
Caffeine production in tobacco plants by simultaneous expression of
three coffee N-methyltrasferases and its potential as a pest repellant
Hirotaka Uefuji
1,3
, Yuko Tatsumi
1
, Masayuki Morimoto
1
, Pulla Kaothien-Nakayama
1
,
Shinjiro Ogita
1,2
and Hiroshi Sano
1,
*
1
Research and Education Center for Genetic Information, Nara Institute of Science and Technology, Nara,
630-0192, Japan (*author for correspondence; e-mail sano@gtc.naist.jp);
2
Biotechnology Research Center,
Toyama Prefectural University, Toyama, 939-0398, Japan;
3
Biotechnology Institute University of Minnesota,
St. Paul, MN, 55108, USA
Received 11 March 2005; accepted in revised form 6 June 2005
Key words
: Caffeine, insect repellant, Nicotiana tabacum, Spodoptera litura, theobromine
Abstract
Caffeine (1,3,7-trimethylxanthine) is derived from xanthosine through three successive transfers of
methyl groups and a single ribose removal in coffee plants. The methyl group transfer is catalyzed by
N
-zmethyltransferases,
xanthosine
methyltransferase
(XMT),
7-methylxanthine
methyltransferase
(MXMT) and 3,7-dimethylxanthine methyltransferase (DXMT). We previously cloned three genes
encoding each of these N-methyltransferases from coffee plants, and reconstituted the final sequence of the
caffeine synthetic pathway in vitro. In the present study, we simultaneously expressed these coffee genes in
tobacco plants (Nicotiana tabacum), using a multiple-gene transfer method, and confirmed successful
caffeine production up to 5 lg g
)1
fresh weight in leaves of the resulting transgenic plants. Their effects on
feeding behavior of tobacco cutworms (Spodoptera litura), which damage a wide range of crops, were then
examined. Leaf disc choice test showed that caterpillars selectively fed on the wild-type control materials, or
positively avoided the transgenic materials. The results suggest a novel approach to confer self-defense by
producing caffeine in planta. A second generation of transgenic crops containing caffeine may save labor
and agricultural costs and also mitigate the environmental load of pesticides in future.
Introduction
Caffeine is naturally produced in certain tropical
and subtropical plants, including coffee, tea, mate´,
guarana´ and cola (Ashihara and Crozier, 2001),
and has long been used as an ingredient of
pharmaceuticals (Schmeller and Wink, 1998). In
addition, it was shown to be effective as a repellant
and pesticide for slugs and snails (Hollingsworth
et al
., 2002) and also for insects (Nathanson, 1984;
Mathavan et al., 1985).
Caffeine is synthesized through multiple meth-
ylation
of
xanthine
derivatives
(Figure 1A)
(Ashihara and Crozier, 2001). The first step is
methylation of xanthosine by xanthosine methyl-
transferase (XMT), yielding 7-methylxanthosine
(Figure 1A, step 1), of which ribose moiety is
removed
by
7-methylxanthosine
nucleosidase
(step 2). The resulting 7-methylxanthine is methy-
lated
by
7-methylxanthine
methyltransferase
(MXMT or theobromine synthase) to produce
3,7-dimethylxanthine
(theobromine)
(step
3),
which is further methylated by 3,7-dimethyl-
xanthine methyltransferase (DXMT or caffeine
synthase) to give caffeine (1,3,7-trimethylxan-
thine) (step 4).
Plant Molecular Biology (2005) 59:221–227
Springer 2005
DOI 10.1007/s11103-005-8520-x
The genes encoding these three N-methyl-
transferases were cloned, and designated as Ca-
XMT1
(accession number AB048793), CaMXMT1
(AB048794), CaMXMT2 (AB084126) and CaD-
XMT1
(AB084125) (Ogawa et al., 2001; Uefuji
et al
., 2003). A combination of these three bacte-
rially expressed proteins (CaXMT1, CaMXMT2
and CaDXMT1) could successfully convert xan-
thosine into caffeine in vitro (Uefuji et al., 2003). It
was thus suggested that caffeine is also synthesized
in planta
via this pathway. Based on these obser-
vations, we
previously proposed that,
since
caffeine inhibits pest feeding (Nathanson, 1984;
Mathavan
et al
.,
1985;
Usher
et al
.,
1988;
Hollingsworth et al., 2002), transgenic plants pro-
ducing caffeine would be expected to be resistant
to herbivorous insects. In order to realize this idea,
we now have focused on construction of transgenic
plants producing sufficient caffeine to repel
herbivores. To this end, we first established a
system
for
multiple
transfer
of
the
three
coffee
N
-methyltransferase
genes,
CaXMT1
,
CaMXMT1/2
and CaDXMT1. We describe here
a successful caffeine production in leaves of the
Figure 1.
