It isn't difficult to form addition polymers from
monomers containing C=C double bonds; many of
these compounds polymerize spontaneously unless
polymerization is actively inhibited.
The simplest way to catalyze the polymerization
reaction that leads to an addition polymer is to add
a source of a free radical to the monomer. The
term free radical is used to describe a family of
very reactive, short-lived components of a reaction
that contain one or more unpaired electrons. In the
presence of a free radical, addition polymers form
by a chain-reaction mechanism that contains
chain-initiation, chain-propagation, and chain-
termination steps.
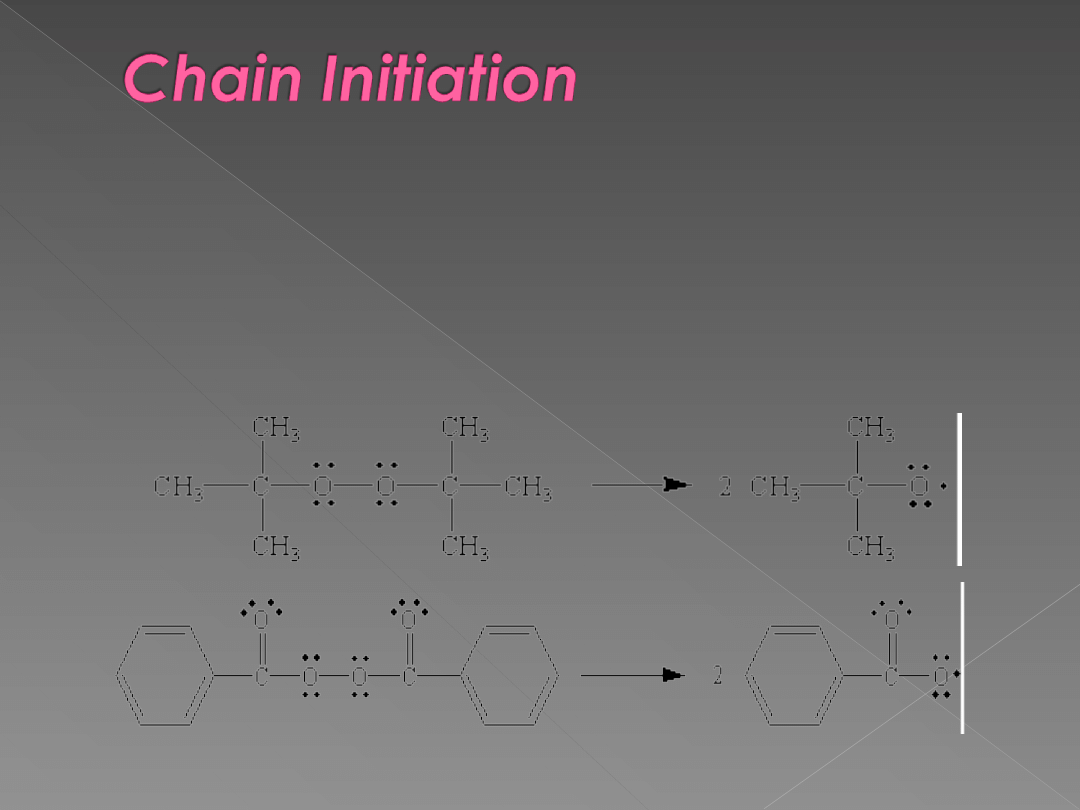
A source of free radicals is needed to initiate the
chain reaction. These free radicals are usually
produced by decomposing a peroxide such as di-
tert-butyl peroxide or benzoyl peroxide, shown
below. In the presence of either heat or light, these
peroxides decompose to form a pair of free radicals
that contain an unpaired electron.
