
BRIEF REPORT
• CID 2003:36 (15 January) • 221
B R I E F R E P O R T
Geographical Differences
in Human Oral Yeast Flora
Jianping Xu
1
and Thomas G. Mitchell
2
1
Department of Biology, McMaster University, Hamilton, Ontario, Canada;
and
2
Department of Molecular Genetics and Microbiology, Duke University
Medical Center, Durham, North Carolina
The oral yeast flora of healthy humans from eastern North
America and China were sampled and compared. Chinese
persons harbored a greater number and diversity of yeast
species in the mouth. Furthermore, Candida albicans, which
is the predominant commensal and etiologic species of can-
didiasis in Europe and the Western Hemisphere, was rela-
tively rare in China.
The most prevalent fungal infection of humans is candidiasis,
which is caused by endogenous species of Candida that are
members of the microbial flora of the oral mucosa and other
body surfaces [1, 2]. Although there is global interest in Candida
species, there is little knowledge about global ecological char-
acteristics of Candida species. Numerous studies of the asso-
ciations between Candida species and human populations in
Europe and the Americas have repeatedly confirmed that the
most common commensal and etiologic species is Candida
albicans [1–5]. However, relatively little is known about the
yeast flora and etiology of yeast infections in developing coun-
tries. Because yeast infections are often caused by endogenous
species and strains, and because species of Candida and other
commensal yeasts differ in their pathogenicity, susceptibility to
antifungal drugs, and other clinically important phenotypes
[1–3], it is prudent to investigate the distribution of these path-
ogenic yeasts in different geographical areas. The objective of
this study was to compare the oral yeast flora of healthy people
in China and eastern North America.
Subjects and methods.
The Chinese samples were obtained
from 239 healthy volunteers from 5 geographically distinct areas
Received 9 July 2002; accepted 8 October 2002; electronically published 19 December
2002.
Financial support: US National Institutes of Health (grant AI 28836).
Reprints or correspondence: Dr. Thomas G. Mitchell, Box 3803, Dept. of Molecular Genetics
and Microbiology, Duke University Medical Center, Durham, NC 27710 (tom.mitchell@
duke.edu).
Clinical Infectious Diseases
2003; 36:221–4
2003 by the Infectious Diseases Society of America. All rights reserved.
1058-4838/2003/3602-0015$15.00
in 3 provinces, which are located in the south-central (Jiangxi),
east-central (Jiangsu), and west-central (Sichuan) regions of
China. The North American samples were obtained from 483
individuals in 3 geographic areas of eastern North America, 2
of which are in the United States (Miami, FL, and Durham,
NC) and 1 of which is in Canada (Wolfville, Nova Scotia).
These Chinese and North American populations were com-
parable in age (range, 15–76 years) and sex (378 men and 344
women). All samples were obtained during the period of Feb-
ruary 1997 through January 1998. No individual had an ap-
parent illness, and none had taken any antifungal medication
in the weeks before samples were obtained. In China, all of the
people who were sampled were Han Chinese, the major ethnic
group. The North American subjects from Miami consisted
entirely of Latin American individuals, the Canadians were all
white, and the racially mixed Durham population was separated
into 3 subgroups (Chinese American, African American, and
white/other).
Sterile swabs were used to sample the upper and lower outer
gingiva of each person. After sampling, the tip of each swab
was immediately severed and submerged in a sterile cryogenic
tube for storage and transport. Each tube contained 0.5 mL of
sterile enrichment broth composed of 2% (weight/volume)
yeast extract, 1% Bacto-peptone (BD), 2% dextrose, 18% glyc-
erol, and chloramphenicol (50 mg/mL). This medium affords
excellent survival and recovery of medical yeasts. All specimens
were processed in the same laboratory. Subcultures were
streaked onto CHROMAgar medium (CHROMAgar), which
selects for yeast growth and permits the direct identification of
C. albicans (green color) and Candida tropicalis (blue color).
This medium does not select for any particular species of yeasts
[6]. Every yeast colony of a different color or morphology was
transferred to nutrient medium and identified with use of the
standard yeast identification system, API 20C (bioMe´rieux).