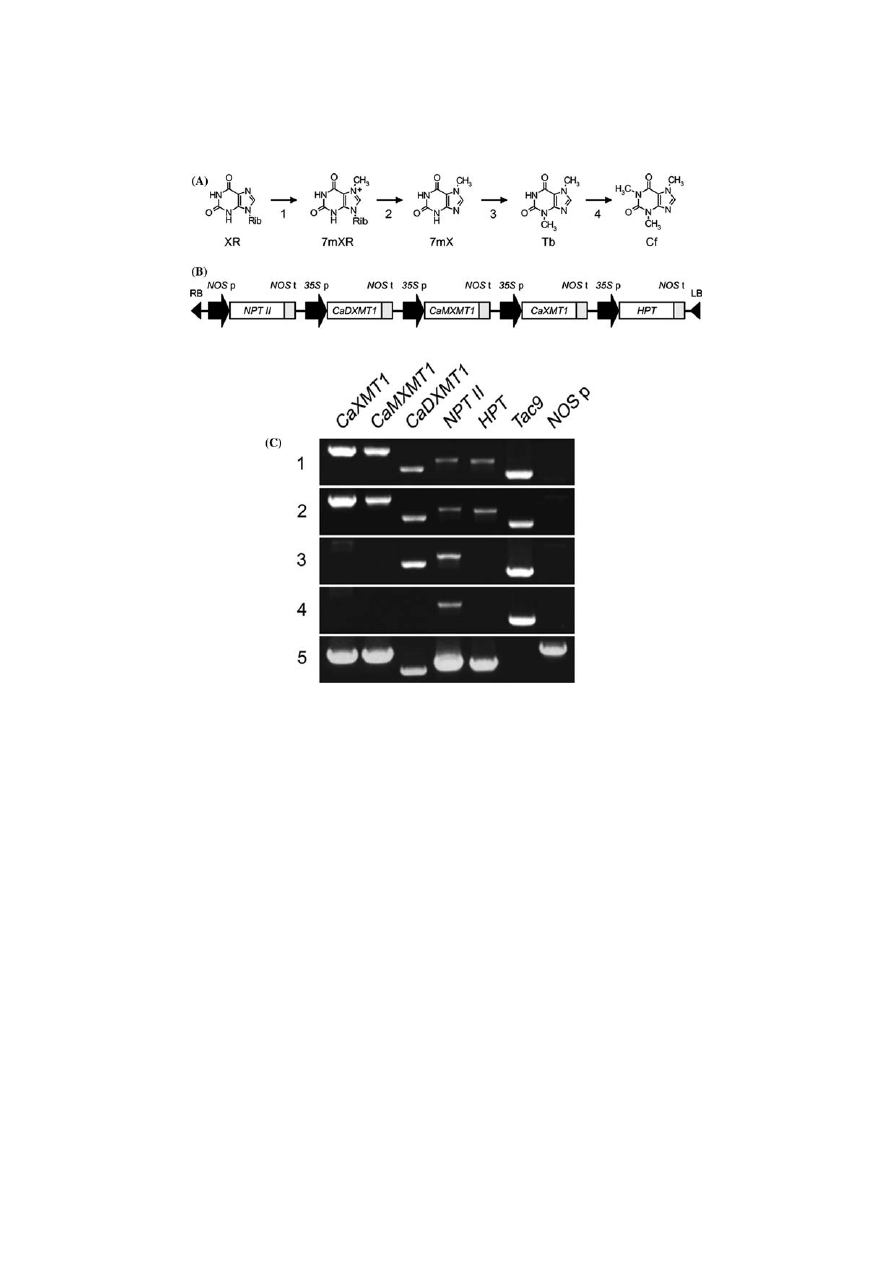
Introduction of the caffeine biosynthetic pathway into tobacco plants. (A) Final sequence of the caffeine biosynthetic
pathway in coffee plants. The first (1), third (3), and fourth (4) steps feature methyl group transfer, and the second (2) step is a
ribose (Rib) removal. XR, xanthosine; 7mXR, 7-methylxanthosine; 7mX, 7-methylxanthine; Tb, theobromine; Cf, caffeine. (B)
Schematic illustration of the T-DNA region of pBIN-NMT777 used in tobacco transformation. LB, left border; RB, right border;
p, promoter; t, terminator; NOS, nopaline synthase; 35S, CaMV 35S RNA; NPT II, neomycin phosphotransferase; HPT, hygro-
mycin phosphotransferase. Gene products of CaXMT1, CaMXMT1 and CaDXMT1 catalyze the first, third, and fourth steps of
the caffeine biosynthetic pathway, respectively. (C) RT-PCR analysis of transgene expression. Total RNA was prepared from
leaves of 23 independent transgenic plants (T
0
) and analyzed for transcripts of the indicated genes by RT-PCR. Four representa-
tive results are shown (columns 1–4). The vector pBIN-NMT777 was used for the control (column 5). Tobacco actin (Tac9) and
NOS
promoter (NOS p) sequences were tested as an endogenous gene and for the untranscriptional T-DNA region, respectively.
222

resulting transgenic plants, which proved unpalat-
able to tobacco cutworms (Spodoptera litura).
