
HEAVY METAL TOXICITY: EFFECT ON PLANT GROWTH AND
METAL UPTAKE BY WHEAT, AND ON FREE LIVING
AZOTOBACTER
RANA ATHAR
1
and MASOOD AHMAD
2
∗
1
Institute of Agriculture, Aligarh Muslim University, Aligarh (U.P.) 202002, India;
2
Department of
Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh (U.P.) 202002, India
(
∗
author for correspondence)
(Received 4 July 2000; accepted 1 August 2001)
Abstract. A pot study was conducted to investigate the toxic effects of certain heavy metals on the
plant growth and grain yield of wheat (Triticum aestivum L.). The results revealed that heavy metals
brought about significant reductions in both parameters, Cd being the most toxic metal followed by
Cu, Ni, Zn, Pb and Cr. Moreover, the presence of Cd in the soil resulted in the maximum inhibition
(84.9%) in the number of free living Azotobacter chroococcum cells over the control. The phytotox-
icity was apparently due to the susceptibility of the free living Azotobacter chroococcum cells to the
toxic doses of heavy metals. Protein content decreased from 19.0–71.4% in metal exposed plants at
metal concentrations equivalent to those found in polluted soil. Metal uptake by grains was directly
related to the applied heavy metal with greater concentrations of metals found in cases where metals
were added separately rather than in combinations. The toxic effects on the plant growth, nitrogen
content in plant parts, and protein content in grains, exerted by two metals in combination were not
additive, but rather only as severe as for the most toxic metal alone.
Keywords: heavy metals, heavy metal uptake, nitrogen fixation, phytotoxicity, wheat
1. Introduction
Industrial wastes are a major source of soil pollution and originate from mining in-
dustries, chemical industries, metal processing industries and others. These wastes
include a variety of chemicals like heavy metals, phenolics etc. (Mueller et al.,
1989; Van Assche and Clijsters, 1990). Use of industrial effluent and sewage sludge
on agricultural land has become a common practice in India as a result of which
these toxic metals can be transferred and concentrated into plant tissues from the
soil. These metals have damaging effects on the plants themselves and may be-
come a health hazard to man and animals. Above certain concentrations and over a
narrow range, the heavy metals turn into toxins (Babich and Stotzky, 1980; Babich
et al., 1982). Moreover, these metals adversely affect natural microbial populations
leading to disruption of vital ecological processes (Sterritt and Lester, 1980; Nriagu
and Nieboer, 1988; Brynhildsen and Rosswall, 1997).
Currently, microorganisms are being used as potential bioindicators for the as-
sessment of chemical risk to the ecosystem (Bitton and Dutka, 1986). The effects
of heavy metals on the growth of plants and microorganisms have been investigated
Water, Air, and Soil Pollution 138: 165–180, 2002.
© 2002 Kluwer Academic Publishers. Printed in the Netherlands.

166
R. ATHAR AND M. AHMAD
TABLE I
Physiochemical properties and heavy metal concentration of the test soil
used in this study
Texture
Sandy clay loam
Type
Alluvial
pH
7.7
Cation exchange capacity (CEC) (cmol kg
−1
)
11.7
Water holding capacity
40.6
Organic matter
0.62
Organic carbon
0.36
Available nitrogen (kg ha
−1
)
170
Anion exchange capacity (AEC) (cmol kg
−1
)
5.1
Lead (Pb)
a
30.0
±2.0
b
Zinc (Zn)
96.0
±2.0
Copper(Cu)
18.6
±2.1
Nickel (Ni)
12.9
±2.5
Cadmium (Cd)
N.D.
c
Chromium (Cr)
15.0
±2.0
a
All metal concentrations are in mg kg
−1
.
b
±Standard deviation.
c
N.D. = Not detected.
by several workers (Skujins et al., 1986; Coppola et al., 1988; Lorenz et al., 1992;
Baccouch et al., 1998). Abiotic stresses like heavy metal stress, air pollutants stress
etc negatively affect processes associated with biomass production and grain yield
in almost all major field grown crops (Agarwal et al., 1999). Every metal and plant
interact in a specific way, which depends on several factors such as type of soil,
growth conditions and the presence of other ions.
The objective of this study was to examine the toxic effect of heavy metals on
a free living nitrogen fixer viz. Azotobacter sp. as well as on the growth of wheat
plants to gain an insight on the loss of agricultural productivity of a very important
cereal crop.
2. Materials and Methods
The soil in which the experiments have been conducted was a sandy clay loam
and had received no exogenous input of metals. The physiochemical properties
and the heavy metal concentrations of the soil are given in Table I. The soil was
sieved (<2 mm) and homogenized and the test heavy metals were added as the
solutions of their chloride salts. The amounts of heavy metals added were equiv-
alent to normal, half and double the concentrations found in the polluted soil in

HEAVY METAL TOXICITY
167
TABLE II
Amounts of heavy metals added to the soil at various
dose levels
Heavy metal
Concentration of heavy metals
(mg kg
−1
) at
0.5
×
1
×
2
×
Pb
97.09
194.18
388.36
(100.6)
a
Zn
2558.7
5117.4
10234.8
(1158.0)
Cu
367.7
735.5
1471.0
(107.2)
Ni
150.9
301.9
603.8
(112.6)
Cd
6.2
12.4
24.8
(5.0)
Cr
37.23
74.46
148.92
(20.1)
a
The values in parentheses indicate the concentrations
of bio-available forms of heavy metals.
Aligarh city receiving industrial effluents of lock manufacturing and plating indus-
tries for over a decade and are given in Table II (R. Athar and M. Ahmad, personal
communication).
2.1. P
OT EXPERIMENTS
A pot experiment was conducted with wheat, Triticum aestivum L. var. PDW 154,
as test crop. Three and a half kilograms of soil were taken in each pot for the
different treatments and three replicates were taken for each treatment. An extra set
of pots which contained no added heavy metals were also taken which served as a
control. The heavy metals Zn, Pb, Ni, Cd, Cr and Cu singly and in combinations
were added once as chloride salts in solution to the soil before sowing. Sufficient
water was added to bring the soil to 50% of its water holding capacity. The soil was
preincubated for 2 weeks before sowing, and was also fertilized with 120:60:50 kg
ha
−1
of nitrogen, phosphorus and potassium (NPK). Wheat seeds obtained from the
Division of Genetics, the Indian Agricultural Research Institute (IARI), New Delhi
were surface sterilized in 0.1% mercuric chloride solution and washed with six
changes of sterile distilled water. The carrier based strain inoculant of Azotobacter
chroococcum (1.5
× 10
10
cells g
−1
) obtained from the Indian Agricultural Research
Institute (IARI), New Delhi was used to treat the seeds of wheat before sowing at

168
R. ATHAR AND M. AHMAD
the rate of 1 g per 5 g of seeds approx. (Vincent, 1970). Four seeds of wheat were
sown in each pot. Seedlings were thinned to two plants per pot, and plants were
watered as and when required. The pots were randomised on alternate weeks to
minimize any positional effects.
