JWH-164, 44, R = C
5
H
11
,
and JWH-159, 45, R = C
5
H
11
), have receptor affinities
comparable to those of the 4-ethoxy compounds (Table 3).
1-Pentyl-3-(4-propyl-1-naphthoyl)indole (36, R = C
5
H
11
,
R
′′
= C
3
H
7
) is a very high affinity CB
1
receptor ligand (K
i
=
0.65 ± 0.03 nM, Table 3), and it was docked in the same
position as JWH-018 (10, R = C
5
H
11
), JWH-122 (27) and
JWH-081 (12, R = C
5
H
11
) [44]. These docking studies
showed that the N-pentyl tail of JWH-182 extends over a
phenylalanine on helix-3 of the CB
1
receptor, and the indole
moiety is between transmembrane helices 5 and 6. The
naphthoyl ring is intracellular to a tryptophan on helix-5 and
another on helix-6 , with the 4-propyl substituent on the
naphthyl ring situated in an open area within the binding
pocket. In this position, both the indole and naphthoyl rings
have stacking interactions with the tryptophans, and the
carbonyl oxygen forms a weak hydrogen-bond with the
tryptophan on helix-6.
In general, cannabimimietic indoles with N-propyl
substituents have significantly less affinity for the CB
1
receptor than the corresponding N-pentyl compounds.
Although a methyl group at C-2 of the indole usually
attenuates CB
1
receptor affinity somewhat, in the case of the
compounds with an unsubstituted naphthoyl group (JWH-
015, 8, JWH-072, 10, R = C
3
H
7
) and the 4-methyl-1-
naphthoyl analogs (JWH-120, 36, R = C
3
H
7
, R
′′
= CH
3
and JWH-148, 37, R = C
3
H
7
, R
′′
= CH
3
), the 2-methyl
compounds have considerably greater CB
1
receptor affinities
than the unsubstituted compounds (Tables 1 and 3). The
situation is similar for the 1-propyl-3-(4-butyl-1-
naphthoyl)indoles (JWH-239, 36, R = C
3
H
7
, R
′′
= C
4
H
9
and JWH-241, 37, R = C
3
H
7
, R
′′
= C
4
H
9
). However, the 2-
methyl analog (JWH-241) has only slightly more than two-
fold greater affinity for the CB
1
receptor than JWH-239.
With the exception of the 4-ethyl- (JWH-211, JWH-212),
and 4-propyl-1-naphthoylindoles (JWH-180, JWH-189),
none of the N-propyl-3-(4-alkyl-1-naphthoylindoles) has a
CB
1
receptor affinity of less than 100 nM. In the N-pentyl
series, the 4-propyl-1-naphthoylindoles (JWH-182, JWH-
181) have exceptionally high affinity for the CB
1
receptor,
respectively (Table 3). These high affinities are reflected in
the N-propyl analogs; JWH-180 (36, R = C
3
H
7
, R
′′
=
C
3
H
7
) has K
i
= 26 ± 2 nM and JWH-189 (36, R = C
3
H
7
)
has K
i
= 70 ± 0.8 nM. In the methoxynaphthoyl series
(Tables 1 and 3), the relative magnitudes of the CB
1
receptor
affinities for the N-propyl indoles parallel those of the N-
pentyl analogs. However, the compounds in this series have
little affinity for the CB
1
receptor with affinities from 204
nM, to >10,000 nM with the exception of JWH-079 (12, R
= C
3
H
7
), which has K
i
= 63 ± 3 nM.
Using the docking position employed for JWH-182, the
consequences of substitution at other positions on the
naphthoyl ring were explored. Substitution at the 2-
naphthoyl position as in 1-pentyl-3-(2-methoxy-1-
naphthoyl)indole (JWH-267, 40, R = C
5
H
11
, Table 3)
causes a large decrease in affinity, relative to the 4-propyl-1-
naphthoyl analog (JWH-182). Docking studies show that the
2-methoxy group in JWH-267 has severe steric conflicts
with the tryptophan on helix-6, causing the ligand to lose
most of its aromatic stacking interactions.
Similar docking studies indicated that various
substituents can be placed at C-4 of the naphthoyl moiety,
and do not cause a significant decrease in affinity, because
there is a fairly wide and deep lipophilic binding pocket in
this region of the receptor. However, substitution at C-6
results in diminished affinity for 1-pentyl-3-(6-methoxy-1-
naphthoyl)indole (JWH-166, 40, R = C
5
H
11
, Table 3)
relative to the 4-propyl-1-naphthoyl analog (JWH-182, 36,
R = C
5
H
11
, R
′′
= C
3
H
7
). In its lowest energy conformation,
a methoxy substituent at C-6 has some steric conflicts with
two amino acids that are alleviated by rotation of the
methoxy group out of the plane of the naphthoyl ring into a
higher energy rotameric state. The necessity for the methoxy
group to assume a higher energy conformation in order to be
accommodated at the binding site, may contribute to the
reduced CB
1
affinity of JWH-166 relative to JWH-182.
Substitution at C-7 of the naphthoyl ring results in only a
slight reduction in affinity for 1-pentyl-3-(7-methoxy-1-
naphthoyl)indole (JWH-164, 44, R = C
5
H
11
, Table 3).
