układ okresowy 3
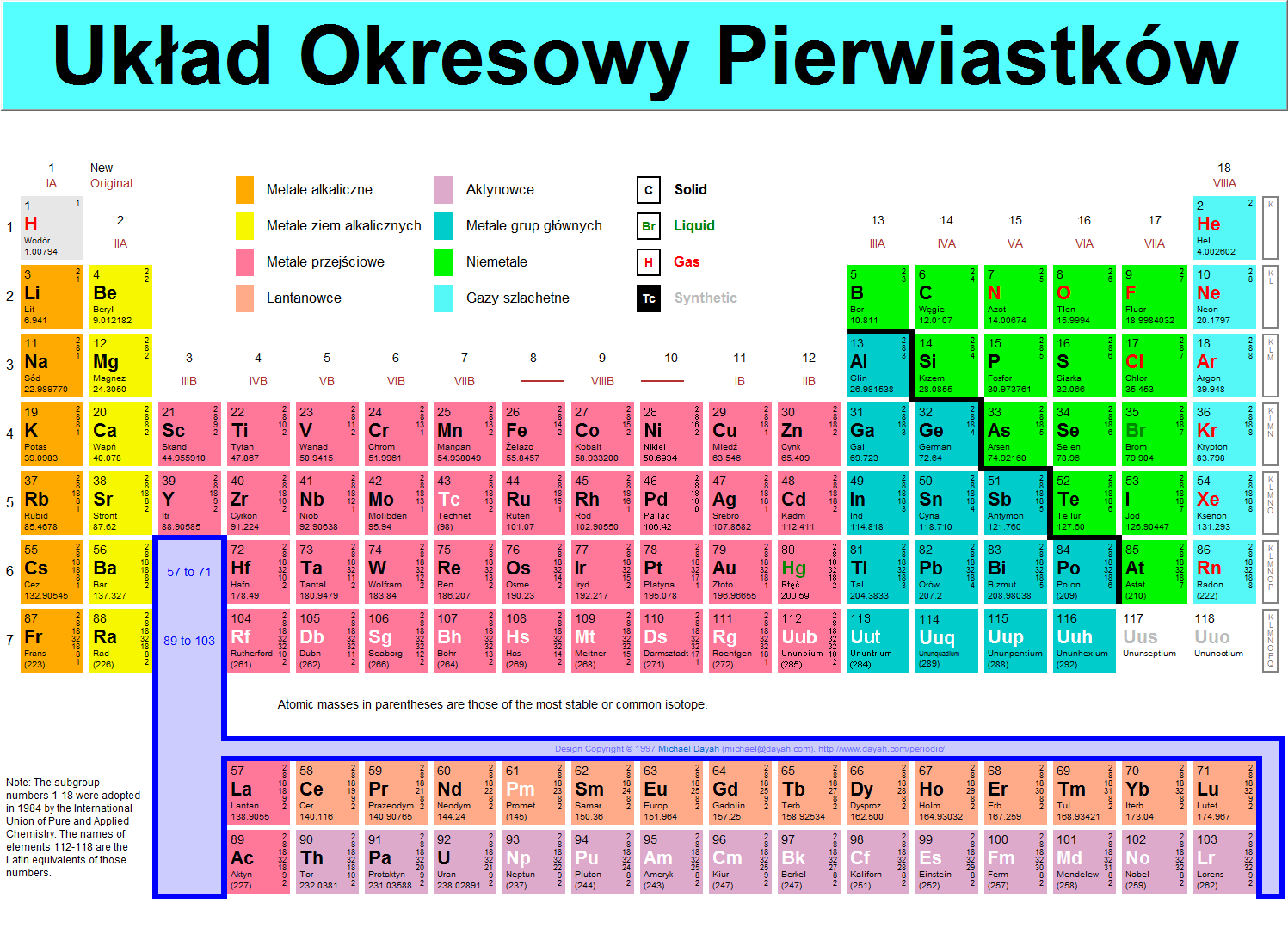
Układ Okresowy Pierwiastków
1
IA
New
Original
1
1 H
Wodór
1.00794
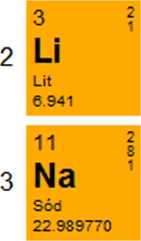
Potas
39.0983
37
Rb
Rubid
85.4678

HA
4
Be
Beryl
9.012182
12
Mg
Magnez
24.3050
20
Ca
Wapń
40.078
38
Sr
Stront
87.62
56
Ba
Bar
137.327
88
Ra
Rad
(226)
3
IIIB
|
Metale alkaliczne |
Aktynowce |
0 |
Solid |
|
Metale ziem alkalicznych |
Metale grup głównych |
h |
Liquid |
|
Metale przejściowe |
Niemetale |
0 |
Gas |
|
La nta nówce |
Gazy szlachetne |
ii |
Synthetic |
18
VIIIA
13
IMA
14
IVA
15
VA
16
VIA
17
VIIA
4
IVB
5
VB
6
VIB
7
VIIB
9
VIIIB
10
11
IB
12
MB
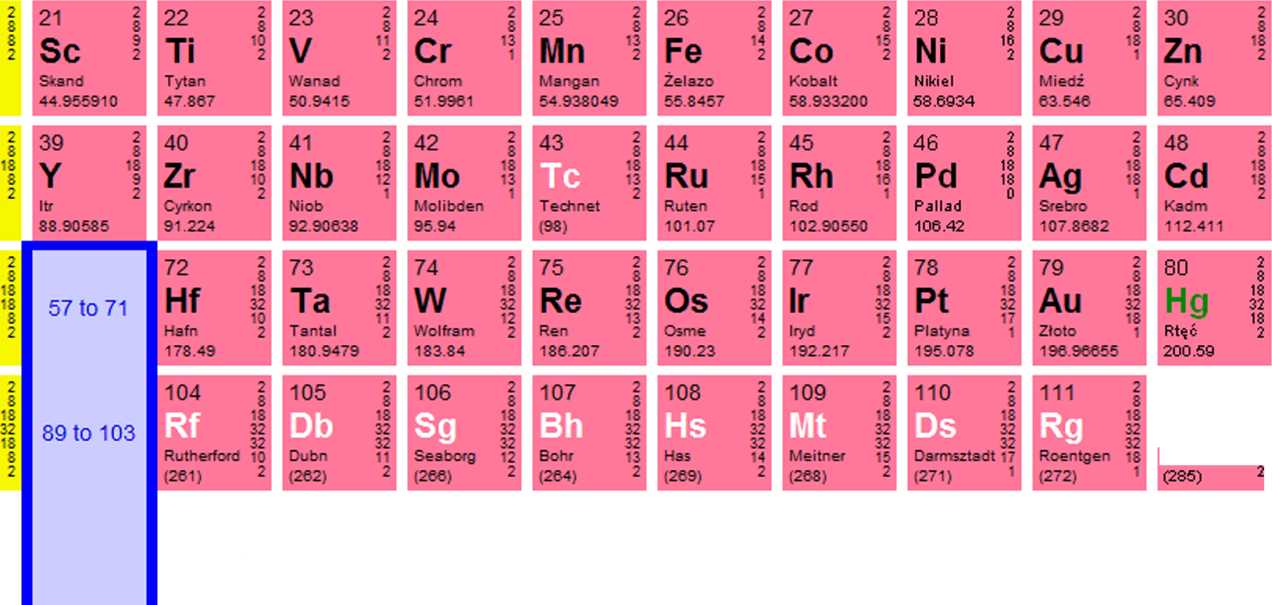
112
1
Ununbium 1$
Atomie masses in parentheses are those of the most stable or common isotope
|
5 1 B Bor 10.811 |
6 5 C Węgiel 12.0107 |
7 i N Azot 14.00674 |
8 1 O Tlen 15.9994 |
9 ? F Fluor 18.9984032 |
|
13 i| Al Glin 26.981538 |
U i Si 4 | Krzem 28.0855 |
p 1 Fosfor 30.973761 |
16 i s Siarka 32.066 |
17 1 Cl Chlor 35.453 |
|
31 1 Ga f Gal 69.723 |
32 || Ge ’? German 72.64 |
33 1 As 1t | Arsen 74.92160 |
34 | Se i Selen 78.96 |
35 § Br ł Brom 79.904 |
|
49 : In ii Ind 114.818 |
50 | Sn ii 4 Cyna 118.710 |
51 1| Sb !| Antymon 121.760 |