2.2. B
IOMASS PRODUCTION
The test plants for biomass production were harvested after 90 days of germination.
Roots and shoots were dried at 80
◦
C for 18 hr and then weighed separately. The
grade of growth inhibition (GGI) was evaluated by the comparison of dry matter
production of metal treated and control plant tissues (Purves, 1985).
2.3. G
RAIN YIELD
Grain yield was also recorded at the harvest of the crop.
2.4. E
STIMATION OF AVAILABLE SOIL NITROGEN
Available soil nitrogen of the treated and control soil was estimated by the Kjeldahl
method using alkaline permanganate (Subbiah and Asija, 1956; Ghosh et al., 1983)
after about four weeks of germination of seeds. The procedure involves distilling
the soil with alkaline potassium permanganate solution and determination of the
ammonia liberated which serves as an index of the available nitrogen status.
2.5. Azotobacter
COUNT IN METAL TREATED AND UNTREATED SOIL
The soil dilution and plate count method of Timonin (1940) was used for count-
ing the Azotobacter population in the rhizosphere region of wheat plants after
about 90 days of sowing of seeds. The wheat plants were carefully uprooted and
brought to the laboratory under aseptic conditions. The roots were removed and
were transferred along with adhering soil particles into a flask having sterilized
distilled water. After thorough shaking of the flask the roots were removed and the
rhizosphere soil sample was serially diluted and a 0.1 mL aliquot from the final
dilution was poured in sterilized petri plates containing 20–25 mL of nitrogen free
Jensen’s agar medium and was spread with a sterilized glass spreader. The plates
were incubated at 28
±2
◦
C for 3–4 days, and the resulting colonies were identified
and scored.
2.6. E
STIMATION OF NITROGEN IN PLANT PARTS
At harvest, the shoots and roots were dried at 80
◦
C for 18 hr, weighed and ground
to pass through a 2 mm pore size stainless steel sieve and the nitrogen in roots and
shoots was determined by the Kjeldahl method (Bremner, 1965).

HEAVY METAL TOXICITY
169
2.7. E
STIMATION OF PROTEIN CONTENT IN GRAINS
At harvest, grains were dried, weighed and ground. The protein content was then
determined by the Kjeldahl method (Bremner, 1965).
2.8. H
EAVY METAL UPTAKE BY GRAINS
The heavy metal concentrations of the most toxic (Cd) and the least toxic (Cr)
metals found in this study in the grains of wheat were determined in hot concen-
trated HNO
3
digests of the ground grain samples. Heavy metals were determined
by Atomic Absorption Spectrophotometer (AAS) model Unichem FP 1900 series.
2.9. S
TATISTICAL ANALYSIS
The results were analysed statistically by analysis of variance and critical differ-
ence (CD) at 5% level according to standard procedures (Chaddha, 1990).
3. Results
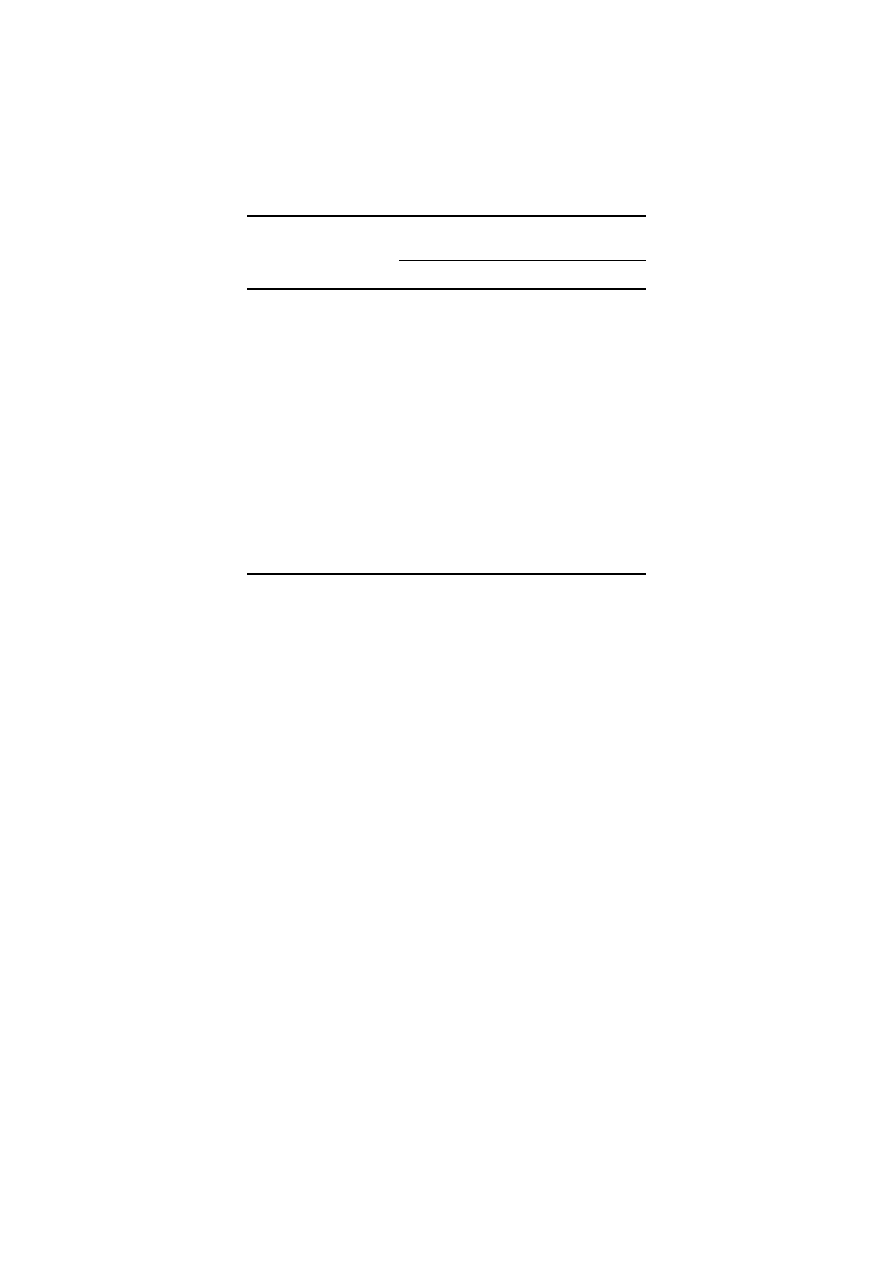
Toxicity of heavy metals on the growth of wheat is presented in Table III. These
data indicate that the heavy metals were toxic to the growth of wheat plants. Shoots
of plants had noticeable and gradual stunted growth. These symptoms were more
obvious in treatments containing Cd alone and a combination of all the heavy
metals (Table III). The reduction in dry weight of wheat plants as a result of
treatment with heavy metals was minimum with Cr and Pb. Phytotoxic effect of
heavy metals was in the following order:
Cd > Cu > Ni > Zn > Pb > Cr
The higher the concentration of heavy metal in the soil, the greater was the toxic
effect on the plant. The results in Table III show that the effects of combinations
of two metals were not additive, rather the effects were only as severe as the most
toxic metal alone.