For the few isolates that were not identified with use of these
2 methods, DNA was extracted, and a portion (D1/D2) of the
28S rRNA was amplified, sequenced, and compared with se-
quences from the GenBank database. Because neither API 20C
nor CHROMAgar distinguishes C. albicans from the closely
related Candida dubliniensis, all strains identified as C. albicans
were further tested for growth at 45
C on Sabouraud glucose
agar, and PCR amplification was performed with C. albicans
species–specific primers [7]. However, no strain of C. dublin-
iensis was isolated. All unique colonies were identified, and,
with a few exceptions, individual oral swabs yielded only a
single species of yeast.
http://cid.oxfordjournals.org/
Downloaded from
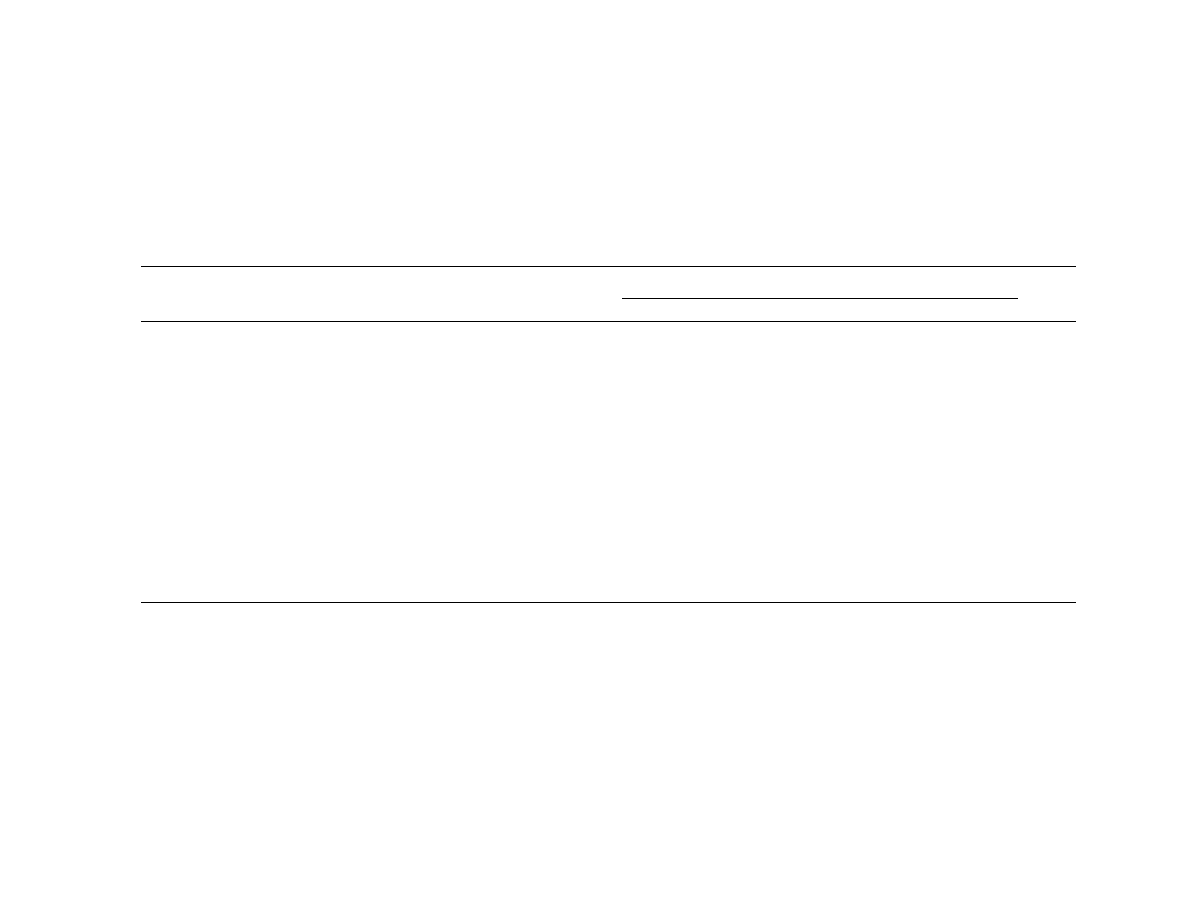
Table 1.
