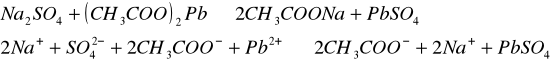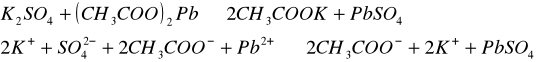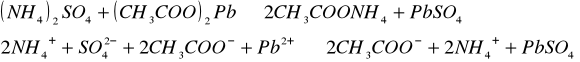[Author ID1: at Tue Mar 31 22:49:00 1998
]Korzeniewicz [Author ID1: at Sat Apr 4 00:59:00 1998
]Łukasz[Author ID1: at Sat Apr 4 01:09:00 1998
] [Author ID1: at Sat Apr 4 00:59:00 1998
]Korzeniewicz Łukasz [Author ID1: at Sat Apr 4 00:59:00 1998
] [Author ID1: at Sat Apr 4 01:09:00 1998
] [Author ID1: at Tue Mar 31 22:49:00 1998
] [Author ID1: at Tue Mar 31 22:50:00 1998
] [Author ID1: at Tue Mar 31 22:49:00 1998
]1998-03-27 [Author ID2: at Mon Apr 10 19:25:00 2000
]
[Author ID1: at Tue Mar 31 22:49:00 1998
][Author ID2: at Mon Apr 10 19:25:00 2000
]IMAb[Author ID0: at Thu Nov 30 00:00:00 1899
]
ĆWICZENIA LABOR[Author ID1: at Tue Mar 31 20:53:00 1998
]L[Author ID1: at Tue Mar 31 20:53:00 1998
]ATORYJNE Z CHEMI NIEORGANICZNEJ.
[Author ID1: at Tue Mar 31 22:49:00 1998
]Temat: DYSOCJACJA ELEKTROLIT [Author ID2: at Mon Apr 10 19:25:00 2000
]YCZNA.[Author ID2: at Mon Apr 10 19:25:00 2000
]
W drugiej połowie XIX[Author ID2: at Mon Apr 10 19:24:00 2000
] [Author ID1: at Fri Mar 27 22:05:00 1998
] w[Author ID1: at Fri Mar 27 22:05:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]w[Author ID1: at Fri Mar 27 22:05:00 1998
]. szwe[Author ID2: at Mon Apr 10 19:24:00 2000
]d[Author ID1: at Fri Mar 27 22:03:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]zki [Author ID2: at Mon Apr 10 19:24:00 2000
]chemik S. A. Arrhenius doświadczalnie udowodnił, [Author ID1: at Fri Mar 27 22:06:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]że substancje chemiczne można podzielić na dwie grupy[Author ID1: at Fri Mar 27 22:07:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]. Do grupy [Author ID1: at Fri Mar 27 22:08:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->I [Author ID1: at Fri Mar 27 22:08:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Tue Mar 31 22:53:00 1998
]można zaliczyć takie, których roztwory wodne przewodzą prąd elektryczny[Author ID1: at Fri Mar 27 22:08:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
], a do grupy [Author ID1: at Fri Mar 27 22:10:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->II[Author ID1: at Fri Mar 27 22:10:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Tue Mar 31 22:53:00 1998
]--> [Author ID1: at Fri Mar 27 22:10:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Tue Mar 31 22:53:00 1998
]te, które w tych samych w[Author ID1: at Fri Mar 27 22:10:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]a[Author ID1: at Fri Mar 27 22:10:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]runkach praktycznie nie przewodzą prądu[Author ID1: at Fri Mar 27 22:10:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]. Substancje grupy [Author ID1: at Fri Mar 27 22:12:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->I[Author ID1: at Fri Mar 27 22:12:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Tue Mar 31 22:53:00 1998
] nazwał [Author ID1: at Fri Mar 27 22:12:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]elektrolitami[Author ID1: at Fri Mar 27 22:13:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
], grupy [Author ID1: at Fri Mar 27 22:13:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->II[Author ID1: at Fri Mar 27 22:13:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Tue Mar 31 22:53:00 1998
]--> [Author ID1: at Fri Mar 27 22:18:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Tue Mar 31 22:53:00 1998
]-[Author ID1: at Fri Mar 27 22:14:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] [Author ID1: at Fri Mar 27 22:18:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]nieelektrolit[Author ID1: at Fri Mar 27 22:14:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]a[Author ID1: at Fri Mar 27 22:14:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]mi.[Author ID1: at Fri Mar 27 22:14:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID0: at Thu Nov 30 00:00:00 1899
][Author ID0: at Thu Nov 30 00:00:00 1899
]
Teorię tą można ująć ogólnie w postaci czter[Author ID1: at Fri Mar 27 22:19:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]ech podstawowych, opartych na doświadczeniu założeń.[Author ID1: at Fri Mar 27 22:20:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