The lowest reduction in the grain yield was recorded with Cr at all the test doses
and the highest was recorded in the plants having been treated with all the test
metals (Table III). Decrease in the grain yield was less than 40% with Cr as against
83.9% by Cd at 2
× concentration. Perusal of the data in Table III clearly indicates
that though Cr appeared to be the least toxic metal, it also led to substantial losses
(>40%) in the dry matter at 2
× concentration. Results in Table III show that the
dry weights of shoot and root of wheat plant, respectively, were reduced 63.4 and
70.5% by Cd; 58.5 and 55.8% by Cu; 51.2 and 46.1% by Ni: 26.3 and 29.1% by Pb;
31.7 and 39.7% by Zn; 17.0 and 13.8% by Cr at 0.5
× concentration. Zn appears to
be less toxic than Cu and Ni despite the relatively higher amount used in the study
(Table II).
170
R. ATHAR AND M. AHMAD
T
A
BLE
III
D
ry
m
at
te
r
and
gr
ai
n
y
ie
ld
of
w
h
eat
pl
ant
exposed
to
v
ar
ious
concent
rat
ions
of
hea
v
y
m
et
al
s
added
ei
th
er
separ
at
el
y
or
in
co
mb
in
atio
n
H
ea
v
y
m
et
al
D
ry
shoot
w
ei
ght
(g
/pot
)
at
D
ry
root
w
ei
ght
(g
/pot
)
at
G
ra
in
w
ei
ght
(g
/pot
)
at
tr
eat
ment
0.
5
×
1
×
2
×
0.
5
×
1
×
2
×
0.
5
×
1
×
2
×
P
b
3.
02
2.
50
1.
80
2.
41
1.
81
1.
30
4.
02
3.
75
3.
03
Z
n
2.
80
2.
10
1.
73
2.
05
1.
71
0.
91
3.
64
3.
16
2.
51
C
u
1.
70
1.
30
1.
00
1.
50
1.
10
0.
68
2.
28
1.
54
1.
05
C
r
3.
40
2.
70
2.
21
2.
93
2.
05
1.
78
4.
71
4.
09
3.
53
N
i
2.
00
1.
70
1.
50
1.
83
1.
60
0.
82
2.
83
2.
34
2.
05
C
d
1.
50
1.
10
0.
90
1.
00
0.
80
0.
63
1.
87
1.
30
0.
85
Ni+
C
d
1
.5
0
1
.1
0
0
.8
6
1
.0
5
0
.8
5
0
.4
9
1
.7
8
0
.9
4
0
.5
5
Ni+
C
r
2
.6
0
2
.0
0
1
.7
5
2
.0
5
1
.4
5
1
.0
8
2
.7
1
2
.3
0
1
.7
7
Cr+
C
d
1
.7
4
1
.4
0
1
.0
1
1
.2
3
0
.9
1
0
.8
5
2
.0
5
1
.7
4
1
.1
6
N
i+C
r+
C
d
1.
05
0.
84
0.
63
0.
83
0.
45
0.
35
1.
06
0.
77
0.
40
Ni+
C
r+
Cd
+
C
u
+
Zn
+
P
b
0
.8
0
0
.7
5
0
.4
3
0
.4
0
0
.3
1
0
.1
9
0
.7
0
0
.3
6
0
.2
9
C
ont
ro
l
–
4.
10
–
–
3.
40
–
–
5.
30
–
S
tat
is
ti
cal
anal
ysi
s
S
ig.
a
Sig
.
Sig
.
Sig
.
Sig
.
Sig
.
Sig
.
Sig
.
Sig
.
(F
test)
CD
b
at
5%
0.
78
0.
71
0.
69
0.
65
0.
65
0.
60
1.
40
1.
40
0.
72
V
al
u
es
are
m
ean
of
th
ree
repl
icat
es.
a
S
ig.
=
S
igni
fi
cant
ly
di
ff
er
ent
o
v
er
cont
ro
l.
b
CD
=
C
ri
ti
cal
di
ff
erence.
HEAVY METAL TOXICITY
171
TABLE IV
Available soil nitrogen (mg kg
−1
) in the rhizosphere soil of wheat under
different metal treatments
Heavy metal
Available soil nitrogen at various
treatments
concentrations of heavy metal treatment
0.5
×
1
×
2
×
Pb
95.0
±5.0
a
92.6
±2.1
89.3
±5.3
Zn
94.4
±5.1
90.2
±1.3
91.0
±2.6
Cu
91.6
±3.6
88.7
±6.2
86.2
±3.7
Cr
96.0
±4.0
94.2
±2.0
92.5
±4.3
Ni
92.2
±1.6
89.3
±5.3
86.5
±3.2
Cd
88.6
±6.3
83.4
±3.0
80.7
±3.0
Cr+Cd
86.5
±3.7
82.1
±1.0
81.0
±2.0
Ni+Cr
90.2
±1.3
88.4
±6.2
80.1
±2.2
Ni+Cd
85.4
±1.7
81.1
±2.1
79.1
±1.8
Ni+Cr+Cd
84.3
±2.0
80.0
±2.0
77.0
±3.0
Ni+Cr+Cd+Cu+Pb+Zn
82.3
±2.5
79.6
±2.5
72.8
±1.0
Control
–
114.0
±6.0
–
Statistical analysis
N.S.
b
N.S.
N.S.
(F test)
Values are mean of three replicates.
a
±Standard deviation.
b
N.S. = Not significant.
