
Recent developments in cannabinoid ligands
Lea W. PadgettT
Howard L. Hunter Chemistry Laboratory, Clemson University, Clemson, SC, 29634-0973, USA
Abstract
Over the past 40 years, much research has been carried out directed toward the characterization of the
cannabinergic system. With the identification of two G-protein coupled receptors and the endogenous ligand,
anandamide, pharmacological targets have expanded to encompass hydrolase and transport proteins as well as
novel classes of cannabinoid ligands. Those ligands that demonstrate high affinity for the receptors and good
biological efficacy are tied together through lipophilic regions repeatedly demonstrated necessary for activity.
This review presents recent developments in the structure–activity relationships of several classes of cannabinoid
ligands.
D
2005 Elsevier Inc. All rights reserved.
Keywords: Cannabinoid; Structure–activity relationship; Pyrazole; Aminoalkylindole
Introduction
Marijuana and hashish, derived from the Indian hemp plant Cannabis sativa L., have long been used
as medicinal agents as well as recreational drugs. The primary psychoactive constituent of marijuana was
identified and its structure elucidated in 1964 as D
9
-tetrahydrocannabinol, 1, (D
9
-THC,
Fig. 1
) by
Gaoni
and Mechoulam (1964)
. Other compounds exhibiting similar psychoactive effects were subsequently
found, including an endogenous ligand, anandamide, 2 (
Devane et al., 1992
). Identification of these
compounds led to the discovery of two G-protein coupled receptors, CB
1
, found in the central nervous
0024-3205/$ - see front matter D 2005 Elsevier Inc. All rights reserved.
doi:10.1016/j.lfs.2005.05.020
T Tel.: +1 864 656 6847; fax: +1 864 656 6613.
E-mail address: leak@clemson.edu.
Life Sciences 77 (2005) 1767 – 1798
www.elsevier.com/locate/lifescie
system (CNS) (
Matsuda et al., 1990
), and CB
2
, which is located in the periphery and is interconnected
with the immune system (
Munro et al., 1993; Howlett, 1998; Pertwee, 1997
). These receptors are part of
the endocannabinoid system, which also consists of long-chain polyunsaturated fatty acids such as
anandamide, 2, and 2-arachidonoyl glycerol (2-AG), 3, as well as metabolizing and transport proteins
(
Khanolkar and Makriyannis, 1999
).
Discovery of the endocannabinoid system has prompted inquiry into the structural features and
biological properties of the receptors. Investigation into the salient structural features of D
9
-THC
and anandamide has led to the development of several structurally diverse classes of compounds
that bind to the receptors. Development of new ligands in different classes aids in the
determination of the structural requisites for receptor activation. The CB
1
receptor has been
implicated in several physiological pathways, including the treatment of neuroinflammatory
diseases, psychological and cognitive disorders, and obesity (
Adam and Cowley, 2002; Pertwee,
2000
). The CB
2
receptor may influence the immune system as it is localized primarily in the
spleen, tonsils, and immune cells (
Martin, 1986
). Structural changes to the ligands permit selective
binding to one receptor subtype, providing controls for developing pharmaceutical agents to target
specific physiological systems.
New compounds are typically evaluated for receptor affinity through in vitro displacement of
radiolabeled ligands with known affinity (
Devane et al., 1988; Compton et al., 1993
). Compounds
showing good receptor affinities can then be evaluated for pharmacological activity and mechanism of
action in a variety of assays. This review focuses on developments since 2002 in ligands that bind with
cannabinoid receptors, both as agonists and as antagonists. New structures and affinity values will be
presented. Ligands that are structurally similar to anandamide and 2-AG (endocannabinoids) will not be
discussed.
NHCH
2
CH
2
OH
O
O
OH
OH
OH
OH
O
N
O
N
O
O
O
OH
OH
1
4
5
2
3
1
2
3
1'
A
B
C
8
9
10
7
Fig. 1. Cannabimimetic ligands of different classes.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1768

Traditional cannabinoids
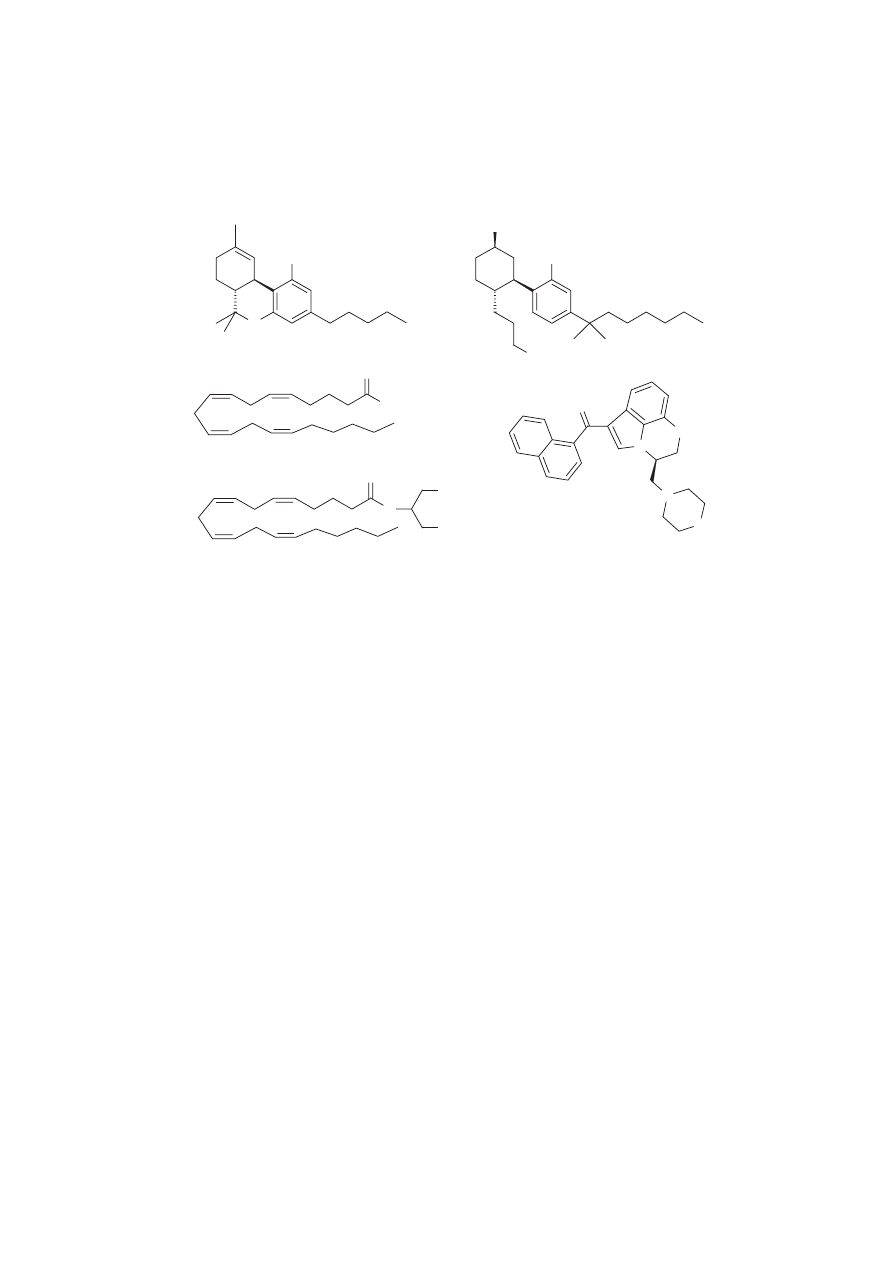
Classical cannabinoids are those containing the tricyclic benzopyran ring system as typified by D
9
-
THC. The structure–activity relationship (SAR) data for this class is very diverse and spans a wide range
of functionalities. Although the movement of the double bond in D
9
-THC to the D
8
position results in a
slight loss in affinity and a small decrease in potency (
Compton et al., 1993; Busch-Petersen et al.,
1996
), the D
8
-THC derivatives exhibit in vitro and in vivo effects similar to D
9
-THC and are
synthetically easier to prepare due to the increased thermodynamic stability of the D
8
double bond
(
Dalzell et al., 1981
). The receptor interaction model that has been developed points to three primary
sites on the molecule: a C3 aliphatic side chain of at least three carbons; a C1 phenolic hydroxyl group;
and a small C9 substituent, usually consisting of a methyl, hydroxymethyl, or hydroxyl (
Howlett et al.,
1988
). The phenolic hydroxyl has been found not to be essential in certain cases, as the synthesis has
been reported of deoxy derivatives that show excellent affinity for the CB
1
receptor and some selectivity
for the CB
2
receptor (
Huffman et al., 1996
). Several analogues that contain cyclic systems have been
prepared and show similar effects (
Reggio et al., 1997
). In depth studies have been made concerning the
nature of the aliphatic side chain. It was shown that a seven-carbon side chain is an optimal length for
affinity (
Edery et al., 1972
). Methylation on the side chain increases potency when close to the aromatic
ring, with the beneficial effects diminishing as the point of substitution moves farther from the ring
system (
Huffman et al., 1995
). Dimethylation to afford 1V, 1V-dimethylheptyl (DMH) is frequently the
side chain of choice due to the high potency of molecules containing this as a functional group and the
ease of DMH synthesis over other dimethyl side chains (
Tius et al., 1995
).
Quantitative structure–activity relationship (QSAR) studies have demonstrated moderate to high
flexibility in the alkyl side chain, pointing to the necessity of a hydrophobic group, but not elucidating
requirements due to bulk (
McAllister et al., 2002
). Side chains with restricted rotation have been
synthesized to determine how much flexibility is required, see
Table 1
. The introduction of 1V double and
triple bonds results in a moderate increase in affinity, where the cis configuration was favored over the
trans (
Busch-Petersen et al., 1996
). Effects arising from unsaturation farther down the side chain are
varied and in vivo effects do not necessarily correlate with in vitro affinities (
Ryan et al., 1995
). Poor
affinity for the CB
1
receptor arises from formation of a ring between the side chain and the C2 position
resulting in a rigid analogue with the side chain forced to project laterally out from the ring system
(
Huffman and Yu, 1998; Lu et al., 1997
). When the side chain is conformationally restricted to project
from the bottom face of the molecule, good affinity is exhibited (
Khanolkar et al., 1999
). Computational
studies suggest that the chain must be able to wrap around in proximity of the phenolic ring (
Keimowitz
et al., 1999
).
To examine the ligand binding pocket of the cannabinoid receptors, analogues containing rings on the
side chain that do not connect back to the benzene ring have been synthesized. The addition of a
dithiolane to the benzylic position affords a ligand with high affinity for both receptors that is
comparable to the 1V,1V-dimethylheptyl derivative, (9) (
Table 1
) (
Papahatjis et al., 1998
). Similarly, the
addition of a cyclopropyl group in the benzylic position affords compounds with high affinity (10).
Functionalization of the cyclopropyl group with gem-dichloro (11) results in slight selectivity for the
CB
2
receptor, while the bulkier gem-dibromo (12) substitution provides compounds that show equally
high affinity for both receptors (
Papahatjis et al., 2002
).
A series of cyclic derivatives was synthesized to examine the size of the pocket into which the side
chain fits. Side chains with 5, 6, and 7 atoms now arranged as a cyclic system were synthesized and
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1769
Table 1
Classical cannabinoid ligands
O
OH
R
K
i
(nM
)
a
Number
R
n–heptyl
1',1'–dimethylheptyl
X = H
X = Cl
CH
3
CH
3
X
X
X = Br
n = 1
n = 2
( )n
n = 3
n = 1
n = 2
S
S
( )n
n = 3
X = CH
3
X = O
X = H
X
X
X = SCH
2
CH
2
S
C
6
H
1 3
S
S
C
6
H
13
O
O
C
6
H
1 3
S
S
C
6
H
13
S
S
C
6
H
1 3
R1 = H
R1 = C
6
H
13
R1 = C
5
H
11
S
S
R1
CB
1
47.6
c, d
28.5
b, e
22
±
4
f
0.83
g
0.44
±
0.07
c, h
1.27
±
0.27
c, h
0.71
±
0.21
c, h
0.34
±
0.04
b, e
0.57
±
0.05
b, e
0.94
±
0.05
b, e
9.49
±
2.42
b, e
1.86
±
0.71
b, e
1.76
±
0.56
b, e
12.3
±
0.61
b, i
297
±
10.6
b, i
67.6
±
2.90
b, i
17.3
±
0.33
b, i
0.45
±
0.07
c, j
32.3
±
4.0
c, j
0.52
±
0.11
c, j
56.9
±
6.8
c, j
1.8
±
0.7
c, j
168
±
18
c, j
0.32
c, k
0.85
±
0.02
b, e
CB
2
39.3
c, d
25.0
b, e
0.49
g
0.86
±
0.16
c, h
0.29
±
0.06
c, h
1.0
±
0.36
c, h
0.39
±
0.06
b, e
0.65
±
0.04
b, e
0.22
±
0.01
b, e
2.74
±
1.10
b, e
1.05
±
0.41
b, e
6.62
±
0.92
b, e
0.91
±
0.08
b, i
23.6
±
1.76
b, i
85.9
±
0.31
b, i
17.6
±
1.03
b, i
1.92
±
0.4
c, j
19.7
±
2.7
c, j
0.22
±
0.06
c, j
257
±
41
c, j
3.6
±
1.3
c, j
103
±
16
c, j
0.52
c, k
0.58
±
0.03
b, e
6,
∆
8–
THC
7
8
10
11
12
13
14
15
16
17
18
19
21
22
20
23
24
25
26
28
27
9
29
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1770

affixed to the C1V position to restrict the orientation and flexibility of the side chain (13–18) (
Nadipuram
et al., 2003
). The disubstitution of the C1V position with a dithiolane or dimethyl was retained to limit
rotation around the C3–C1V bond. The cyclohexyl dithiolane compound (17), while exhibiting good
affinity, shows a decrease in receptor affinity with the cyclic group present relative to the same length
straight carbon chain (9). The cyclic dimethyl analogues (13–15) demonstrate similar affinities within
the series and when compared to the DMH side chain (8), implying that the decrease in affinity of the
dithiolane series may be due to steric effects.
An additional series was synthesized examining the placement of an aromatic moiety on the
cannabinoid side chain. A phenyl ring replaced the cyclohexyl group of the previous series and was
connected to the tricyclic ring system by a methylene, dithiolane, dimethyl, and ketone at the C1V
position (19–22) (
Krishnamurthy et al., 2003
). This series preserves the size and mobility restrictions of
the cyclohexyl analogue but significantly changes the electronic effects. The dimethyl compound (19)
shows good affinity with selectivity for the CB
2
receptor. This is in contrast to the cyclohexyl derivative
(14), which demonstrates no selectivity for receptor subclass. The dithiolane (20) was unselective and
showed less affinity than its aliphatic partner (17). The presence of the ketone moiety (21) affords good
selectivity for the CB
2
receptor, but this compound as well as the methylene compound (22) have lower
affinities on all counts when compared with D
8
-THC (6).