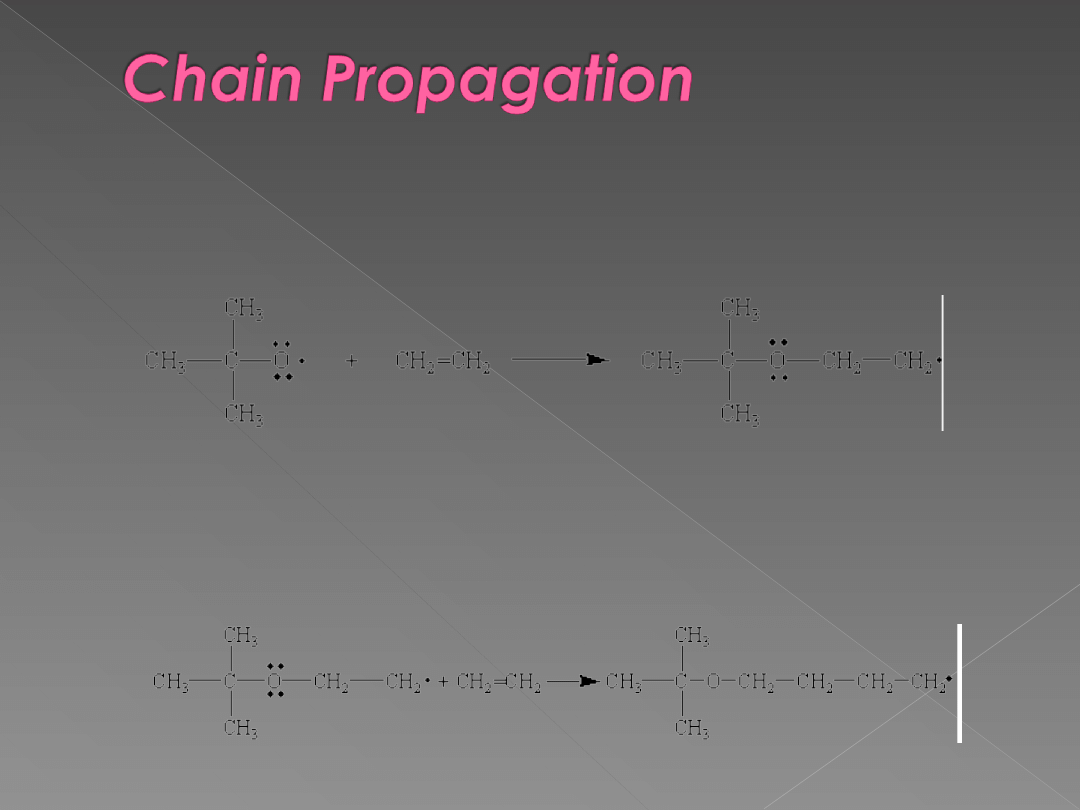
The free radical produced in the chain-initiation
step adds to an alkene to form a new free
radical.
The product of this reaction can then add
additional monomers in a chain reaction.
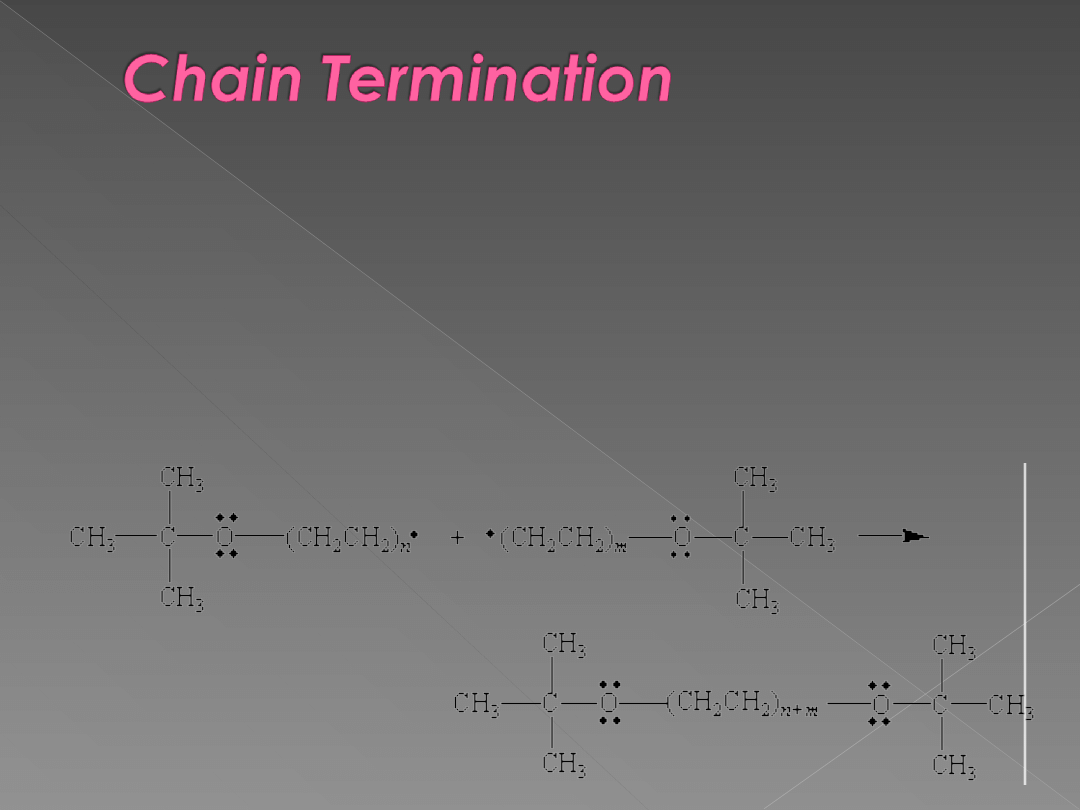
Whenever pairs of radicals combine to
form a covalent bond, the chain
reactions carried by these radicals are
terminated.