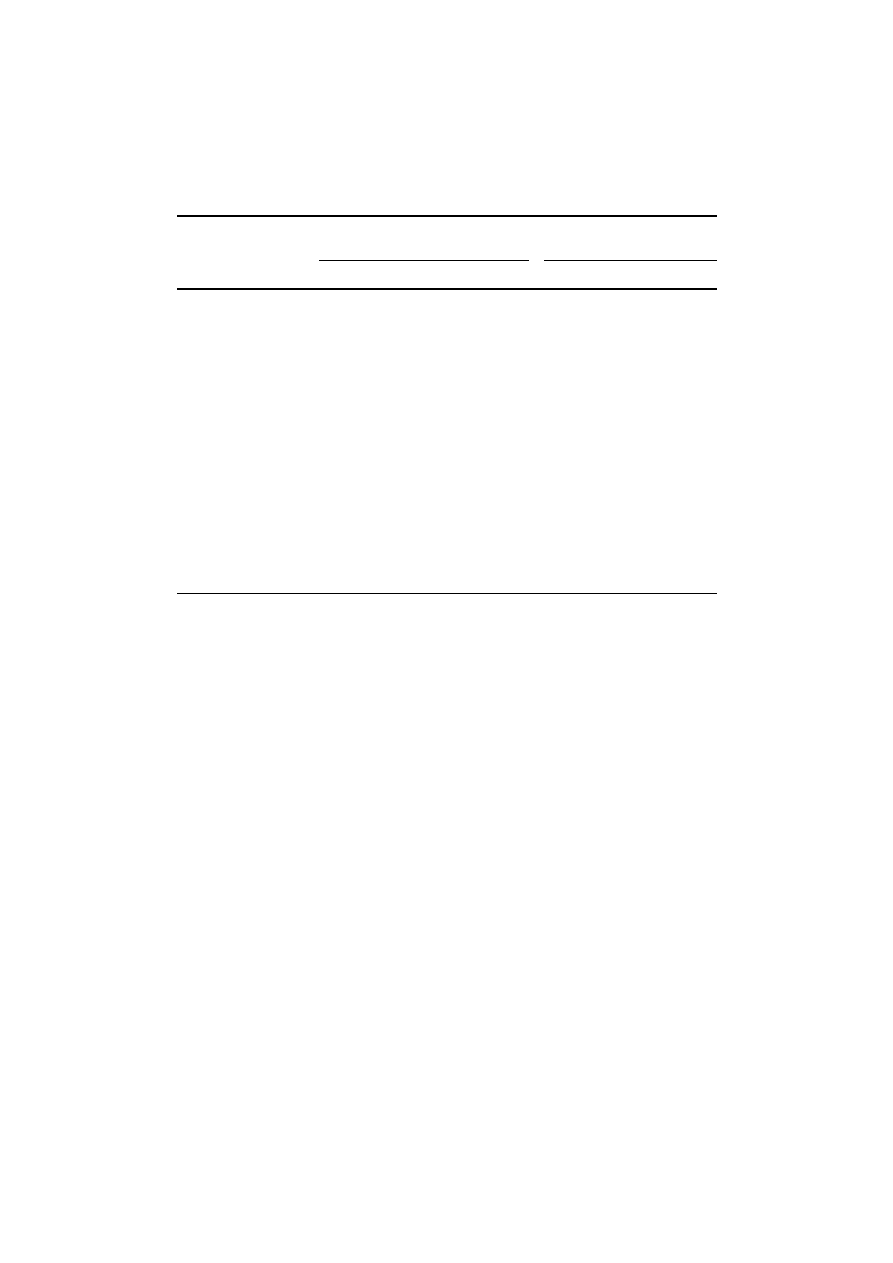
Results in Table IV show that the reduction in nitrogen content in the soil
with metal treatments at all the three concentrations was found to be statistically
insignificant.
There was hundred percent inhibition of Azotobacter population with the treat-
ment having the combination of six metals at all the test doses, though some
cells were present in the soil treated with the combination of three metals i.e. Ni
+ Cr + Cd. (Table V). A marginal toxicity towards the Azotobacter population
was observed with Cr while the highest bactericidal effect was obtained in case
of Cd. Even at concentrations equal to half the usual concentrations detected in
contaminated soil (i.e. 0.5
×), the metals Cd, Cu and Ni significantly inhibited the
Azotobacter population. Although Cr did not exhibit the microbiocidal behaviour
at the levels it was maximally present in the polluted soil, it could bring about
pronounced effect in the Azotobacter count (TAC) at 2
× concentration (Table V).
The order of toxicity of various heavy metals was as under:
Cd > Cu > Ni > Zn > Pb > Cr
172
R. ATHAR AND M. AHMAD
TABLE V
Total number of Azotobacter chroococcum cells in the rhizosphere of wheat expressed in terms of
standard deviation and mean log values
Heavy metal
TAC (
× 10
2
CFU g
−1
) at the
TAC (log
10
CFU g
−1
) at the
treatment
metal concentrations of
metal concentrations of
0.5
×
1
×
2
×
0.5
× 1 ×
2
×
Pb
40.9
±6.6
a
28.4
±3.8 20.7±5.2
3.54
3.40
3.16
Zn
39.0
±8.8
22.2
±3.9 15.3±3.5
3.53
3.30
3.16
Ni
36.4
±3.8
19.9
±2.8 13.8±4.7
3.40
3.20
3.10
Cu
26.7
±5.3
16.3
±2.0 12.3±1.6
3.30
3.16
3.04
Cd
21.3
±5.9
15.2
±3.3
9.2
±2.4
3.26
3.13
2.90
Cr
44.0
±5.3
35.6
±3.7 28.4±3.3
3.56
3.43
3.36
Ni+Cr
35.0
±6.9
19.4
±6.8 11.3±1.7
3.50
3.20
3.00
Cr+Cd
20.4
±3.3
11.0
±4.1
7.0
±3.1
3.20
3.00
2.76
Ni+Cd
11.1
±1.8
8.2
±2.1
3.8
±1.7
3.00
2.83
2.50
Ni+Cr+Cd
8.5
±2.6
5.5
±2.0
2.8
±0.9
2.70
2.66
2.36
Ni+Cr+Cd+Zn+Pb+Cu
N.D.
b
N.D.
N.D.
N.D.
N.D.
N.D.
Control
–
61.0
±7.6
–
–
3.70
Statistical analysis
Sig.
c
Sig.
Sig.
Sig.
Sig.
Sig.
(F test)
CD
d
at 5%
21.4
18.7
16.4
0.29
0.23
0.23
Values are mean of three replicates.
a
± Standard deviation.
b
N.D. = Not detected.
c
Sig. = Significantly different over control.
d
CD = Critical difference.
The nitrogen content in shoots and roots decreased in metal treated plants. Data in
Table VI indicates that there was a significant reduction in nitrogen (%) in shoots
and roots of wheat plants compared with the control. In both the shoots and roots,
the percent of nitrogen varied inversely with the amounts of metals added, Cd
and Cu causing the greatest effect. The percent of nitrogen in shoot and root was
reduced by 68.6 and 79.4% in the presence of Cd at 2
× concentration.
Data in Table VII indicates that heavy metal treatment under different con-
centrations resulted in decreased protein content in grains with Cr recording the
highest protein content in grains. Protein content was significantly lower in metal
treated grains compared with the control.