A series of D
8
-THC analogues with rings at the benzylic position was produced (
Papahatjis et al.,
2003
). Cyclopropyl and dithiolane systems (9–10) have already been described. A cyclopentyl (23) and
dioxolane (25) functionality were each synthesized and show good affinity for both receptors with a mild
selectivity for the CB
2
subtype. Enlargement of the ring to a six-membered dithiane (28) decreases the
affinity slightly and shows a mild selectivity for the CB
1
receptor. The five-membered dithiolane without
the hexyl group attached (27) shows a marked decrease in affinity for both receptors. Increasing the bulk
of the dithiolane to contain vicinal dimethyl or benzodithiolane moieties also results in a decrease in
affinity (24, 26). The CB
2
receptor shows greater susceptibility to the steric bulk of the substituents,
implying a greater steric limitation in the binding pocket of this receptor.
Bicyclics
While attempting to simplify the cannabinoid structure necessary for binding, the group at Pfizer
synthesized a number of bicyclic cannabinoid ligands that lack the pyran ring of traditional cannabinoids
(
Little et al., 1988
). The prototypical compound for this class of non-classical cannabinoids is CP-
55,940, (4,
Fig. 2
), a compound now used for radiolabeled displacement assays. It has been shown that
the aliphatic side chain and the phenolic hydroxyl are necessary for affinity, but the removal of the
cyclohexyl hydroxyl affords decreased affinity, and the removal of the cyclohexyl ring results in a
complete loss in affinity (
Howlett et al., 1988
).
Notes to Table 1:
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the mean
of three values with standard error of mean.
b
Data collected with cell membranes from HEK293 cells transfected with the
human CB
1
cannabinoid receptor and membranes from CHO-K1 cells transfected with the human CB
2
cannabinoid receptor.
c
Affinity determined using rat brain (CB
1
) or mouse spleen (CB
2
) membranes.
d
Busch-Petersen et al. (1996)
,
e
Nadipuram et al.
(2003)
,
f
Huffman and Yu (1998)
,
g
Khanolkar et al. (2000)
,
h
Papahatjis et al. (2002)
,
i
Krishnamurthy et al. (2003)
,
j
Papahatjis et
al. (2003)
,
k
Papahatjis et al. (1998)
.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1771

Based on a proposed alignment similar to that suggested by
Tong et al. (1998)
, Huffman designed a
pyridone ligand (31) where the benzenoid ring of CP-55,940 was replaced with a heterocycle and the
side chain was an n-pentyl like that found on D
9
-THC (
Huffman et al., 2001
). Although it was believed
that this compound would serve as a rigid analogue of anandamide, both stereoisomers (9h-OH, JWH-
168; 9a-OH, JWH-183) prepared demonstrated poor affinity for the CB
1
receptor subtype. The poor
affinity may arise from the inability of the amide carbonyl to substitute for a phenolic hydroxyl, since
CP-47,497 (30) is structurally very similar and possesses very good affinity. These two compounds may
not be good models for anandamide, or it may be a different conformation that gives rise to biological
activity. These compounds do, however, exhibit good CB
2
selectivity, and may demonstrate salient
features for a binding profile at the peripheral receptor subtype as the removal of the phenolic hydroxyl
of D
8
-THC also results in increased CB
2
selectivity.
Cannabidiol (54), a naturally occurring compound in the Marijuana plant, is also bicyclic, but has
poor affinity for CB
1
and does not exhibit the same in vivo profile. In an effort to determine why this is
the case,
Wiley et al. (2002)
prepared a number of resorcinols on the cannabidiol template (
Table 2
).
These compounds demonstrate many of the same trends shown by other classes of ligands. The single
most significant feature is the lipophilic side chain, which can vary in length and branching. The C3 side
chain has been demonstrated to be necessary for high CB
1
affinity in the traditional cannabinoid series
and with anandamide and in the indole series (
Seltzman et al., 1997
). Those compounds shown in
Table
2
without a dimethylheptyl side chain (43, 52) demonstrate reduced affinity for the CB
1
receptor
corresponding to the degree of difference between the side chain and the preferred DMH. The CB
2
affinities are affected, although to a lesser degree. This is in accordance with data that show that greater
CB
2
affinity is retained over a range of side chains in the D
8
-THC series (
Huffman et al., 1999
).
In the cannabidiol series, the C2 resorcinol substituent was also important for determining receptor
binding. The standard substituent at this position is a cyclohexyl group (33), originally chosen because
of the C ring in the traditional cannabinoids. Decreasing the size of this ring to a cyclopentyl results in a
decrease in affinity (32). Increasing the ring size to a cycloheptyl or adamantyl group (34, 35) provides a
small increase in affinity. The addition of a heteroatom to the ring results in a significant decrease in
affinity (36, 37), and in the case of nitrogen (38), a total loss of affinity for both receptors and the loss of
all in vivo activity. Hydrocarbon additions to this ring also result in a small to moderate decrease in
affinity (40–42) compared to 33, although 44 shows a slight increase in affinity. As has been seen in the
D
8
-THC and anandamide series (
Showalter et al., 1996; Compton et al., 1993
), unsaturations often result
in a decrease in affinity (39).
O
H
OH
OH
DMH
N
C
3
H
7
O
OH
OH
OH
DMH
4
30
31
-168, 183
CP-55940
CP-47497
JWH
Fig. 2. Bicyclic ligands.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1772
Table 2
Resorcinol derivatives (
Wiley et al., 2002
)
R2
R1
R1
R3
Affinty, K
i
(nM)
Number
R2
CB
1
CB
2
32
cyclopentyl
95
±
6
33
cyclohexyl
11
±
2
34
cycloheptyl
18
±
1
35
adamantyl
7
±
1
36
n = 2
153
±
17
37
S
( )n
n = 1
138
±
4
38
N
C
H
3
> 10,000
39
1–cyclohexenyl
97
±
5
40
2–methylcyclohexyl
16
±
2
41
4–methylcyclohexyl
45
±
1
42
4–phenylcyclohexyl
144
±
22
43
3–methylcyclohexyl
Dimethylbutyl
96
±
4
44
3,3–dimethylcyclohexyl
2
±
0.3
45
3–methylcyclohexyl
> 10,000
46
O
> 10,000
47
O
> 10,000
48
O
OH
> 10,000
49
OH
5820
±
662
50
OH
OH
1990
±
77
51
OH
7515
±
721
52
OH
> 10,000
53
R1
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OCH
3
OCH
3
OCH
3
OCH
3
OCH
3
OCH
3
OCH
3
OCH
3
OCH
3
OH
R3
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
DMH
CH
3
DMH
3201
±
141
7
±
0.4
1.5
±
0.1
2
±
0.2
3
±
0.8
12
±
2
28
±
12
5424
±
1103
28
±
5
1
±
0.3
5
±
0.9
9
±
2
13
±
1
0.3
±
0.01
466
±
110
> 10,000
911
±
116
342
±
22
105
±
19
101
±
14
161
±
24
> 10,000
64
±
8
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the mean
of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from CHO-
K1 cells transfected with the human CB
2
cannabinoid receptor.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1773
Methylation of the phenols provides compounds with no appreciable CB
1
affinity and that are
CB
2
selective (45–47). This effect has been observed previously in the D
8
-THC series when there is
no free phenolic hydroxyl (
Huffman et al., 1999
). The CB
2
affinity of these compounds was
increased by the addition of a tertiary alcohol in the position where the resorcinol is attached (48,
49, 53). Additional substitution to the cyclohexyl ring did not produce any significant beneficial
effects (50–52) and the incorporation of an oxygen atom into the ring structure greatly attenuated
affinity (46–48).
Although cannabidiol is not itself a psychoactive compound and shows poor affinity for the
cannabinoid receptors, it has been shown to act as an antagonist against WIN-55212-2 and CP-55940
Table 3
Cannabidiol derivatives (
Thomas et al., 2004
)
R3
R1
R2
Number
R1
R2
R3
CB
1
K
i
a
54, ( )-CBD
OH
OH
C
5
H
11
4.9 AM
55
OH
OH
CH
2
CQC(CH
2
)
2
CH
2
N
3
114 nM
56
OH
OCH
3
C
5
H
11
N 10 AM
57
OCH
3
OCH
3
C
5
H
11
N 10 AM
58 Abnormal-cbd
OH
C
5
H
11
OH
N 30 AM
b
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the
mean of three values with standard error of mean. Affinity determined using mouse brain membranes.
b
Offertaler et al. (2003)
.
Table 4
Two enantiomeric classical cannabinoids (
Thakur et al., 2002
)
O
C
6
H
13
OH
OH
HO
Stereochemistry
Affinity K
i
(nM)
a
CB
1
CB
2
59, 6R, 6aS, 9S, 10aS
94.82
124.80
60, 6S, 6aR, 9R, 10aR
0.16
1.15
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the
mean of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from
CHO-K1 cells transfected with the human CB
2
cannabinoid receptor.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1774
(
Pertwee et al., 2002
). It does this in a manner that implies an as yet undetermined interaction
mechanism, as the antagonism occurs at concentrations far below its binding values (
Pertwee et al.,
2002
). Recent investigations by
Thomas et al. (2004)
are directed at determining the mechanism
through which this antagonism occurs. Four compounds, shown in
Table 3
, were developed and
tested in the mouse vas deferens protocol and evaluated for K
i
values against [
3
H]CP55940. It was
determined that these changes were sufficient to point to possible therapeutic targets, as subtle
changes resulted in dramatic differences in ability to antagonize WIN-55212-2 in the mouse vas
deferens assay and to attenuate contractions induced by phenylephrine, the a
1
-adrenoceptor agonist.
As each molecule gives different results on these tests, it is likely that more than one mechanism is at
work. The antagonism of WIN-55212-2 is competitive, but does not appear to act through direct
competition for the CB
1
binding site. More work is required to determine what mechanisms are
taking place, how to improve the selectivity, and if the cannabidiol derivatives are functioning as
neutral antagonists.
Two enantiomeric hybrid cannabinoids have been prepared (59–60) that demonstrate a
stereochemical preference in binding (
Thakur et al., 2002
). These compounds and their affinities
for both receptor subtypes are shown in
Table 4
. These compounds have the southern aliphatic
hydroxyl of CP-55940, but are conformationally restricted due to the pyran ring and the unsaturation
of the alkyl chain.
Aminoalkylindoles and related compounds
While searching for non-steroidal anti-inflammatory drugs (NSAIDs) the Sterling-Winthrop group
prepared a group of compounds that inhibit adenylate cyclase activity, are antinociceptive, and are not
blocked by naloxone (
Bell et al., 1991
). The lead compound in this series was pravadoline (61,
Fig. 3
)
and other compounds were developed with increased cannabinoid potency such as WIN-55,212-2 (5),
but with the cost of NSAID efficacy (
Compton et al., 1992
). A detailed review of the
aminoalkylindole (AAI) SAR has been previously presented (
Huffman, 1999
). The salient structural
features for this class are a C3 naphthoyl group and a lipophilic group attached to the indole nitrogen,
N
N
O
O
OMe
O
N
OH
C
5
H
11
O
OH
OH
Pravadoline
61
JWH-161
122
HU-210
123
Fig. 3. Cannabinoid ligands.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1775
although the aminoalkyl group can be replaced with an alkyl group of suitable length with no loss in
affinity (
Wiley et al., 1998; Huffman et al., 1994; Kumar et al., 1995
). It has been proposed that AAIs
interact with the receptor differently than the classical cannabinoids (
Song and Bonner, 1996
). The
carbonyl, believed to be necessary for hydrogen-bonding, has been demonstrated unnecessary through
the synthesis of cannabimimetic indenes (
Reggio et al., 1998
). A model wherein AAIs bind to the
cannabinoid receptor through aromatic stacking has been advanced and is well supported by
computational data (
Reggio et al., 1998
). Experimental evidence shows a decrease in affinity for
pyrroles, which do not contain the benzenoid moiety, in relation to the corresponding indoles (
Lainton
et al., 1995
).
Working within these hypotheses, several indoles have recently been prepared to examine the
effect of hydrogen-bonding sites on the receptor affinity of ligands. A series of 3-(1-
pentylindole)-1-naphthylmethanes and their corresponding 2-methyl analogues have been produced
by
Huffman et al. (2003)
containing no sites for hydrogen-bonding interaction, shown in
Table
5
. The 3-(1-pentylindole)-1-naphthylmethane (62) and its 4-methyl-naphthyl (63) and 4-
Table 5
3-Substituted indoles (
Huffman et al., 2003
)
N
R
X
R2
R1
R
R2
X
K
i
CB
1
(nM)
a
C
5
H
11
H
H
22
±
2
C
5
H
11
H
23
±
6
C
5
H
11
OCH
3
H
17
±
3
C
5
H
11
H
H
151
±
18
C
5
H
11
H
127
±
19
C
5
H
11
OCH
3
H
323
±
28
C
5
H
11
H
O
9
±
5
C
5
H
11
O
0.69
±
0.05
C
5
H
11
OCH
3
O
1.2
±
0.1
C
5
H
11
H
O
9.5
±
4.5
C
5
H
11
O
5.0
±
2.1
C
5
H
11
OCH
3
O
4.5
±
0.1
K
i
CB
2
(nM)
a
2.9
±
2.6
1.2
±
1.2
12.4
±
2.2
2.9
±
2.6
0.73
±
0.03
1.9
±
0.3
C
5
H
11
H
O
52
±
5
H
H
113
±
28
H
41
±
13
OCH
3
H
20
±
2
H
O
42
±
5
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
O
6
±
1
Number
63, JWH–184
62, JWH–175
64, JWH–185
65, JWH–196
66, JWH–194
67, JWH–197
68, JWH–018
69, JWH–122
70, JWH–081
71, JWH–007
72, JWH–149
73, JWH–098
74, JWH–116
75, JWH–195
76, JWH–192
77, JWH–199
78, JWH–200
79, JWH–193
80, JWH–198
N
O
R1
H
H
H
H
H
H
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
C
2
H
5
H
H
H
H
H
H
OCH
3
O
10
±
2
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the mean
of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from CHO-
K1 cells transfected with the human CB
2
cannabinoid receptor.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1776

methoxynaphthyl (64) analogues show receptor affinities that are the same within the limits of
experiment (K
i
= 17–23 nM). This is slightly less affinity than the corresponding naphthoyl
analogues (68–70). The 2-methyl analogues containing the carbonyl (71–73) differ only slightly
from the non-methylated indoles (68–70), but the 2-methyl analogues of 3-(1-pentylindole)-1-
naphthylmethane (65–67) exhibit greatly reduced receptor affinity. Modeling studies indicate that
this difference may arise from a disruption of aromatic stacking interactions by the 2-methyl
group orienting the halves of the indole molecule into a non-active conformation. The ability of
the naphthoyl groups to hydrogen bond can account for the small decrease in affinity for the 3-
(1-pentylindole)-1-naphthylmethane series relative to the naphthoyl series, but the relatively high
affinity shown by these compounds even in the absence of hydrogen bonding substituents
supports the hypothesis that aromatic stacking is the more important interaction mechanism.