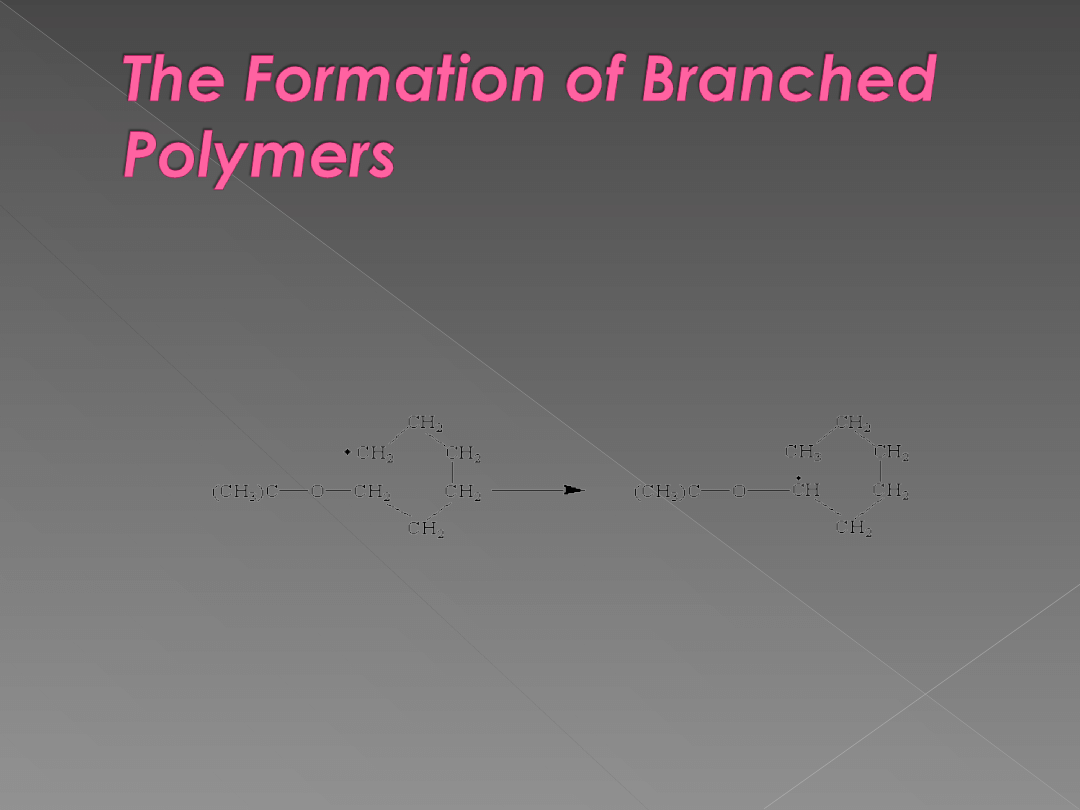
At first glance we might expect the product of the free-radical
polymerization of ethylene to be a straight-chain polymer. As the chain
grows, however, it begins to fold back on itself. This allows an
intramolecular reaction to occur in which the site at which polymerization
occurs is transferred from the end of the chain to a carbon atom along the
backbone.
