100443
Imidazofl.2-ajpyrazine Dertuatiues Journal of Medianal Chemutry, 1984, Vol. 27, No. 2 207
TaMe I. ‘H NMR Spectra of Imidazo[2,2-o]pyrazine and 5//-Pyrrolo{2,3-6Jpyrazine Deriyatlye*0
|
compć |
Chemical ahifw, ppm |
coupling constonts, Hz | |||||||
|
Hj |
H, |
H, |
H, |
H. |
R |
A.. | |||
|
1 |
7.76 |
7.66 |
8.08 |
7.81 |
9.07 |
4.5 |
1.5 | ||
|
2 |
7.8 |
7.8 |
8.26 | ||||||
|
3 |
7.42 |
7.9S |
7.75 |
8.92 |
2.45 |
4.25 |
1.5 | ||
|
4 |
7.74 |
8.05 |
7.87 |
9.04 |
4.77 |
4.5 |
1.6 | ||
|
5 |
8.29 |
8.18 |
7.93 |
9.16 |
4.47-1.43 |
4.5 |
1.5 | ||
|
6 |
7.81 |
7.95 |
8.28 |
7.99 |
4.6-1.5 |
4.5 | |||
|
7 |
8.39 |
8.43 |
4.47-1.42 | ||||||
|
8 |
8.41 |
8.29 |
7.95 |
9.14 |
3.9S |
4.5 |
1.5 | ||
|
9 |
7.87 |
8.2 |
4.21-1.28-3.96 | ||||||
|
10 |
9.09 |
8.04 |
9.06 |
4.47-1.55-2.74 |
4.5 | ||||
|
11 |
8.65 |
8.15 |
9.05 |
2.66 |
2.5 |
0.8 | |||
|
12 |
8.25 |
8.6 |
4.45-1.28-3.96 |
2.75 | |||||
“ Solution in CDCl„ except for thc compound 11 (CDCl, and MCjSO-d*).
Scheme III
Scheme II
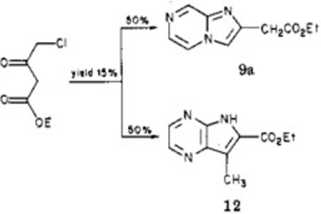
the reactivity in thc ortho position of nuclear pyiazinic amines.13 Likewise, during the synthesis of 6/f-pyrrolo* [2,3-6]pyrazine derivatives,u imidazo|l,2-a]pyrazine de-rivatives appeared simultancously.
From a structural point of view, the NMR spectra of the synthesized compounds were consistent:5 the Hs proton of compound 10 was strongly deshielded hecause of the proximity of the carboxylate group in the 3-position. Compound 11 had no signals corresponding to imidazolic protons hecause of an acetyl group in the 3-position and a chlorine atom in the 2*position. The presence of the chlorinc atom was confirmed by raicroanalysis and mass spectrometry studies (see Experimental Section and Table
ID.
Compound 12 showed two pyrazinic coupled protons: tho absence of a strongly deshielded signal (approximately 9 ppm), characteristie of H„ in imidazo[l,2-n]pyrazine
(9) Baldwin, J. J.; Lutnma, W. C., Jr. (Merck and Co., Inc.) Ger. Offen.. 2820938 (CL Co7 D487/04). 1978; U.S. Appl. 796 958, 1977.
(10) Lumrea, W. C. (Merck and Co. Inc.), Bur. Pat. Appl 13914 (CL C07D 487/04), 1980; U.S. Appl. 4 970 22.1979. Lumna, W. C. (Merck and Co. Inc.) 4242344 (Cl. 424251: AG1K31/ 495). 1980; Appl. 4970, 1979-
(11) Abignente, E.; Arena. F.; De Caprariie, P.; Parcntc, L. Far-maco., Ed. Sei. 1975. 30, 815-22.
(12) Abignente, E.; Arena, F.; Da Caprarii*, P.; Nuretti, R.; Mamo, E.; Lampa, E.; Rowtti, F.; Ottavo, R. Farmaco 1981,36,61-80.