Elektrolity, a więc k[Author ID1: at Fri Mar 27 22:22:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]wasy, zasady i sole podczas rozpuszczania w wodzie rozpadają się na elementy naładowane elektrycznie, czyli ulegają tzw. [Author ID1: at Fri Mar 27 22:22:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]dysocj[Author ID1: at Fri Mar 27 22:25:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]a[Author ID1: at Fri Mar 27 22:25:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]cji elektrolitycznej.[Author ID1: at Fri Mar 27 22:25:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]Elementy te nazwano [Author ID1: at Fri Mar 27 22:26:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]jonami.[Author ID1: at Fri Mar 27 22:26:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]Jony naładowane doda[Author ID1: at Fri Mar 27 22:26:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]t[Author ID1: at Fri Mar 27 22:26:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]nio nazywają się [Author ID1: at Fri Mar 27 22:26:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]kationami, [Author ID1: at Fri Mar 27 22:27:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]a ujemne[Author ID1: at Fri Mar 27 22:27:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ][Author ID1: at Fri Mar 27 22:28:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]anionami.[Author ID1: at Fri Mar 27 22:28:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]-->[Author ID1: at Fri Mar 27 22:28:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]Suma ładunków elektrycznych kationów i anionów, powstałych na skutek dysocjacji elektrolitycznej [Author ID1: at Fri Mar 27 22:28:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]elektrolitów, jest [Author ID1: at Fri Mar 27 22:31:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]zawsze [Author ID1: at Fri Mar 27 22:32:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]równa [Author ID1: at Fri Mar 27 22:31:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]zeru.[Author ID1: at Fri Mar 27 22:32:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]-->[Author ID1: at Fri Mar 27 22:32:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]Nieelektrolity[Author ID1: at Fri Mar 27 22:32:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ], tzn. substancje, które w roztworach i w stanie stopionym nie p[Author ID1: at Fri Mar 27 22:33:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]rzewodzą prądu elektrycznego, nie ulegają dysocjacji [Author ID1: at Fri Mar 27 22:36:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]elektrolitycznej.[Author ID1: at Fri Mar 27 22:36:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]-->[Author ID1: at Fri Mar 27 22:36:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]Własności [Author ID1: at Fri Mar 27 22:37:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]chemiczne jonów różnią się zupełnie od własności obojętnych atomów lub cząsteczek. Z [Author ID1: at Fri Mar 27 22:38:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]tego względu obecność jonów w roztworach nadaje im ch[Author ID1: at Fri Mar 27 22:39:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]a[Author ID1: at Fri Mar 27 22:39:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]rakterystyczne [Author ID1: at Fri Mar 27 22:39:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]cechy chemiczne i fizyczne.[Author ID1: at Fri Mar 27 22:41:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ]-->[Author ID1: at Fri Mar 27 22:42:00 1998 ][Author ID2: at Mon Apr 10 19:24:00 2000 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]
Kwasami [Author ID1: at Fri Mar 27 22:42:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] [Author ID1: at Fri Mar 27 22:42:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]s[Author ID1: at Fri Mar 27 22:59:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]ą [Author ID1: at Fri Mar 27 22:42:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]to [Author ID1: at Fri Mar 27 22:59:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]związki chemiczne[Author ID1: at Fri Mar 27 22:42:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
], które podcza[Author ID1: at Fri Mar 27 22:43:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]s rozpuszczania w wodzie dysocjują ca[Author ID1: at Fri Mar 27 22:43:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]ł[Author ID1: at Fri Mar 27 22:43:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]kowicie lub częściowo na kationy wodorowe i aniony reszt kwasowych.[Author ID1: at Fri Mar 27 22:43:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:00:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