Replacement of the pentyl group with a morpholinoethyl group (75–80) to add additional
hydrogen bonding sites results in compounds that have moderate to good affinities for the
receptor. However, the pentyl group still provides compounds with greater affinities. Modeling
results support the chain length experimentally found to be preferred, namely 4–6 carbons in the
alkyl chain, with the maximum occurring at the pentyl. A hydrophobic pocket has been
postulated that requires at least three carbons to interact but lengthening the chain to seven
carbons results in a van der Waals overlap. Aromatic residues are arranged near the hydrophobic
pocket in such a way as to prefer the s-trans ligand conformation around the indole–naphthoyl
bond. The presence of a 2-methyl group in the absence of a carbonyl creates a strong energetic
preference for the s-cis conformer resulting in the observed loss of affinity.
It has been demonstrated that the presence of a 7-methyl group on the naphthoyl substituent does
not significantly affect the affinity of N-pentyl-3-(1-naphthoyl)indole (
Aung et al., 2000
). New
indoles have been prepared to expand on the known SAR concerning substituted 3-naphthoyl
groups. A series of alkyl substituted indoles has recently been prepared by Huffman et al.
(unpublished data) and the data are presented in
Table 6
. The addition of an ethyl or propyl
substituent to the four position of the naphthoyl in both N-propyl- and N-pentylindole series (85,
86, 96, 97) results in an increase in affinity for both receptors compared with 68 and 83. In the N-
propyl series a 4-butylnaphthoyl substituent results in an increase in affinity (87), but provides a
decrease in the N-pentyl series (98), although this compound still possesses high affinity. For the N-
propylindoles, addition of a methyl group in the seven-naphthoyl position (81) results in a five-fold
increase in CB
1
affinity, and an ethyl group (93) gives a three-fold increase. Both substituents
afford an increase in CB
2
receptor affinity. If the indole has a 2-methyl substituent, however,
substitution at the 7-naphthoyl position to give 82 results in a decrease in affinity when compared
with 88. Substitution in the seven-naphthoyl position appears to have no significant effect on
receptor affinity in the N-pentyl series.
Indoles, shown in
Table 7
, have been synthesized to evaluate the effects of 2, 4, 6, and 7-alkoxy-1-
naphthoyl substituents on receptor affinity. Substitution in the 4 position with methoxy or ethoxy
generally increases the receptor affinity for CB
1
in both the N-pentyl (70, 120) and N-propylindoles
(105, 112). In the presence of the indole 2-methyl group, the affinities decrease for both N-alkyl series
(109, 113, 121), with the exception of analogue 73, which increases slightly. Substitution in the 6
position affords a decrease in CB
1
affinity, regardless of a 2-methyl group on the indole (106, 110,
115, 118). Groups in the 2 position of the naphthoyl give rise to significant reductions in affinity (104,
108, 114, 117). Substitution at the 7-naphthoyl position resulted in an increase in affinity for 107 and
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1777
116, but a decrease in CB
1
affinity with the indole 2-methyl (111, 119). Substitution of an alkoxy
group anywhere on the naphthoyl in the presence of the indole 2-methyl group results in a decrease in
CB
2
receptor affinity. The notable exception to this is again compound 73. In the N-propyl series,
when no 2-methyl substituent is present on the indole, substitution at all positions on the naphthoyl
with an alkoxy group gives rise to increased affinity for CB
2
. In the N-pentyl series in the absences of
the indole 2-methyl, substitution at any position other than 6-naphthoyl (115) results in a decrease in
CB
2
affinity. The 2-methyl-3-(2-methoxy-1-naphthoyl)-N-pentylindole analogue (117) also shows
good CB
2
selectivity and has a moderate affinity. This series as a whole provides a significant number
Table 6
3-(Alkyl-1-naphthoyl)indoles (
Huffman et al., 2003
)
N
O
R1
R
R3
R2
Compound
R
R1
R2
R3
K
i
CB
1
(nM)
a
K
i
CB
2
(nM)
a
81, JWH-076
C
3
H
7
H
H
CH
3
214 F 11
106 F 46
82, JWH-046
C
3
H
7
CH
3
H
CH
3
343 F 38
16 F 5
83, JWH-072
C
3
H
7
H
H
H
1050 F 55
170 F 54
84, JWH-120
C
3
H
7
H
CH
3
H
1054 F 31
6.1 F 0.7
85, JWH-212
C
3
H
7
H
C
2
H
5
H
33 F 0.9
10 F 1.2
86, JWH-180
C
3
H
7
H
C
3
H
7
H
26 F 2
9.6 F 2.0
87, JWH-239
C
3
H
7
H
C
4
H
9
H
342 F 20
52 F 6
88, JWH-015
C
3
H
7
CH
3
H
H
164 F 22
13.8 F 4.6
89, JWH-148
C
3
H
7
CH
3
CH
3
H
123 F 8
14 F 1.0
90, JWH-211
C
3
H
7
CH
3
C
2
H
5
H
70 F 0.8
12 F 0.8
91, JWH-189
C
3
H
7
CH
3
C
3
H
7
H
52 F 2
12 F 0.8
92, JWH-241
C
3
H
7
CH
3
C
4
H
9
H
147 F 20
49 F 7
93, JWH-235
C
3
H
7
H
H
C
2
H
5
338 F 34
123 F 34
94, JWH-236
C
3
H
7
CH
3
H
C
2
H
5
1351 F 204
240 F 63
68, JWH-018
C
5
H
11
H
H
H
9 F 5
2.9 F 2.6
95, JWH-048
C
5
H
11
CH
3
H
CH
3
10.7 F 1.0
0.49 F 0.1
96, JWH-210
C
5
H
11
H
C
2
H
5
H
0.46 F 0.03
0.69 F 0.01
97, JWH-182
C
5
H
11
H
C
3
H
7
H
0.65 F 0.03
1.1 F 0.1
98, JWH-240
C
5
H
11
H
C
4
H
9
H
14 F 1
7.2 F 1.3
99, JWH-213
C
5
H
11
CH
3
C
2
H
5
H
1.5 F 0.2
0.42 F 0.05
100, JWH-181
C
5
H
11
CH
3
C
3
H
7
H
1.3 F 0.1
0.62 F 0.04
101, JWH-242
C
5
H
11
CH
3
C
4
H
9
H
42 F 9
6.5 F 0.3
102, JWH-234
C
5
H
11
H
H
C
2
H
5
8.4 F 1.8
3.8 F 0.6
103, JWH-262
C
5
H
11
CH
3
H
C
2
H
5
28 F 3
5.6 F 0.7
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the
mean of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from
CHO-K1 cells transfected with the human CB
2
cannabinoid receptor.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1778
of compounds with selectivity for the CB
2
receptor, although the affinities for many of them are only
moderate.
Pyrroles
As a result of the suggested overlaps of traditional cannabinoids and the aminoalkylindoles
indicating how these two different structural classes interact with the cannabinoid receptors, it was
concluded that the benzenoid moiety of the indole may not be necessary for activity. A series of 3-(1-
Table 7
3-(Alkoxy-1-naphthoyl)indoles
N
O
R1
R
R5
R3
R4
R2
Compound
R
R1
R2
R3
R4
R5
K
i
CB
1
(nM)
a
K
i
CB
2
(nM)
a
83, JWH-072
C
3
H
7
H
H
H
H
H
1050 F 55
170 F 54
104, JWH-265
C
3
H
7
H
OCH
3
H
H
H
3788 F 323
80 F 13
105, JWH-079
C
3
H
7
H
H
OCH
3
H
H
63 F 3
32 F 6
106, JWH-163
C
3
H
7
H
H
H
OCH
3
H
2358 F 215
138 F 12
107, JWH-165
C
3
H
7
H
H
H
H
OCH
3
204 F 26
71 F 8
88, JWH-015
C
3
H
7
CH
3
H
H
H
H
164 F 22
13.8 F 4.6
108, JWH-266
C
3
H
7
CH
3
OCH
3
H
H
H
N 10,000
455 F 55
109, JWH-094
C
3
H
7
CH
3
H
OCH
3
H
H
476 F 67
97 F 3
110, JWH-151
C
3
H
7
CH
3
H
H
OCH
3
H
N 10,000
30 F 1.1
111, JWH-160
C
3
H
7
CH
3
H
H
H
OCH
3
1568 F 201
441 F110
112, JWH-259
C
3
H
7
H
H
OC
2
H
5
H
H
220 F 29
74 F 7
113, JWH-261
C
3
H
7
CH
3
H
OC
2
H
5
H
H
767 F 105
221 F14
68, JWH-018
C
5
H
11
H
H
H
H
H
9 F 5
2.9 F 2.6
114, JWH-267
C
5
H
11
H
OCH
3
H
H
H
381 F16
7.2 F 0.14
70, JWH-081
C
5
H
11
H
H
OCH
3
H
H
1.2 F 0.1
12.4 F 2.2
115, JWH-166
C
5
H
11
H
H
H
OCH
3
H
44 F 10
1.9 F 0.08
116, JWH-164
C
5
H
11
H
H
H
H
OCH
3
6.6 F 0.7
6.9 F 0.2
71, JWH-007
C
5
H
11
CH
3
H
H
H
H
1.2 F 0.1
12.4 F 2.2
117, JWH-268
C
5
H
11
CH
3
OCH
3
H
H
H
1379 F 193
40 F 0.6
73, JWH-098
C
5
H
11
CH
3
H
OCH
3
H
H
4.5 F 0.1
1.9 F 0.3
118, JWH-153
C
5
H
11
CH
3
H
H
OCH
3
H
250 F 24
11 F 0.5
119, JWH-159
C
5
H
11
CH
3
H
H
H
OCH
3
45 F 1
10.4 F 1.4
120, JWH-258
C
5
H
11
H
H
OC
2
H
5
H
H
4.6 F 0.6
10.5 F 1.3
121, JWH-260
C
5
H
11
CH
3
H
OC
2
H
5
H
H
29 F 0.4
25 F 1.9
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the
mean of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from
CHO-K1 cells transfected with the human CB
2
cannabinoid receptor.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1779
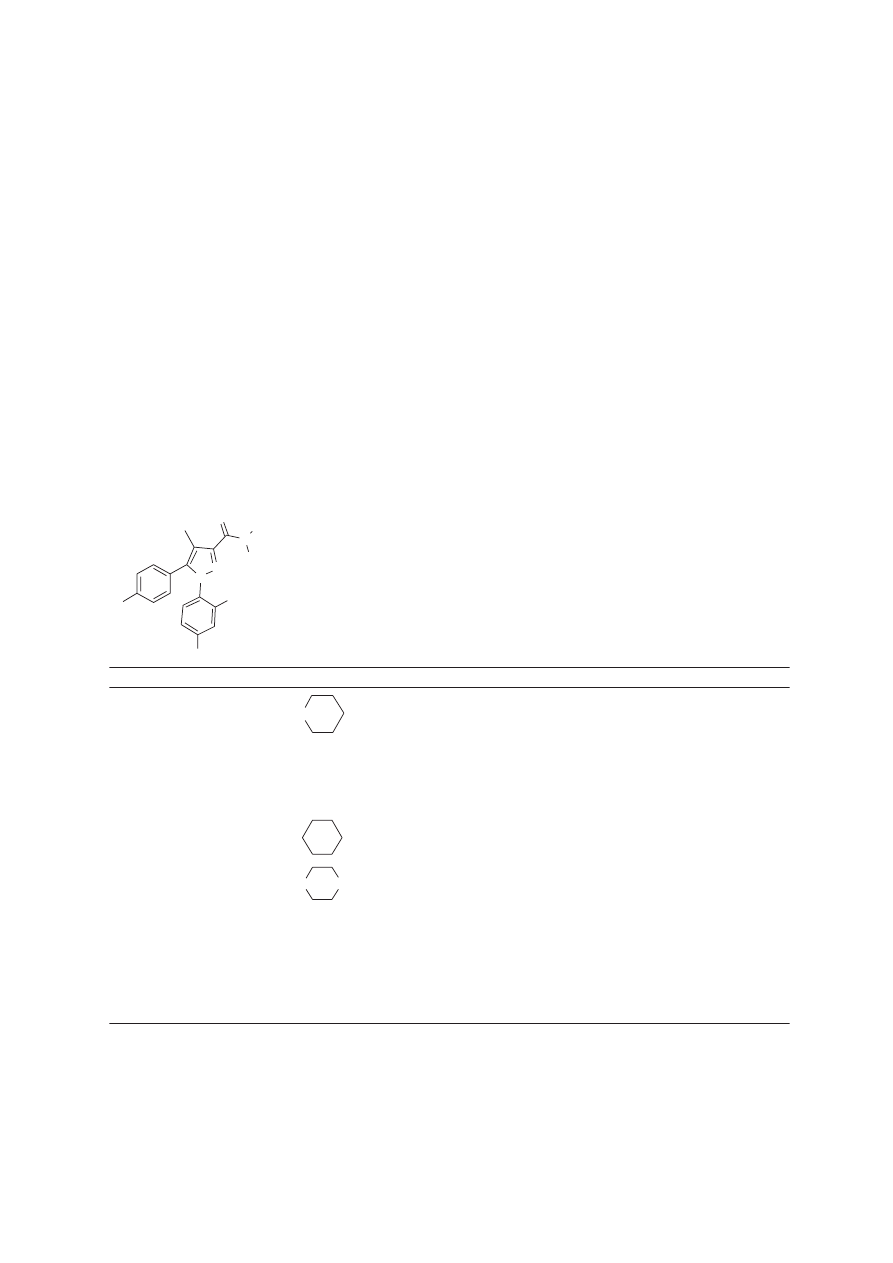
naphthoyl)-alkylpyrroles was prepared to evaluate this hypothesis, and it was determined that the
pyrroles possess less affinity for the cannabinoid receptor than do the corresponding indoles (
Lainton
et al., 1995
). It was also observed that the effect of alkyl chain length on binding affinity is similar to
that observed in the 3-(1-naphthoyl)-alkylindole series where the optimal length is five carbons. The
affinity decreases rapidly as the chain is lengthened or shortened. To expand the SAR concerning
pyrroles, several substituted derivatives have been prepared. A hybrid compound, JWH-161 (122),
combines the traditional D
8
-THC structure with that of an alkylindole. The high affinity this
compound shows for CB
1
(K
i
= 19 F 3 nM) provides a model for the directed substitution of the
pyrrole nucleus (
Huffman et al., 2000
).