When this happens, branches are introduced onto the polymer chain.
Free-radical polymerization of ethylene produces a polymer that contains
branches on between 1 and 5% of the carbon atoms. Of these branches,
10% contain two carbon atoms, 50% contain four carbon atoms, and 40%
are longer side chains.
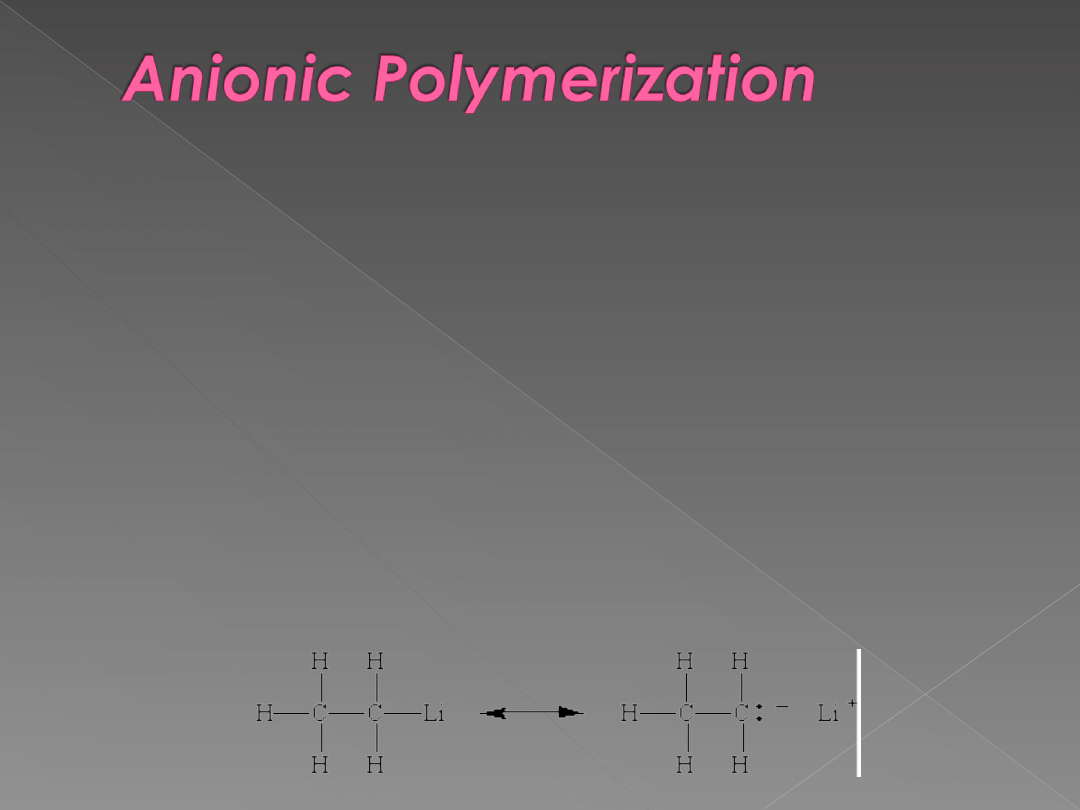
Addition polymers can also be made by chain
reactions that proceed through intermediates that
carry either a negative or positive charge.
When the chain reaction is initiated and carried by
negatively charged intermediates, the reaction is
known as anionic polymerization. Like free-
radical polymerizations, these chain reactions take
place via chain-initiation, chain-propagation, and
chain-termination steps.
The reaction is initiated by a Grignard reagent or
alkyllithium reagent, which can be thought of a
source of a negatively charged CH
3-
or CH
3
CH
2-
ion.