Zasady [Author ID1: at Fri Mar 27 23:01:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]są to związki chemiczne, które [Author ID1: at Fri Mar 27 23:02:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]podczas rozpuszczania w wodzie dysocjują całkow[Author ID1: at Fri Mar 27 23:03:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]i[Author ID1: at Fri Mar 27 23:03:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]cie lub częściowo [Author ID1: at Fri Mar 27 23:03:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]na aniony wodorotlenowe OH[Author ID1: at Fri Mar 27 23:05:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-[Author ID1: at Fri Mar 27 23:06:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] i kationy metali.[Author ID0: at Thu Nov 30 00:00:00 1899
][Author ID0: at Thu Nov 30 00:00:00 1899
]
Sole [Author ID1: at Fri Mar 27 23:07:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]są produktami reakcji kwasów z zasadami. [Author ID1: at Fri Mar 27 23:07:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]Związki [Author ID1: at Fri Mar 27 23:08:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]te w temperaturze pokojowej występują na ogół w stanie stałym, krystalicznym i mają budowę jonową, czyli składają się z kationów [Author ID1: at Fri Mar 27 23:09:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]metali lub kationu [Author ID1: at Fri Mar 27 23:12:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]amonowego [Author ID1: at Fri Mar 27 23:13:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]i anionów[Author ID1: at Fri Mar 27 23:09:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] reszt kwasowych[Author ID1: at Fri Mar 27 23:13:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
].[Author ID1: at Fri Mar 27 23:09:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:13:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
Do porównywania mocy elektrolitów wprowadzono pojęcie stopnia i stałej dysocjacji ele[Author ID1: at Fri Mar 27 23:14:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]k[Author ID1: at Fri Mar 27 23:14:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]trolitycznej.[Author ID1: at Fri Mar 27 23:14:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
Stopień dysocjacji [Author ID1: at Fri Mar 27 23:17:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]określa [Author ID1: at Fri Mar 27 23:17:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]się[Author ID1: at Fri Mar 27 23:18:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] stosunkiem liczby moli cząsteczek zdysocjowanych[Author ID1: at Fri Mar 27 23:20:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] na jony do liczby moli cząsteczek substancji rozpuszczonej:[Author ID1: at Fri Mar 27 23:21:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
[Author ID1: at Fri Mar 27 23:23:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:23:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID2: at Mon Apr 10 19:24:00 2000
]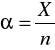
[Author ID1: at Fri Mar 27 23:23:00 1998
][Author ID1: at Fri Mar 27 23:23:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
], [Author ID1: at Fri Mar 27 23:25:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:28:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
gdzie[Author ID1: at Fri Mar 27 23:25:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->:[Author ID1: at Fri Mar 27 23:25:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:30:00 1998
]--> [Author ID1: at Fri Mar 27 23:25:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:29:00 1998
]-->α[Author ID1: at Fri Mar 27 23:26:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:29:00 1998
] [Author ID1: at Tue Mar 31 22:54:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->-[Author ID1: at Fri Mar 27 23:26:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:29:00 1998
] stopień dysocjacji[Author ID1: at Fri Mar 27 23:26:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->, X[Author ID1: at Fri Mar 27 23:26:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:29:00 1998
] [Author ID1: at Tue Mar 31 22:54:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]- liczba moli czą[Author ID1: at Fri Mar 27 23:26:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]steczek zdysocjowanych na jony, [Author ID1: at Fri Mar 27 23:28:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-->n[Author ID1: at Fri Mar 27 23:28:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:29:00 1998