The metal (Cd and Cr) uptake pattern by wheat grains is given in Table VIII.
It was observed that as the concentration of Cd and Cr in the grains increased,
the yield was reduced and the metal accumulation in grains was also found to be
directly related to that applied to the soil. The concentrations of individual metals
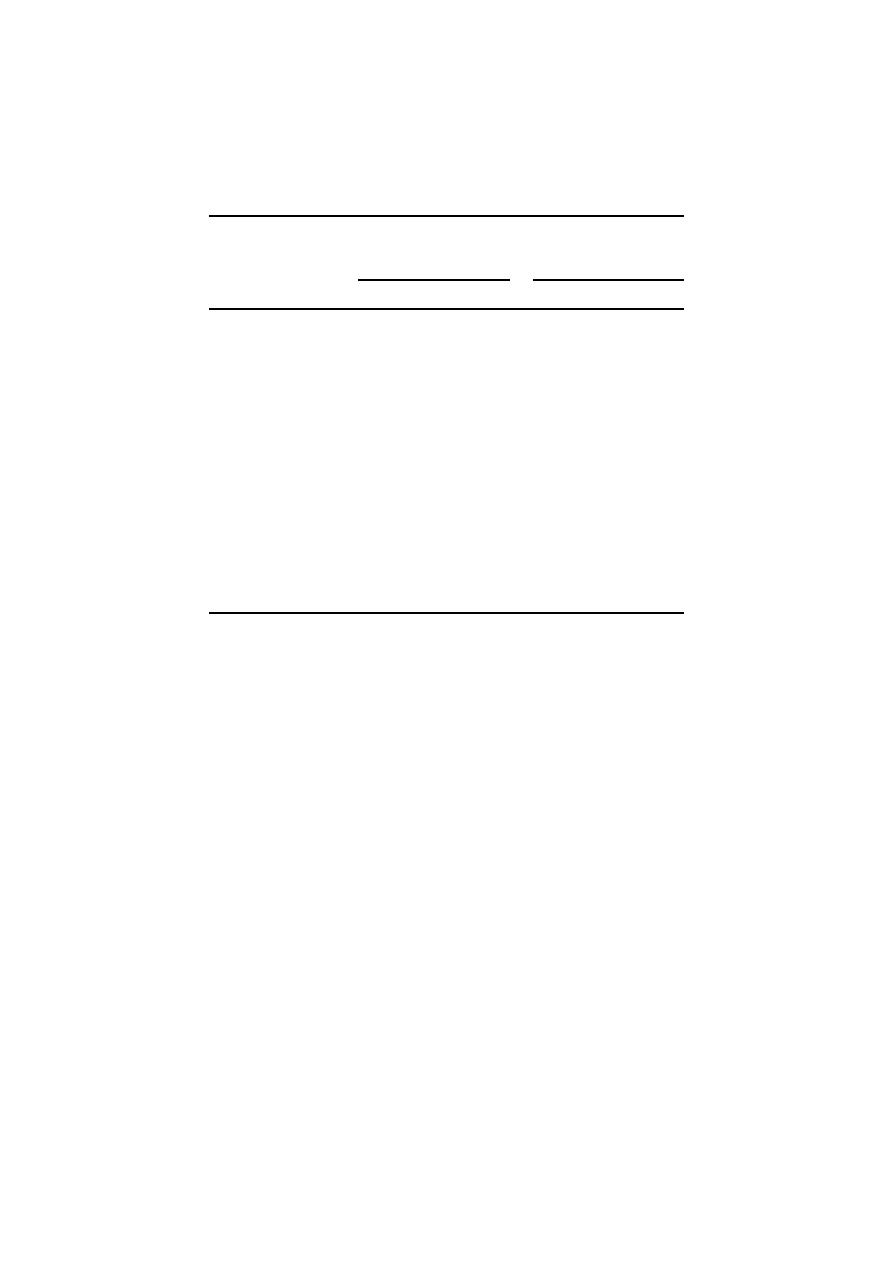
HEAVY METAL TOXICITY
173
TABLE VI
Percent nitrogen content in shoots and roots of wheat plant as influenced by different
heavy metal treatments
Heavy metal
% N in shoot at various
% N in root at various
treatment
concentrations of metal
concentrations of metal
treatments
treatments
0.5
×
1
×
2
×
0.5
×
1
×
2
×
Pb
1.84
1.64
1.42
1.50
1.10
0.62
Zn
1.52
1.32
1.20
1.30
0.66
0.50
Ni
1.42
1.20
0.94
0.97
0.53
0.46
Cu
1.34
1.01
0.90
0.90
0.62
0.40
Cd
1.22
0.90
0.72
0.86
0.53
0.35
Cr
2.00
1.86
1.70
1.60
1.40
1.00
Ni+Cr
2.00
1.70
1.51
1.10
0.91
0.82
Cr+Cd
1.33
1.00
0.92
0.96
0.73
0.60
Ni+Cd
1.40
1.20
0.88
0.44
0.18
0.15
Ni+Cr+Cd
1.00
1.10
0.70
0.42
0.35
0.16
Ni+Cr+Cd+Pb+Zn+Cu
0.72
0.53
0.33
0.28
0.20
0.11
Control
–
2.30
–
–
1.70
–
Statistical analysis
Sig.
a
Sig.
Sig.
Sig.
Sig.
Sig.
(F test)
CD
b
at 5%
0.85
0.63
0.50
0.50
0.37
0.29
Values are mean of three replicates.
a
Sig. = Significantly different over control.
b
CD = Critical difference.
in grains were usually found to be greater for metals added separately than in
combinations of all metals in the pot.
4. Discussion
Treatment of wheat plant with heavy metals resulted in decreased dry matter and
grain yield, reduced nitrogen content in plant tissues and lowered protein content
in grains.
The data in Table III indicates that heavy metals exerted an adverse effect on the
growth and yield of wheat plants substantiating the reported phytotoxicity of these
metal ions (Woolhouse, 1983). The effects on plants of environmental stresses
are determined by the responses of the individual cells in which the integrity of
structure and function is affected (Ciamporova and Mistrik, 1993). In the present
investigation, Cr was found to be the least but significantly phytotoxic metal as
174
R. ATHAR AND M. AHMAD
TABLE VII
Protein content in wheat grains as influenced by different heavy
metal treatments
Heavy metal
Protein (%) in wheat grains at
treatments
0.5
×
1
×
2
×
Pb
18.0
15.6
13.1
Zn
16.2
12.5
10.0
Ni
14.5
11.5
8.0
Cu
13.0
11.2
9.5
Cd
10.7
8.1
6.2
Cr
19.0
17.0
15.0
Ni+Cr
15.6
12.5
10.0
Cr+Cd
10.0
8.7
7.0
Ni+Cd
9.3
7.2
5.6
Ni+Cr+Cd
9.5
8.0
6.2
Ni+Cr+Cd+Zn+Pb+Cu
7.2
6.0
4.0
Control
–
21.0
–
Statistical analysis (F test)
Sig.
a
Sig.