162 | Te !i Tellur 127.60 |
53 1 1 18 Jod 126.90447 |
|
81 1 Tl 1 Tal 3 204.3833 |
82 Pb li 18 Ołów 4 207.2 |
83 I Ri '8 Bl s Bizmut 5 208.98038 |
84 || po 8 Polon 61 (209) | |
85 1 At 1 Astat 7 | (210) |
|
113 Ununtrium (284) |
wnurwuadUm |
115 Ununpentium (288) |
116 Ununhexium (292) |
117 Uus Ununseptium |
10
Ne
Neon
20.1797
18
Ar
Argon
39.948
36
Kr
Krypton
83.798
54
Xe
Ksenon
131.293
86
Rn
Radon
(222)
118
Uuo
Ununoctium
|
57 | La ii Lantan 2 138.9055 |
58 1 Ce i| Cer 2 140.116 |
59 1 Pr 3 Prazeodym 2 140.90765 |
60 l Nd 3 Neodym 2 144.24 |
61 i Ą Promet 2 (145) |
62 Sm a Samar 2 150.36 |
63 | Eu 3 Europ 2 151.964 |
64 i Gd 3 Gadolin 2 157.25 |
65 1 Tb 3 Terb 2 158.92534 |
66 \ Dy 3 Dysproz 2 162.500 |
67 i Ho 3 Holm 2 164.93032 |
68 1 Cr 18 Er 1 Erb 2 167.259 |
69 1 Tm 3 Tul 2 168.93421 |
70 i Yb Ą Iterb 2 173.04 |
71 i Lu li Lutet 2 174.967 | |
|
89 | Ac I Aktyn 9 (227) 2 |
90 i Th 1 Tor 10 232.0381 2 |
91 I Pa 1 Protaktyn 9 231.03588 2 |
92 1 u g Uran 9 238.02891 2 |
93 1 i Neptun 9 (237) 2 |
94 1 i Pluton 8 (244) 2 |
95 l ES31 Ameryk 8 (243) 2 |
96 1 I Kiur 9 (247) 2 |
97 I i Berkel 8 (247) 2 |
98 1 B i Kaliforn 8 (251) 2 |
Einstein 8 (252) 2 |
100 1 1 Ferm 8 (257) 2 |
I Mendelew 8 (258) 2 |