] [Author ID1: at Tue Mar 31 22:54:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]- [Author ID1: at Fri Mar 27 23:28:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]liczba moli cząsteczek substancji rozpuszczonej.[Author ID1: at Fri Mar 27 23:30:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] Stopień dysocjacji jest odwrotnie [Author ID1: at Fri Mar 27 23:33:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:36:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
proporcjonalny do[Author ID1: at Fri Mar 27 23:33:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] [Author ID1: at Fri Mar 27 23:34:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]stę[Author ID1: at Fri Mar 27 23:33:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]ż[Author ID1: at Fri Mar 27 23:34:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]enia i [Author ID1: at Fri Mar 27 23:33:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]w rozcieńczeniu nieskończenie wielkim zbliża się do 100%[Author ID1: at Fri Mar 27 23:35:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]. Jest [Author ID1: at Fri Mar 27 23:36:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]on również zależny od temperatury i rośnie wraz z jej wzrostem. [Author ID1: at Fri Mar 27 23:37:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]Dysocjacja elektrolityczna jest procesem odwracalnym, więc w roztworze elektrolitów istnieje równowaga[Author ID1: at Fri Mar 27 23:42:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
],[Author ID1: at Fri Mar 27 23:45:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] dla kt[Author ID1: at Fri Mar 27 23:42:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]órej można napisać wyrażenie na stałą równowagi [K[Author ID1: at Fri Mar 27 23:45:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]c[Author ID1: at Fri Mar 27 23:46:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]]:[Author ID1: at Fri Mar 27 23:46:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
[Author ID1: at Fri Mar 27 23:47:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:47:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID2: at Mon Apr 10 19:24:00 2000
]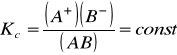
[Author ID1: at Fri Mar 27 23:47:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
], jeże[Author ID1: at Fri Mar 27 23:51:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]li [Author ID1: at Fri Mar 27 23:51:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]t=const[Author ID1: at Fri Mar 27 23:52:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
], [Author ID1: at Fri Mar 27 23:52:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID1: at Fri Mar 27 23:53:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
gdzie [Author ID1: at Fri Mar 27 23:52:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
](A[Author ID1: at Fri Mar 27 23:52:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]+[Author ID1: at Fri Mar 27 23:52:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]),(B[Author ID1: at Fri Mar 27 23:53:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]-[Author ID1: at Fri Mar 27 23:53:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
])[Author ID1: at Fri Mar 27 23:53:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] [Author ID1: at Tue Mar 31 22:53:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]- rzeczywiste[Author ID1: at Fri Mar 27 23:53:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] stężenie jonów w stanie równowagi, [Author ID1: at Fri Mar 27 23:54:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
](AB)[Author ID1: at Fri Mar 27 23:54:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] - rzeczywiste stężenie cząsteczek niezdysocjowanych[Author ID1: at Fri Mar 27 23:54:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
] [Author ID1: at Fri Mar 27 23:56:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]w [Author ID1: at Fri Mar 27 23:54:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]stanie równowagi.[Author ID1: at Fri Mar 27 23:56:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID0: at Thu Nov 30 00:00:00 1899
][Author ID0: at Thu Nov 30 00:00:00 1899
]