Materials and methods
Tranformation vectors
In order to construct a plasmid containing multi-
ple genes, each gene was initially independently
introduced into pBI221 (Clontech) by replacing
the GUS coding sequence with coffee cDNA
clones, CaMXMT1 (Ogawa et al., 2001), CaXM-
T1
(Uefuji et al., 2003) and CaDXMT1 (Uefuji
et al
., 2003). Each expression cassette containing
the CaMV 35S promoter, cDNA clone and NOS
terminator of pBI221 was removed and succes-
sively inserted into the multiple cloning site of
pBluescript II SK (-) (Stratagene). The three
connected cassettes were finally replaced with the
GUS coding sequence and NOS terminator of
pIG121Hm (Hiei et al., 1994) and designated as
pBIN-NMT777 (Figure 1B).
Transgenic tobacco plants
Leaf discs from tobacco (Nicotiana tabacum cv
Xanthi) plants were transformed with pBIN-
NMT777 via Agrobacterium tumefaciens LBA4404
(Hiei et al., 1994). The pIG121Hm was also used for
transformation to serve as a control. Transformants
were selected on media containing kanamycin
sulfate (50 mg l
)1
) and confirmed for transgene
expression by RT-PCR using leaf total RNA and
appropriate marker genes (see below). Resulting
plantlets were grown in a growth chamber at 28
C
under a 14 h/10 h of light/dark photoperiod at a
photon flux rate of 70 lmol m
)2
s
)1
with a relative
humidity of 60%. The T
0
plants were propagated by
cutting method. Briefly, appropriate young stem
with 2–3 leaves was cut out from the mature T
0
plants, placed in water to allow adventitious roots to
regenerate for 2–3 weeks. The resulting plantlet was
transferred onto soil in a pot, grown under the same
conditions as the original transformants, and used
for further experiments.
RT-PCR
Expression of marker and introduced methylta-
ransferase genes were examined with RT-PCR
(Uefuji et al., 2003). Total RNAs were prepared from
leaves using an RNeasy Plant Mini Kit (Qiagen) and
subjected to RT-PCR with sets of gene specific
primers. Primer sets were 5
¢-GGAGAGGCTA
TTCGGCTATG- 3
¢/5¢- TCAAGAAGGCGATAGA
AAGGC-3
¢ (for NPT II); 5¢-GCGTGACCTATTG
CATCTCC-3
¢/5¢-TTCTACACAGCCATCGGTCC-
3
¢ (for HPT); 5¢-GTGATGGTGTCAGCCACACT-
3
¢/5¢-GCTGAGGGAAGCCAAGATAG-3¢ (for ac
tin gene, Tac9); 5
¢-ATGCTCCACTGACGTTCCAT
-3
¢/5¢- TCAAGAAGGCGATAGAAGGC-3¢ (for
NOS
promoter sequence); 5
¢-ATCAACTGGTTCTC
GCCAAG-3
¢/5¢-CTGCTCTAACGGAAGATGCA-
3
¢
(for
CaXMT1
);
5
¢-TCCTACAATCTGGCT
CTTGC-3
¢/5¢-TGCTTTAATTTGTTCATGGGAT
C-3
¢ (for CaMXMT1) and 5¢-TCATTCTACAA
TCTGTTTCTCATCAG-3
¢/5¢-TATGGAATTCG
GGTTCTCGA-3
¢ (for CaDXMT1). PCR was car-
ried out using an RNA PCR Kit (AMV) Ver.2.1
(Takara) under the condition of a 28-cycle of
denaturation at 94
C for 30 s, annealing at 58 C
for 30 s and extension at 72
C for 1 min. After
fractionation on agarose gel electrophoresis, prod-
ucts were identified by visualization with ethidium
bromide staining.
Quantification of purine alkaloids
Appropriate parts of transgenic plants were har-
vested at the vegetative phase (before bud forma-
tion within 6 weeks after regeneration of cuttings)
or at the reproductive phase (after bud formation
later than 8 weeks after cutting regeneration).
Samples were dried at 80
C for 24 h, and purine
alkaloids (caffeine and theobromine) were extracted
and purified from dried plant parts as described
(Baumann et al., 1995). Suspension of purine alka-
loids in ultra pure water was dried, resolved in
200 ll of 25% methanol, and subjected to HPLC
using a Puresil C18 column (Waters). The column
was developed at a flow-rate of 1 ml min
)1
with the
solvent by multisolvent delivery system (Waters)
and
reaction
products
were
densitometrically
monitored at 270 nm with a tunable absorbance
detector (Waters) (Uefuji et al., 2003).
Leaf disc choice test
The palatability of transgenic tobacco leaves was
assessed with tobacco cutworms (Spodoptera litura
Fabricius). S. litura larvae were reared on an
223
artificial diet (Insecta LFS, Nosan, Corporation,
Yokohama, Japan) in a climate chamber at 27
C,
relative humidity 60% and an 8 h/16 h light/dark
photoperiod. Individual third-instar larva was
confined to Petri dishes containing wet filter paper
and deprived of diet for 3–6 h in the dark. A set of
three leaf discs of 10 mm-diameter was prepared
from transgenic plants producing caffeine due to
the introduced pBIN-NMT777. Another set of leaf
discs was prepared from plants transformed with
the pIG121Hm vector alone to serve as the
control. The two sets were placed in Petri dishes
with the individual larva which was then allowed
to feed for 3 h in the dark. Digital photographs of
the leaf discs were taken at start and end points of
the feeding time. Analyzing the photographs by
Scion Image (Scion), leaf areas consumed were
calculated. Twenty replicate tests were made per
transgenic plant line, and tests where no feeding
was observed were excluded.
