100444
Journal of Medicinal Chemistry ART1CLE
Journal of Medicinal Chemistry ART1CLE
fluma/enil (3)
Chart 1. Structures of Refercncc Compounds
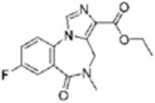
DMCM (1) Kj-7142 (2)
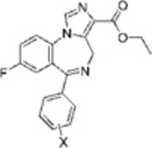
4*. X ■ 3'-NOł 4t>. X - 4--NO:
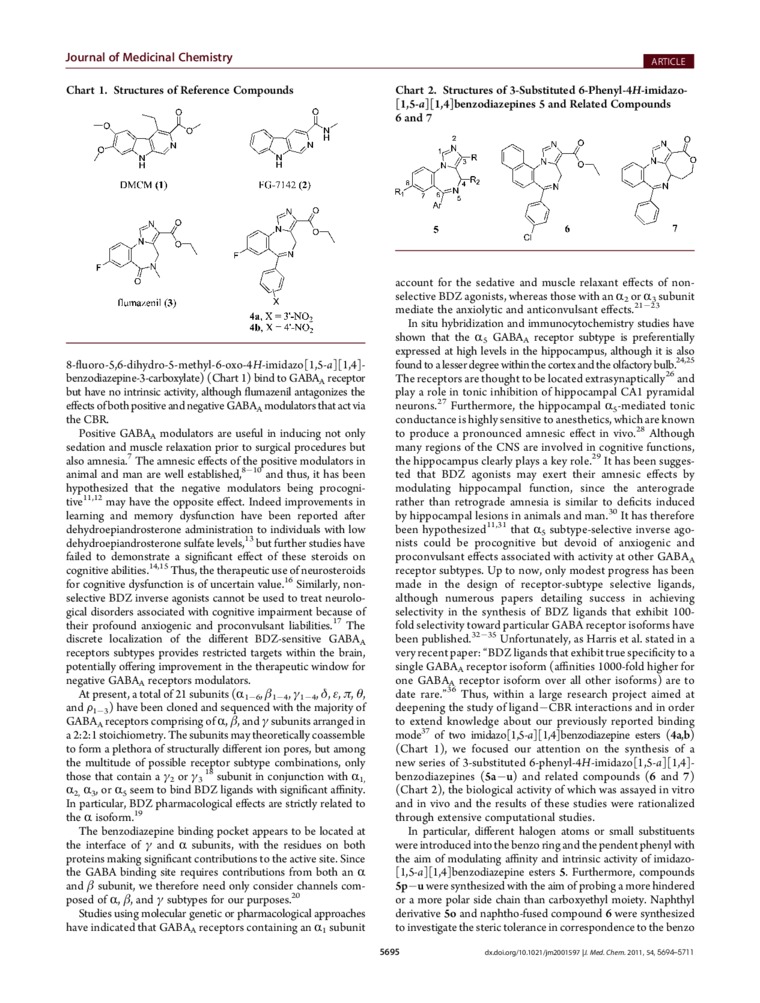
8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[l,$-«i][l,4]-benzodiazepinc-.Vcarboxylate) (Chart 1) bind to GABAa receptor but have no intrinsic activity, although fluma/enil anlagoniz.es the effects ofboth positńre and ncgative GABAa modulators that act via the CBR.
Positivc GABAa modulators arc uschli in inducing not only scdation and musdc rclaxation prior to surgical proccdurcs but also amnesia. The amnesic effects of the positive modulators in animal and man arc well cstablishcd,* 'and thus, it has bcen hypothesized that the negative modulators being procogni-tive11’12 may have the opposite effect. Indeed improwments in leaming and memory dysfunction have bcen reported aftcr dchydrocpiandrostcronc administration to individuals with Iow dehydrocpiandrostcronc sulfatc lcvds,'' but furthcr studics havc failcd to dcmonstrate a significant effect of thesc steroids on cognitwc abilitics.14 1' Thus, the thenipeutie usc ofncurostcroids for cognitive dysfunction is of uncertain valuc.lf‘ Similarly, non-selective BDZ inverse agonists cannot be used to treat neurolo-gical disorders associated with cognitive impairment because of their profound anxiogenic and proconvulsant liabilities.1 The discrete locali/ation of the different BDZ-sensitive GABAa reccptors subtypes provides rcstricted targets within the brain, potentially offering improvcmcnt in the therapcutic window for ncgativc GABAa reccptors modulators.