Teoria kwasów i zasad według Br[Author ID1: at Tue Mar 31 20:55:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]önsteda mówi, że cząsteczka kwasu po oddaniu protonu staje się cząsteczką lub jonem zasady i odwrotnie tzn. że cząsteczka zasady po przejęciu protonu staje się cząstką kwasu. Według [Author ID1: at Tue Mar 31 22:42:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]tej teori[Author ID1: at Tue Mar 31 22:45:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]i, kwas może wyk[Author ID1: at Tue Mar 31 22:46:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]a[Author ID1: at Tue Mar 31 22:46:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]zywać swoje właściwości kwasowe tylko wobec zasad[Author ID1: at Tue Mar 31 22:46:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]y, która przyjmuje proton i o[Author ID1: at Tue Mar 31 22:47:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]d[Author ID1: at Tue Mar 31 22:47:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]wrotnie zasada może nią być tylko w obecności kwasu, który oddaje proton.[Author ID1: at Tue Mar 31 22:47:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
]
[Author ID1: at Tue Mar 31 22:49:00 1998
][Author ID2: at Mon Apr 10 19:24:00 2000
][Author ID0: at Thu Nov 30 00:00:00 1899
]
Arkadiusz Kasprzyk IMCa[Author ID0: at Thu Nov 30 00:00:00 1899 ]
Temat: Dysocjacja elektrolityczna.[Author ID2: at Mon Apr 10 19:26:00 2000 ]
Doświadczenie 1.[Author ID0: at Thu Nov 30 00:00:00 1899 ]
![]()
[Author ID1: at Tue Mar 31 23:28:00 1998
]Dysocjacja chlorku miedziowego w obecności [Author ID1: at Tue Mar 31 22:51:00 1998
]H[Author ID1: at Tue Mar 31 22:52:00 1998
]2[Author ID1: at Tue Mar 31 22:52:00 1998
]O.[Author ID1: at Tue Mar 31 22:52:00 1998
][Author ID1: at Tue Mar 31 22:54:00 1998
]
Przebieg reakcji zależy od stałej dielektrycznej rozpuszczal[Author ID1: at Tue Mar 31 23:37:00 1998 ]nika [Author ID1: at Tue Mar 31 23:37:00 1998 ]-->ε[Author ID1: at Tue Mar 31 23:38:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ]-->.[Author ID1: at Tue Mar 31 23:38:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ] Gdy [Author ID1: at Tue Mar 31 23:38:00 1998 ]-->ε[Author ID1: at Tue Mar 31 23:38:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ]--><10[Author ID1: at Tue Mar 31 23:39:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ] dysocjacja nie zachodzi. Gdy [Author ID1: at Tue Mar 31 23:40:00 1998 ]10<[Author ID1: at Tue Mar 31 23:40:00 1998 ]ε[Author ID1: at Tue Mar 31 23:41:00 1998 ]<[Author ID1: at Tue Mar 31 23:40:00 1998 ]40[Author ID1: at Tue Mar 31 23:41:00 1998 ] dysocjacja zachodzi częściowo, gdy[Author ID1: at Tue Mar 31 23:41:00 1998 ] [Author ID1: at Tue Mar 31 23:43:00 1998 ]ε[Author ID1: at Tue Mar 31 23:43:00 1998 ]<40[Author ID1: at Tue Mar 31 23:43:00 1998 ] dysocjacja zachodzi całkowicie. Następuje tu zmiana barwy z żółtej na niebieską. Natomiast [Author ID1: at Tue Mar 31 23:43:00 1998 ]dysocjacja [Author ID1: at Tue Mar 31 23:44:00 1998 ]-->CuCl[Author ID1: at Tue Mar 31 23:45:00 1998 ][Author ID1: at Tue Mar 31 23:45:00 1998 ]-->2[Author ID1: at Tue Mar 31 23:45:00 1998 ][Author ID1: at Tue Mar 31 23:45:00 1998 ]--> [Author ID1: at Tue Mar 31 23:41:00 1998 ][Author ID1: at Tue Mar 31 23:45:00 1998 ]w obecności acetonu, [Author ID1: at Tue Mar 31 23:46:00 1998 ]zachodzi częściowo. Na [Author ID1: at Tue Mar 31 23:47:00 1998 ]dnie znajdują się niebieskie jony [Author ID1: at Tue Mar 31 23:48:00 1998 ]Cu[Author ID1: at Tue Mar 31 23:48:00 1998 ]2+[Author ID1: at Tue Mar 31 23:48:00 1998 ], a u góry żółte niezdysocjowane [Author ID1: at Tue Mar 31 23:49:00 1998 ]CuCl[Author ID1: at Tue Mar 31 23:49:00 1998 ]2[Author ID1: at Tue Mar 31 23:49:00 1998 ]. Oznacza to, że w obecności acetonu dysocjacja zachodzi częściowo.[Author ID1: at Tue Mar 31 23:50:00 1998 ]
Doświadczenie 2.[Author ID1: at Tue Mar 31 23:15:00 1998 ]
Dysocjacja chlorku kobaltowego w obecności [Author ID1: at Tue Mar 31 23:16:00 1998 ]H[Author ID1: at Tue Mar 31 23:17:00 1998 ]2[Author ID1: at Tue Mar 31 23:17:00 1998 ]O.[Author ID1: at Tue Mar 31 23:17:00 1998 ][Author ID1: at Tue Mar 31 23:28:00 1998 ]
[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID1: at Tue Mar 31 23:28:00 1998
]![]()
[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]
Stała równowagi dla tej reakcj[Author ID1: at Tue Mar 31 23:28:00 1998 ]i:[Author ID1: at Tue Mar 31 23:28:00 1998 ]
[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID1: at Tue Mar 31 23:28:00 1998
]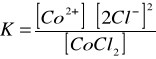
[Author ID1: at Tue Mar 31 23:28:00 1998
].[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID1: at Tue Mar 31 23:35:00 1998
]
Dodatek wspólnego jonu chlorkowego [Author ID1: at Tue Mar 31 23:36:00 1998 ]Cl[Author ID1: at Tue Mar 31 23:36:00 1998 ]-[Author ID1: at Tue Mar 31 23:36:00 1998 ] cofnął dysocjacje. Roztwór zmienił barwę z różowej na niebieską. Nadmiar jonu chlorkowego przesuwa równowagę w stronę zdysocjowanego [Author ID1: at Tue Mar 31 23:36:00 1998 ]CoCl[Author ID1: at Tue Mar 31 23:36:00 1998 ]2[Author ID1: at Tue Mar 31 23:36:00 1998 ].[Author ID1: at Tue Mar 31 23:36:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]