Docking studies show that a methoxy substituent at C-7
encounters no steric problems in its minimum energy
conformation. However, the methoxy group blocks the
aromatic stacking interaction between the naphthoyl ring and
the tryptophan on helix-5, which is present in the 4-propyl
analog. This loss of an aromatic stacking interaction may
account for the 10-fold reduction in affinity of the 7-methoxy
compound (JWH-164, 44, R = C
5
H
11
) relative to the 4-
propyl analog (JWH-182, 36, R = C
5
H
11
, R
′′
= C
3
H
7
).
To gain insight into the receptor interactions responsible
for the SAR of these cannabimimetic indoles at the CB
1
receptor, molecular modeling and receptor docking studies
were carried out. These studies were similar to those
described above for naphthoylindoles JWH-018 (10, R =
C
5
H
11
), JWH-122 ( 27) and JWH-081 (12, R = C
5
H
11
) [44].
The set of 3-(4-propyl-1-naphthoyl)indoles (JWH-180, 36, R
= C
3
H
7
, R
′′
= C
3
H
7
, JWH-189, 37, R = C
3
H
7
, R
′′
=
C
3
H
7
, JWH-182, 36, R = C
5
H
11
, R
′′
= C
3
H
7
, JWH-181,
37, R = C
5
H
11
, R
′′
= C
3
H
7
Table 3) and the set of 3-(6-
methoxy-1-naphthoyl)indoles (JWH-163, 42, R = C
3
H
7
,
JWH-151, 43, R = C
3
H
7
, JWH-166, 42, R = C
5
H
11
, JWH-
153, 43, R = C
5
H
11
Table 3) were chosen. In addition, the
N-pentyl-3-(2-methoxy-1-naphthoyl)indoles (JWH-267, 40,
R = C
5
H
11
and JWH-268, 41, R = C
5
H
11
, Table 3) were
examined.
Based on a study of rigid naphthylidene-substituted
aminoalkylindene analogs of cannabimimetic indoles that
mimic the s-cis or s-trans conformation of the
cannabimimetic indoles, it was concluded that that the s-
trans conformation is probably the preferred conformation for
the interaction of cannabimimetic indoles at both the CB
1
and CB
2
receptors [16]. For this reason, the lowest energy s-
trans
conformer of 2-methyl-1-pentyl-3-(4-propyl-1-
1406 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
N
O
NO
2
I
N
CH
3
N
O
CH
3
N
O
OCH
3
I
49
50
Fig. (13). Structures of AM1241 and AM630.
naphthoyl)indole (JWH-181, 37, R = C
5
H
11
, R
′′
= C
3
H
7
).),
rather than its global minimum energy s-cis conformer was
used in the docking studies. Because of the use of the s-trans
conformer as the bioactive conformation for the C-2 methyl
indoles, the affinities of ligands in this series can, in general,
be expected to be reduced relative to those of the
corresponding indoles without a C-2 methyl group for which
the global minimum energy conformers are s-trans
conformers. Such a general reduction is, in fact, seen in this
series (Tables 1 and 3).
receptor has 82-fold selectivity for the CB
2
receptor [48].
This compound has been found to produce antinociception
to thermal stimuli, an effect which is blocked by the CB
2
receptor antagonist AM630 (50) [48, 49]. In another study it
was found that the antihyperalgesic and antialloldynic effects
of AM1241 were blocked by the CB
2
antagonist SR144528,
but not by the CB
1
antagonist SR141716 [50]. These data
indicate that these effects are mediated through the CB
2
receptor. Similar effects were noted in capsaicin induced
hyperalgesia and aalodynia [51].
Compared to their N-pentyl congeners, each analog in the
N-propyl series shows reduced CB
1
receptor affinity. In the
N-pentyl series, the pentyl tail resides in a hydrophobic
binding pocket which appears to orient the aromatic rings of
the ligand for aromatic stacking interactions with the
receptor. The N-propyl tail is too short to access this
hydrophobic pocket and simultaneously allow the ligand to
engage in aromatic stacking interactions. As a result, ligands
with the propyl substituent may have more difficulty in
assuming the correct aromatic region orientation necessary
for productive binding at the CB
1
receptor. The importance
of an alkyl chain of certain length is very reminiscent of the
classical cannabinoids for which it has been shown that C-3
alkyl chains shorter than pentyl have severely reduced CB
1
affinities [2-5].
Very recently, a group at Bristol-Myers Squibb has
described two new groups of indole based cannabinoids. One
series of compounds was comprised of amides derived from a
substituted indole 3-carboxylic acid, several of which show
selectivity for the CB
2
receptor [52]. The most highly
selective compound in this series is phenylalanine derived
amide 51, which has excellent affinity for the CB
2
receptor
(K
i
= 8 nM) and little affinity for the CB
1
receptor (K
i
=
4000 nM). The second series of cannabimimetic indoles are
pyridones, derived from compounds similar to 51 [53]. One
of these indolopyridones (52) has very high affinity for the
CB
2
receptor (K
i
= 1.0 ± 0.2 nM), and also has high affinity
for the CB
1
receptor (K
i
= 16 ± 4 nM). In addition,
indolopyridone 52 is orally effective in a mouse model of
inflammation.