At prescnt.a total of 21 subunits (di-*,/f| 4, y, ą,Ó, f, .7, 0, and p, j) have bcen doncd and scqucnccd with the majority of GABAa receptors comprising of a, fi, and y subunits arranged in a 2:2:1 stoichiometry. The subunits may theoretically coassemble to form a plethora of structurally different ion pores, but among the multitude of possible receptor subtypc combinations, only those that contain a >s or y$ subunit in conjunction with (1|, CU av or as seem to bind BDZ ligands with significant affinity. In particular, BDZ pharmacological effects arc strictly related to the tt isoform.w
The benzodiazepine binding pocket appears to be located at the interface of y and a subunits, with the residues on both proteins muking significant contributions to the active site. Since the GABA binding site requircs contributions from both an a and fi subunit, we therefore need oni)’ consider channels com-posed of O, fi, and y subtypes for our purposes.20
Studics using moleeular genetic or pharmacological approachcs have indicated that GABAa receptors containing an (l| subunit
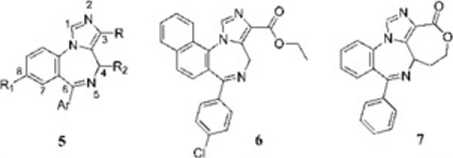
Chart 2. Structures of 3-Substituted 6-Phenyl-4H-imidazo-[l,5-d][l,4]benzodiazepines 5 and Related Compounds 6 and 7
account for the sedative and muscle relaxant effects of non-sclectivc BDZ agonists, whercas those with an a> or (i; subunit mediate the anxiolytic and anticonvulsant effects.'1 2'
In situ hybridization and immunocytochemistry studics have shown that the a?; GABAa receptor subtypc is preferentially expressed at high lcvels in the hippocampus, although it is also found to a lesser degree within the cortex and the olfactory bulb.‘4'' The receptors are thought to be located extrasynaptically''’ and play a role in tonie inhibition of hippocampal CA1 pyramidal neurons.2 Furthermore, the hippocampal a5-mediated tonie conductancc is highly sensitive to anesthetics, which are known to produce a pronouneed amncsic cffect in snyc.'11 Although many regions of the CNS are involved in cognitive functions, the hippocampus clearly plays a key role.29 It has been sugges-ted that BDZ agonists may excrt their amncsic effects by modulating hippocampal function, sińce the anterograde rather than retrograde amnesia is similar to deficits induced by hippocampal lesions in animals and man.w It has therefore bcen hypothesized11'1 that <ls subtypc-sclcctivc invcrsc agonists could be procognitive but devoid of anxiogcnic and proconvulsant effects associated with activity at other GABAa receptor subtypes. Up to now, only modest progress has bcen madę in the design of reccptor-subtypc sclectivc ligands, although numerous papers detailing succcss in achieving selectmty in the synthesis of BDZ ligands that exhibit 100-fold selectivity toward particular GABA receptor isoforms have been published.'2 's Unfortunately, as Harris et al. stated in a very recent paper: ’BDZ ligands that exhibit true specificity to a single GABAa receptor isoform (affinitics 1000-fold higher for one GABAa receptor isoform ovcr all other isoforms) arc to datę rarc.“'f’ Thus, within a large rcscarch project aimed at dcepening the study of ligand—CBR intcractions and in order to extend knowledgc about our prcviously reported binding modę' of two imidazo[l3-<»][l.4]benzodiazepine esters (4a,b) (Chart l), we focused our attention on the synthesis of a new series of 3-substituted 6-phenyl-4H-imidazo[l,5-a][l,4]-benzodiazepines (5a—u) and related compounds (6 and 7) (Chart 2), the biological activity of which wxs assayed in vitro and in \ivo and the rcsults of these studies were rationalized through cxtcnsive computational studics.
In particular, different halogen atoms or smali substituents were introduced intothe benzo ring and the pendent phenyl with the aim of modulating affinity and intrinsic activity of imidazo-(l,S-<i](l,4]benzodiazepine esters 5. Furthermore, compounds 5p—u were synthesized with the aim of probing a morę hindered or a morę polar side chain than carboxyethyl moiety. Naphthyl dcrivative 5o and naphtho-fused compound 6 were synthesized to invcstigate the sterie tolerancc in correspondcncc to the benzo
5695 d*A»U>fyl0.1021/)m2001S97 \l AM Chem 2011. S< 5694-5711
Wyszukiwarka
Podobne podstrony:
CSG237 226 Complete Spanish Grammar Forms of the Subject Pronouns The following chart reviews los pr
Environmental Medicine Journal of Institute of Occupational Medicine and Environmental Health in
nu NEW ENGLAND JOURNAL of MEDICIN E A Pmpectń* Siudy of Walking as Coniparedwith Vigorous Exercisc i
Journal of Clinical Medicine MDPI Article Diverse Action of Selected Statins on Skeletal Muscle Cell
1188 Journal of Medicinal Chemittry, 1991, Vol. 34, No. 3 Scheme I Cristolli et al. CN HjSCHCjH,
63 Effect in Melanoma Cells. Journal of Agricultural and Food Chemistry: 53, p. 4728-4735. Trapp, S.
OF CHEMISTRYMedChemCommRESEARCH ARTICLE View Article Online Vi«w Journal K) Chock for updatcs Cite t
NOWOŚCI dedykowane Farmaceutom PHARMACEUTICSAccess ►Pharmacy ^ ROYAL SOCIETY ^ ^ OF CHEMISTRY Journa
diagnostyka laboratoryjna Journal of Laboratory Diagnostics 2011 • Volume 47 • Number 2 • 197-203 Pr
INTERNET JAKO PRZEDMIOT I OBSZAR BADAŃ PSYCHOLOGII SPOŁECZNEJ 199 participation. Journal of Personal
STUDIA I MATERIAŁY TOWARZYSTWA NAUKOWEGO NIERUCHOMOŚCI JOURNAL OF THE POLISH REAL ESTATE SCIENTIFIC
STUDIA I MATERIAŁY TOWARZYSTWA NAUKOWEGO NIERUCHOMOŚCI JOURNAL OF THE POLISH REAL ESTATE SCIENTIFIC
Zdj?cie038 SNOMED - Systematized NOmenclatuie of MEDicine -Nor ( S NOntendature c
więcej podobnych podstron