[Author ID1: at Fri Apr 3 21:07:00 1998 ]Doświadczenie 3.[Author ID1: at Fri Apr 3 21:06:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]
Do probówki zawierającej trzy krople (siarczanu żelaza III) [Author ID1: at Fri Apr 3 22:45:00 1998 ]Fe[Author ID1: at Fri Apr 3 22:46:00 1998 ]2[Author ID1: at Fri Apr 3 22:46:00 1998 ](SO[Author ID1: at Fri Apr 3 22:46:00 1998 ]4[Author ID1: at Fri Apr 3 22:46:00 1998 ])[Author ID1: at Fri Apr 3 22:46:00 1998 ]3[Author ID1: at Fri Apr 3 22:46:00 1998 ] dol[Author ID1: at Fri Apr 3 22:47:00 1998 ]aliśmy[Author ID1: at Fri Apr 3 22:50:00 1998 ] [Author ID1: at Fri Apr 3 22:47:00 1998 ]([Author ID1: at Fri Apr 3 22:49:00 1998 ]ti[Author ID1: at Fri Apr 3 22:47:00 1998 ]o[Author ID1: at Fri Apr 3 22:48:00 1998 ]cyjani[Author ID1: at Fri Apr 3 22:47:00 1998 ]a[Author ID1: at Fri Apr 3 22:48:00 1998 ]nu[Author ID1: at Fri Apr 3 22:47:00 1998 ] potasowego[Author ID1: at Fri Apr 3 22:48:00 1998 ]) [Author ID1: at Fri Apr 3 22:49:00 1998 ]KSCN[Author ID1: at Fri Apr 3 22:49:00 1998 ], otrzymany roztwór zabarwiony był na czerw[Author ID1: at Fri Apr 3 22:49:00 1998 ]o[Author ID1: at Fri Apr 3 22:49:00 1998 ]no. Po [Author ID1: at Fri Apr 3 22:49:00 1998 ]dodaniu wody destylowanej nastąpiła dysocjacja elektrolityczna, w wyniku kt[Author ID1: at Fri Apr 3 22:51:00 1998 ]ó[Author ID1: at Fri Apr 3 22:51:00 1998 ]rej [Author ID1: at Fri Apr 3 22:51:00 1998 ]roztwór odbarwił się na pomarańczowo.[Author ID1: at Fri Apr 3 22:52:00 1998 ]
Dysoc[Author ID1: at Fri Apr 3 22:58:00 1998 ]jacja roztworu [Author ID1: at Fri Apr 3 22:58:00 1998 ]Fe(SCN)[Author ID1: at Fri Apr 3 22:59:00 1998 ]3[Author ID1: at Fri Apr 3 23:00:00 1998 ]:[Author ID1: at Fri Apr 3 23:00:00 1998 ]-->[Author ID1: at Fri Apr 3 22:58:00 1998 ][Author ID1: at Fri Apr 3 23:00:00 1998 ]
-->[Author ID1: at Fri Apr 3 22:54:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]-->[Author ID1: at Fri Apr 3 22:54:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]-->[Author ID1: at Fri Apr 3 23:52:00 1998
]-->![]()
[Author ID1: at Fri Apr 3 23:52:00 1998
]-->[Author ID1: at Fri Apr 3 22:54:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]-->.[Author ID1: at Fri Apr 3 23:00:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]
Do zdysocjowanowego rodanku w pierwszym przypadku dodaliśmy [Author ID1: at Fri Apr 3 23:00:00 1998 ]Fe[Author ID1: at Fri Apr 3 23:02:00 1998 ]2[Author ID1: at Fri Apr 3 23:02:00 1998 ](SO[Author ID1: at Fri Apr 3 23:02:00 1998 ]4[Author ID1: at Fri Apr 3 23:02:00 1998 ])[Author ID1: at Fri Apr 3 23:02:00 1998 ]3[Author ID1: at Fri Apr 3 23:02:00 1998 ] co spowodowało [Author ID1: at Fri Apr 3 23:03:00 1998 ]to zabarwienie roztworu na kolor krwist[Author ID1: at Fri Apr 3 23:05:00 1998 ]o[Author ID1: at Fri Apr 3 23:06:00 1998 ] czerwoną[Author ID1: at Fri Apr 3 23:05:00 1998 ]. Obecność wspólnego jonu [Author ID1: at Fri Apr 3 23:08:00 1998 ]Fe[Author ID1: at Fri Apr 3 23:09:00 1998 ]3+[Author ID1: at Fri Apr 3 23:10:00 1998 ] cofnęła dysocjację na stronę [Author ID1: at Fri Apr 3 23:10:00 1998 ]Fe(SCN)[Author ID1: at Fri Apr 3 23:11:00 1998 ]3[Author ID1: at Fri Apr 3 23:11:00 1998 ]. W [Author ID1: at Fri Apr 3 23:11:00 1998 ]drugim przypadku [Author ID1: at Fri Apr 3 23:11:00 1998 ]dodaliśmy [Author ID1: at Fri Apr 3 23:12:00 1998 ]KSCN[Author ID1: at Fri Apr 3 23:13:00 1998 ], miało to taki sam [Author ID1: at Fri Apr 3 23:13:00 1998 ]wynik jak w pierwszym przypadku. [Author ID1: at Fri Apr 3 23:14:00 1998 ]Wspólnym jonem [Author ID1: at Fri Apr 3 23:11:00 1998 ]w tym przypadku był [Author ID1: at Fri Apr 3 23:15:00 1998 ]SCN[Author ID1: at Fri Apr 3 23:16:00 1998 ]-[Author ID1: at Fri Apr 3 23:16:00 1998 ]. Również tutaj obecność cofnęła[Author ID1: at Fri Apr 3 23:16:00 1998 ] dysocjację[Author ID1: at Fri Apr 3 23:17:00 1998 ] [Author ID1: at Fri Apr 3 23:18:00 1998 ]w stronę [Author ID1: at Fri Apr 3 23:17:00 1998 ]Fe(SCN)[Author ID1: at Fri Apr 3 23:17:00 1998 ]3[Author ID1: at Fri Apr 3 23:17:00 1998 ].[Author ID1: at Fri Apr 3 23:17:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]