Results
Construction of transgenic tobacco
T-DNA of a binary vector, pBIN-NMT777, con-
taining five expression cassettes for the three
N
-methyltransferase genes and two selection mar-
ker genes (Figure 1B) was introduced into tobacco
plants via Agrobacterium-mediated transforma-
tion. After appropriate culture and selection, 23
kanamycin-resistant
transgenic
plantlets
were
obtained, among which 15 were confirmed by
RT-PCR to express all three N-methyltransferase
genes (Figure 1C). The remaining lines expressed
only
one
or
two
of
the
introduced
genes
(Figure 1C). Transcript levels of each gene were
found to be comparable that of endogenous actin
gene (Tac9) in most transgenic lines, suggesting the
introduced genes to be actively transcribed. For
further analysis, we selected eight independent
lines, which expressed all three genes at a high level.
Production of caffeine
The selected lines were grown to maturity, and
accumulation of purine alkaloids in leaves was
examined by HPLC (Figure 2). Initial analysis
using mature leaf samples showed the transgenic
plants to efficiently accumulate caffeine and
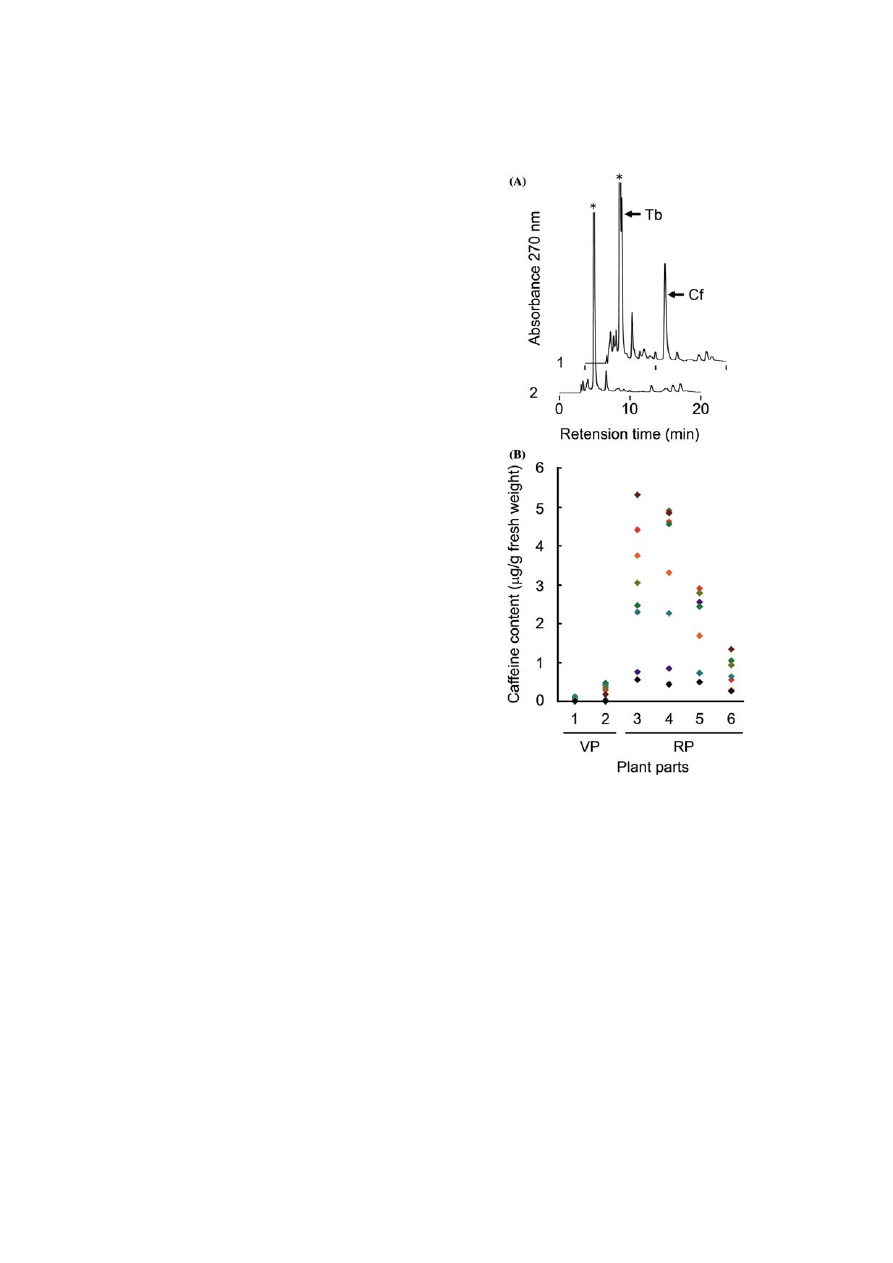
Figure 2.