Sig.
CD
b
at 5%
8.3
7.4
6.5
Values are mean of three replicates.
a
Sig. = Significantly different over control.
b
CD = Critical difference.
compared to other metals added separately in the soil. Low levels of phytotoxicity
of Cr(III) have been attributed to its insolubility under most soil conditions (James
and Bartlet, 1984), and it did not affect the plant growth unless the concentrations
were very large (Smith et al., 1992).
Previous investigations indicated that Cr
3
+
added in sand culture of wheat under
glasshouse conditions brought about significant reduction in biomass, chlorophyll
and activities of catalase and peroxidase while it enhanced the acid phosphatase
and ribonuclease activities (Sharma and Sharma, 1996). Cd at all levels tested was
found to be the most toxic metal for the free living nitrogen fixer i.e. Azotobacter
chroococcum as well as for the wheat crop and caused the most severe reduction
in the dry weight of shoot, root and grain yield followed in order by Cu, Ni and
Zn. Previous studies have also demonstrated a relatively higher phytotoxicity of
Cd and Cu than that of Zn (Kalyanaraman and Sivagurunathan, 1993). In general,
the reduction in the dry weight of roots was more severe than the dry weight of
shoots following treatment with heavy metals added separately or in combination
(Table III). This is supported by the findings of Karataglis et al. (1991) who re-
ported that the influence of relatively higher amounts of Cu, Zn, Pb, Ni, Cr and
Cd in wheat cv. Vergina resulted in depressed shoot growth but the most evident

HEAVY METAL TOXICITY
175
T
A
BLE
V
III
Cd
and
C
r
cont
ent
s
(mg
k
g
−
1
)
in
w
heat
gr
ai
ns
as
in
fl
u
enced
by
met
al
amendment
s
added
si
ngl
y
o
r
in
combi
nat
io
n
Hea
v
y
m
et
al
Cd
concent
rat
io
n
at
C
r
concent
rat
io
n
at
tr
eat
ment
0.
5
×
1
×
2
×
0.
5
×
1
×
2
×
C
d
0.
22
±
0.
03
a
0.
42
±
0.
07
0.
70
±
0.
17
–
–
–
Cr
–
–
–
0
.9
3±
0.
12
3.
70
±
0.
20
10.
0±
0.
1
Ni+Cr+Cd+Z
n+P
b
+Cu
0
.1
1±
0.
01
0.
30
±
0.
08
0.
51
±
0.
18
0.
72
±
0.
40
0.
64
±
0.
14
9.
6±
3.
0
Co
n
tro
l
–
N.D.
b
–
–
0.
11
±
0.
1
–
V
al
u
es
are
m
ean
of
th
ree
repl
icat
es.
a
±
S
tandar
d
de
vi
at
ion.
b
N.
S
.
=
N
ot
det
ect
ed.

176
R. ATHAR AND M. AHMAD
symptoms were on roots. Baccouch et al. (1998) showed that the accumulation of
carbohydrates in maize shoots treated with Ni might, at least in part be the cause
of root growth inhibition.
Amendment of soil with the heavy metals at concentrations higher than the
normal levels resulted in a striking decrease of root and shoot biomass expressed in
terms of dry weight (Table III). It has earlier been reported that increasing Cu sup-
ply resulted in decreased root biomass indicating the alterations of physiology and
metabolism of test plants (Ouzounidou et al., 1995). Biomass loss (fresh weight)
under metal treatment has also been reported by many workers (Lolkema et al.,
1984; Verkleij and Prast, 1989).
When two heavy metals were added in combinations, instead of an additive
effect on the phytotoxicity, the effect was only as severe as for the most toxic metal
alone. This might be due to the antagonistic effect of the two metals. Cd is reported
to antagonize the inhibitory effect of Zn on the total amount of mineralised carbon
(Bewley and Stotzky, 1983).
The use of non-symbiotic nitrogen fixer, Azotobacter sp. as a bioinoculant is
known to benefit a wide variety of crops, due to its properties like nitrogen fixation,
secretion of growth promoting substances, vitamins, anti-fungal metabolites and
phosphate solubilization (Mishustin and Shilkinova, 1971; Brown, 1972; Martinez
Toledo et al., 1988).
Nitrogen fixing capacity of biological nitrogen fixing organisms (BNFs) has
been found to be sensitive to small concentrations of heavy metals added exper-
imentally or in connection with mining (Letunova et al., 1985; Skujins et al.,
1986).
Table V shows that there was a significant depletion in the number of Azoto-
bacter cells with complete absence of Azotobacter population in the rhizospheric
soil containing all the test metals. This is due to their high sensitivity to heavy
metals (Maliszewska et al., 1985). Azotobacter sp. were found to be sensitive to the
heavy metals present in the sludge following its application to soil (Martensson and
Torstensson, 1996). Previous studies have also shown that as the pH was increased
from acidic (e.g. pH 5) to alkaline (pH 8 and 9) value, the toxicity of Cd to bacteria
and fungi was increased, suggesting that Cd OH
+
, which was formed at these
alkaline pH levels, was more toxic than was divalent Cd
2
+
(Babich and Stotzky,
1977a, b). A significant decrease in the grain protein content was observed with
heavy metal treatment in this study (Table VII). This is in accordance with the
findings of Salgare and Acharekar (1992) who reported that growth performance,
as well as pigment, carbohydrate and protein content showed a decreasing trend
with increase in the level of industrial pollution. Decreased levels of protein content
in heavy metal exposed tissues have been reported by many workers (Gupta, 1986;
Satyakala and Jamil, 1997). Relatively strong affinities of heavy metal ions for side
chain ligands of protein indicate that enzyme and other functional proteins are one
of the primary targets of metal toxicity (Hampp et al., 1976).

HEAVY METAL TOXICITY
177
Our results on the metal uptake by wheat grains are given in Table VIII. The
uptake of metals in the grains was greater at least in the case of 1
× concentration
when the metals were added separately than in combination. This is in accordance
with the findings of Smilde (1981) who demonstrated that the total amounts of
metals in plant tissues were higher for metals added separately than for combined
metals. It is an established fact that the soils and plants under waste water irrigation
from various industries contained higher concentrations of heavy metals than those
irrigated with tubewell water (Bansal, 1998). Moreover, the heavy metals deposited
in soil were bound preferentially to interaggregate soil material, and accumulation
preferentially occurred in parts of the soil where plant roots were concentrated and
in the forms easily accessible for plants (Wilcke et al., 1998). Cd is of particular
concern to the human health as it is concentrated by many cereal and vegetables
(leafy and roots) as well as fruits (Wagner, 1993; Cieslinski et al., 1996) which can
lead to unexpected human intoxication when it is consumed (Jarup et al., 1998).