Do trzech probówek wprowadziliśmy następujące związ[Author ID1: at Fri Apr 3 23:19:00 1998 ]ki: [Author ID1: at Fri Apr 3 23:19:00 1998 ]Pb(NO[Author ID1: at Fri Apr 3 23:20:00 1998 ]3[Author ID1: at Fri Apr 3 23:21:00 1998 ])[Author ID1: at Fri Apr 3 23:21:00 1998 ]2[Author ID1: at Fri Apr 3 23:21:00 1998 ],[Author ID1: at Fri Apr 3 23:21:00 1998 ] (CH[Author ID1: at Fri Apr 3 23:21:00 1998 ]3[Author ID1: at Fri Apr 3 23:22:00 1998 ]COO)[Author ID1: at Fri Apr 3 23:22:00 1998 ]2[Author ID1: at Fri Apr 3 23:22:00 1998 ]Pb[Author ID1: at Fri Apr 3 23:22:00 1998 ] oraz [Author ID1: at Fri Apr 3 23:22:00 1998 ]PbCl[Author ID1: at Fri Apr 3 23:23:00 1998 ]2[Author ID1: at Fri Apr 3 23:23:00 1998 ]. Następnie kolejno do każdej z nich nala[Author ID1: at Fri Apr 3 23:23:00 1998 ]liśmy kwasu siarkowego (po dwie krople). W [Author ID1: at Fri Apr 3 23:24:00 1998 ]pierwszej probówce [Author ID1: at Fri Apr 3 23:25:00 1998 ]roztwór [Author ID1: at Fri Apr 3 23:27:00 1998 ]zmienił konsystencję i w[Author ID1: at Fri Apr 3 23:25:00 1998 ]y[Author ID1: at Fri Apr 3 23:25:00 1998 ]dzielił się z nieg[Author ID1: at Fri Apr 3 23:25:00 1998 ]o osad. W [Author ID1: at Fri Apr 3 23:27:00 1998 ]drugiej także wydzielił się osad[Author ID1: at Fri Apr 3 23:28:00 1998 ], to samo, ale z mniejszą i[Author ID1: at Fri Apr 3 23:29:00 1998 ]n[Author ID1: at Fri Apr 3 23:29:00 1998 ]tensywnością, zaszło w trzeciej probówce.[Author ID1: at Fri Apr 3 23:29:00 1998 ]
-->[Author ID1: at Fri Apr 3 23:31:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:31:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->
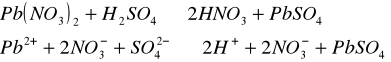
[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:31:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->
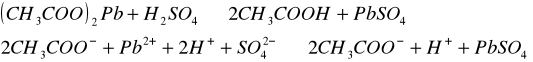
[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->
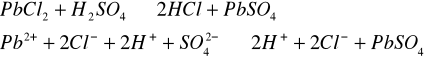
[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]
W drugiej [Author ID1: at Fri Apr 3 23:52:00 1998 ]części doświadczenia do kolejnych trzech probówek wl[Author ID1: at Fri Apr 3 23:53:00 1998 ]aliśmy[Author ID1: at Fri Apr 3 23:56:00 1998 ] [Author ID1: at Fri Apr 3 23:57:00 1998 ]kolejno [Author ID1: at Fri Apr 3 23:53:00 1998 ]Na[Author ID1: at Fri Apr 3 23:54:00 1998 ]2[Author ID1: at Fri Apr 3 23:54:00 1998 ]SO[Author ID1: at Fri Apr 3 23:54:00 1998 ]4[Author ID1: at Fri Apr 3 23:54:00 1998 ], [Author ID1: at Fri Apr 3 23:54:00 1998 ]K[Author ID1: at Fri Apr 3 23:54:00 1998 ]2[Author ID1: at Fri Apr 3 23:54:00 1998 ]SO[Author ID1: at Fri Apr 3 23:54:00 1998 ]4[Author ID1: at Fri Apr 3 23:54:00 1998 ], [Author ID1: at Fri Apr 3 23:54:00 1998 ](NH[Author ID1: at Fri Apr 3 23:55:00 1998 ]4[Author ID1: at Fri Apr 3 23:55:00 1998 ])[Author ID1: at Fri Apr 3 23:55:00 1998 ]2[Author ID1: at Fri Apr 3 23:55:00 1998 ]SO[Author ID1: at Fri Apr 3 23:55:00 1998 ]4[Author ID1: at Fri Apr 3 23:56:00 1998 ]. Do każdej z nich doda[Author ID1: at Fri Apr 3 23:56:00 1998 ]liśmy[Author ID1: at Fri Apr 3 23:57:00 1998 ] po[Author ID1: at Fri Apr 3 23:56:00 1998 ] dwie [Author ID1: at Fri Apr 3 23:57:00 1998 ]krople [Author ID1: at Fri Apr 3 23:57:00 1998 ]Pb(NO[Author ID1: at Fri Apr 3 23:57:00 1998 ]3[Author ID1: at Fri Apr 3 23:57:00 1998 ])[Author ID1: at Fri Apr 3 23:57:00 1998 ]2[Author ID1: at Fri Apr 3 23:57:00 1998 ],[Author ID1: at Fri Apr 3 23:57:00 1998 ] [Author ID1: at Fri Apr 3 23:57:00 1998 ]co spowodowało wytrącenie się osadu w każdej probówce.[Author ID1: at Fri Apr 3 23:58:00 1998 ][Author ID1: at Sat Apr 4 00:00:00 1998 ]
Doświadczenie 5.[Author ID1: at Sat Apr 4 00:15:00 1998 ]
Do dwóch[Author ID1: at Sat Apr 4 00:17:00 1998 ] probówek zawierających (kwas octowy) [Author ID1: at Sat Apr 4 00:18:00 1998 ]CH[Author ID1: at Sat Apr 4 00:19:00 1998 ]3[Author ID1: at Sat Apr 4 00:19:00 1998 ]COOH[Author ID1: at Sat Apr 4 00:19:00 1998 ] dodaliśmy oranż metylowy[Author ID1: at Sat Apr 4 00:19:00 1998 ]. Otrzymany [Author ID1: at Sat Apr 4 00:22:00 1998 ]roztwór miał barwę pomarańczową. Do jednej probówki dodaliśmy [Author ID1: at Sat Apr 4 00:23:00 1998 ]CH[Author ID1: at Sat Apr 4 00:24:00 1998 ]3[Author ID1: at Sat Apr 4 00:24:00 1998 ]COONa[Author ID1: at Sat Apr 4 00:24:00 1998 ] przez co roztwór zmienił barwę na lekko żółtą. Obecność [Author ID1: at Sat Apr 4 00:24:00 1998 ]wspólnego jonu[Author ID1: at Sat Apr 4 00:25:00 1998 ] [Author ID1: at Sat Apr 4 00:27:00 1998 ] CH[Author ID1: at Sat Apr 4 00:26:00 1998 ]3[Author ID1: at Sat Apr 4 00:26:00 1998 ]COO[Author ID1: at Sat Apr 4 00:26:00 1998 ]-[Author ID1: at Sat Apr 4 00:27:00 1998 ] cofnęła dysocjację[Author ID1: at Sat Apr 4 00:25:00 1998 ] w stronę [Author ID1: at Sat Apr 4 00:27:00 1998 ] CH[Author ID1: at Sat Apr 4 00:26:00 1998 ]3[Author ID1: at Sat Apr 4 00:26:00 1998 ]COOH[Author ID1: at Sat Apr 4 00:26:00 1998 ].[Author ID1: at Sat Apr 4 00:27:00 1998 ]