A computational overlay of JWH-161 (122) and a number of pyrrole derivatives target the distal ring of
the naphthoyl moiety, the a-positions of the pyrrole, and the alkylhydroxy substituent of the potent
cannabinoid HU-210 (K
i
= 0.73 F 0.11) (123) as prime sites for further investigation (
Huffman et al.,
2000
). Several new ligands are shown in
Table 8
(
Tarzia et al., 2003
). The concomitant presence of methyl
substituents at both a-positions of the pyrrole (125) results in a moderate decrease in affinity for the CB
1
Table 8
Pyrrole cannabinoid analogues (
Tarzia et al., 2003
)
N
R3
O
R5
R4
R2
R1
Number
R1
R2
R3
R4
R5
Affinity K
i
(nM)
a
rCB1
hCB2
124
C
5
H
11
H
1-naphthyl
H
H
30.5 F 4.7
552 F 314
125
C
5
H
11
CH
3
1-naphthyl
H
CH
3
45.3 F 7.5
9.85 F 2.1
126
C
3
H
7
CH
3
1-naphthyl
H
CH
3
N 1000
309.7 F 20.8
127
pClC
6
H
4
CH
2
CH
3
1-naphthyl
H
CH
3
83.7 F 17.8
55.6 F 26.5
128
C
5
H
11
CH
3
1-naphthyl
Br
CH
3
13.3 F 0.5
6.8 F 1.0
129
C
3
H
7
CH
3
1-naphthyl
Br
CH
3
780 F 326
691.3 F 101.3
130
pClC
6
H
4
CH
2
CH
3
1-naphthyl
Br
CH
3
38 F 7.2
194.5 F 27.5
131
C
5
H
11
H
1-naphthyl
(CH
2
)
4
235.8 F 6.2
139 F 55
132
C
5
H
11
CH
3
C
6
H
5
H
CH
3
N 1000
N 1000
133
C
5
H
11
CH
3
C
6
H
5
Br
CH
3
N 1000
N 1000
134
pClC
6
H
4
CH
2
CH
3
C
6
H
5
H
CH
3
N 1000
N 1000
135
C
5
H
11
CH
3
HO(CH
2
)
3
H
CH
3
N 3000
N 10,000
136
C
5
H
11
CH
3
o(CH
3
CO)C
6
H
4
NH
H
CH
3
367.3 F 31.2
N 1000
137
C
5
H
11
CH
3
c-C
6
H
11
NH
H
CH
3
415.5 F 79.5
483.5 F 211
138
b
C
5
H
11
H
1-naphthyl
H
C
6
H
5
11.6
139
c
C
5
H
11
H
1-naphthyl
H
1-naphthyl
40.83 F 3.32
49.2 F 7.1
140
c
C
5
H
11
H
1-naphthyl
H
2-naphthyl
333.7 F 17.0
169.3 F 17.0
a
Data from displacement of [
3
H]WIN55212-2 in at least three independent experiments run in duplicate and expressed as the
mean of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from
CHO-K1 cells transfected with the human CB
2
cannabinoid receptor.
b
Knight et al. (2003)
.
c
Knight et al. (2004)
.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1780

receptor and a large increase in affinity for the CB
2
receptor when compared to 3-(1-naphthoyl)-N-
pentylpyrrole (124). This characteristic is also demonstrated in several other ligands, although the effect is
less significant (127, 128, 130). The addition of a bromine atom to the 4-position of the pyrrole also results
in a small increase in affinity for CB
1
and a large increase in affinity for the CB
2
receptor (128). The
addition of a cyclohexyl ring connecting the 4 and 5 position greatly reduces CB
1
affinity (131), however,
even though it is assumed to occupy the same location as the benzenoid moiety of the corresponding
indoles. As with the indoles, addition of a propyl chain to the nitrogen in place of a pentyl group results in
significantly attenuated affinity for CB
1
(126) or both receptor subtypes (129), although in some cases it
provides a degree of CB
2
selectivity, a trend that has been previously observed (
Wiley et al., 1998
). The
successful substitution of a para-chlorobenzyl substituent (127, 130) to the nitrogen yielding compounds
with moderate affinity is unexpected in light of the bulk of evidence for a lipophilic binding pocket of finite
size. The para-chlorobenzyl substituent with a benzoyl instead of a naphthoyl gives a compound with no
affinity for either receptor (134). These compounds may interact with the receptor through a different
mechanism, although there exists little evidence on which to base a hypothesis at this time.
Alterations to the 3-aroyl substituent provide compounds with dramatically attenuated potency,
speaking strongly for the distal naphthyl ring interacting directly with the ligand binding pocket
(
Tarzia et al., 2003
). The use of a benzoyl substituent gives compounds with no appreciable affinity
(132–134). The compounds that have structural features such that an aliphatic ring may occupy the
same spatial location as the naphthyl ring, and thus the cyclohexene ring of D
8
-THC, still show some
binding potential, although it is greatly reduced. This is demonstrated by N-(2-acetylphenyl)
carboxamido and N-cyclohexyl carboxamido groups (136, 137). The addition of a propanol
substituent (135) to mimic the northern aliphatic hydroxyl on many traditional cannabinoids was
also performed in an effort to target the binding site that the traditional cannabinoids appear to utilize.
This compound exhibited no appreciable affinity, indicating that this may not be an important binding
interaction, at least for the pyrroles. This lends support to the belief that the pyrroles bind with the
receptor in a mode different to that of the traditional cannabinoids.
While the proposed alignment (
Huffman et al., 1994
) does not indicate the benzenoid moiety of the
indoles as essential to binding, its removal results in a decrease in potency. Indeed, if the AAIs interact
primarily through aromatic stacking by a different mode than that of the traditional cannabinoids, this
ring may play a less obvious but essential role. To probe the decrease in affinity, a series of pyrroles has
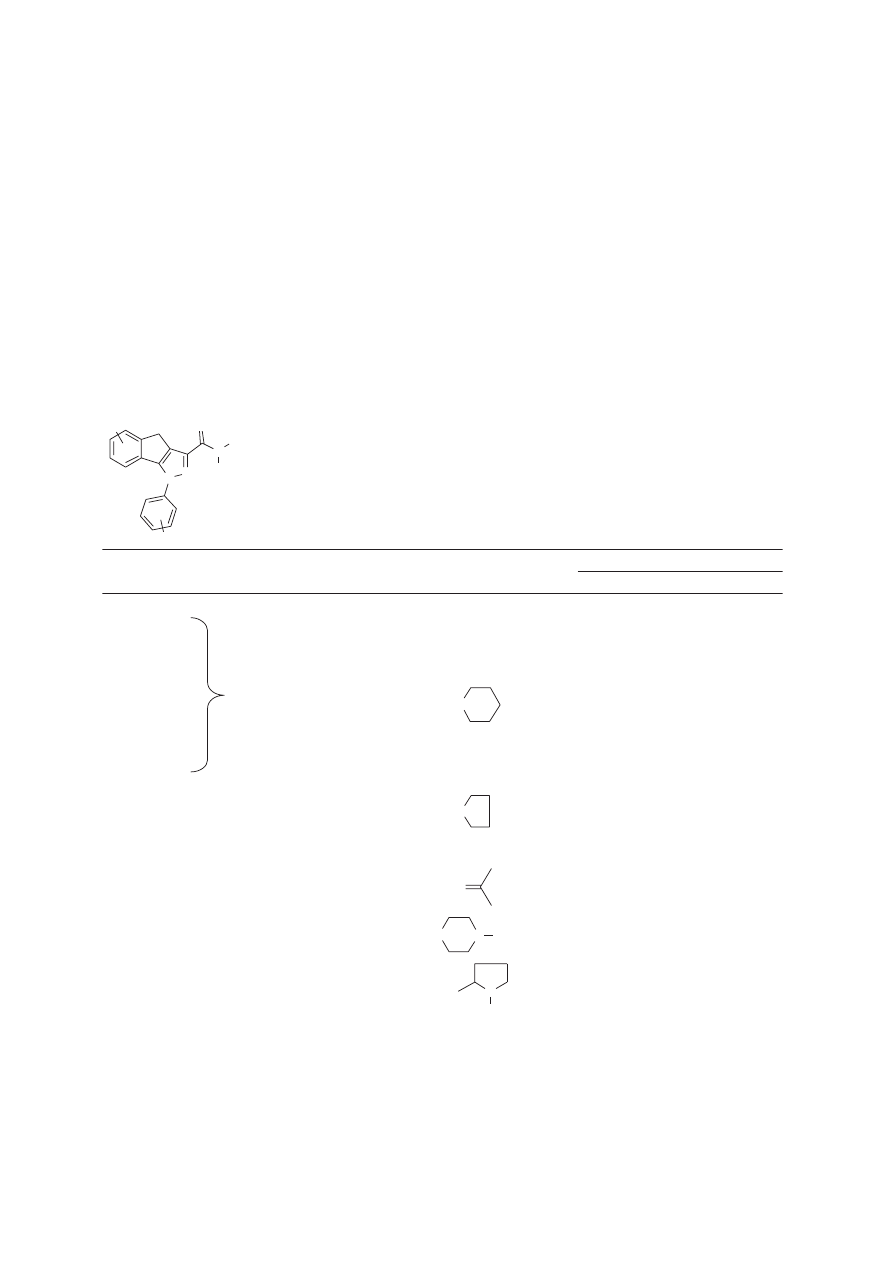
been prepared that replace the fused benzenoid moiety with a conformationally flexible aromatic ring in
N
O
H
N
Cl
Cl
Cl
N
N
N
O
H
N
Cl
Cl
Cl
N
N
N
O
H
Cl
SR141716
141
SR144528
142
143
1
2
3
4
5
Fig. 4. Pyrazole antagonists and phenyl analogue.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1781
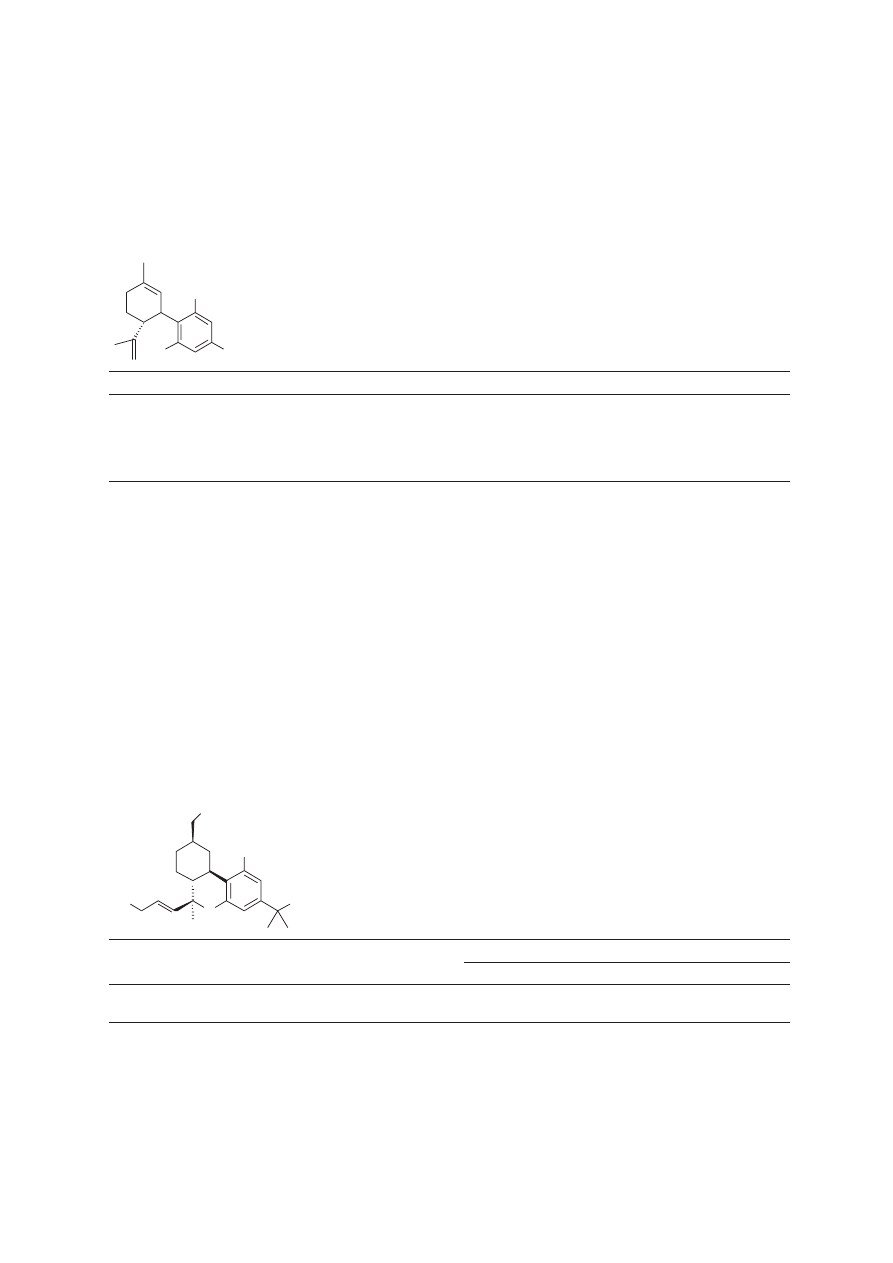
the 2-position of the pyrrole nucleus. The addition of a phenyl substituent (138) affords a compound
with CB
1
affinity comparable to that of 3-(1-naphthoyl)-N-pentylindole (124) (
Knight et al., 2003
).
Derivatization of the phenyl substituent provides compounds with a range of affinities, which, when
taken with compound 138, points to more than electronic effects for determining receptor interaction.
Replacement of the phenyl with 1-naphthyl (139) results in a 5-fold decrease in affinity, but substitution
with 2-naphthyl (140) results in a 43-fold decrease in affinity for CB
1
. The drop in affinity between these
three compounds is likely due to steric limitations in the binding pocket, but no docking studies have
been performed to support this hypothesis.
