CCF20130319�015

Research and Science Articles published in this section have been reviewed by three members of the Editorial Review Board
Adrian Lussi’
Elmar Hellwig2 Joachim Klimek3
1 Department of Preventive, Restorative and Pediatrie Dentistry, School of Dental Medicine, University of Bern, Switzerland
2 Department of Operative Dentistry and Periodontology, Unh/ersity Clinic of Dentistry, Freiburg, Germany
5 Department of Conservative and Preventive Dentistry, Dental Ginie, Justus Liebig University, Giessen, Germany
Address for correspondence:
Prof. Dr. Adrian Lussi Department of Preventive, Restorative and Pediatrie Dentistry School of Dental Medidne University of Bem Freiburgstrasse 7 CH-3010 Bem Switzerland
Fluorides - Modę of Action and Recommendations for Use
effective both in. promoting remineralization and inhibiting derrrireraiization. Considering the fact that the caries deciine occurred at >:r,e same time that local fluoridation measures becarńe widely used, the conclusion seenns justified that regular application of F can inhibit caries.
Summary Various authors have shown tire daclina i" the indvstp’al‘Z2d coun-
of which local fiuorde application in ferm of fluoridated toothpastes is of prś.nary importance. The caries-protective po-tentiai of fluorapatite is quite Iow; in contrast, disso!ved fluorides in the vicinity of enamel are
Schweiz Monatsschr Zahnmed 122: 1030-1036 (2012)
Accepted for publication:
6 0ctober 2011
protficlń/e facCors
promoling faclors
c.t^łniryj
calciurr* phCiphAte r ar-hfacter.Di sufcstsnces
■ ruinoftfcntc fc,vcTtrf<3 - ro-ijced saliv3 F-*<xk.:nc^
«• ilteT -lDCt C.lfb-0
hydratem
prob.ot cs
Introduction
Dental hard tissue consists of highly mineralized enamel, as well as dentin and cementum, which contain a much greater proportion of organie matrix. The minerał phase of the dental hard tissues is not pure hydroxyapatite (HAP = Ca,0 (P04)ć OH,), but rather is a calcium-deficient biomateriał in which numerous other ions are incorporated. Building hydrogen phosphate, carbonate and magnesium ions into the HAP lattice leads to a less stable, morę soluble apatite. The greater proportion of carbonate in dentin (5.5%) than in enamel (3%) makes dentin crystals morę susceptible to acid attack. However, the partial substitution of fluoride ions for OH groups in the crystal lattice can stabilize the apatite structure to a certain degree.
In healthy human enamel, fluorhydroxyapatite (FHAP) or fluorapatite (FAP) are present in addition to HAP, although in the outermost enamel iayer, less than an average of 5% of the OH groups of HAP are replaced by fluoride. At a depth of 50 pm, this percentage drops further.
The present article discusses the importance of fluoride for caries prevention (Featherstone 2000, Lussi 2010) (Fig. 1) and presents practical recommendations for the use of fluoride.
Acid attack
Enamel is a calcium-deficient. carbonate-rich hydroxyapatite. In its stable State, there are sufficient Ca2\ P045-, OH" and F
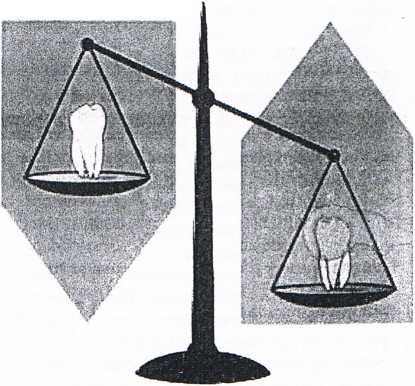
fig. 1 Tire caries balance. Pathological and protedwe feciuis wfiiui simt the caries balance between de and remineralization (modified from Feather stone 2000)
1030 Schweiz Monatsschr Zahnmed Vol. 122 11/2012
Wyszukiwarka
Podobne podstrony:
CCF20130319�019 ■? Research and Science Articles published in this section have been reviewed by thr
CCF20130319�017 <® Research and Science Artides published in this section have been reviewed by t
22 (899) Ir is a good idea to start by decorating a smali fiat surface, so in this section I have in
DIPLOMA Centre of Rehabilitation^ intermedicus This course has been approved by Polish Society of
CCF20130319�020 Fluorides - Modę of Action and Recommendations for Use Research and Sciencpossible a
img004 2 Tytuł oryginału Stylish Decoupage. 15 step-by-step projects to dazzle and delight First pub
Bndging By default, one bridge (brO) is defined and active. In this section you can define additiona
1 In this section we briefly discuss why it benefits your PhD program to work according to a Schedul
9 Tanzimat and “Mecelle” 115 published. In the following years further translations into variou
they are smaller and are not systematic. In this case, instead of differences, we may talk about tre
img004(2) Tytuł oryginału Stylish Decoupage. 75 step-by-step projects to dazzle and delight First pu
CCF20110521�007 Heart and blood disorders Fili in the crossword. Across 5 Irregula
00139 >858430acb91880687589de10557be5 140Simpson & KeatsEconomic-Statistical Approach Using Tra
195WELDED JOINTS Distribution of stresses in v/elaed and riveted connections, by W. Hovgaarć. Procee
więcej podobnych podstron