102 1 -1® i Nobel 8 (259) 2 |
103 | B i Lorens 9 (262) 2 |
Notę: The subgroup numbers 1-18 were adopted in 1984 bythe International Union of Pure and Applied Chemistry. The names of elements 112-118 are the Latin equivalents ofthose numbers.
Wyszukiwarka
Podobne podstrony:
układ okresowy Układ Okresowy Pierwiastków 1 IA 11
Ukl okres (2) kolor 1 IA 18 O • 1 2,1 I-I 1312 n UKŁAD OKRESOWY PIERWIASTKÓW 2372
układ okresowy 1 IA 1.00794 • 1 2.1 1312 1 H ___________ ( (UKŁAD OKRESOWY PIERWIASTKÓW 18 4.00
Image2 18C 8 Układ okresowy pierwiastków 2 He Widok Podstawowe informacje Rozpowszechnienie Kalkulat
Image3 18C 8 Układ okresowy pierwiastków 2 He Widok Podstawowe informacje Rozpowszechnienie Kalkulat
Image4 C 5 Układ okresowy pierwiastków 18 2 He Widok Podstawowe informacje Rozpowszechnienie Kalkula
I i I UKŁAD OKRESOWY PIERWIASTKÓW — , aWA łaVISB/ « ™ m<A ’ J‘s W®.5 S4 ji? 3.5- .•
UKŁAD OKRESOWY PIERWIASTKÓW CHEMICZNYCH f>7 -25 • Symbol ■ piorwlaatka
UKŁAD OKRESOWY PIERWIASTKÓW E 8 nip głównych [e7] l 0Gmi U ■!II ■ » »• r
imię i nazwisko kl.vta ocena WERSJA ASprawdzian: Budowa atomu a układ okresowy pierwiastków
UKŁAD OKRESOWY PIERWIASTKÓW »»«•» Motl ir--- ^ Mm i* lAR # 1 n»»-«
więcej podobnych podstron