Production
of
purine
alkaloids
in
transgenic
tobacco plants. (A) HPLC chromatograms of purine alkaloids
accumulated in transgenic plants transfected with pBIN-
NMT777 (1) and pIG121Hm (2). Purine alkaloids were puri-
fied from mature leaves located far from the inflorescence of
transgenic plants in the reproductive phase. Arrows indicate
peak positions of caffeine (Cf) and theobromine (Tb). The
asterisk indicates an impurity. (B) Accumulation pattern of
caffeine in transgenic plants transfected with pBIN-NMT777.
Caffeine was purified from leaves of the transgenic plants in
the vegetative phase (VP) or the reproductive phase (RP).
Samples were immature (1), mature (2), mature near inflores-
cence (3), mature far from inflorescence (4) and senescent (5)
leaves, respectively. Immature fruits (6) were also examined.
Eight independent lines were analyzed, and data from one
individual line are indicated by same color diamonds.
224
theobromine, in contrast to control plants con-
taining an empty vector (Figure 2A). Subsequently
caffeine content was determined in individual
leaves
at
different
developmental
stages
(Figure 2B). Plants in the vegetative growth phase
contained limited amounts of caffeine. In imma-
ture leaves, the alkaloid was often undetectable. In
mature leaves, however, the average caffeine
content was 0.2 lg per g fresh weight and when
plants aged and entered the reproductive stage to
form flower buds, caffeine content increased to
over 5 lg per g fresh weight. Immature fruits
contained caffeine at a rather low level, up to
1.3 lg per g fresh weight. No caffeine was detected
in any parts of control plants (data not shown).
The results thus indicated that caffeine was indeed
synthesized in transgenic tobacco leaves, and that
its content was higher in the older leaves of plants
in the reproductive growth phase.
Anti-herbivore effects
Using transgenic lines which produced different
levels of caffeine, we next tested their effects on
feeding behavior of tobacco cutworms (Spodoptera
litura
), which damage a wide range of crops.
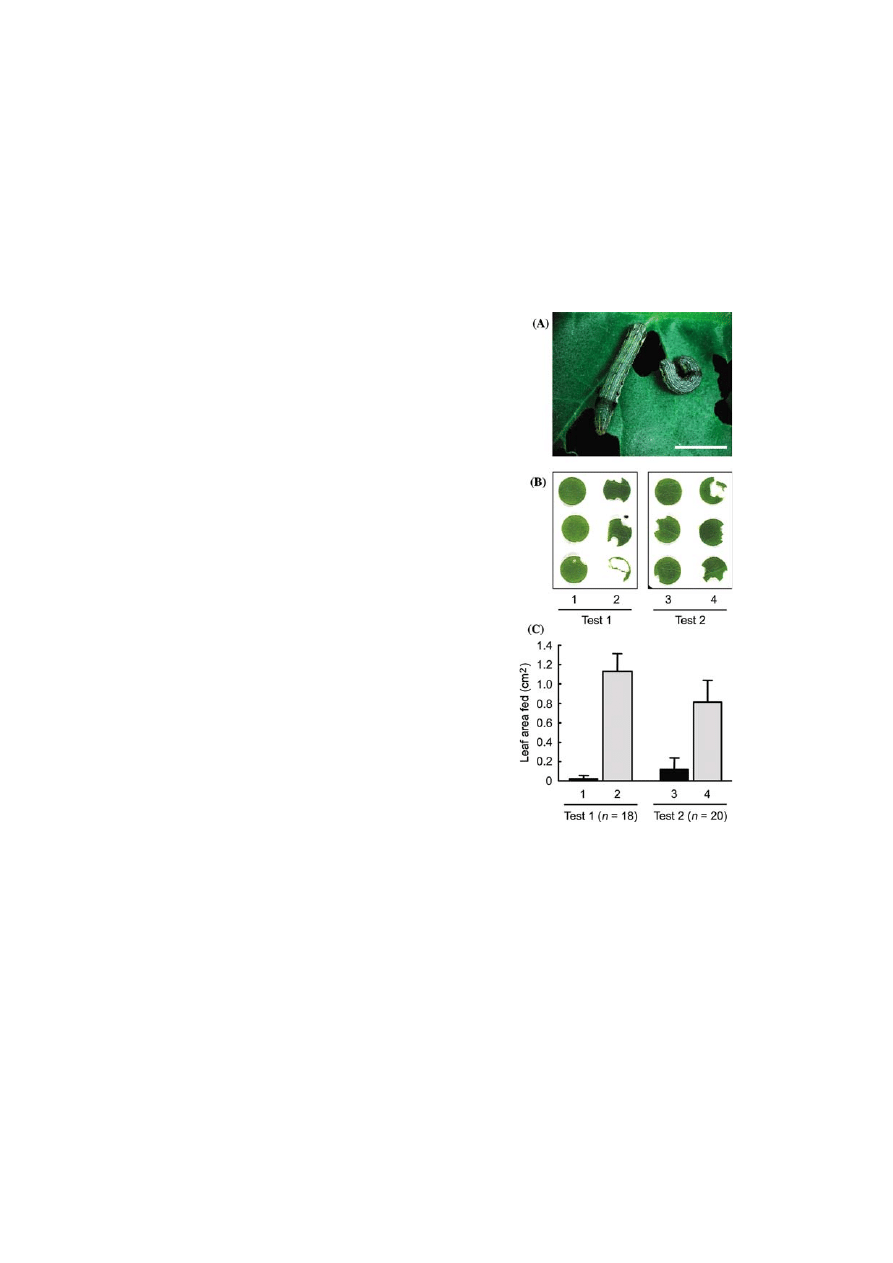
Caterpillars at the third-instar (Figure 3A) were
starved for several hours and then allowed to select
and feed on leaf discs prepared from transgenic or
control plants. Two independent experiments
showed that insects selectively fed on the control
materials, or positively avoided the transgenic
materials (Figure 3B). A quantitative estimation
confirmed this observation, indicating that eaten
areas were up to 1.1 cm
2
for the control, while less
than 0.02 cm
2
for the transgenic discs containing
caffeine at 4.9 lg per g fresh weight (Test 1;
Figure 3C). A similar result was obtained with
transgenic discs accumulating less caffeine at
0.4 lg per g fresh weight (Test 2; Figure 3C).
These observations clearly indicated caffeine to be
efficient as a pest repellant.
Discussion
Regarding the physiological function of caffeine, it
has been proposed to constitute a chemical defense
system against pathogen attack and herbivores
(Ashihara and Crozier, 1999). In a previous non-
choice test, spraying of tomato leaves with over
1% caffeine solution drastically deterred feeding
by tobacco hornworms (Nathanson, 1984). Caf-
feine also reduced the reproductive potential in