Yeast species isolated from the oral cavities of healthy volunteers in regions of China and North America.
Country
Province
or state
City or
community
Ethnicity
a
No. of
hosts
No. (%)
of hosts
with yeasts
No. (%) of each species isolated
Species
diversity
b
Ca
Cf
Cgu
Cl
Cp
Ct
Other
China
Jiangxi
Sitou Village
Chinese
58
47 (81.0)
5
5
3
2
18
4
Cgl, 1; Ch, 1;
Ha, 1; Rg, 1;
Sc, 6
0.846
Huangtugang Chinese
90
61 (67.8)
3
10
15
1
29
2
Tc, 1
0.694
Nanchang
Chinese
35
23 (65.7)
4
4
4
2
6
2
Ha, 1
0.862
Jiangsu
Nanjing
Chinese
23
11 (47.8)
0
0
3
2
5
1
0
0.745
Sichuan
Chengdu
Chinese
33
18 (54.5)
3
0
9
0
5
0
Rr, 1
0.679
Total for China
239
160 (66.9)
15 (9.4)
19 (11.9) 34 (21.3) 7 (4.4) 63 (39.4) 9 (5.6)
13 (8.1)
0.791
Canada
Nova Scotia
Wolfville
White
29
18 (62.1)
17
0
0
1
0
0
0
0.112
USA
Florida
Miami
Latin American
34
14 (41.2)
9
1
0
0
1
2
Ck, 1
0.593
North Carolina Durham
Chinese American
58
17 (29.3)
16
0
0
0
1
0
0
0.118
African American
48
17 (35.4)
17
0
0
0
0
0
0
0
White, other
314
125 (39.8)
114
3
0
0
4
3
Cgl, 1
0.167
Total for North
America
483
191 (39.5)
173 (90.6)
4 (2.1)
0
1 (0.5)
6 (3.1)
5 (2.6)
2 (1.0)
0.178
NOTE.
Ca, Candida albicans; Cf, Candida famata; Cgl, Candida glabrata; Cgu, Candida guilliermondii; Ch, Candida humicola; Ck, Candida krusei; Cl, Candida lusitaniae; Cp, Candida parapsilosis; Ct,
Candida tropicalis; Ha, Hansenula anomala; Rr, Rhodotorula rubra; Rg, Rhodotorula glutinus; Sc, Saccharomyces cerevisiae; Tc, Trichosporon cutaneum; USA, United States of America.
a
No significant difference was observed in the oral yeast carriage rate and species composition between male and female hosts or among different age groups either within a sample or for the
whole collection.
b
Species diversity was calculated as
, where N is the sample size and p
i
is the frequency of a particular species in the sample. Species diversity has a maximum value of 1.0 when
2
(1
⫺ Sp )N/(N ⫺ 1)
i
all hosts have different yeast species, and it has a minimum value of 0 when all hosts harbor the same yeast species.
by guest on December 13, 2013
http://cid.oxfordjournals.org/
Downloaded from

BRIEF REPORT
• CID 2003:36 (15 January) • 223
Results.
The resulting yeast isolates and carriage rates are
listed in table 1. In eastern North America, the recovery of
yeasts was similar for all 3 geographic areas and different racial
groups. The overall rate of carriage was 39.5% (range, 29.3%–
62%), and species diversity was low (0.178): C. albicans was
the predominant isolate, with an overall frequency of 90.6%
(range, 64%–100%). The other species isolated from the mouth
were Candida parapsilosis (3.1% of species recovered), Candida
tropicalis (2.6%), Candida famata (2.1%), and a single isolate
each (0.5%) of Candida glabrata, Candida krusei, and Candida
lusitaniae. These rates of carriage and the species isolated are
similar to those noted in previous studies of oral yeasts recov-
ered from healthy individuals and outpatients in European and
Western nations [1, 4, 7].