In addition to the new cannabimimetic indoles reported
by the Clemson group, several other new compounds have
been described, some of which are very promising, highly
selective ligands for the CB
2
receptor. One indole derivative,
AM1241, (2-iodo-5-nitrophenyl)-[1-(1-methylpiperidin-2-
ylmethyl)-1H-indol-3-yl]methanone (49) with K
i
= 3.4 ± 0.5
nM at the CB
2
receptor and K
i
= 280 ± 41 nM at the CB
1
Two studies of the in vitro metabolism of
cannabimimetic indoles have been carried out by Zhang et
al. [54, 55]. Both of these studies employed rat liver
microsomes, and the metabolites were characterized by a
combination of mass spectrometry and NMR spectroscopy.
In the initial study, WIN-55,212-2 (4) provides two major
and at least six minor metabolites [54]. The major
N
O
CH
3
N
O
N
H
CO
2
CH
3
OCH
3
N
O
N
O
OCH
3
N
CH
3
CH
3
H
3
C
51
52
Fig. (14). Bristol-Myers Squibb cannabimimetic indoles.
Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1407
CO
2
H
CH
3
SOCH
3
F
N
CO
2
H
CH
3
Cl
H
3
CO
O
N
H
Ar
O
53
54
55
Fig (15). Structures of sulindac, indomethacin, and cannabimimetic indenes.
metabolites are dihydrodiols, derived by arene oxidation of
the naphthalene ring of 4 [56]. The major metabolites were
the only compounds present in sufficient quantity for NMR
studies, the other metabolites were characterized only by
mass spectrometry. The minor products included two
monohydroxy compounds and metabolites derived by
oxidation of the morpholine ring. The second study was an
investigation of the metabolism of AM630 (50), in which
the metabolites were characterized by mass spectrometry
[55]. A total of 17 metabolites were identified, which
included cleavage of the methyl ether, aromatic
hydroxylation and a variety of products resulting from
oxidation of the morpholine ring, with and without ether
cleavage.
nM). The CB
1
receptor affinities of the corresponding Z
isomers are significantly lower.
Careful preparation of the pure E- (7) and Z-isomers of 4-
[2-[1-(1-naphthalenylmethylene)-1H-inden-3-yl]ethyl]mor-
pholine and 4-[2-[2-methyl-1-(1-naphthalenylmethylene)-1H-
inden-3-yl]ethyl]morpholine by Reggio et al. afforded an
opportunity to study which stereoisomer was responsible for
biological activity [16]. The compounds described by
Reggio's group were carefully purified by chromatography,
and the structures assigned by
1
H NMR techniques,
primarily NOE experiments. Molecular modeling studies
demonstrated that the naphthyl group of WIN-55212-2 and
the p-methoxyphenyl group of pravadoline occupied the
same region of space. Comparison of WIN-55212-2 with the
indenes indicated that only a small amount of energy was
required to overlay the naphthyl rings of the two classes of
ligands. These studies support the appropriateness of using
these rigid analogs as models for the s-cis and s-trans
conformers of cannabimimetic indoles. The E isomers were
found to have high affinity for both receptor subtypes,
whereas the Z-isomers exhibit poor affinity. Since the E-
isomers of the indenes are a model for the s-trans conformer
of the indoles, this evidence suggests that s-trans is the
bioactive conformation.
R
R
1
56-59
Fig. (16). Structures of Indenes 56-59.
INDENES
Studies involving mutant receptors and molecular
modeling strongly suggest that cannabimimetic indoles
interact with the cannabinoid receptors primarily through
aromatic stacking [39, 40, 43]. The importance of aromatic
group orientation in the indene series supports this
hypothesis and implies that the indenes interact with the
CB
1
receptor through the same mechanism as the indoles
[16]. In order to exclude a possible hydrogen-bonding
mechanism of these ligands with the receptor, a series of
indenes was prepared with an alkyl group in place of the
ethylmorpholino found in 7 and 55. [44 and R. Mabon,
unpublished work].
Cannabimimetic indenes were first prepared by the
Sterling-Winthrop group, while studying the effects of
pravadoline (3) on the central nervous system [15]. It was
observed that sulindac (53), an indene analog of
indomethacin, (54), has anti-inflammatory activity
comparable to that of indomethacin, but lacks the CNS side
effects of 54. Several 1-(2-(4-morpholino)ethyl)-3-arylidene
derivatives were prepared as mixtures of E and Z isomers.
Derivatives in which the appended aryl group was a
substituted phenyl ring, exhibited low affinities. Two E-
naphthylidene analogs, (55), showed good affinity (Ar = 1-
naphthyl, IC
50
= 1.0 nM; 4-methoxy-1-naphthyl, IC
50
= 0.9
The affinities of several of these compounds as mixtures
of E- and Z- isomers for the CB
1
receptor were determined
Table 5.