several species of moths (Mathavan et al., 1985). A
recent study showed that slugs fed significantly less
on ‘Napa’ cabbage leaves sprayed with only 0.01%
Figure 3.
Anti-herbivore effects of transgenic plants. (A)
Tobacco cutworm (S. litura) larvae at the third instar were
allowed to feed on six leaf dics, three from caffeine produc-
ing, and three from control plants. Bar indicates 5 mm. (B)
Leaf disc choice test. After feeding for 3 h in the dark, each
disc was collected and photographed. Two transgenic lines
were tested; lines #1 (Test 1) and #2 (Test 2) contained caf-
feine at 5 lg and 0.4 lg per g fresh weight, respectively. Disc
samples are from caffeine containing (1 and 3) and control
leaves (2 and 4). (C) Quantification of feeding behavior.
Twenty replicate tests were performed for one transgenic
plant as described above, and fed leaf areas (vertical axis)
were calculated with the aid of an image analyzer. The hori-
zontal axis indicates the duplicated test (Test 1 and Test 2)
with discs from caffeine containing (1 and 3) and control
leaves (2 and 4), respectively. Some tests which showed no
feeding were excluded from the evaluation.
225

caffeine solution, and topical treatment with over
0.1% caffeine solution was lethal to snails
(Hollingsworth et al., 2002). These results suggest
that caffeine is generally effective as a pesticide.
In the present study, we found transgenic
tobacco plants expressing genes of the caffeine
biosynthetic pathway to produce up to 5 lg
caffeine per g fresh weight of leaf tissues. When
subjected to a choice test, tobacco cutworms
apparently avoided the transgenic leaves, indicat-
ing caffeine to have a clear repelling effect on pest
insects. However, this amount was not high
enough to confer a lethal effect, as they apparently
grew normally with these materials up to the
pupating stage (unpublished observation). Preli-
minary experiments subsequently showed that over
10 mg of caffeine per g fresh weight of artificial
food was necessary to kill caterpillars of this moth
(unpublished observation). These findings indicate
that, although caterpillars positively avoid leaves
containing low amount of caffeine, if there is no
choice, they are able to feed on such materials.
This is consistent with a previous report, describ-
ing that tobacco cutworms accepted their normal
host plant, Ricinus communis, that was soaked up
to 0.3% caffeine solution (Mathavan et al., 1985).
In the case of another Noctuidae moth, Pseudaletia
unipuncta
, initial application of caffeine was appar-
ently deterrent, but after prolonged exposure,
caterpillars habituated to caffeine (Usher et al.,
1988). These observations implied that caffeine
might not be a powerful pest controller in agricul-
ture. However, careful assays revealed that, even
caterpillars fed apparently normally with caffeine
containing diets, at maturity, their reproductive
potential has greatly reduced, laying less eggs with
lower protein content in comparison with the
control (Mathavan et al., 1985). It can be con-
cluded that caffeine expression in plants has
implications for assessing the overall pest suppres-
sion, serving as a repellant even at a low concen-
tration, and as a toxicant at higher concentrations.
