100437
1188 Journal of Medicinal Chemittry, 1991, Vol. 34, No. 3
Scheme I
Cristolli et al.
|
CN |
HjSCHCjH, 3 | |
|
CNKN, |
1 + CM OH |
unijCi |
|
COOEt |
CH, |
0
1
Ito-C
NH.OH
0
II
H2N -C H2N
2
3
N«N0, / NCI /
N*NO,

O 0 0
|
"■':u |
EtO-C X? | |
|
A |
i |
A |
|
♦ |
6 |
4 |
|
A |
***/ j IMM, | |
|
-CMC,M„ 1 CH OH 1 |
TT |
HOHjC N |
|
CH, |
A 9 |
A a |
o
MlN-C. N
|
s, |
Sal |
' A |
|
Cp |
R, | |
|
5 |
H |
H |
|
!• |
M |
CH |
|
Sb |
H |
CM |
|
Se |
H |
"CM |
|
Sd |
H |
OvCHQH |
|
Se |
M |
tWHjCMtOMKK |
|
sr |
H |
1»JC*CCH|0M|CM. |
|
99 |
H |
<C,H |
|
9h |
H |
CHAH |
|
Si |
CH |
|
no. |
X |
Y |
Z |
K,.M |
|
la* |
N |
N |
N |
0.7 X 10 • |
|
lb* |
CH |
N |
N |
1.6 x 10-T |
|
lc« |
N |
CH |
N |
1.0 x 10 * |
|
ld* |
N |
N |
CH |
4.0 X 10 4 |
|
le* |
CH |
CH |
N |
7.1 X 10*s |
|
lf |
CH |
N |
N |
6.6 x 1CT |
|
la |
N |
CH |
N |
3.6 x 10 * |
|
lh |
N |
N |
CH |
not active |
|
U |
CH |
CH |
N |
U x 10* |
|
11 |
CH |
N |
CH |
not active |
* Mialurc of two isomers: 2'S.VR and 2'R&S.
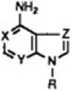
of EHNA itself, and 1-deaza-EHNA (lb), though less po-tent, is a good inhibitor. On the contrary, substitution of a raethine group for the nitrogen atom in the 7*position of the purine moiety of EHNA produces a dramatic drop in the inhibitory activity (ld). Also the contemporary substitution of pyrimidine nitrogens by carbons brings about a conaiderable reduction of activity (le). In order to introduce additional simplifications to the EHNA Chemical atructure, a series of er>Ł/iro-l-(2-hydroxy-3-nonyl)imidazole derivatives have been synthesized.
Chemlstry
Condenaation of ethył 2-amino-2-cyanoacetate: with eo'fńro-3-amino-2-nonanol# in the preaence of triethyl orthoformate gave ethył eryf/iro5-amino*l-(2-hydroxy-3-nonyl)imidazule-4-carboxylate (2) (Scheme I). Ammo-nolysis of 2 in a sealed tubę at 110 °C for 3 days afforded eryfhro-5-amino-l-(2-hydroxy-3-nonyl)imidazole-4-carboxamide (3).
(7) Lagemann, F. I.; Shaw. G. B. Chem. Ind. {London) 1980, Ml.
(8) Shaeffer. H. J.. Schwender. C. F. J. Med. Chem. 1974. 17.6.
Table I. tnhibition Con&tanU of Calf Intestinal Adenoeine Deaminase5
|
CHC«H„ |
HOCH, |
|
CHOW 1 |
- lę |
|
CH, |
HO |
|
ia-a |
1«-l |
Treatment of the same compound (2) with sodium ni-trite at -20 °C in the preaence of 50% hypophosphorous acid gave ethył enfbro-l-(2-hydroxy-3*nonyl)imidazole-4-carboxylate (4), which was converted by methanolic ammonia at 110 °C to eryfhro-l-(2-hydroxy-3-nonyl)-iraidazole-4-carboxainide (5). Treatment of ethył ester 4 with the appropriate aminę provided N-substituted amides 5a-ł. Nitrosation of compound 2 at --25 °C in the presence of 6 N hydrochloric acid and cuprous chloride afforded ethył er>(/jro-5-chloro-l-(2-hydroxy-3*nonyl)imidazole-4-carboxylate (6) which was comerted by 30% ammonium hydroxide at 110 °C to erythro-5-chloro-l-(2-hydroxy-3-nonyl)imidazo)e-4-carboxamide (7). Reduction of 4 with LiAłH4 in THF provided eryt/iro-l-(2-hydroxy-3-nonyl)-4-(hydroxymethyl)imidazole (8).