Table 9
Selected pyrazole analogues (
Wiley et al., 2001
)
N
N
Cl
Cl
R1
R
Number
R
R1
K
i
CB
1
(nM)
a
141 SR141716
4–Clphenyl
N
N
H
O
144
4–Clphenyl
O
F
145
4–Clphenyl
N
H
O
146
4–Clphenyl
N
H
O
147
4–Clphenyl
O
148
4–Clphenyl
N
H
O
149
4–Clphenyl
OH
150
4–Clphenyl
151
Ph
N–(piperidin–1–yl)–amido
152
N–(piperidin–1–yl)–amido
6.2
b
54
±
2
32
±
5
48
±
12
82
±
10
167
±
32
657
±
21
422
±
40
2.1
±
0.08
233
±
3
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the mean
of three values with standard error of mean. Affinity determined using rat brain homogenate.
b
Thomas et al. (1998)
.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1782

Diarylpyrazoles
The search for cannabinoid receptor antagonists was largely unsuccessful until the development of
a new family of ligands by the Sanofi group in 1994 (
Rinaldi-Carmona et al., 1994
). This class is
based on a diarylpyrazole, of which SR141716 (141,
Fig. 4
) is the archetype. This compound is
selective for the CB
1
receptor and antagonizes the actions of D
9
-THC, CP-55940, and WIN-55212-2
in vivo (
Rinaldi-Carmona et al., 1995, 1998
). Early uses of these compounds in pharmacology
testing and initial development of SAR and pharmacophores for these compounds have been
previously reviewed (
Barth and Rinaldi-Carmona, 1999
). Modeling data points to a possible overlap
of the para position on the 5-aryl substituent with the side chain of D
9
-THC (
Thomas et al., 1998
).
A series of analogues prepared by
Wiley et al. (2001)
agrees with this alignment, see
Table 9
. These
analogues demonstrate the necessity of the 5-aryl substituent, as the receptor affinity sharply
decreases if this position is substituted with an alkyl chain (152). They also show that replacement
of the amide with a ketone, alkyl, or alkyl ether results in an attenuation of affinity (147, 149, 150).
Interestingly, the substitution of the amide nitrogen with a pentyl or heptyl chain (145, 146) gives
compounds with good affinity that exhibit agonist tendencies in vivo (
Wiley et al., 2001
). A
thorough presentation of the SAR of these compounds has been previously reported (
Howlett et al.,
2002
).
Studies concerning the effects of structural features employed in the compounds shown in
Table 9
and others shown in
Table 10
(
Bass et al., 2002
) demonstrate that the inverse agonism of SR141716
and the affinity values for the receptor do not correlate with the ability to stimulate locomotor
activity. The synthesis of rigid analogues with an indazole nucleus has provided compounds with
poor affinity that are capable of locomotor stimulation. The compounds described by
Wiley et al.
(2001)
with bulky groups on the 1-phenyl substituent are unable to promote locomotor activity as
are compounds where the carboxyamide piperidine functionality has been replaced with either a
ketone or an alkyl chain exchanged for the piperidine. Sterically hindered groups on the 5-aryl
Table 10
Rigid analogues of SR141716 (
Bass et al., 2002
)
NO
2
Cl
Br
OH
Cl
Cl
H
CH
3
153
154
155
156
157
158
H
3533 ± 170
475 ± 6
881 ± 44
592 ± 27
>10,000
1487 ± 99
Number
R1
R1
K
i
CB
1
(nM)
a
N
N
R1
R2
N
O
N
H
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the mean
of three values with standard error of mean. Affinity determined using mouse brain membranes.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1783
substituent do not appear to change the activity, although the 4-methyl substituent of the pyrazole
appears to play a role in determining if the compound will stimulate or depress activity. These
compounds display a wide range of affinities for the receptor, which appears to bear no direct
connection to this particular in vivo effect. An analogue has been prepared wherein the pyrazole is
replaced by a phenyl ring, (143) (
Bass et al., 2002
). This compound shows a decreased ability to
stimulate locomotor activity and has a moderate affinity for the CB
1
receptor (K
i
= 113 F 20) relative
to [
3
H]CP55940.
A study of the effect of the aminopiperidine region on binding and antagonism has been conducted by
Francisco et al. (2002)
. Alkyl, hydroxyalkyl, and alkylhydrazine derivatives of SR141716 were prepared
and their affinities determined against [
3
H]CP55940, see
Table 11
. This series examines two primary
features: substituent size and the presence of heteroatoms. Relative to SR141716, replacement of the
piperidine nitrogen with a methylene group (164) provides a compound with high affinity and good
Table 11
Amide and hydrizide derivatives (
Francisco et al., 2002
)
N
O
H
Cl
N
N
Cl
Cl
R
141 SR141716
159
160
161
162
163
164
165
166
167
168
169
170
171
172
173
N
N
O
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
(CH
2
)
3
CH
3
CH
2
(CH
2
)4CH
3
OH
CH
2
CH
2
OH
CH
2
CH
2
CH2OH
NH
2
NHCH
3
NHCH2CH
3
NH(CH
2
)
2
CH
3
NH(CH
2
)
3
CH
3
5.6
b
46.3
±
1.5
29.9
±
0.6
13.4
±
1.0
11.4
±
0.5
18.1
±
4.0
2.46
±
0.10
22.9
±
2.2
1690
±
480
385
±
13
160
±
19
374
±
27
555
±
86
143
±
9
74.8
±
11.5
50.9
±
6.4
>000
b
3110
±
610
2960
±
2100
1600
±
430
1110
±
240
6870
228
±
2
2400
±
780
7820
4270
±
570
1250
±
280
12,100
±
170
6660
±
930
6061
±
900
2620
±
440
2850
±
160
R
K
i
CB
1
(nM)
a
K
i
CB
2
(nM)
a
Number
a
Data from displacement of [
3
H]CP55940 in at least three independent experiments run in duplicate and expressed as the mean
of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from CHO-
K1 cells transfected with the human CB
2
cannabinoid receptor.
b
Rinaldi-Carmona et al. (1994)
.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1784
antagonistic behavior. Substitution with a morpholino group (165) results in a decrease in affinity, as
does the presence of a terminal hydroxyl group (166–168). Throughout all the derivatives, affinity
increases as the carbon chain length increases up to pentyl. After this, the affinity begins to decrease
again. This effect is seen even over the addition of the heteroatoms. QSAR studies suggest that
preferential binding occurs when the amide substituent is no more than 3 A
˚ in length and there is a
positive charge density on the substituent (
Francisco et al., 2002
). This computational modeling is
supported by the structure binding data.
Table 12
1,4-Dihydroindeno[1,2-c]pyrazole-based ligands (
Mussinu et al., 2003
)
N
N
N
O
H
R2
R
R1
N
N
N(CH
3
)
2
NH
2
N
N
N
CH
3
N
Et
CH
2
K
i
(nM)
a
Number
R
R1
R2
CB
1
2050
±
90
1268
±
51
1570
±
15
333
±
0.5
825
±
74
723
±
53
1152
±
65
363
±
30
399
±
24
1787
±
85
>5000
3035
±
13.5
798
±
48
1881
±
119
2183
±
123
2789
±
19
>5000
>5000
CB
2
0.34
±
0.06
0.225
±
0.02
0.27
±
0.02
5.5
±
0.5
0.23
±
0.036
6.788
±
0.47
0.385
±
0.04
0.037
±
0.003
12.3
±
1
0.9
±
0.09
48
±
5
120
±
15
9.9
±
0.52
144
±
20
455
±
44
978
±
35
>5000
>5000
174
175
176
177
178
179
180
181
182
183
184
185
186
187
188
189
190
191
6Cl
6F
6Br
6I
5Cl
7Cl
H
6CH
3
6OCH
3
6Cl
6Cl
6Cl
6Cl
6Cl
6Cl
6Cl
6Cl
6Cl
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
4 Cl
H
4 OCH
3
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
2,4 Cl
2
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the mean
of three values with standard error of mean. Affinity determined using mouse brain homogenate (CB
1
) and mouse spleen
membranes (CB
2
).
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1785
Another series of rigid diarylpyrazoles was synthesized and shows affinities similar to that of
SR144528 (142), a potent and selective ligand for CB
2
receptor subtype (
Mussinu et al., 2003
). The
prototypical compound of this system, shown in
Table 12
, is substituted similarly to SR141716 but with
a five-membered ring closed between the 4-position of the pyrazole and the 5-aryl substituent (174).
This compound demonstrates a high potency and selectivity for the CB
2
receptor. Differences in the aryl
substituent, R, are well tolerated, although a 6-OMe (182) and 6-I (177) substituent results in a decrease
in potency and selectivity, but maintains the trend. Special attention should be paid to the 6-Me
compound (181), which is very potent and has a 9810-fold selectivity over the CB
1
receptor. Changes to
the 1-aryl pyrazole substituent result in some attenuation of these effects, but maintain the trend.
Exchanging the piperidine of the carboxyamido group for other nitrogen-containing groups results in a
marked decrease in potency and selectivity, although selectivity for the CB
2
receptor is maintained.
These data suggest that enforcing this rigid conformation on the molecule locks its conformation into
that which is preferred by the CB
2
receptor, although there is no conclusive evidence presented to
indicate why. These compounds were not evaluated for antagonist activity.
An additional rigid analogue (192) (
Fig. 5
) containing a seven-membered ring as the connection
between the 5-phenyl substituent and the pyrazole of SR141716 was prepared. This compound was
found to have extremely high affinity and selectivity for the CB
1
receptor in a competitive assay
against [
3
H]CP55940 (CB
1
K
i
= 350 F 5 fM; CB
2
K
i
= 21 F 0.5 nM), a selectivity of 60,000-fold (
Ruiu
et al., 2003
). This compound, dubbed NESS-0327, was found to inhibit WIN-55212-2 induced
analgesia and antinociception, and also to behave as a competitive antagonist in the mouse vas
deferens assay. As the compound does not itself display antinociceptive action, it may be a true
antagonist and not an inverse agonist, although further study is necessary.
Synthesis of several analogues and their evaluation in comparative molecular field analysis
(CoMFA) modeling studies has been performed by
Shim et al. (2002)
. Steric contour images
demonstrate that both the N1 and C5 pyrazole aryl substituents are of significant consequence for
receptor binding. As was illustrated in some compounds initially presented by
Wiley et al. (2001)
,
the addition of hydrophobic substituents to these two aryl groups increases affinity for the CB
1
receptor until a certain size is reached. Then a sharp decrease in affinity seems to indicate that a
steric overlap occurs, exceeding the size of the binding pocket. Simple alkyl chains on the N1
position (195–198) may be able to bend around and mimic the size and shape of the 2,4-
dichlorophenyl substituent, implying an interaction with certain residues. These data, shown in
Table 13
, suggest a superposition of the N1 substituent and the C3 side chain of traditional
N
N
Cl
N
O
H
N
Cl
Cl
Fig. 5. NESS-0327 (192).
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1786
cannabinoids that has been shown to possess a maximum optimal size (
Melvin et al., 1993
). The
5-aryl moiety, which exceeds beyond the superposition with known agonists, may interact with the
receptor and prevent its conformation from changing to the active state. This overlap is in contrast
Table 13
Selected pyrazole analogues (
Shim et al., 2002
)
N
N
R2
R1
R3
Number
R1
R2
R3
K
i
CB
1
(nM)
a
193
2,4-dichlorophenyl
N-(hydroxymethyl)amido
Br
165
194
2,4-dichlorophenyl
N-(morpholin-4-yl)amido
Br
19
195
n-propyl
N-(piperidin-1-yl)amido
H
771
196
n-pentyl
N-(piperidin-1-yl)amido
H
23
197
n-hexyl
N-(piperidin-1-yl)amido
H
21
198
n-heptyl
N-(piperidin-1-yl)amido
H
47
199
2,4-dichlorophenyl
(piperidin-1-yl)ethoxymethyl
Cl
232
200
2,4-dichlorophenyl
(cyclohexyl)methoxymethyl
Cl
100
201
2,4-dichlorophenyl
4-fluorobenzoxymethyl
Cl
6
202
4-n-pentylphenyl
N-(piperidin-1-yl)amido
Cl
1360
203
2,4-dichlorophenyl
N-(piperidin-1-yl)amido
n-pentyl
1
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the
mean of three values. Affinity determined using rat brain homogenate.
Table 14
Cycloalkyl analogues (
Krishnamurthy et al., 2004
)
N N
R1
R
N
O
H
N
Number
R
R1
K
i
(nM)
a
CB
1
CB
2
204
p-chlorophenyl
Cyclopentyl
1560 F 77
1020 F 22
205
p-chlorophenyl
Cyclohexyl
351 F1.5
3210 F 45
206
p-chlorophenyl
Cycloheptyl
275 F 67
2197 F 21
207
p-chlorophenyl
3-methylcyclohexyl
494 F 57
281 F11
208
p-chlorophenyl
4-methylcyclohexyl
264 F 26
479 F 50
209
p-methylphenyl
2,4-dichlorophenyl
39 F 2.0
2490 F 102
210
Cyclohexyl
p-chlorophenyl
318 F 8.5
133 F 30
211
Cycloheptyl
p-chlorophenyl
273 F 19
410 F 10
212
Cyclohexyl
cyclohexyl
5110 F 110
N 2.5 10
5
a
Data from displacement of [
3
H]CP-55940 in at least three independent experiments run in duplicate and expressed as the
mean of three values with standard error of mean. Affinity determined using rat brain homogenate (CB
1
) and membranes from
CHO-K1 cells transfected with the human CB
2
cannabinoid receptor.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1787
to that presented by
Thomas et al. (1998)
, in which the C5 aryl group is overlaid with the C3 side
chain. Both models stress the importance of the 4-chloro group as an extension beyond the
molecular volume shared with agonists, and assign one aryl group to be the antagonist-conferring
Table 15
Diaryldihydropyrazole derivatives (
Lange et al., 2004
)
N
N
R2
N
S
O
O
R3
R
R1
Number
R
R1
R2
R3
K
i
CB
1
(nM)
a
213
H
4-CH
3
NH
2
4-Cl
197 F 152
214
H
4-Cl
NH
2
4-Cl
16.1 F 6.6
215
H
4-OCH
3
NH
2
4-Cl
196 F 107
216
H
2,4,6-(CH
3
)
3
NH
2
4-Cl
24.2 F 13.0
217
H
4-F
NH
2
4-Cl
52.6 F 10.5
218
H
4-CF
3
SCH
3
4-Cl
16.6 F 11.6
219
H
4-Cl
N(CH
3
)
2
4-Cl
280 F 178
220
H
4-F
N(CH
3
)
2
4-Cl
N1000
221
H
2-Cl
NHCH
3
4-Cl
75.4 F 12.3
222
H
3-Cl
NHCH
3
4-Cl
13.9 F 7.9
223
H
4-CF
3
NHCH
3
4-Cl
221 F130
224
H
4-Cl
NHCH
3
4-Cl
25.2 F 7.4
225
4-F
4-Cl
NHCH
3
4-Cl
584 F 220
226
H
4-Cl
NHCH
3
4-F
214 F 55
227
4-Cl
4-Cl
NHCH
3
4-Cl
255 F 105
228
H
H
NHCH
3
4-Cl
170 F 44
229
H
4-F
NHCH
3
4-Cl
338 F 170
230
H
4-CH
3
NHCH
3
4-Cl
119 F 40
231
H
3-CF
3
NHCH
3
4-Cl
36.5 F 21.7
232
H
2,4,6-(CH
3
)
3
NHCH
3
4-Cl
54.2 F 17.7
233
H
4-OCH
3
NHCH
3
4-Cl
22.9 F 11.0
234
H
3,4-benzo
NHCH
3
4-Cl
21.8 F 3.4
235
b
H
4-CF
3
NHCH
3
4-Cl
35.9 F 10.8
236
c
H
4-CF
3
NHCH
3
4-Cl
293 F 120
237
b
H
4-Cl
NHCH
3
4-Cl
7.8 F 1.4
238
c
H
4-Cl
NHCH
3
4-Cl
894 F 324
a
Data from displacement of [
3
H]arachadonic acid in at least three independent experiments run in duplicate and expressed as
the mean of three values with standard error of mean. Affinity determined with CHO-K1 cells overexpressing the human
cannabinoid receptor.