In contrast to the homogeneity of yeasts among the sites in
North America, the overall carriage of oral yeasts was higher
and the array of yeast species was significantly different in China
(
for both comparisons). C. albicans was not the most
P
!
.01
frequently isolated species in any of the 5 regions. Indeed, C.
albicans ranked fourth in prevalence and accounted for only
9.4% (range, 0%–17.4%) of all yeasts isolated in China. The 3
most common yeast species were C. parapsilosis (39.4%), Can-
dida guilliermondii (21.3%), and C. famata (11.9%). These 3
species generally occurred at very low frequency or not at all
in other surveyed geographic regions [1]. At 4 of the sites in
China, C. parapsilosis was the most prevalent species; at the
other site, C. guilliermondii was the most common isolate. Sev-
eral studies have implicated a strong association of C. para-
psilosis colonization of the hands and attachment to catheters
with opportunistic candidemia among neonates [5, 8, 9].
Discussion.
The oral yeast microflora of healthy Chinese
persons contrasts with the current conceptions about the ecol-
ogy of yeasts in humans. The oral yeast microflora were not
determined by ethnicity: the rates of carriage and yeast species
were similar among Chinese individuals and persons from other
racial groups in eastern North America, but they differed from
the rates and species isolated from Chinese people living in
China. The differences between China and eastern North Amer-
ica are not related to the recent shifts in yeast flora that have
resulted from the widespread use of antifungal drugs in North
American patient populations. All 722 of the volunteers sam-
pled here were healthy, and none had taken any commercial
antifungal drugs before samples were obtained. Indeed, the
usual azole-resistant species, C. glabrata, C. lusitaniae, and C.
krusei, were recovered in very low numbers in both China and
eastern North America (table 1).
There are several possible explanations for the observed dif-
ferences in the prevalences and types of oral yeast microflora
between China and eastern North America. The higher carriage
rate in China may be related to poor oral hygiene. For example,
toothbrushing is not commonly practiced in 2 of the 5 com-
munities that were sampled in China (Sitou Village and Huang-
tugang), and these 2 communities had the highest rates of yeast
carriage (81% and 68%, respectively). Alternatively, the wider
spectrum of yeast species isolated from people in China may
reflect an ancestral human-yeast association, whereas, in North
America, undetermined societal factors, which perhaps relate
to industrialization, globalization, lifestyle, diet, regular dental
care, or other factors, may favor the selection of C. albicans
over other species. This hypothesis is supported by studies of
the yeast flora among Chinese persons in Hong Kong, who
represent the most westernized population of China. Similar
to the findings from eastern North America, Chinese persons
in Hong Kong had lower rates of yeast carriage (range,
13%–24% of persons), and C. albicans predominated (range,
77%–84% of isolates) [3, 10, 11].
It is also possible that the people from whom we obtained
samples were not representative of their respective geographic
populations. However, this is unlikely for several reasons. Par-
ticipation was voluntary, and the volunteers in each area were
not selected or subject to selective bias. Within each region,
the sex ratios were close to 1, and the age ranges were broad
and evenly distributed. Furthermore, the different geographic
areas within both eastern North America and China were sep-
arated by great distances, and travel by natives residing in these
locations was not common.
In addition, the results could not be attributed to the in-
vestigators or methods because the same procedures were used
to obtain and process the cultures. The specimens from China
and Canada and approximately one-half of the specimens from
North Carolina were obtained by one of us (J.X.), the remaining
samples from North Carolina were obtained by the other
(T.G.M.), and the swabs from Miami were collected by a student
in our laboratory (A. R. Ramos). All of the isolates were cul-
tured and identified in our laboratory at Duke (Durham, NC).
Unfortunately, there is scant information about which spe-
cies of Candida most commonly cause candidiasis in China. If
C. albicans is more pathogenic than other species, it may cause
the majority of opportunistic infections in China, as elsewhere.
However, the incidence of candidiasis may be comparatively
lower in China. Conversely, if the incidence and etiology of
opportunistic candidiasis reflect the endogenous yeast flora,
which is the current consensus, this report would predict a high
incidence of candidiasis in China, predominated by species
other than C. albicans.