Basic hydrołysis of the same ester 4 gave erythro-l-(2-hydroxy-3-nonyl)imidazole-4-carboxylic acid (9) in 66% yield. Condenaation of aminomalonitriłe with erythro-3-amino-2-nonanol5 in the presence of triethyl orthoformate gave eryfhro-5-amino-l-(2-hydroxy-3-nonyl)imidazołe-4-carbonitrile (10). Compound 10 was diazotized with iso-pentyl nitrite in tetrahydrofuran (THF) at reflux to give 50% erythro- l-(2-hvdroxv-3-nonyl)imidazole-4-carbonitrile (13).
Amidine 12 was prepared from 10 in two steps: treatment of nitrile 10 with dry hydrogen chloride in methanoł at 0 °C provided 83% methyl eryf/iro-5-araino-l-(2* hydroxy-3-nonyl)imidazole-4-carboximidate hydrochloride (11) (Scheme II). The latter compound was further con-verted into eryf/tro-5-amino-l-(2-hydroxy-3-nonyl)-imidazole-4-carboxamidine hydrochloride (12) when treated with methanolic ammonia at 80 °C for 4 h. The same reactions did not work well in the case of nitrile 13. The alternative route includcs synthesis of amidoxime 14 by treatment of 13 with hydroxylamine, followed by reduction with hydrogen and Raney Ni catalyst at 45 psi for 6 h to give eryf/jro-l-(2-hydroxy-3-nonyl)imidazole-4-carboxamidine hydrochloride (15). Treatment of 13 with hydrogen sulfide in dry pyridir.e and triethylamine at room temperatura gave eryt/iro-l-(2-hydroxy-3-nonyl)-imidazole-4-thiocarboxamide (16). Hydrogenation ofthe same carbonitrile 13 in the presence of 5% Pd/C tri-
Wyszukiwarka
Podobne podstrony:
Zarządzanie i Finanse Journal of Management and Finance Vol. K3, No. 3/2/2015 Wojciech
IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010
12 IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010 www.IJCSI
IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010
14 IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010 www.IJCSI
15 IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010 www.IJCSI
Imidazofl.2-ajpyrazine Dertuatiues Journal of Medianal Chemutry, 1984, Vol. 27, No
Zarządzanie i Finanse Journal of Management and Finance Vol. 12, No. 1/2014 Tomasz Jurkiewicz1 Agnie
The Bulletin of The Canadian Association of Physicists Vol. 34 No. 6 1978 Bulletin de I As
Journal of Chromatugraphic Sci«?nce, Vol. 47, August 2009 Table II. Seven Volalile Compounds Most Fr
nu NEW ENGLAND JOURNAL of MEDICIN E A Pmpectń* Siudy of Walking as Coniparedwith Vigorous Exercisc i
Cross-Cultural Validation, Journal of Computer-Mediated Communication, vol. 5, no.
Journal of Medicinal Chemistry ART1CLE Journal of Medicinal Chemistry ART1CLE fluma/enil (3) Chart 1
ASSAULT, Journal of Armored & Heliborne Warfare Vol 1 (2002) Hj 1 A&JŁ A m&Ji m ¥ t* *r
http://jci.sciedupress.com Journal of Curriculum and Teaching Vol. 8. No. 1; 2019Assessment of
więcej podobnych podstron