b
( )-Enantiomer.
c
(+)-Enantiomer.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1788
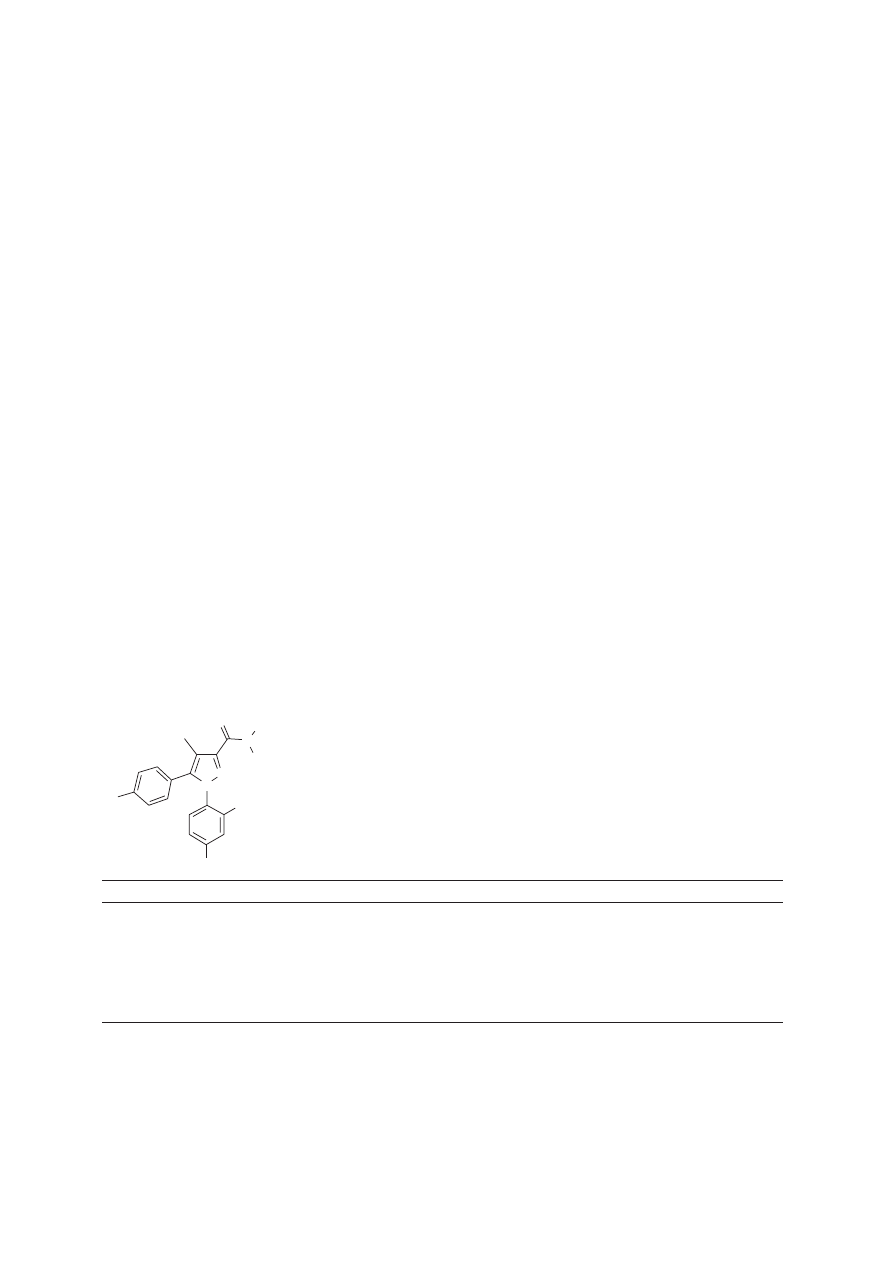
group. A comparison of the affinities for compounds 202 and 203 implies that the N1 group is
more sterically restricted, since an alkyl extension in this position resulted in a significant loss of
affinity.
Based on the findings that the N1 substituent does not have to be an aromatic ring to maintain
appreciable receptor affinity and that cyclohexyl groups can be isosteric to phenyl rings in biological
systems (
Hashimoto et al., 2002
), a series of pyrazoles with cycloalkyl groups in the C5 and N1
positions were prepared and evaluated for receptor affinity, shown in
Table 14
(
Krishnamurthy et al.,
2004
). The replacement of these aryl groups was detrimental to receptor affinity for both subtypes.
Only the C5 p-methylphenyl substituent showed any significant binding affinity and there
appears to be no consistent selectivity for receptor subtype within this series.
A new series of 3,4-diarylpyrazolines has been developed loosely based on the structure of
SR141716 (
Lange et al., 2004
). A significant number of derivatives have been synthesized in an effort
to develop a useful SAR picture of this ligand class, shown in
Table 15
. Several key features in this
series present themselves. Those compounds with good CB
1
affinities also generally present
antagonistic effects when evaluated in vivo for CP55940 induced hypotension and WIN-55212-2
induced hypothermia. All of the compounds tested for CB
2
affinity had K
i
values of 1000 nM or
greater. Affinity is increased when the aryl sulfonyl group is substituted with a halide in the 4 position
(214, 217) or is substituted with three methyl groups in a 2,4,6 pattern (216). Higher affinity was
obtained when the amidine possessed at least one hydrogen atom (in an NH
2
or NHCH
3
functionality). The presence of one methyl group significantly increases bioavailability. X-ray
crystallography of one potent analogue showed the positioning for an intramolecular hydrogen bond
between the amidine hydrogen and the N2 of the dihydropyrazole. Molecular modeling calculations
Table 16
Selected pyrazole derivatives (
Dyck et al., 2004
)
N
N
N
O
R2
R1
Cl
Cl
Cl
Number
R1
R2
K
i
CB
1
(nM)
a
239
H
3-Azabicyclo[3.3.0]octan-3-yl
5 F 1
240
CH
3
Cyclohexyl
100 F 32
241
H
1-Homopiperidinyl
14 F 5
242
H
CH(Me)CHMe2
41 F 3
243
H
2-(4-Fluorophenyl)ethyl
91 F 35
244
H
4-Pyridyl
85 F 5
245
H
2-(Dimethylamino)ethyl
(70%)
b
a
Measuring displacement of [
3
H]CP-55,940 from HEK EBNA cells expressing human CB
1
receptor. Data from three
independent experiments expressed as the mean of three values with standard error of mean.
b
Percent inhibition at 20 AM.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1789
also indicate the presence of this interaction in a low-energy conformation of the molecules. X-ray
crystallography also indicated that the absolute configuration of the 4-position was 4S. Both the R and
S stereoisomer were resolved for two potent ligands, and it was determined that the R isomers (236,
238) exhibited significantly lower affinity for the receptor and displayed no activity in vivo. This
demonstrates a distinct stereospecificity in the receptor–ligand interaction.
With the exception of 143, the SR141716 analogues discussed so far have been designed on a pyrazole
nucleus. Three new series by
Dyck et al. (2004)
begin to examine the impact of the pyrazole ring, whether
as a scaffold or as an essential part of the receptor interaction. Selected pyrazoles were synthesized to
evaluate the effects of certain amide substituents on receptor affinity. The receptor affinities for these
compounds are shown in
Table 16
. These data are consistent with those already reported. Replacement of
the hydrazine with a simple amine results in a reduction in affinity. Disubstitution of the amide nitrogen
also results in lowered affinity (240). Polar substituents in this region are not well tolerated. The slightly
larger bicyclooctyl group (239), however, gives a slightly increased affinity over the piperidyl substiutent
of SR141716.
A series of triazoles with similar amide substituents was synthesized, see
Table 17
. These
compounds as a whole demonstrated lower affinities for the receptor than the pyrazoles. It is believed
that the absence of the C4 methyl group may be the cause for this. An attempt to spatially occupy the
site of the absent methyl group by adding ortho-substituents to the neighboring aryl group was
unsuccessful, instead resulting in a further loss of affinity (252–256). The isomeric orientation of the
Table 17
Selected triazole derivatives (
Dyck et al., 2004
)
N
N
N
N
O
R2
R1
Y
Cl
X
Cl
Number
X
Y
R1
R2
K
i
CB
1
(nM)
a
246
Cl
H
H
3-Azabicyclo[3.3.0]octan-3-yl
137 F 35
247
Cl
H
H
4-Methylcyclohexyl
95 F 34
248
Cl
H
H
1-(4-Chlorophenyl)ethyl
66 F 17
249
Cl
H
H
1-Indanyl
101 F 34
250
Cl
H
H
2-(Dimethylamino)ethyl
(49)
b
251
Cl
H
H
1-Benzylpyrrolidin-3-yl
29 F10
252
H
Cl
H
3-Azabicyclo[3.3.0]octan-3-yl
164 F 60
253
H
Cl
H
1-Homopiperidinyl
(43)
b
254
H
Cl
CH
2
CH
2
N(3-CF
3
C
6
H
4
)CH
2
CH
2
32 F 5
255
OMe
Cl
H
3-Azabicyclo[3.3.0]octan-3-yl
270 F 5
256
OMe
Cl
H
Isopropyl
350 F 137
a
Measuring displacement of [
3
H]CP-55,940 from HEK EBNA cells expressing human CB
1
receptor. Data from three
independent experiments expressed as the mean of three values with standard error of mean.
b
Percent inhibition at 20 AM.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1790

triazole ring has little effect on the interaction of the molecule with the receptor. The same trends
regarding amide substituents are seen in the triazoles as were seen in the pyrazoles.
An analogous imidazole series was produced and evaluated for CB
1
receptor affinity, shown in
Table
18
. These compounds are less potent than the pyrazoles, but show better affinity than the triazoles. With a
methyl (264, 266), cyano (263), or bromo (262) substituent in the position corresponding to the C4 methyl
of SR141716, the affinities are comparable to the pyrazoles. The presence of a small substituent in this
position seems to be essential for effective binding to occur, although an acetylene group (265) eliminates
the affinity. As can be inferred from previous data, the presence of increasingly lipophilic side chains on
the amide result in an increase in affinity in all three of these series.
A series of hydantoin-based ligands was prepared and evaluated for their receptor affinity and
lipophilicity (
Ooms et al., 2002
). These compounds were first evaluated for their ability to displace
SR141716 at a concentration of 10 AM. Three compounds (272, 273, 275) displaced approximately
90% of the SR141716 and were examined for their affinity for the human CB
1
receptor against
SR141716. These compounds are shown in
Table 19
. Compounds with aryl substituents other than
bromine displayed a weakened ability to displace SR141716 (267–271). Lipophilicity is not the only
factor in displacement, since many compounds with modest lipophilicity also show good displacement
of SR141716. The compounds tested for CB
1
affinity also demonstrated neutral antagonist activity.
Table 18
Imidazole derivatives (
Dyck et al., 2004
)
N
N
N
O
R2
R1
R3
Cl
Cl
Cl
Number
R1
R2
R3
K
i
CB
1
(nM)
a
257
H
1-Piperidinyl
H
85 F 16
258
H
3-Azabicyclo[3.3.0]octan-3-yl
H
66 F 11
259
H
1-Homopiperidinyl
H
78 F 14
260
H
Cyclohexyl
H
48 F 19
261
H
2-(Dimethylamino)ethyl
H
(48)
b
262
H
3-Azabicyclo[3.3.0]octan-3-yl
Br
11 F 4
263
H
3-Azabicyclo[3.3.0]octan-3-yl
CN
9 F 1
264
H
3-Azabicyclo[3.3.0]octan-3-yl
Me
14 F 4
265
H
3-Azabicyclo[3.3.0]octan-3-yl
CCH
770 F 206
266
H
1-(4-Chlorophenyl)ethyl
Me
33 F 9
a
Measuring displacement of [
3
H]CP-55,940 from HEK EBNA cells expressing human CB
1
receptor. Data from three
independent experiments expressed as the mean of three values with standard error of mean.
b
Percent inhibition at 20 AM.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1791
Miscellaneous classes
A novel, CB
2
selective ligand, JTE-907 (277) (
Fig. 6
) was reported in 2001 (
Iwamura et al., 2001
).
This compound shows the biological activity of an inverse agonist and good affinity and selectivity for
the human CB
2
receptor (K
i
hCB
1
= 2370 F 297 nM; hCB
2
= 35.9 F 7.32 nM). This compound is the first
1,2-dihydroquinone-3-carboxyamide reported as a selective cannabinoid ligand. The 3-carboxyamide
group is seen in the arylpyrazoles and may play an important role in the binding of this compound. It
was noted that this compound displays anti-inflammatory effects in vivo.
A series of CB
2
selective 1,8-naphthyridines was synthesized after the development of 277
(
Ferrarini et al., 2004
). These compounds also have the 3-carboxyamide of the arylpyrazoles, but
contain an alkyl or arylalkyl substituent on the N1 position similar to that of the AAIs. These
compounds, shown in
Table 20
, display poor affinity for both receptors if there is no N1 substituent
(278, 279) or if there is a methylene spacer (304–309) between the ring system and the carboxyamide
group. The presence of an ethylmorpholino group as the N1 substituent conveys some affinity, but the
Table 19
Hydantoin derivatives (
Ooms et al., 2002
)
N
N
H
R1
R1
O
(CH
2
)nR
2
O
Number
R1
n
R2
K
i
CB
1
a
Lipophilicity
b
267
H
2
N(CH
2
CH
2)2
O
N/D
2.16
268
CH
3
2
N(CH
2
CH
2)2
O
N/D
3.49
269
CH
3
6
CH
3
N/D
7.04
270
F
2
N(CH
2
CH
2)2
O
N/D
2.81
271
OCH
3
2
N(CH
2
CH
2)2
O
N/D
2.73
272
Br
2
N(CH
2
CH
2)2
O
70.3 F 4.3
3.86
273
Br
3
OH
103.2 F 68
3.76
274
Br
5
CH
3
N/D
6.87
275
Br
6
CH
3
97.9 F 5.5
7.45
276
Br
7
CH
3
N/D
7.99
a
N/D = not determined; data from three independent experiments expressed as the mean of three values with standard error of
mean. Measured against [
3
H]SR141716 in CHO expressed human CB
1
.
b
Calculated using the CLIP method.