Dysocjacja kwasu octowego[Author ID1: at Sat Apr 4 00:45:00 1998 ] (CH[Author ID1: at Sat Apr 4 00:48:00 1998 ]3[Author ID1: at Sat Apr 4 00:48:00 1998 ]COOH)[Author ID1: at Sat Apr 4 00:48:00 1998 ]:[Author ID1: at Sat Apr 4 00:48:00 1998 ][Author ID1: at Sat Apr 4 00:30:00 1998 ]
[Author ID1: at Sat Apr 4 00:30:00 1998
][Author ID1: at Sat Apr 4 00:30:00 1998
]![]()
[Author ID1: at Sat Apr 4 00:30:00 1998
].[Author ID0: at Thu Nov 30 00:00:00 1899
]
Stała dys[Author ID1: at Sat Apr 4 00:32:00 1998 ]ocjacji[Author ID1: at Sat Apr 4 00:32:00 1998 ] kwasu octowego [Author ID1: at Sat Apr 4 00:33:00 1998 ]([Author ID1: at Sat Apr 4 00:49:00 1998 ]CH[Author ID1: at Sat Apr 4 00:33:00 1998 ]3[Author ID1: at Sat Apr 4 00:33:00 1998 ]COOH[Author ID1: at Sat Apr 4 00:33:00 1998 ])[Author ID1: at Sat Apr 4 00:49:00 1998 ]:[Author ID1: at Sat Apr 4 00:33:00 1998 ]
[Author ID1: at Sat Apr 4 00:34:00 1998
][Author ID1: at Sat Apr 4 00:34:00 1998
]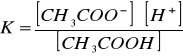
[Author ID1: at Sat Apr 4 00:34:00 1998
].[Author ID0: at Thu Nov 30 00:00:00 1899
]
W drugiej części [Author ID1: at Sat Apr 4 00:37:00 1998 ]doświadczenia do dwóch probówek wprowadziliśmy [Author ID1: at Sat Apr 4 00:38:00 1998 ]NH[Author ID1: at Sat Apr 4 00:39:00 1998 ]4[Author ID1: at Sat Apr 4 00:39:00 1998 ]OH[Author ID1: at Sat Apr 4 00:39:00 1998 ], który zabarwiliśmy fenoloftaleiną (kolor malinowy). Dodanie [Author ID1: at Sat Apr 4 00:39:00 1998 ]do jednej z dwóch probówek [Author ID1: at Sat Apr 4 00:41:00 1998 ]NH[Author ID1: at Sat Apr 4 00:42:00 1998 ]4[Author ID1: at Sat Apr 4 00:42:00 1998 ]Cl[Author ID1: at Sat Apr 4 00:42:00 1998 ]2[Author ID1: at Sat Apr 4 00:42:00 1998 ] spowodowało całkowite odbarwieni[Author ID1: at Sat Apr 4 00:42:00 1998 ]e roztworu.[Author ID1: at Sat Apr 4 00:42:00 1998 ] Również [Author ID1: at Sat Apr 4 00:43:00 1998 ]tu obecność wspólnego jonu cofnęła dysocjację.[Author ID0: at Thu Nov 30 00:00:00 1899 ]
[Author ID1: at Sat Apr 4 00:44:00 1998 ]Dysocjacja wodorotlenku amonowego[Author ID1: at Sat Apr 4 00:45:00 1998 ] (NH[Author ID1: at Sat Apr 4 00:49:00 1998 ]4[Author ID1: at Sat Apr 4 00:49:00 1998 ]OH)[Author ID1: at Sat Apr 4 00:49:00 1998 ]:[Author ID1: at Sat Apr 4 00:45:00 1998 ]-->[Author ID1: at Sat Apr 4 00:33:00 1998 ][Author ID1: at Sat Apr 4 00:39:00 1998 ]
[Author ID1: at Sat Apr 4 00:28:00 1998
][Author ID1: at Sat Apr 4 00:28:00 1998
]![]()
[Author ID1: at Sat Apr 4 00:28:00 1998
].[Author ID1: at Sat Apr 4 00:47:00 1998
]
Stała dysocjacji tej zasady wynosi:[Author ID1: at Sat Apr 4 00:48:00 1998 ]
[Author ID1: at Sat Apr 4 00:51:00 1998
][Author ID1: at Sat Apr 4 00:51:00 1998
]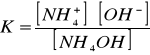
[Author ID1: at Sat Apr 4 00:51:00 1998
].[Author ID1: at Sat Apr 4 00:51:00 1998
]
[Author ID1: at Fri Apr 3 23:56:00 1998 ]-->[Author ID1: at Fri Apr 3 23:51:00 1998 ][Author ID1: at Fri Apr 3 23:56:00 1998 ]
[Author ID1: at Tue Mar 31 23:18:00 1998
][Author ID1: at Tue Mar 31 23:18:00 1998
]![]()
[Author ID1: at Tue Mar 31 23:18:00 1998
]-->[Author ID1: at Tue Mar 31 23:01:00 1998
][Author ID1: at Tue Mar 31 23:14:00 1998
]
[Author ID1: at Tue Mar 31 22:45:00 1998 ]-->[Author ID1: at Fri Mar 27 22:52:00 1998 ][Author ID1: at Fri Mar 27 23:54:00 1998 ]
![]()
Wyszukiwarka
Podobne podstrony:
Ćw.ch.3, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Ćw.ch.4, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Ćw.ch.5, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Ćw.ch.4(1), Szkoła, penek, Przedmioty, Chemia, Laboratoria
Pierwiastki poprawione, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Ćw.ch.3, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Chemia kataliza, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Roztwory, Szkoła, penek, Przedmioty, Chemia, Laboratoria
KOROZJA1, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Hydroliza, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Szybkość reakcji, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Pierwiastki 2, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Redox2, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Elektroliza, Szkoła, penek, Przedmioty, Chemia, Laboratoria
w03 Dysocjacja elektrolity, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Sprawozdanie 5 ćwiczenia 4 i 5, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Reakcje redoks, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Korozja, Szkoła, penek, Przedmioty, Chemia, Laboratoria
więcej podobnych podstron