Receptor Affinities of Indenes Tested as Mixtures of E and Z Stereoisomers
Compound
R
R1
K
i
(nM) CB
1
JWH-171, 56
pentyl
H
51
±
2
JWH-170, 57
propyl
H
698
±
27
JWH-173, 58
pentyl
CH
3
108
±
12
JWH-172, 59
propyl
CH
3
140
±
8
1408 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
N
R
O
60-64
N
Ar
O
65-70
N
R1
R5
R2
O
R3
R4
71-84
Fig. (17). Structures of Cannabimimetic Pyrroles.
and are shown in Table 5. The introduction of a pentyl
group (56, JWH-171) afforded a mixture of E and Z isomers
that showed good affinity (K
i
= 51
±
2 nM) for the CB
1
receptor, however, this is somewhat less than that of indene
7. The E-isomer of 56 (35, JWH-176) shows increased
affinity for the CB
1
receptor, with K
i
= 26
±
4 nM. [44] The
introduction of a propyl group in JWH-170 (57) results in
significantly attenuated CB
1
affinity, a trend which has been
seen repeatedly in the indole series. The presence of a 2-
methyl substituent results in reduced CB
1
affinity for the
pentyl compound (58, JWH-173), but increased affinity for
determined that the benzene moiety of the indole was not
necessary for biological activity [13]. Since it appeared
possible that these pyrrole derivates would show
cannabimimetic activity, the synthesis of a series of alkyl
pyrroles (6) analogous to previously prepared indoles was
undertaken. These compounds showed reduced affinity
relative to their indole counterparts, but demonstrated a
similar trend with regard to the N-alkyl chain length, where
CB
1
receptor affinity peaks at around five carbons. 3-(1-
Naphthoyl)-N-pentylpyrrole is relatively potent in the
spontaneous activity and tail flick assays, and causes a dose-
Table 6. Receptor Affinities of 2-Phenyl-4-(1-naphthoyl)-N-alkylpyrroles
Compound
R
K
i
(nM) CB
1
JWH-156, 60
propyl
890
±
364
JWH-150, 61
butyl
59.7
±
1.0
JWH-145, 62
pentyl
11.6
JWH-147, 63
hexyl
9.4
JWH-146, 64
heptyl
19.0
the propyl compound (59, JWH-172) relative to JWH-171
(56).
dependent inhibition of electrically evoked contractions of
the mouse vas deferens that could be antagonized by
SR141716 [13, 57].
PYRROLES
Based upon the hypothesis that cannabimimetic indoles,
and thus the corresponding pyrroles, interact with the
receptor largely through aromatic stacking, a series of 2-
phenyl-4-(1-naphthoyl)-N–alkyl pyrroles (60-64) was
synthesized. With the exception of the N-propyl derivative
(JWH-156, 60), these compounds exhibited good affinity for
Based on the alignment proposed by Huffman et al.[12],
in which the ketonic carbonyl of WIN-55212-2 (4) and the
phenolic hydroxyl of THC (1) are overlaid, as are the
naphthalene moiety of 4 and the A-ring of 1, it was
Table 7. Receptor Affinities 2-Aryl-4-(1-naphthoyl)-N-pentyl pyrroles, 65-70
Compound
Aryl
K
i
(nM) CB
1
JWH-309, 65
1-naphthyl
40.83
±
3.32
JWH-347, 66
2-naphthyl
333.7
±
17.0
JWH-243, 67
p-methoxyphenyl
285
±
40.3
JWH-292, 68
o-methoxyphenyl
29
±
0.7
JWH-308, 69
p-fluorophenyl
41
±
1.4
JWH-307, 70
o-fluorophenyl
7.7
±
1.8
Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1409
Table 8.
CB
1
and CB
2
Affinities of pyrroles 71-84
Compound
R1
R2
R3
R4
R5
Affinity K
i
(nM)
rCB1
hCB2
71
C
5
H
11
H
1-naphthyl
H
H
30.5
±
4.7
552
±
314
72
C
5
H
11
CH
3
1-naphthyl
H
CH
3
45.3
±
7.5
9.85
±
2.1
73
C
3
H
7
CH
3
1-naphthyl
H
CH
3
>1000
309.7
±
20.8
74
pClC
6
H
4
CH
2
CH
3
1-naphthyl
H
CH
3
83.7
±
17.8
55.6
±
26.5
75
C
5
H
11
CH
3
1-naphthyl
Br
CH
3
13.3
±
0.5
6.8
±
1.0
76
C
3
H
7
CH
3
1-naphthyl
Br
CH
3
780
±
326
691.3
±
101.3
77
pClC
6
H
4
CH
2
CH
3
1-naphthyl
Br
CH
3
38
±
7.2
194.5
±
27.5
78
C
5
H
11
H
1-naphthyl
(CH
2
)
4
235.8
±
6.2
139
±
55
79
C
5
H
11
CH
3
C
6
H
5
H
CH
3
>1000
>1000
80
C
5
H
11
CH
3
C
6
H
5
Br
CH
3
>1000
>1000
81
pClC
6
H
4
CH
2
CH
3
C
6
H
5
H
CH
3
>1000
>1000
82
C
5
H
11
CH
3
HO(CH
2
)
3
H
CH
3
>3000
>10000
83
C
5
H
11
CH
3
o(CH
3
CO)C
6
H
4
NH
H
CH
3
367.3
±
31.2
>1000
84
C
5
H
11
CH
3
c- C
6
H
11
NH
H
CH
3
415.5
±
79.5
483.5
±
211
the CB
1
receptor (Table 6). Retaining the pentyl group,
several analogs of JWH-145 (62) with substituted phenyl
substituents (65-70) have been prepared, and their receptor
affinities have been determined (Table 7). These compounds
exhibit a range of affinities for the CB
1
receptor, with some
compounds exhibiting affinities similar to that of 62, and
others displaying little or no affinity. Initial results indicate
that para-substituents provide decreased receptor affinity
when compared with 62. This appears to be the case,
regardless of the electronic nature of the substituent, although
there is not enough evidence to rule out electronic effects.