Albering et al. (1999) showed that the legal standard for Cd as endorsed by com-
modities act was exceeded in wheat crops grown in soil contaminated with heavy
metals and the main exposure pathways for the general population was through the
consumption of food crops grown in these soils.
The different heavy metals used in this study were found to vary in their phyto-
toxic effects with Cd being the most toxic and Cr the least toxic. Most of the
desirable soil microbiological activities of Azotobacter and nodule forming bac-
teria of legumes are adversely affected as the acidity increases. Therefore, the
alkaline pH of the test soil in the present system presumably make it easier to
monitor the toxicity of heavy metals alone.
We can conclude that soils contaminated by heavy metals bring about a marked
depletion of non-symbiotic nitrogen fixers and interfering with nitrogen uptake
mechanism in plants which probably leads to substantial losses in dry matter and
grain yield of wheat plant.
References
Agarwal, S. K., Agarwal, M. and Grover, A.: 1999, ‘Emerging trend in agricultural biotechnology
research; use of abiotic stress induced promorter to drive expression of a stress resistance gene in
the transgenic system leads to high level stress tolerance associated with minimal negative effects
on growth’, Curr. Sci. 77, 1577–1579.
Albering, H. J., Van Leusen, S. M., Moonen, E. J., Hoogewerff, J. A. and Kleinjans, J. C.: 1999,
‘Human health risk assessment: A case study involving heavy metal soil contamination after the
flooding of the river Meuse during the winter of 1993–1994’, Environ. Health Perspect. 107,
37–43.
Babich, H. and Stotzky, G.: 1977a, ‘Sensitivity of various bacteria, including actinomycetes and fungi
to cadmium and the influence of pH on sensitivity’, Appl. Environ. Microbiol. 33, 681–695.
Babich, H. and Stotzky, G.: 1977b, ‘Effect of cadmium on fungi and on interactions between fungi
and bacteria in soil: Influence of clay minerals and pH’, Appl. Environ. Microbiol. 33, 1059–1066.

178
R. ATHAR AND M. AHMAD
Babich, H. and Stotzky, G.: 1980, ‘Environmental factors that influence the toxicity of heavy metal
and gaseous pollutants to microorganisms’, CRC Crit. Rev. Microbiol. 8, 99–145.
Babich, H., Schiffenbauer, M. and Stotzky, G.: 1982, ‘Comparative toxicity of trivalent and
hexavalent chromium to fungi’, Bull. Environ. Contam. Toxicol. 28, 193–202.
Baccouch, S., Chaovi, A. and El Ferjani, E.: 1998, ‘Nickel toxicity : effects on growth and
metabolism of maize’, J. Plant Nutr. 21, 577–588.
Bansal, O.P.: 1998, ‘Heavy metal pollution of soils and plants due to sewage irrigation’, Indian J.
Environ. Health 40, 51–57.
Bewley, R. J. F and Stotzky, G.: 1983, ‘Effects of cadmium and zinc on microbial activity in soil;
Influence of clay minerals. Part II: Metal added simultaneously’, Sci. Tot. Environ. 31, 57–69.
Bitton, G. and Dutka, B. J.: 1986, ‘Introducing and Review of Microbial and Toxicity Screening
Procedures’, in B. J. Dutka and G. Bitton (eds), Toxicity Testing using Microorganisms, Vol. I,
CRC Press, Boca Raton, Florida, pp. 1–26.
Bremner, J. M.: 1965, ‘Total Nitrogen’, in C. A. Black (ed.), Methods of Soil Analysis, American
Society of Agronomy Madison, Part 2, pp. 1149–1178.
Brown, M. E.: 1972, ‘Plant growth substances produced by microorganisms of soil and rhizosphere’,
J. Appl. Bacteriol. 35, 443–451.
Brynhildsen, L. and Rosswall, T.: 1997, ‘Effects of metals on the microbial mineralization of organic
acids’, Water, Air, and Soil Pollut. 94, 45–57.
Chaddha, A.: 1990, Agricultural Statistics in India, Commonwealth Publishers, New Delhi, India.
Ciamporova, M. and Mistrik, I.: 1993, ‘The ultra-structural response of root cells to stressful
conditions’, Environ. Exp. Bot. 33, 11–26.
Cieslinski, G., Van Rees, K. C. J., Huang, P. M., Kozak, L. M., Rostad, H. P. W. and Knott, D. R.:
1996, ‘Cadmium uptake and bioaccumulation in selected culitvars of durum wheat and flax as
affected by soil type’, Plant Soil 182, 115–124.
Coppola, S., Dumontet, S., Portonio, M., Basile, G. and Marino P.: 1988, ‘Effect of cadmium bearing
sewage sludge on crop plants and microorganisms in two different soils’, Agric. Ecosyst. Environ.
20, 181–194.
Ghosh, A. B., Bajaj, J. C., Hasan, R. and Singh, D.: 1983, Soil and Water Testing Method, Indian
Agricultural Research Institute, New Delhi.
Gupta, S. L.: 1986, ‘Copper uptake and inhibition of growth, photosynthetic pigments and macro-
molecules in the cyanobacterium Anacystis ridulans’, Phytosynthetica 20, 447–452.
Hampp, R., Beulich, K. and Ziegler, H.: 1976, ‘Effects of zinc and cadmium on photosynthetic CO
2
fixation and hill activity of isolated spinach chloroplasts’, Z. Pflanzer Physioal. 77, 336–344.
James, B. R. and Bartlet, R. J.: 1984, ‘Plant–soil interactions of chromium’, J. Environ. Qual. 13,
67–70.
Jarup, L., Berglund, M., Elinder, C.G., Nordberg, G. and Vahter, M.: 1998, ‘Health effects of cad-
mium exposure – A review of the literature and a risk estimate’, Scand. J. Work Environ. Health
24(1), 1–51.
Kalyanaraman, S. B. and Sivagurunathan, P.: 1993, ‘Effect of cadmium, copper and zinc on the
growth of blackgram’, J. Plant Nutr. 16, 2029–2042.
Karataglis, S., Moustakas, M. and Symeonidis, L.: 1991, ‘Effect of heavy metals on isoperoxidases
of wheat’, Biologia Plantarum 33, 3–9.
Letunova, S. V., Umarov, M. M., Niyazova and Melekhin, Y. I.: 1985, ‘Nitrogen fixation activity as a
possible criterion for determining permissible concentrations of heavy metals in soil’, Soviet Soil
Science 17, 88–92.