(13) KatriUky, A. R.; Boullon, A. J. Ado. Htterocyel Clum. 1972, 14, 1.
(14) Clark, B. A. J.; Parrick, J.; Dorgan, R. J. J. J. Chem. Soc., Perkin Tront. 1 1976.13. 1361-3.
Scheme IV
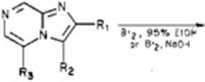
1, R, = R, = R, = H 5, R, = CO.Et; R. = R, = H 18, R, = CONH;; R, * R, * H
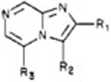
13, R, = H; R: = Br; R, = H
14, R, = H; R. = H; R, = Br
15, R. H; Rt * R, ■ Br
16, R, = COjEt; R. = H; R, = Br
25, R, = NH,; R, = Br; R, = H
derivatives, confirmed this structure. As well, the ISC NMR data dcmonstratcd the presence of four quaternary car bon atoms and only two carbon atoms in the ortho poeition carrying one proton (Jcj^ - Jc-k, m 184 Hz; Jc#: and Jc,H, • 10 and 12 Hz). Thi9 structure was consistent with the molecular weight measured by mass spectrometry.
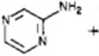
The use of ethyl 4-chloroacetoacetate as reagent in the former reactions also supplied. though with a smali yield, an imidazo[ 1,2-a ]pyrazine derivative, 9a, and compound 12, resulting from partial reagent isomerization of the chlorine derivative in the 2-position (Scheme III).
The use of the 3,5 dibromoaminopyrazine14 with ethyl 2-chloroacetoacetate or ethyl 4-chloroacetoacetate resulted only in the compound 9. There was no formation of the óf/-pyrrolo[2,3-6]pyrazine derivativc, likcly bccausc of the blockage of the poeition of cyclization by a bromine atom
(Ló) Camcrino, B.; PalamidcNi, G. Gazz. Chim. Ilal. 1960, 90, 1807-14.
Wyszukiwarka
Podobne podstrony:
Zarządzanie i Finanse Journal of Management and Finance Vol. K3, No. 3/2/2015 Wojciech
IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010
12 IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010 www.IJCSI
IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010
14 IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010 www.IJCSI
15 IJCSI International Journal of Computer Science Issues, Vol. 7, Issue 3, No 9, May 2010 www.IJCSI
1188 Journal of Medicinal Chemittry, 1991, Vol. 34, No. 3 Scheme I Cristolli et al. CN HjSCHCjH,
Zarządzanie i Finanse Journal of Management and Finance Vol. 12, No. 1/2014 Tomasz Jurkiewicz1 Agnie
Journal of Chromatugraphic Sci«?nce, Vol. 47, August 2009 Table II. Seven Volalile Compounds Most Fr
Cross-Cultural Validation, Journal of Computer-Mediated Communication, vol. 5, no.
Turystyka i Rozwój Regionalny 2019, nr 12, 85-94 Journal of Tourism and Regional Development 2019, N
Journal of KONES Internal Combustion Engines 2002 No. 1-2 ISSN 1231 - 4005AN ATTAUPT EXPLAIN IMPROVE
ASSAULT, Journal of Armored & Heliborne Warfare Vol 1 (2002) Hj 1 A&JŁ A m&Ji m ¥ t* *r
http://jci.sciedupress.com Journal of Curriculum and Teaching Vol. 8. No. 1; 2019Assessment of
246 247 (6) 246 Liirralura q/iou<am Wilkcs K. (1984), Iscotisdousness itnporłattł?, „Brihsh Journ
diagnostyka laboratoryjna Journal of Laboratory Diagnostics 2011 • Volume 47 • Number 2 • 197-203 Pr
INTERNET JAKO PRZEDMIOT I OBSZAR BADAŃ PSYCHOLOGII SPOŁECZNEJ 199 participation. Journal of Personal
więcej podobnych podstron