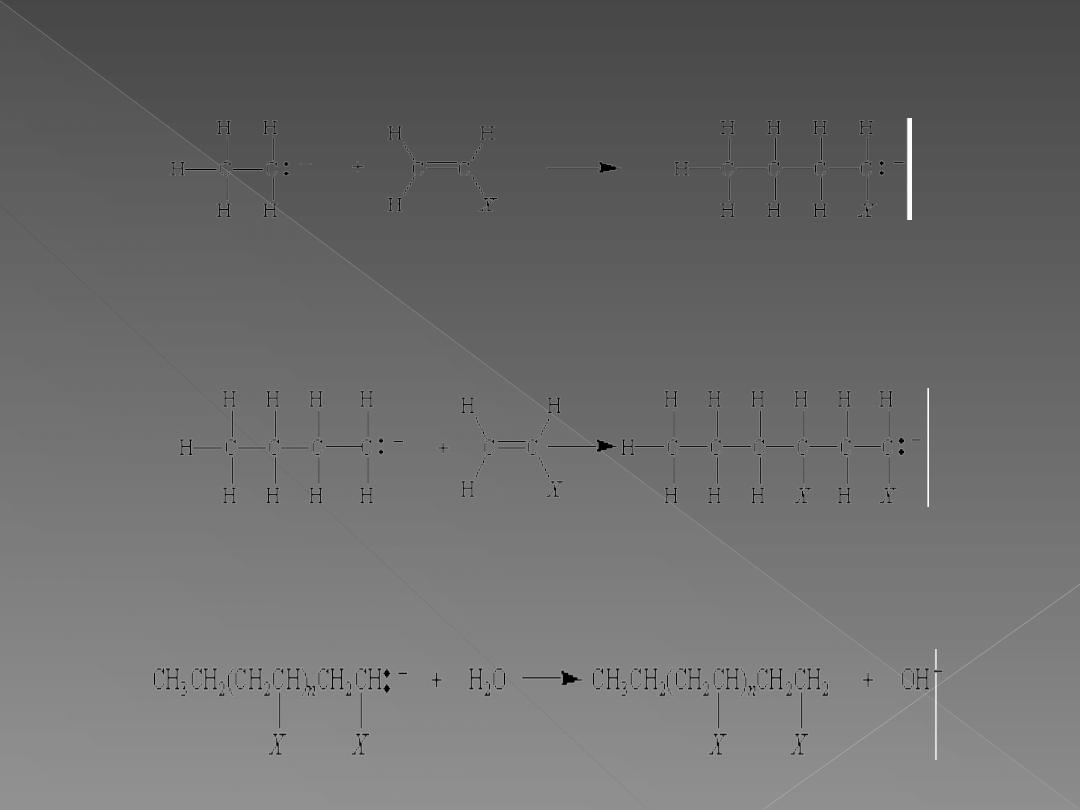
The CH
3-
or CH
3
CH
2-
ion from one of these metal alkyls
can attack an alkene to form a carbon-carbon bond.
The product of this chain-initiation reaction is a new
carbanion that can attack another alkene in a chain-
propagation step.
The chain reaction is terminated when the carbanion
reacts with traces of water in the solvent in which the
reaction is run.