Great variation in levels of caffeine was evident
in the various transgenic lines. Several causes of
low caffeine production in tobacco plants are
conceivable. First, the pool of the starting mate-
rial,
xanthosine,
might
have
been
limited.
Currently, the exact concentration of xanthosine
in tobacco is not known, but it could be low, since
plants generally possess a purine salvage pathway
(Stasolla et al., 2003), in which xanthosine is
actively metabolized. It is thus likely that the
xanthosine pool is a bottle-neck for caffeine
synthesis. Second, 7-methylxanthosine nucleosi-
dase activity, which removes a ribose moiety from
7-methylxanthosine, might have been a limiting
factor. Using a crude extract from tobacco leaves,
we found a low but distinct activity of ribose
removal from 7-methylxanthosine in vitro (unpub-
lished
observation).
Although
this
indicated
tobacco plants to contain a nucleosidase with
broad substrate specificity, it is also likely that the
absence of a specific 7-methylxanthosine nucleos-
idase in tobacco plants can result in low caffeine
synthesis. Third, caffeine synthase activity borne
by CaDXMT1 might have been rather low, since
the enzyme demonstrated a relatively low K
m
toward
theobromine
(Uefuji
et al
.,
2003)
compared with other caffeine synthases from
coffee (Mizuno et al., 2003) and tea (Kato et al.,
2000). Thus conversion of theobromine into
caffeine may not be efficient in transgenic plants.
Fourth, intracellular translocation of synthesized
caffeine might have been problem. In coffee plants,
caffeine is thought to be transported into vacuoles
(Mosli Waldhauser and Baumann, 1996). If
tobacco plants are not equipped with such a
system, large deposits of caffeine might not be
feasible due to its cytotoxicity. Fifth, the possibil-
ity cannot be excluded that tobacco plants are able
to rapidly degrade undesirable compounds includ-
ing caffeine and its precursors. Finally, the grow-
ing condition might have affected the caffeine
production, since expression of secondary plant
compounds including alkaloids is known to be
influenced by environmental factors such as levels
and quality of light (Aerts and De Luca, 1992).
Although the introduced genes were driven by the
CaMV 35S promoter, which is supposed to
function independently on light, it is possible that
our transgenic plants could produce little level of
caffeine due to artificial culture conditions with a
relatively low light intensity in a growth cabinet.
Whatever the cause of low caffeine contents may
be, in order to practically use caffeine in planta, its
production should be increased up to 2- to 3-fold the
order in the present tobacco plants. Several
approaches to accomplish this are conceivable.
For instance, a pool of the starting material,
xanthosine, could be increased by manipulating
purine metabolic pathways. Application of another
caffeine synthase, CCS1, which exhibits a 10-fold
226

higher affinity for theobromine than CaDXMT1
(Mizuno et al., 2003), may be of help in constructing
a powerful caffeine synthesis pathway. Introduction
of an effective 7-methylxanthosine hydrolase/phos-
phorylase is another option. A bacterial gene
encoding a xanthosine phosphorylase (XapA)
(Seeger et al., 1995) was shown to possess such
activity (unpublished observation), and its use
together with the three N-methyltransferases may
be practical. In addition, a careful estimation of
caffeine contents is necessary for resulting trans-
genic plants, which could be grown under different
environments, including both laboratory and field
conditions.
Caffeine is classified by the US Food and Drug
Administration as a GRAS (‘generally recognized
as safe’) compound (US code of Federal Regula-
tions, 2001), and has been used as an ingredient of
many pharmaceuticals (Schmeller and Wink,
1998). It is also suggested to be a useful pest
controller for crop protection (Nathanson, 1984;
Hollingsworth et al., 2002), due to its specific
lethal effects in insects by inhibiting phosphodies-
terase activity (Croteau et al., 2000). Our present
results suggest the possibility to produce relatively
large amounts of caffeine in useful crops, thereby
simultaneously conferring herbivore resistance and
supplying pharmaceutical materials.
Acknowledgements
The authors thank T. Imura and H. Asao (Nara
Prefectural Agricultural Experiment Station), and
M. Morimoto (Kinki University) for comments
on the assessment of palatability. This work was
supported by grants from the New Energy and
Industrial Technology Development Organization
(NEDO) and the Research for the Future Pro-
gram of the Japan Society for the Promotion of
Science (JSPS-RFTF00L01604). H.U. was the
recipient of a postdoctoral research fellowship of
the NEDO during the performance of the studies.
References
Aerts, R.J. and De Luca, V. 1992. Phytochrome is involved in
the light-regulation of vindoline biosynthesis in Catharan-
thus
. Plant Physiol. 100: 1029–1032.
Ashihara, H. and Crozier, A. 1999. Biosynthesis and metabo-
lism of caffeine and related purine alkaloids in plants. Adv.
Bot. Res. 30: 118–205.
Ashihara, H. and Crozier, A. 2001. Caffeine: a well known but
little mentioned compound in plant science. Trends Plant
Sci. 6: 407–413.