N
N
H
O
O
O
O
O
O
Fig. 6. JTE-907 (277).
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1792
presence of a benzyl or n-alkyl group results in a significant increase in affinity. Increased affinities
are observed if the carboxyamide group contains a cycloalkyl substituent over an aryl substituent. The
compound displaying the highest affinity for both receptors is 295, although better CB
2
selectivity is
Table 20
1,8-Naphthyridine derivatives (
Ferrarini et al., 2004
)
N
N
R2
R
O
(CH
2
)n
O
NHR
1
Number
R
R1
R2
n
K
i
a
(nM)
CB
1
CB
2
278
H
Cyclohexyl
CH
3
0
1000
1000
279
H
Benzyl
CH
3
0
1000
1000
280
ethylmorpholino
Cyclohexyl
CH
3
0
1000
100 F 8
281
ethylmorpholino
Morpholino
CH
3
0
1000
1000
282
ethylmorpholino
CH
2
cyclohexyl
CH
3
0
1000
117 F 15
283
ethylmorpholino
N-CH
3
pipz
CH
3
0
1000
1000
284
ethylmorpholino
Benzyl
CH
3
0
1000
475 F 25
285
ethylmorpholino
4-CH
3
-cyclohexyl
CH
3
0
537 F 24
30 F 2
286
ethylmorpholino
Cyclopentyl
CH
3
0
1000
50 F 4
287
ethylmorpholino
Cycloheptyl
CH
3
0
560 F 33
22 F 2
288
ethylmorpholino
Isopentyl
CH
3
0
1000
50 F 3
289
ethylmorpholino
p-Cl-Benzyl
CH
3
0
1000
1000
290
benzyl
Cyclohexyl
CH
3
0
127 F 13
10 F 0.5
291
Benzyl
Benzyl
CH
3
0
1000
1000
292
Benzyl
p-Cl-Benzyl
CH
3
0
1000
1000
293
o-F-benzyl
Cyclohexyl
CH
3
0
208 F 17
44 F 2
294
o-F-benzyl
Benzyl
CH
3
0
1000
600 F 60
295
p-F-benzyl
Cyclohexyl
CH
3
0
15 F 1.8
5.5 F 0.4
296
p-F-benzyl
Benzyl
CH
3
0
457 F 40
65.3 F 6
297
n-hexyl
Cyclohexyl
CH
3
0
95 F 3
8.0 F 0.2
298
n-hexyl
Benzyl
CH
3
0
1000
325 F 25
299
n-butyl
Cyclohexyl
CH
3
0
262 F 10.4
17.5 F 1
300
n-butyl
Benzyl
CH
3
0
1000
1000
301
ethylmorpholino
Benzyl
NH
2
0
1000
1000
302
ethylmorpholino
Cyclohexyl
NH
2
0
1000
1000
303
ethylmorpholino
Cyclohexyl
Cl
0
1000
25 F 1.8
304
ethylmorpholino
Benzyl
CH
3
1
1000
1000
305
Benzyl
Benzyl
CH
3
1
1000
729 F 82
306
ethylmorpholino
Cyclohexyl
CH
3
1
1000
1000
307
Benzyl
Cyclohexyl
CH
3
1
1000
530 F 50
308
n-hexyl
Cyclohexyl
CH
3
1
1000
1000
309
n-butyl
Cyclohexyl
CH
3
1
1000
1000
a
Affinity of compounds for CB
1
receptor was evaluated using mouse cerebellum membranes and [
3
H]-CP 55,940. Affinity
for CB
2
receptor was assayed using mouse spleen homogenate and [
3
H]-CP 55,940. K
i
values were obtained from five
independent experiments carried out in triplicate and are expressed as the mean standard error.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1793

exhibited by several other compounds in this series. Decreased affinity is observed if the 7-methyl
group is replaced with an NH
2
group (301, 302), but replacement with Cl (303) gives a four-fold
increase in affinity for CB
2
.
Conclusion
The analogues produced in the last 2 years have filled gaps in the understanding of cannabinoid SAR
and posed many new questions. The pyrazoles are typically CB
1
selective, but the many of the analogues
shown in
Table 12
are highly CB
2
selective. Compound 174 is especially noteworthy since it is substituted
as SR141716, but is held rigid by the presence of a bridging methylene to form a cyclopentyl ring. This
opens up a new area of study for controlling receptor subtype specificity. As yet, there has been little
published in the way of CB
1
selective agonists, although there are CB
2
selective agonists in all the classes
discussed here. The indole and pyrrole series have many avenues left to be pursued regarding substitution
of the aryl systems and control of receptor selectivity. Particularly interesting would be an investigation of
derivatization of the naphthyl system of the pyrroles. The structure of the pyrroles is suggestive of the
pyrazoles; similar substitution patterns to the pyrazoles may prove fruitful on the pyrroles. It may be that
some of the pyrrole analogues emerge as antagonists or inverse agonists.
In summary, a significant number of exogenous ligands have been developed over the last 2 years,
largely focusing on developments in the pyrazole class. SR141716 is currently in clinical trials for
treatment of obesity, and this has fueled interest in the development of antagonists as therapeutic agents.
Repetition of previously observed themes is present in these compounds: benefits arising from lipophilic
groups up to a finite size, intramolecular hydrogen-bonding to establish low-energy conformations and the
availability of those conformations for binding. Also the effect of aromaticity on the cannabimimetic
effects of the AAI structural class has been investigated. Improved techniques in computational modeling
and pharmacological assays are providing more insight into directed ligand synthesis. There are many
questions yet to be answered concerning these structural classes and their abilities to not only interact with
the receptors but to generate biological activity that can be used therapeutically.
Acknowledgements
The author thanks Drs. John W. Huffman and Julia Brumaghim of Clemson University and Dr.
Clifford Padgett of North Carolina State University for helpful discussions concerning this review. The
work carried out at Clemson University included in this review was supported by Grants DA 03590, DA
03671, and DA 15579 from the National Institute on Drug abuse.
References
Adam, J., Cowley, P., 2002. Recent advances in the cannabinoids. Expert Opinions in Therapeutics 12, 1475 – 1489.
Aung, M.M., Griffin, G., Huffman, J.W., Wu, M.-J., Keel, C., Yang, B., Showalter, V.M., Abood, M.E., Martin, B.R., 2000.
Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug and Alcohol
Dependence 60, 133 – 140.
Barth, F., Rinaldi-Carmona, M., 1999. The development of cannabinoid antagonists. Current Medicinal Chemistry 6, 745 – 755.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1794

Bass, C.E., Griffin, G., Grier, M., Mahadevan, A., Razdan, R.K., Martin, B.R., 2002. SR141716A induced stimulation of
locomotor activity—A structure activity relationship study. Pharmacology, Biochemistry, and Behavior 74, 31 – 40.
Bell, M.R., D’Ambra, R.E., Kumar, V., Eissenstat, M.A., Herrmann Jr., J.L., Wetzer, J.R., Rosi, D., Philion, R.E., Daum, S.J.,
Hlasta, D.J., Kullnig, R.K., Ackerman, J.H., Haubrich, D.R., Luttinger, D.A., Baizman, E.R., Miller, M.S., Ward, S.J., 1991.
Antinociceptive (Aminoalkyl)indoles. Journal of Medicinal Chemistry 34, 1099 – 1110.
Busch-Petersen, J., Hill, W.A., Fan, P., Khanolkar, A., Xie, X.Q., Tius, M.A., Makriyannis, A., 1996. Unsaturated side chain h-
11-Hydroxyhexahydrocannabinol analogs. Journal of Medicinal Chemistry 39, 3790 – 3796.
Compton, D.R., Gold, L.H., Ward, S.J., Balster, R.L, Martin, B.R., 1992. Aminoalkylindole analogs: cannabimimetic activity of
a class of compounds structurally distinct from D
9
-tetrahydrocannabinol. Journal of Pharmacology and Experimental
Therapeutics 263, 1118 – 1126.
Compton, D.R., Rice, K.C., De Costa, B.R., Razdan, R.K., Melvin, L.S., Johnson, M.R., Martin, B.R., 1993. Cannabinoid
structure–activity relationships: correlation of receptor binding and in vivo activities. Journal of Pharmacology and
Experimental Therapeutics 265, 218 – 226.
Dalzell, H.C., Uliss, D.B., Handrick, G.R., Razdan, R.K., 1981. Hashish. 26. Factors influencing double-bond stability in
cannabinoids. Journal of Organic Chemistry 46, 949 – 953.
Devane, W.A., Dysarz III, F.A., Johnson, M.R., Melvin, L.S., Howlett, A.C., 1988. Determination and characterization of a
cannabinoid receptor in rat brain. Molecular Pharmacology 34, 605 – 613.
Devane, W.A., Hanus, L., Breuer, A., Pertwee, R.G., Stevenson, L.A., Grin, G., Gibson, D., Mandelbaum, A., Etinger, A., Mechoulam,
R., 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946– 1949.
Dyck, B., Goodfellow, V.S., Phillips, T.P., Grey, J., Mustapha, H., Rowbottom, M., Naeve, G.S., Brown, B., Saunders, J., 2004.
Potent imidazole and triazole CB1 receptor antagonists related to SR141716. Bioorganic and Medicinal Chemistry Letters
14, 1151 – 1154.
Edery, J., Grunfeld, Y., Parath, G., Ben-Zvi, Z., Shani, A., Mechoulam, R., 1972. Structure–activity relations in the
tetrahydrocannabinol series. Modifications on the aromatic ring and in the side-chain. Arzneimittel-Forschung 22,
1995 – 2003.
Ferrarini, P.L., Calderone, V., Cavallini, T., Maera, C., Saccomanni, G., Pani, L., Ruiu, S., Gessa, G.L., 2004. Synthesis and
biological evaluation of 1,8-naphthyridin-4(1H)-on-3-carboxamide derivatives as new ligands of cannabinoid receptors.
Biological and Medicinal Chemistry 12, 1921 – 1933.
Francisco, M.E.Y., Seltzman, H.H., Gilliam, A.F., Mitchell, R.A., Rider, S.L., Pertwee, R.G., Stevenson, L.A., Thomas, B.F.,
2002. Synthesis and structure activity relationships of amide and hydrazide analogues of the cannabinoid CB1 receptor
antagonist N-(piperidinyl)-5-(4-chlorophenyl)-1-(2,3-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716).
Journal of Medicinal Chemistry 45, 2708 – 2719.
Gaoni, Y., Mechoulam, R., 1964. Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of the
American Chemical Society 86, 1646 – 1647.
Hashimoto, H., Maeda, K., Ozawa, K., Haruta, J., Wakitani, K., 2002. 4-Aryl/cycloalkyl-5-phenyloxazole derivatives as
selective COX-2 inhibitors. Bioorganic and Medicinal Chemistry Letters 12, 65 – 68.
Howlett, A.C., 1998. The CB1 cannabinoid receptor in the brain. Neurobiology of Disease 5, 405 – 416.
Howlett, A.C., Johnson, M.R., Melvin, L.S., Milne, G.M., 1988. Nonclassical cannabinoid analgetics inhibit adenylate cyclase:
development of a cannabinoid receptor model. Molecular Pharmacology 33, 297 – 302.
Howlett, A.C., Barth, F., Bonner, T.I., Cabral, G., Casellas, P., Devane, W.A., Felder, C.C., Herkenham, M., Mackie, K., Martin,
B.R., Mechoulam, R., Pertwee, R.G., 2002. International union of pharmacology. XXVII. Classification of cannabinoid
receptors. Pharmacology Reviews 54, 161 – 202.
Huffman, J.W., 1999. Cannabimimetic indoles, pyrroles and indenes. Current Medicinal Chemistry 6, 705 – 720.
Huffman, J.W., Yu, S., 1998. Synthesis of a tetracyclic, conformationally constrained analogue of delta8-THC. Bioorganic and
Medicinal Chemistry 6, 2281 – 2288.
Huffman, J.W., Dai, D., Martin, B.R., Compton, D.R., 1994. Design, synthesis, and pharmacology of cannabimimetic indoles.
Bioorganic and Medicinal Chemistry Letters 4, 563 – 566.
Huffman, J.W., Lainton, J.A.H., Dai, D., Jordan, R.D., Duncan, S.G., 1995. Variation of the alkyl side chain in delta 8-THC.
Life Science 56, 2021 – 2024.
Huffman, J.W., Yu, S., Showalter, V., Abood, M.E., Wiley, J.L., Compton, D.R., Martin, B.R., Bramblett, R.D., Reggio, P.H.,
1996. Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2
receptor. Journal of Medicinal Chemistry 39, 3875 – 3877.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1795

Huffman, J.W., Liddle, J., Yu, S., Aung, M.M., Abood, M.E., Wiley, J.L., Martin, B.R., 1999. 3-(1V,1V-Dimethylbutyl)-1-deoxy-
(8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorganic and Medicinal Chemistry 7,
2905 – 2914.
Huffman, J.W., Lu, J., Dai, D., Kitaygorodskiy, A., Wiley, J.L., Martin, B.R., 2000. Synthesis and pharmacology of a hybrid
cannabinoid. Bioorganic and Medicinal Chemistry 8, 439 – 447.
Huffman, J.W., Lu, J., Hynd, G., Wiley, J.L., Martin, B.R., 2001. A pyridone analogue of traditional cannabinoids. A new class
of selective ligands for the CB2 receptor. Biological and Medicinal Chemistry 9, 2863 – 2870.
Huffman, J.W., Mabon, R., Wu, M.-J., Lu, J., Hart, R., Hurst, D.P., Reggio, P.H., Wiley, J.L., Martin, B.R., 2003. 3-Indolyl-1-
naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB
1
cannabinoid receptor. Bioorganic and Medicinal Chemistry 11, 539 – 549.
Iwamura, H., Suzuki, H., Ueda, Y., Kaya, T., Inaba, T., 2001. In vitro and in vivo pharmacological characterization of JTE-907, a
novel selective ligand for cannabinoid CB2 receptor. Journal of Pharmacology and Experimental Therapeutics 296, 420 – 425.
Keimowitz, A.R., Martin, B.R., Razdan, R.K., Crocker, P.J., Mascarella, S.W., Thomas, B.F., 2000. QSAR analysis of D
8
-THC
analogues: relationship of side-chain conformation to cannabinoid receptor affinity and pharmacological potency. Journal of
Medicinal Chemistry 43, 59 – 70.
Khanolkar, A.D., Makriyannis, A., 1999. Structure–activity relationships of anandamide, an endogenous cannabinoid ligand.
Life Science 65, 607 – 616.
Khanolkar, A.D., Lu, D., Fan, P., Tian, X., Makriyannis, A., 1999. Novel conformationally restricted tetracyclic analogs of D
8
-
tetrahydrocannabinol. Bioorganic and Medicinal Chemistry Letters 9, 2119 – 2124.