The intermediate that carries the chain reaction during polymerization
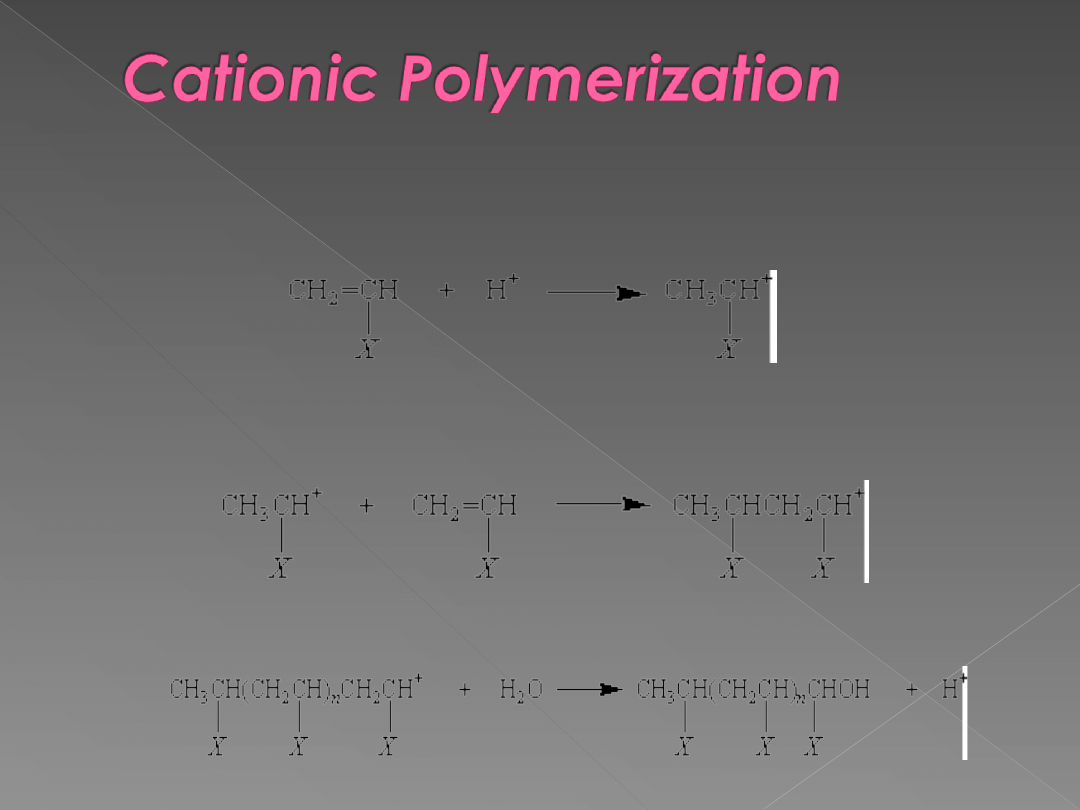
can also be a positive ion, or cation. In this case, the cationic
polymerization reaction is initiated by adding a strong acid to an
alkene to form a carbocation.
The ion produced in this reaction adds monomers to produce a
growing polymer chain.
The chain reaction is terminated when the carbonium ion reacts with
water that contaminates the solvent in which the polymerization is
run.
The initiation step of ionic polymerization reactions has a
much smaller activation energy than the equivalent step for
free-radical polymerizations. As a result, ionic polymerization
reactions are relatively insensitive to temperature, and can be
run at temperatures as low as -70
o
C. Ionic polymerization
therefore tends to produce a more regular polymer, with less
branching along the backbone, and more controlled tacticity.
Because the intermediates involved in ionic polymerization
reactions can't combine with one another, chain termination
only occurs when the growing chain reacts with impurities or
reagents that can be specifically added to control the rate of
chain growth. It is therefore easier to control the average
molecular weight of the product of ionic polymerization
reactions.
Ionic polymerizations are more difficult to carry out on an
industrial scale than free-radical polymerizations. Ionic
polymerization is therefore only used for monomers that don't
polymerize by the free-radical mechanism or to prepare
polymers with a regular structure.
In 1963 Karl Ziegler and Giulio Natta received the Nobel prize in
chemistry for their discovery of coordination compound catalysts for
addition polymerization reactions. These Ziegler-Natta catalysts
provide the opportunity to control both the linearity and tacticity of
the polymer.
Free-radical polymerization of ethylene produces a low-density,
branched polymer with side chains of one to five carbon atoms on up
to 3% of the atoms along the polymer chain. Ziegler-Natta catalysts
produce a more linear polymer, which is more rigid, with a higher
density and a higher tensile strength. Polypropylene produced by
free-radical reactions, for example, is a soft, rubbery, atactic polymer
with no commercial value. Ziegler-Natta catalysts provide an isotactic
polypropylene, which is harder, tougher, and more crystalline.
A typical Ziegler-Natta catalyst can be produced by mixing solutions
of titanium(IV) chloride (TiCl
4
) and triethylaluminum [Al(CH
2
CH
3
)
3
]
dissolved in a hydrocarbon solvent from which both oxygen and
water have been rigorously excluded. The product of this reaction is
an insoluble olive-colored complex in which the titanium has been
reduced to the Ti(III) oxidation state.