Increase in substituent size from fluoro to methoxy results in
a rapid decline of affinity. In both the ortho and para
positions, higher receptor affinity is provided by the smaller
fluoro substituent with the difference more pronounced in the
para position. This trend is observed with 2-(1-naphthyl)
and 2-(2-naphthyl)pyrrole substituents. The 2-naphthyl
substituent has one ring oriented such that it is equivalent to
a meta and para “substituent” on the aryl ring attached to
the pyrrole. Thus, the 2-naphthyl compound, (66), has a
significantly lower affinity for the CB
1
receptor than the
pyrrole with a 1-naphthyl substituent at C-2, (61).
These compounds also show little affinity for either receptor,
although their affinities are somewhat enhanced relative to
pyrroles 79-81. Replacement of the C-3 aromatic system
with a 3-hydroxypropane (82) in an attempt to mimic the
northern aliphatic hydroxyl of some successful traditional
cannabinoids results in a complete loss of affinity [58].
Substitution of both
α
-positions of the pyrrole with methyl
groups has little effect on affinity for either receptor, and the
introduction of a bromine atom to the unsubstituted
β
-
position results in a slight increase in binding for both
receptor subtypes. The addition of a cyclohexyl ring (78)
connecting the 4- and 5-positions greatly reduces affinity,
although it is assumed that the substituent occupies the
same location as the benzenoid moiety of the corresponding
indoles.
CONCLUSION
Modeling studies of the receptor and results obtained
with mutant CB
1
receptors strongly suggest that
cannabimimetic indoles, and presumably the pyrroles and
indenes, interact at a different site in the receptor than
traditional cannabinoids and endogenous cannabinoids, such
as anandamide. These studies also indicate that these classes
of cannabinoid receptor ligands interact with the CB
1,
and
probably the CB
2
receptor, primarily by aromatic stacking
interactions. These interactions with the CB
1
receptor are
considerably different than those of the traditional
cannabinoids, and it now appears unlikely that it will be
possible to develop a universal pharmacophore for the
cannabimimetic indoles and the traditional cannabinoids.
The experiments with mutant CB
1
receptors combined with
modeling studies have shed considerable light on the nature
of the interactions of various classes of cannabinoids with the
Due to the relatively high affinity displayed by the hybrid
cannabinoid JWH-161 (20) [42] pyrrole derivatives with
other substituents appended to the pyrrole nucleus have been
synthesized [58]. Replacement of the naphthoyl ring system
resulted in decreased affinity for the CB
1
and CB
2
receptors,
shown in Table 8. The presence of a benzoyl substituent at
C-3 gives compounds 79-81, which have no appreciable
affinity for either the CB
1
or CB
2
receptor. Two compounds,
83 and 84, with carboxamido groups at C-3, were also
prepared. It was predicted that the carboxamido group would
occupy the same spatial location as the naphthyl ring of the
cannabimimetic indoles or the cyclohexene ring of
∆
8
-THC.

1410 Current Medicinal Chemistry, 2005, Vol. 12, No. 12
Huffman and Padgett
CB
1
receptor, and in the future should assist in providing a
firm basis for the continued development of the SAR of both
classical and indole based cannabinoids.
[10]
Bell, M. R.; D'Ambra, T. E.; Kumar, V.; Eissanstat, M. A.;
Herrmann, J. L.; Wetzel, J. R.; Rosi, D.; Philion, R. E.; Daum, S. J.;
Hlasta, D. J.; Kullnig, R. K.; Ackerman, J. H.; Haubrich, D. R.;
Luttinger, D. A.; Baizman, E. R.; Miller, M. S.; Ward, S. J. J. Med.
Chem. 1991, 34, 1099.
Although much has been accomplished in developing the
medicinal chemistry of the cannabimimetic indoles, pyrroles
and indenes in the nearly 15 years that the biological activity
of these compounds has been recognized, a great deal
remains to be done. Inter alia, these include further study of
the SAR of the indenes and pyrroles, which should shed
additional light upon the detailed interactions of these
ligands with the cannabinoid receptors. Also, the
development of additional ligands which are highly specific
for each receptor should be carried out in order to develop
further insight into the physiological role of each receptor,
and with the ultimate goal of developing clinically useful
compounds. In the past few years, the significance of the
CB
2
receptor has become apparent, and it will be necessary
to identify additional ligands that show a high degree of
affinity for CB
2
relative to CB
1
. The in vivo pharmacology
of such selective agonists should be informative in terms of
ultimately identifying the role of endogenous cannabinoids
in animal physiology. Finally, although a great deal of work
has been carried out concerning the SAR of the
cannabimimetic indoles, additional systematic studies of the
effects of various substituents on the indole nitrogen and ring
carbons, as well as on the naphthalene ring need to be carried
out.