Khanolkar, A.D., Palmer, S.L., Makriyannis, A., 2000. Molecular probes for the cannabinoid receptors. Chemistry and Physics
of Lipids 108, 37 – 52.
Knight, L.W., Huffman, J.W., Isherwood, M.L., 2003. Pyrrole-based non-traditional cannabinoids. Symposium on the
Cannabinoids, Cornwall, Ontario, Canada, June 25–28, 2003. International Cannabinoid Research Society, vol. 69.
Knight, L.W., Huffman, J.W., Isherwood, M.L., 2004. Synthesis and pharmacology of pyrrole-based cannabinoids. Symposium
on the Cannabinoids, Paestum, Italy, June 22–27, 2004. International Cannabinoid Research Society, vol. 97.
Krishnamurthy, M., Ferreira, A.M., Moore, B.M., 2003. Synthesis and testing of novel phenyl substituted side-Chain analogues
of classical cannabinoids. Bioorganic and Medicinal Chemistry Letters 13, 3487 – 3490.
Krishnamurthy, M., Li, W., Moore, B.M., 2004. Synthesis, biological evaluation, and structural studies on N1 and C5
substituted cycloalkyl analogues of the pyrazole class of CB1 and CB2 ligands. Bioorganic and Medicinal Chemistry 12,
393 – 404.
Kumar, V., Alexander, M.D., Bell, M.R., Eissenstat, M.A., Casiano, F.M., Chippari, S.M., Haycock, D.A., Lutinger, D.A.,
Kuster, J.E., Miller, M.S., Stevenson, J.I., Ward, S.J., 1995. Morpholinoalkylindenes as antinociceptive agents: novel
cannabinoid receptor agonists. Bioorganic and Medicinal Chemistry Letters 5, 381 – 386.
Lainton, J.A.H., Huffman, J.W., Martin, B.R., Compton, D.R., 1995. 1-Alkyl-3-(1-naphthoyl)pyrroles: a new class of
cannabinoid. Tetrahedron Letters 36, 1401 – 1404.
Lange, J.H.M., Coolen, H.K.A.C., van Stuivenberg, H.H., Dijksman, J.A.R., Herremans, A.H.J., Ronken, E., Keizer, H.G., Tipker,
K., McCreary, A.C., Veerman, W., Wals, H.C., Stork, B., Verveer, P.C., den Hartog, A.P., de Jong, N.M.J., Adolfs, T.J.P.,
Hoogendoorn, J., Kruse, C.G., 2004. Synthesis, biological properties, and molecular modeling investigations of novel 3,4-
diarylpyrazolines as potent and selective CB1 cannabinoid receptor antagonists. Journal of Medicinal Chemistry 47, 627 – 643.
Little, P.J., Compton, D.R., Johnson, M.R., Melvin, L.S., Martin, B.R., 1988. Pharmacology and stereoselectivity of structurally
novel cannabinoids in mice. Journal of Pharmacology and Experimental Therapeutics 247, 1046 – 1051.
Lu, D., Khanolkar, A., Meng, Z., Fan, P., Reggio, P.H., Makriyannis, A., 1997. Presented at., 1997. International Cannabinoid
Research Society, 1997 Symposium on the Cannabinoids, Stone Mountain, GA, ICRS, p. 1.
Martin, B.R., 1986. Cellular effects of cannabinoids. Pharmacology Reviews 38, 45 – 74.
Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C., Bonner, T.I., 1990. Structure of a cannabinoid receptor and
functional expression of the cloned cDNA. Nature 346, 561 – 564.
McAllister, S.D., Tao, Q., Barnett-Norris, J., Buehner, K., Hurst, D.P., Guarnieri, F., Reggio, P.H., Nowell Harmon, K.W.,
Cabral, G.A., Abood, M.E., 2002. A critical role for a tyrosine residue in the cannabinoid receptors for ligand recognition.
Biochemical Pharmacology 63, 2121 – 2136.
Melvin, L.S., Milne, G.M., Johnson, M.R., Subramaniam, B., Wilken, G.H., Howlett, A.C., 1993. Structure–activity
relationships for cannabinoid receptor binding and analgesic activity: studies of bicyclic cannabinoid analogs. Molecular
Pharmacology 44, 1008 – 1015.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1796

Munro, S., Thomas, K.L., Abu-shaar, M., 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature
365, 61 – 64.
Mussinu, J.-M., Ruiu, S., Mule, A.C., Pau, A., Carai, M.A.M., Loriga, G., Murineddu, G., Pinna, G.A., 2003. Tricyclic
pyrazoles: Part 1. Synthesis and biological evaluation of novel 1,4-dihydroindeno[1,2-c]pyrazole-based ligands for CB1 and
CB2 cannabinoid receptors. Bioorganic and Medicinal Chemistry 11, 251 – 263.
Nadipuram, A.K., Krishnamurthy, M., Ferreira, A.M., Wei, L., Moore, B.M., 2003. Synthesis and testing of novel classical
cannabinoids: exploring the side chain ligand binding pocket of the CB1 and CB2 receptors. Bioorganic and Medicinal
Chemistry 11, 3121 – 3132.
Offertaler, L., Mo, F.-M., Batkai, S., Liu, J., Begg, M., Razdan, R.K., Martin, B.R., Bukoski, R.D., Kunos, G., 2003.
Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Molecular Pharmacology
63, 699 – 705.
Ooms, F., Wouters, J., Oscari, O., Happaerts, T., Bouchard, G., Carrupt, P.-A., Testa, B., Lambert, D.M., 2002. Exploration of the
pharmacophore of 3-alkyl-5-arylimidazolidinediones as new CB1 cannabinoid receptor ligands and potential antagonists:
synthesis, lipophilicity, affinity, and molecular modeling. Journal of Medicinal Chemistry 45, 1748 – 1756.
Papahatjis, D.P., Kourouli, T., Abadji, V., Goutopoulos, A., Makriyannis, A., 1998. Pharmacophoric requirements for
cannabinoid side chains: multiple bond and C1V-substituted D
8
-tetrahydrocannabinols. Journal of Medicinal Chemistry 41,
1195 – 1200.
Papahatjis, D.P., Nikas, S.P., Andreou, T., Makriyannis, A., 2002. Novel 1V,1V-chain substituted D
8
-tetrahydrocannabinols.
Bioorganic and Medicinal Chemistry Letters 12, 3583 – 3586.
Papahatjis, D.P., Nikas, S.P., Kourouli, T., Chari, R., Xu, W., Pertwee, R.G., Makriyannis, A., 2003. Pharmacophoric
requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1V. Journal of Medicinal
Chemistry 46, 3221 – 3229.
Pertwee, R.G., 1997. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacology and Therapeutics 74,
129 – 180.
Pertwee, R.G., 2000. Neuropharmacology and therapeutic potential of cannabinoids. Addiction Biology 5, 37 – 46.
Pertwee, R.G., Ross, R.A., Craib, S.J., Thomas, A., 2002. ( )-Cannabidiol antagonizes cannabinoid receptor agonists and
noradrenaline in the mouse vas deferens. European Journal of Pharmacology 456, 99 – 106.
Reggio, P.H., Wang, T., Brown, A.E., Fleming, D.N., Seltzman, H.H., Griffin, G., Pertwee, R.G., Compton, D.R., Abood,
M.E., Martin, B.R., 1997. Importance of the C-1 substituent in classical cannabinoids to CB2 receptor selectivity:
synthesis and characterization of a series of O,2-propano-D
8
-tetrahydrocannabinol analogs. Journal of Medicinal Chemistry
40, 3312 – 3318.
Reggio, P.H., Basu-Dutt, S., Barnett-Norris, J., Castro, M.T., Hurst, D.P., Seltzman, H.H., Roche, M.J., Gilliam, A.F.,
Thomas, B.F., Stevenson, L.A., Pertwee, R.G., Abood, M.E., 1998. Derivation of a pharmacophore model for anandamide
using constrained conformational searching and comparative molecular field analysis. Journal of Medicinal Chemistry 41,
5177 – 5187.
Rinaldi-Carmona, M., Barth, F., Heaulme, M., Shire, D., Calandra, B., Congy, C., Martinez, S., Maruani, J., Neliat, G., Caput,
D., Ferrara, P., Soubrie, P., Breliere, J.C., Le Fur, G., 1994. SR141716A, a potent and selective antagonist of the brain
cannabinoid receptor. FEBS Letters 350, 240 – 244.
Rinaldi-Carmona, M., Barth, F., Heaulme, M., Alonso, R., Shire, D., Congy, C., Soubrie, P., Breliere, J.C., Le Fur, G., 1995.
Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor
antagonist. Life Sciences 56, 1941 – 1947.
Rinaldi-Carmona, M., Barth, F., Millan, J., Derocq, J.M., Casellas, P., Congy, C., Oustric, D., Sarran, M., Bouaboula, M.,
Calandra, M., Portier, M., Shire, D., Breliere, J.C., Le Fur, G., 1998. SR 144528, the first potent and selective antagonist of the
CB2 cannabinoid receptor. Journal of Pharmacology and Experimental Therapeutics 284 (2), 644 – 650.
Ruiu, S., Pinna, G.A., Marchese, G., Mussinu, J.-M., Saba, P., Tambaro, S., Casti, P., Vargiu, R., Pani, L., 2003. Synthesis and
characterization of NESS 0327: a novel putative antagonist of the CB1 cannabinoid receptor. Journal of Pharmacology and
Experimental Therapeutics 306, 363 – 370.
Ryan, W., Singer, M., Razdan, R.K., Compton, D.R., Martin, B.R., 1995. A novel class of potent tetrahydrocannabinols
(THCS): 2’-yne-delta 8- and delta 9-THCS. Life Sciences 56, 2013 – 2020.
Seltzman, H.H., Fleming, D.N., Thomas, B.F., Gilliam, A.F., McCallion, D.S., Pertwee, R.G., Compton, D.R., Martin, B.R.,
1997. Synthesis and pharmacological comparison of dimethylheptyl and pentyl anandamide analogs. Journal of Medicinal
Chemistry 40, 3626 – 3634.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1797

Shim, J.-Y., Welsh, W.J., Cartier, E., Edwards, J.L., Howlett, A.C., 2002. Molecular interaction of the Antagonist N-(piperidin-
1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide with the CB1 cannabinoid receptor.
Journal of Medicinal Chemistry 45, 1447 – 1459.
Showalter, V.M., Compton, D.R., Martin, B.R., Abood, M.E., 1996. Evaluation of binding in a transfected cell line expressing a
peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. Journal of
Pharmacology and Experimental Therapeutics 278, 989 – 999.
Song, Z.H., Bonner, T.I., 1996. A lysine residue of the cannabinoid receptor is critical for receptor recognition by several
agonists but not WIN55212-2. Molecular Pharmacology 49, 891 – 896.
Tarzia, G., Duranti, A., Tontini, A., Spadoni, G., Mor, M., Tivara, S., Plazzi, P.V., Kathuria, S., Piomelli, D., 2003. Synthesis
and structure–activity relationships of a series of pyrrole cannabinoid receptor agonists. Bioorganic and Medicinal
Chemistry 11, 3965 – 3973.
Thakur, G.A., Palmer, S.L., Harrington, P.E., Stergiades, I.A., Tius, M.A., Makriyannis, A., 2002. Enantiomeric resolution of a
novel chiral cannabinoid receptor ligand. Biochemical and Biophysical Methods 54, 415 – 422.
Thomas, B.F., Gilliam, A.F., Burch, D.F., Roche, M.J., Seltzman, H.H., 1998. Comparative receptor binding analyses of
cannabinoid agonists and antagonists. Journal of Pharmacology and Experimental Therapeutics 285, 285 – 292.
Thomas, A., Ross, R.A., Saha, B., Mahadevan, A., Razdan, R.K., Pertwee, R.G., 2004. 6U-Azidohex-2U-yne-cannabidiol: a
potential neutral, competitive cannabinoid CB1 receptor antagonist. European Journal of Pharmacology 487, 213 – 221.
Tius, M.A., Hill, W.A., Zou, G., Busch-Petersen, X.L., Kawakami, J., Fernandez-Garcia, J.K., Drake, M.C., Abadji, D.J.,
Makriyannis, V., 1995. Classical/non-classical cannabinoids hybrids; stereochemical requirements for the southern
hydroxyalkyl chain. Life Sciences 56, 2007 – 2012.
Tong, W., Collantes, E.R., Welsh, W.J., Berglund, B.A., Howlett, A.C., 1998. Derivation of a pharmacophore model for
anandamide using constrained conformational searching and comparative molecular field analysis. Journal of Medicinal
Chemistry 41, 4207 – 4215.
Wiley, J.L., Compton, D.R., Dai, D., Lainton, J.A.H., Phillips, M., Huffman, J.W., Martin, B.R., 1998. Structure–activity
relationships of indole- and pyrrole-derived cannabinoids. Journal of Pharmacology and Experimental Therapeutics 285,
995 – 1004.
Wiley, J.L., Jefferson, R.G., Grier, M.C., Mahadevan, A., Razdan, R.K., Martin, B.R., 2001. Novel pyrazole cannabinoids:
insights into CB1 receptor recognition and activation. Journal of Pharmacology and Experimental Therapeutics 296,
1013 – 1022.
Wiley, J.L., Beletskaya, I.D., NG, E.W., Dai, Z., Crocker, P.J., Mahadevan, A., Razdan, R.K., Martin, B.R., 2002. Resorcinol
Derivatives: A novel template for the development of cannabinoid CB1/CB2 and CB2 selective agonists. Journal of
Pharmacology and Experimental Therapeutics 301, 679 – 689.
L.W. Padgett / Life Sciences 77 (2005) 1767–1798
1798
Wyszukiwarka
Podobne podstrony:
recent developments in the med chem of cannabimimetic indoles pyrroles and indenes curr med chem 12
Miller Recent Developments In Slab A Software Based System For Interactive Spatial Sound Synthesis
1998 Recent developments in superstring theory Schwarz
Recent Developments in Cosmology
hallucinogenic botanicals of america minireview life sci 78 519 526 (2005) jlfs 2005 09 005
adamantyl cannabinoids a novel class of cannabinergic ligands j med chem 48 4576 4585 (2005)
Developments in computer aided dryer selection (Baker, Lababidi)
deRegnier Neurophysiologic evaluation on early cognitive development in high risk anfants and toddl
New Developments in HBV Treatment
Balancing Disappointment and Enthusiasm Developments in EU Balkans relations during 2003
Method Development in High Performance Liquid Chromatography
Lee Cognitive Development in Bilingual Children
Rethinking SSL Development in an Appified World
Developments in seismic structural analysis and design
Microbiota is essential for social development in the mouse
Bernays, Edward L , Recent Trends in Public Relations Activities (1937)
więcej podobnych podstron