Acknowledgments
We are grateful to all of the volunteers who participated in
this study. We thank Adela R. Ramos, for obtaining the samples
from Florida, and Dee Amick, for helping with identification
of yeast isolates. We are indebted to Jun Zhou, Xing Wei, Ting
http://cid.oxfordjournals.org/
Downloaded from

224
• CID 2003:36 (15 January) • BRIEF REPORT
Chen, and Huamou Zhou, who helped arrange the sample
collections in China.
References
1. Odds FC. Candida and candidosis. London: Bailliere Tindall, 1988.
2. Hazen KC. New and emerging yeast pathogens. Clin Microbiol Rev
1995; 8:462–78.
3. Leung WK, Dassanayake RS, Yau JY, Jin LJ, Yam WC, Samaranayake
LP. Oral colonization, phenotypic, and genotypic profiles of Candida
species in irradiated, dentate, xerostomic nasopharyngeal carcinoma
survivors. J Clin Microbiol 2000; 38:2219–26.
4. Kleinegger CL, Lockhart SR, Vargas K, Soll DR. Frequency, intensity,
species, and strains of oral Candida vary as a function of host age. J
Clin Microbiol 1996; 34:2246–54.
5. Kam AP, Xu J. Diversity of commensal yeasts within and among healthy
hosts. Diagn Microbiol Infect Dis 2002; 43:19–28.
6. Pfaller MA, Houston A, Coffmann S. Application of CHROMagar
Candida for rapid screening of clinical specimens for Candida albicans,
Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata.
J Clin Microbiol 1996; 34:58–61.
7. Xu J, Boyd CM, Livingston E, Meyer W, Madden JF, Mitchell TG.
Species and genotypic diversities and similarities of pathogenic yeasts
colonizing women. J Clin Microbiol 1999; 37:3835–43.
8. Levy I, Rubin LG, Vasishtha S, Tucci V, Sood SK. Emergence of Candida
parapsilosis as the predominant species causing candidemia in children.
Clin Infect Dis 1998; 26:1086–8.
9. Huang YC, Lin TY, Leu HS, Peng HL, Wu JH, Chang HY. Outbreak
of Candida parapsilosis fungemia in neonatal intensive care units: clin-
ical implications and genotyping analysis. Infection 1999; 27:97–102.
10. Sedgley CM, Samaranayake LP. The oral prevalence of aerobic and
facultatively anaerobic gram-negative rods and yeasts in Hong Kong
Chinese. Arch Oral Biol 1994; 39:459–66.
11. Sedgley CM, Samaranayake LP, Chan JC, Wei SH. A 4-year longitudinal
study of the oral prevalence of enteric gram-negative rods and yeasts
in Chinese children. Oral Microbiol Immunol 1997; 12:183–8.
http://cid.oxfordjournals.org/
Downloaded from
Wyszukiwarka
Podobne podstrony:
PEAR, Gender Differences In Human Machine Anomalies
Changes in human gut flora with age
Lößner, Marten Geography education in Hesse – from primary school to university (2014)
Cultural Differences in Television?vertising
Gender and Racial Ethnic Differences in the Affirmative Action Attitudes of U S College(1)
Differences In Sexual Harassment
MNS in human brain
Dane Rudhyar THE PRACTICE OF ASTROLOGY AS A TECHNIQUE IN HUMAN UNDERSTANDING
Lößner, Marten Geography education in Hesse – from primary school to university (2014)
Aspden CYCLOTRON RESONANCE IN HUMAN BODY CELLS (1997)
Differences in the note taking skills of students with high achievement,
(autyzm) Hadjakhani Et Al , 2005 Anatomical Differences In The Mirror Neuron System And Social Cogn
Statistical testing of individual differences in sensory profiling
(psychology, self help) 10 Little Things That Can Make a Big Difference in Your Marriage
25 Because of the Angels – Angelic Intervention in Human Lives
MRS of limbic structures display metabolite differences in young unaFFECTED RELATIVES OF SCHISOPHREN
Stimulation of Collagen Production in Human Fibroblasts
więcej podobnych podstron