[11]
D'Ambra, T. E.; Estep, K. G.; Bell, M. R.; Eissenstat, M. A.; Josef,
K. A.; Ward, J.; Haycock, D. A.; Baizman, E. R.; Casiano, F. M.;
Beglin, N. C.; Chippari, S. M.; Grego, J. D.; Kullnig, R. K.; Daley,
G. T. J. Med. Chem. 1992, 35, 124.
[12]
Huffman, J. W.; Dai, D.; Martin, B. R.; Martin, B. R.; Compton, D.
R. Bioorg. Med. Chem. Lett. 1994, 4, 563.
[13]
Lainton, J. A. H.; Huffman, J. W.; Martin, B. R.; Compton, D. R.
Tetrahedron Lett. 1995, 36, 1401.
[14]
Wiley, J. L.; Compton, D. R.; Dai, D.; Lainton, J. A. H.; Phillips,
M.; Huffman, J. W.; Martin, B. R. J. Pharmacol. Exp. Ther. 1998,
285, 995.
[15]
Kumar, V.; Alexander, M. D.; Bell, M. R; Eissenstat, M. A.;
Casiano, F. M.; Chippari, S. M.; Haycock, D. A.; Luttinger, D. A.;
Kuster, J. E.; Miller, M. S.; Stevenson, J. I.; Ward, S. J. Bioorg.
Med. Chem. Lett. 1995, 5, 381.
[16]
Reggio, P. H.; Basu-Dutt, S.; Barnett-Norris, J.; Castro, M. T.;
Hurst, D. P.; Seltzman, H. H.; Roche, M. J.; Gilliam, A. F.;
Thomas, B. F.; Stevenson, L. A.; Pertwee, R. G.; Abood, M. E. J.
Med. Chem. 1998, 41, 5177.
[17]
Huffman, J. W. Curr. Med. Chem. 1999, 6, 705.
[18]
Devane, W. A.; Dysarz, F. A.; Johnson, M. R.; Melvin, L. S.;
Howlett, A. C. Mol. Pharmacol. 1988, 34, 605.
[19]
Matsuda, L. A.; Lolait, S. J.; Brownstein, M. J.; Young, A. C.;
Bonner, T. H. Nature 1990, 346, 561.
[20]
Gérard, C. M.; Mollereau, C.; Vassart. G.; Parmentier, M.
Biochem. J. 1991, 279, 129.
[21]
Little, P. J.; Compton, D. R.; Johnson, M. R.; Melvin, L. S.; Martin,
B. R. J. Pharmacol. Exp. Ther. 1988, 247, 1046.
[22]
Compton, D. R.; Rice, K. C.; De Costa, B. R.; Razdan, R. K.;
Melvin, L. S.; Johnson, M. R.; Martin, B. R. J. Pharmacol Exp.
Ther. 1993, 265, 218.
ACKNOWLEDGEMENTS
[23]
Munro, S.; Thomas. K. L.; Abu-Shar, M. Nature 1993, 365, 61.
[24]
Pertwee, R. G. Pharmacol. Ther. 1997, 74, 129.
The author thanks Dr. Patricia H. Reggio of the
University of North Carolina at Greensboro for many helpful
discussions over a several year period. The work carried out
at Clemson University which is included in the review was
supported by grants DA03590 and DA15340 to JWH and
DA15579 to LWP, all from the National Institute on Drug
Abuse. The author also thanks Drs. Billy R. Martin, Jenny
L. Wiley, David R. Compton, Dana E. Selley and Mary E.
Abood of Virginia Commonwealth University for the
pharmacological evaluation of the compounds prepared in
our laboratory. Thanks is also extended to the graduate
students and postdoctorals at Clemson University who
carried out the work from our group described in this review.
[25]
Showalter, V. M.; Compton, D. R.; Martin, B. R.; Abood, M. E. J.
Pharmacol. Exp. Ther. 1996, 278, 989
[26]
Felder, C. C.; Joyce, K. E.; Briley, E. M.; Mansouri, J.; Mackie,
K.; Blond, O.; Lai, Y.; Ma, A. L.; Mitchell, R. L. Mol.Pharmacol .
1995, 48, 443.
[27]
Busch-Petersen, J.; Hill, W. A.; Fan, P.; Khanolkar, A.; Xie, X.-
Q.; Tius, M. A.; Makriyannis, A. J. Med. Chem. 1996, 39, 3790.
[28]
Pertwee, R. G. Gen. Pharmacol. 1993, 24, 811.
[29]
Selley, D. E.; Stark, S.; Sim, L. J.; Childers, S. R. Life Sci. 1996, 59,
659.
[30]
Howlett, A. C.; Barth, F.; Bonner, T. I.; Cabral, G.; Casellas, W.