Lolkema, P. C., Donker, M. H., Schouten, A. J. and Ernst, W. H. O.: 1984, ‘The possible role of
metallothioneins in copper tolerance of Silence Cucubaius’, Planta 162, 174–179.
Lorenz, S. E., McGrath, S. P. and Giller, K. E.: 1992, ‘Assessment of free living nitrogen fixation
activity as a biological indicator of heavy metal toxicity in soil’, Soil Biol. Biochem. 24, 601–606.

HEAVY METAL TOXICITY
179
Maliszewska, W., Dec, S., Wierzbicka, H. and Wozniakowska, A.: 1985, ‘The influence of vari-
ous heavy metal compounds on the development and activity of soil microorganisms’, Environ.
Pollut. 37, 195–215.
Martensson, A. M. and Torstensson, L.: 1996, ‘Monitoring sewage sludge using heterotrophic
nitrogen fixing microorganisms’, Soil Biol. Biochem. 28, 1621–1630.
Martinez Toledo, M. V., Gonzalez Lopez, J., de la Rubia, T., Mureno, J. and Ramos Cormenzana,
A.: 1988, ‘Grain yield response of zea mays (hybrid AE 703) to Azotobacter chroococcum H23’,
Biol. Fertil. Soils 6, 352–353.
Mishustin, E. N. and Shilkinova, V. K.: 1971, Biological Nitrogen Fixation of Atmospheric Nitrogen,
Macmillan Press Ltd., London.
Mueller, J. G., Chapman, P. J. and Pritchard, P. H.: 1989, ‘Creosote contaminated sites’, Environ. Sci.
Technol. 23, 1197–1201.
Nriagu, J. O. and Nieboer, E.: 1988, Chromium in the Natural and Human Environment, John Wiley
& Sons, New York.
Ouzounidou, G., Ciamporova, M., Moustakas, M. and Karataglis, S.: 1995, ‘Responses of Maize
(Zea mays L.) plants to copper stress I. Growth, mineral content and ultrastructure of roots’,
Environ. Exp. Bot. 35, 167–176.
Purves, D.: 1985, Trace Element Contamination of the Environment, Elsevier Science Publisher,
Amsterdam, The Netherlands.
Salgare, S. A. and Acharekar, C.: 1992, ‘Effect of industrial pollution on growth and content of
certain weeds’, J. Nature Conserve. 4, 1–6.
Satayakala, G. and Jamil, K.: 1997, ‘Studies on the effect of heavy metal pollution on Pistia statiotes
statiotes L., (Water lettuce)’, Indian. J. Environ. Health 39, 1–7.
Sharma, D. C. and Sharma, C. P.: 1996, ‘Chromium uptake and toxicity effects on growth and
metabolic activities in wheat, Tritiicum aestivum L. cv. UP 2003’, Indian J. Exp. Biol. 34,
689–691.
Skujins, J., Nohrstedt, H. O. and Oden, S.: 1986, ‘Development of a sensitive biological method for
the determination of a low level toxic contamination in soils’, Swed. J. Agric. Res. 16, 113–118.
Smilde, K. W.: 1981, ‘Heavy metal accumulation in crops grown on sewage sludge amended with
heavy metals’, Plant Soil 62, 3–14.
Smith, S. R., Sweet, N. R., Davies, G. K. and Hallett, J. E.: 1992, ‘Uptake of chromium and mercury
by crops. Sites with a long history of sludge disposal (phase II EHA 9019)’, Final Report to the
Department of Environment, WRC Medminham, Marlow, pp. 53–73.
Sterritt, R. M. and Lester, J. N.: 1980, ‘Interactions of heavy metals with bacteria’, Sci. Tot. Environ.
14, 5–17.
Subbiah, B. V. and Asija, G. L.: 1956, ‘A rapid procedure for the determination of available nitrogen’,
Curr. Sci. 25, 259–260.
Timonin, M. I.: 1940, ‘The interaction of higher plants and soil microorganisms I. Microbial
population of rhizosphere of seedlings of certain cultivated plants’, Can. J. Res. 18, 307–317.
Van Assche, F. and Clijsters, H.: 1990, ‘Effects of metals on enzyme activity in plants’, Plant Cell
Environ. 13, 195–206.
Verkleij, J. A. C. and Prast, J. E.: 1989, ‘Cadmium tolerance and co-tolerance in Silene vulagris
(Moench.) Gracke [= S. Cucubalus (L) Wibs.]’, New Phytol. 111, 637–645.
Vincent, J. M.: 1970, A Manual for the Practical Study of the Root Nodule Bacteria, International
Biological Programme Handbook, No. 15, Blackwell Scientific Publications, Oxford.
Wagner, G.J.: 1993, ‘Accumulation of cadmium in crop plants and its consequences to human health’,
Adv. Agron. 51, 173–212.
Wilcke, W., Mosbach, J., Kobza, J. and Zech, W.: 1998, ‘Distribution of Al and heavy metals in bulk
soil and aggregates at three sites contaminated by the emissions of a central Slovak Al smelter’,
Water, Air, and Soil Pollut. 106, 389–402.

180
R. ATHAR AND M. AHMAD
Woolhouse, H. M.: 1983, ‘Toxicity and Tolerance in the Responses of Plants to Metals’, in O. L.
Lange, P. S. Nobel, C. B. Osmond and H. Ziegler (eds), Encyclopaedia of Plant Physiology,
Springer Verlag, Berlin, pp. 245–262.
Wyszukiwarka
Podobne podstrony:
Dialectical Behavioral Therapy and BPD Effects on Service Utilisation and Self Reported Symptoms
ties, leaders and time in teams strong interference about network structure effect on teamt vialibil
the effect of sowing date and growth stage on the essential oil composition of three types of parsle
the effect of water deficit stress on the growth yield and composition of essential oils of parsley
EFFECTS OF CAFFEINE ON GROWTH AND METAMORPHOSIS OF MOTH FLY
The effects of plant flavonoids on mammalian cells implication for inflammation, heart disease, and
4 Plant Structure, Growth and Development, before ppt
[30]Dietary flavonoids effects on xenobiotic and carcinogen metabolism
Changes in passive ankle stiffness and its effects on gait function in
33 437 452 Primary Carbides in Spincast HSS for Hot Rolls and Effect on Oxidation
9 Inhibitory effect of AgNPs on microbial growth
48 671 684 Cryogenic Treatment and it's Effect on Tool Steel
Genetic and environmental effects on polyphenols
71 1021 1029 Effect of Electron Beam Treatment on the Structure and the Properties of Hard
4 Plant Structure, Growth and Development, before ppt
[30]Dietary flavonoids effects on xenobiotic and carcinogen metabolism
Changes in passive ankle stiffness and its effects on gait function in
EFFECTS OF EATING AND NOT EATING ON ENERGY STORES AND BODY WEIGHT
więcej podobnych podstron