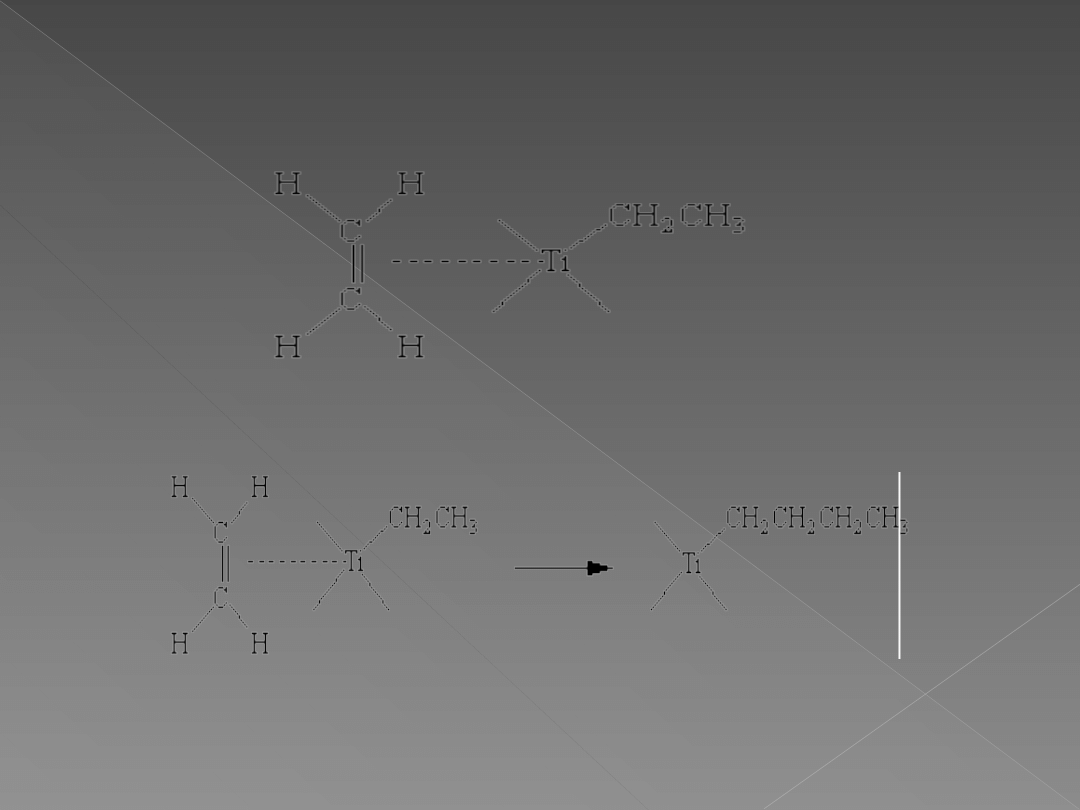
The catalyst formed in this reaction can be described as coordinately
unsaturated because there is an open coordination site on the titanium
atom. This allows an alkene to act as a Lewis base toward the titanium
atom, donating a pair of electrons to form a transition-metal complex.
The alkene is then inserted into a Ti-CH
2
CH
3
bond to form a growing
polymer chain and a site at which another alkene can bond.
Thus, the titanium atom provides a template on which a linear polymer
with carefully controlled stereochemistry can grow.
Document Outline
Wyszukiwarka
Podobne podstrony:
Polymerization Reactions
A protocol for polymerase chain reaction detection of Enterococcus faecalis and Enterococcus faec
4 3 Polymerase Chain Reaction (PCR)
Degradable Polymers and Plastics in Landfill Sites
Development of Carbon Nanotubes and Polymer Composites Therefrom
Polymer Processing With Supercritical Fluids V Goodship, E Ogur (Rapra, 2004) Ww
Inorganic Polymers
Propylene Polymers
Fundamentals of Polymer Chemist Nieznany
Polymer Supported Reagents
Electrochemical properties for Journal of Polymer Science
Dendronized Polymers
Modeling of Polymer Processing and Properties
Metal Containing Polymers
Ethylene Polymers, HDPE
Amorphous Polymers
Ethylene Polymers, LLDPE
10Imprint Polymers
więcej podobnych podstron