A.; Devane, W. A.; Felder, C. C.; Herekenham, M.; Mackie, K.;
Martin, B. R.; Mechoulam, R.; Pertwee, R. G. Pharmacol. Rev.
2002, 54, 161.
[31]
Eissenstat, M. A.; Bell, M. R.; D’Ambra, T. E.; Alexander, E. J.;
Daum, S. J.; Ackerman, J. H.; Gruett, M.D.; Kumar, V.; Estep, K.
G.; Olefirowicz, E. M.; Wetzel, J. R.; Alexander, M. D.; Weaver,
J. D.; Haycock, D. A.; Luttinger, D. A.; Casiano, F. M.; Chippari,
S. M.; Kuster, J. E.; Stevenson, J. I.; Ward, S. J. J. Med. Chem.
1995, 3094.
REFERENCES
[32]
Kuster, J. E.; Stevenson, J. I.; Ward, S. J.; D’Ambra, T. E.;
Haycock, D. A. J. Pharmacol. Exp. Ther. 1993, 264, 1352.
[1]
Gaoni, Y.; Mechoulam, R. J. Am. Chem. Soc. 1964, 86, 1646.
[2]
Razdan, R. K. Pharmacol. Rev. 1986, 38, 75.
[33]
Compton, D. R.; Gold, L. H.; Ward, S. J.; Balster, R. L.; Martin, B.
R. J. Pharmacol. Exp. Ther. 1992, 263, 1118.
[3]
Rapaka, R. S.; Makriyanis, A. Structure-Activity Relationships of
the Cannabinoids, NIDA Research Monograph 79; National
Institute on Drug Abuse, Rockville, MD, 1987.
[34]
D'Ambra, T. E.; Eissenstat, M. A.; Abt, J.; Ackerman, J. H.;
Bacon, E. R.; Carabateas, P. M.; Josef, K. A.; Kumar, V.;
Weaver, J. D.; Arnold, R.; Casiano, F. M.; Chippari, S. M.;
Haycock, D. A.; Kuster, J. E.; Luttinger, D. A.; Stevenson, J. I.;
Ward, S. J.; Hill, W. A.; Khanolkar, A.; Makriyannis, A. Bioorg.
Med. Chem. Lett. 1996, 6, 17.
[4]
Mechoulam, R.; Devane, W. A.; Glaser, R. In
Marijuana/Cannabinoids: Neurobiology and Neurophysiology;
Murphy, L; Bartke, A . Ed.; CRC Press, Boca Raton 1992; pp 1-33.
[5]
Seltzman, H. H. Curr. Med. Chem. 1999, 6, 685.
[6]
Melvin, L. S.; Johnson, M. R.; Herbert, C. A.; Milne, G. M.;
Weissman, A. A. J. Med. Chem. 1984, 27, 67.
[35]
Shim, J.-Y.; Collantes, E. R.; Welsh, W. J.; Subramaniam, B.;
Howlett, A. C.; Eissenstat, M. A.; Ward, S. J. J. Med. Chem. 1998,
41, 4521.
[7]
Johnson, M. R.; Melvin, L. S.; Milne, G. M. Life Sci. 1982, 31,
1703.
[36]
Huffman, J. W.; Wu, M.-J.; Lainton, J. A. H.; Dai, D.; Phillips, M.;
Keel, C.; Wiley, J. L.; Compton, D. R.; Showalter, V.; Abood, M.
E.; Martin, B. R. 1997 Symposium on the Cannabinoids
International Cannabinoid Research Society; Burlington, VT,
1997, p. 8.
[8]
Johnson, M. R.; Melvin, L. S. In Cannabinoids as Therapeutic
Agents; Mechoulam, R., Ed; CRC Press; Boca Raton, FL, 1986; pp.
121-145.
[9]
Melvin, L. S.; Milne, G. M.; Johnson, M. R.; Subramian, B.;
Wilken, G. H.; Howlett, A. C. Mol. Pharmacol. 1993, 44. 1008.

Recent Developments in the Medicinal Chemistry of Cannabimimetic
Current Medicinal Chemistry, 2005, Vol. 12, No. 12 1411
[37]
Aung, M. M.; Griffin, G.; Huffman, J. W.; Wu, M.-J.; Keel, C.;
Yang, B.; Showalter, V. M.; Abood, M. E.; Martin, B. R. Drug
Alcohol Depend. 2000, 60, 133.
[49]
Malan, T. P. ; Ibrahim, M.; Deng, H.; Liu, Q.; Mata, H. P.;
Vanderah, T.; Porreca, F.; Makriyannis, A. Pain 2001, 93, 239.
[50]
Nackley, A. G.; Makriyannis, A.; Hohmann, A. G. Neurosci.
2003, 119, 747.
[38]
Huffman, J. W. Curr. Pharm. Des.2000, 6, 1323.
[39]
Song, Z. H.; Bonner, T. I. Mol. Pharmacol. 1996, 49, 891.
[51]
Hohmann, A. G.; Farthing, J. N.; Zvonok, A. M.; Makriyannis, A.
J. Pharmacol. Exp. Ther. 2004, 308, 445.
[40]
Chin, C.; Lucas-Lenard, J.; Abadji, V.; Kendall, D. A. J.