Baumann, T.W., Schulthess, B.H. and Ha¨nni, K. 1995. Guarana´
(Paullinia cupana) rewards seed dispersers without intoxicat-
ing them by caffeine. Phytochemistry 39: 1063–1070.
Croteau, R., Kutchan, T.M. and Lews, N.G. 2000. Natural
products (Secondary metabolites). In: B.B. Buchanan, W.
Gruissem and R.L. Jones (Eds.), Biochemistry and Molecular
Biology of Plants, American Society of Plant Physiologists,
Rockville, pp. 1250–1318.
Hiei, Y., Ohta, S., Komari, T. and Kumashiro, T. 1994.
Efficient transformation of rice (Oryza sativa L.) mediated
by Agrobacterium and sequence analysis of the boundaries of
the T-DNA. Plant J. 6: 271–282.
Hollingsworth, R.G., Armstrong, J.W. and Campbell, E. 2002.
Caffeine as a repellent for slugs and snails: at high
concentrations this stimulant becomes a lethal neurotoxin
to garden pests. Nature 417: 915–916.
Kato, M., Mizuno, K., Crozier, A., Fujimura, T. and Ashihara,
H. 2000. Caffeine synthase gene from tea leaves. Nature 406:
956–957.
Mathavan, S., Premalatha, Y. and Christopher, M.S.M. 1985.
Effects of caffeine and theophylline on the fecundity of four
lepidopteran species. Exp. Biol. 44: 133–138.
Mizuno, K., Okuda, A., Kato, M., Yoneyama, N., Tanaka, H.,
Ashihara, H. and Fujimura, T. 2003. Isolation of a new dual-
functional caffeine synthase gene encoding an enzyme for the
conversion of 7-methylxanthine to caffeine from coffee
(Coffea arabica L.). FEBS Lett. 534: 75–81.
Mosli Waldhauser, S.S. and Baumann, T.W. 1996. Compart-
mentation of caffeine and related purine alkaloids depends
exclusively on the physical chemistry of their vacuolar complex
formation with chlorogenic acids. Phytochemistry 42: 985–996.
Nathanson, J.A. 1984. Caffeine and related methylxanthines:
possible naturally occurring pesticides. Science 226: 184–187.
Ogawa, M., Herai, Y., Koizumi, N., Kusano, T. and Sano, H.
2001. 7-Methylxanthine methyltransferase of coffee plants:
gene isolation and enzymatic properties. J. Biol. Chem. 276:
8213–8218.
Schmeller, T. and Wink, M. 1998. Utilization of alkaloids in
modern medicine. In: M.F. Roberts and M. Wink (Eds.),
Alkaloids: Biochemistry, Ecology, and Medicinal Applica-
tions, Prenum Press, New York, pp. 435–459.
Seeger, C., Poulsen, C. and Dandanell, G. 1995. Identification
and characterization of genes (xapA, xapB, and xapR)
involved in xanthosine catabolism in Escherichia coli. J. Bac-
teriol. 177: 5506–5516.
Stasolla, C., Katahira, R., Thorpe, T.A. and Ashihara, H. 2003.
Purine and pyrimidine nucleotide metabolism in higher
plants. J. Plant Physiol. 160: 1271–1295.
Uefuji, H., Ogita, S., Yamaguchi, Y., Koizumi, N. and Sano,
H. 2003. Molecular cloning and functional characterization
of three distinct N-methyltransferases involved in the
caffeine biosynthetic pathway in coffee plants. Plant Physiol.
132: 372–380.
US Code of Federal Regulations 2001. Title 21-Food and
Drugs (21CFR182), US Government, p. 455.
Usher, B.F., Bernays, E.A. and Barbehenn, R.V. 1988. Anti-
feedant tests with larvae of Pseudaletia unipuncta: viability of
behavioral response. Entomol. Exp. Appl. 48: 203–212.
227
Wyszukiwarka
Podobne podstrony:
Producing proteins in transgenic plants and animals
Food production in the mediterranean area
Imaging of Water Flow in Porous Media by Magnetic Resonance
Palaikastro Shells and Bronze Age Purple Dye Production in the Mediterranean Basin
Dragland 2005 Oil production in tansy
ecdltest, produkty ryżowe, List of Rice Products in Stock
In these promises broken, deep below each word gets lost in the echo by Witness
Analysis of nonvolatile species in a complex matrix by heads
A Voyage in the Sunbeam by Annie Allnut Brassey
Rapid and efficient purification and refolding of a (His) tagged recombinant protein produced in E c
Cosmic Law Patterns in the Universe by Dean Brown
Angel in the Attic by closettwilighter1
Experimental study of drying kinetics by forced convection of aromatic plants
David Icke The Hidden Codes in the Bible by Roy Reinhold
Crime and Law Enforcement in Poland edited by Andrew Siemaszko
Preludium in G z suity wiolonczelowej by Bach
ProblemSolver in metal stamping by Dayton
Kruczkowska, Joanna Kings and Poets Self Irony in Selected Poems by George Seferis and Derek Mahon
więcej podobnych podstron