Neurochem. 1998, 70, 363.
[52]
Hynes, J.; Leftheris, K.; Wu, H.; Pandit, C.; Chen, P.; Norris, D. J.;
Chen, B.-C.; Zhao, R.; Kiener, P. A.; Chen, X.; Turk, L. A.; Patil-
Koota, V.; Gillooly, K. M.; Shuster, D. J.; McIntyre, K. W. Bioorg.
Med. Chem. Lett. 2002, 12, 2399.
[41]
Song, A. H.; Slowey, C.-A.; Hurst, D. P.; Reggio, P. H. Mol.
Pharmacol. 1999, 56, 834.
[42]
Huffman, J. W.; Lu, J.; Kitaygorodskiy, A.; Wiley, J. L.; Martin,
B. R. Bioorg. Med. Chem. 2000, 8, 439.
[53]
Wrobleski, S. T.; Chen, P.; Hynes, J.; Lin, S.; Norris, D. J.; Pandit,
C. R.; Spergel, S.; Wu, H.; Tokarski, J. S.; Chen, X.; Gillooly, K.
M.; Kiener, P. A.; McIntyre, K. W. Patil-Koota, V.; Shuster, D. J.;
Turk, L. A.; Yang, G.; Leftheris, K. J. Med. Chem. 2003, 46,
2110.
[43]
Bramblett, R. D.; Reggio, P. H. 1995 Symposium on the
Cannabinoids, International Cannabinoid Research Society;
Burlington, VT, 1995, pp. 16.
[44]
Huffman, J. W.; Mabon, R.; Wu, M.-J.; Lu, J.; Hart, R.; Hurst, D.
P.; Reggio, P. H.; Wiley, J. L.; Martin, B. R. Bioorg. Med. Chem.
2003, 11, 539.
[54]
Zhang, Q.; Ma, P.; Iszard, M.; Cole, R. B.; Wang, W.; Wang, G.
Drug Met. Dispos. 2002, 30, 1077.
[45]
Xie, X.-Q.; Eissenstat, M.; Makriyannis, A. Life Sci. 1995, 56,
1963.
[55]
Zhang, Q.; Ma, P.; Wang, W.; Cole, R. B.; Wang, G. J. Mass
Spec. 2004, 39, 672.
[46]
Huffman, J. W.; Zengin, G.;Wu, M.-J.; Lu, J.; Hynd, G.; Bushell,
K.; Thompson, A. L. S.; Bushell, S.; Tartal, C.; Hurst, D. P.;
Reggio, P. H.; Selley, D. E.; Cassidy, M. P.; Wiley, J. L.; Martin, B.
R. Bioorg. Med. Chem. 2005, 13, 89.
[56]
Jerina, D.; Daly, J.; Witkop, B.; Zaltzman-Nirenberg, P.;
Udenfreind, S. Arch. Biochem. Biophys. 1968, 128, 176.
[57]
Pertwee, R. G.; Griffin, G.; Lainton, J. A. H.; Huffman, J. W. Eur.
J. Pharmacol., 1995, 284, 241.
[47]
Huffman, J. W.; Wu, M.-J.; Lu, J. J. Org. Chem. 1998, 63, 4510.
[58]
Tarzia, G.; Duranti, A.; Tontini, A.; Spadoni, G.; Mor, M.; Tivara,
S.; Plazzi, P. V.; Kathuria, S.; Piomelli, D. Bioorg. Med. Chem.
2003, 11, 3965.
[48]
Ibrahim, M.; Deng, H.; Zvonok, A.; Cockayne, D. A.; Kwan, J.;
Mata, H.; Vanderah, T. W.; Lai, J.; Porreca, F.; Makriyannis, A.;
Malan, T. P. Proc. Natl. Acad. Sci. USA 2003, 100, 10529.
Wyszukiwarka
Podobne podstrony:
1 pentyl 3 phenylacetylindoles a new class of cannabimimetic indoles bioorg med chem lett 15 4110 41
recent developments in cannabinoid ligands life sci 77 1767 1798 (2005)
[Mises org]Raico,Ralph The Place of Religion In The Liberal Philosophy of Constant, Toqueville,
Homosexuals in the Military Analysis of the Issue
Blood in the Trenches A Memoir of the Battle of the Somme A Radclyffe Dugmore
Increased diversity of food in the first year of life may help protect against allergies (EUFIC)
Knudsen, 3rd Hand in the Angers Fragment of Saxo Grammaticus
Aspects of the development of casting and froging techniques from the copper age of Eastern Central
Haranas Redshift Calculations in the Dynamic Theory of Gravity
In the Key Chords of Dawn
Robert P Smith, Peter Zheutlin Riches Among the Ruins, Adventures in the Dark Corners of the Global
NLP How MetaStates Fill In The Missing Pieces of NLP
Sipperl The Machine in the Pastoral Imagery of 18th century utopia
Holt Edward Out of many One The voice(s) in the crusade ideology of Las Navas de Tolosa Thesis final
Stephen King A Bedroom In The Wee Hours Of The Morning
Production networks and consumer choice in the earliest metal of Western Europe
Microbiota is essential for social development in the mouse
więcej podobnych podstron