Electrophilic Aromatic Substitution
The Nitration of Methyl Benzoate
Aromatic compounds react with electrophiles to give products resulting in substitution of
a hydrogen atom by the electrophile. This is in contrast to the reaction of alkenes with
electrophiles, which typically give products of addition, rather than substitution.
Br
2
FeBr
3
Br
+
HBr
Br
2
Br
Br
The reaction of aromatic systems with electrophiles results in substitution because
abstraction of an H
+
from the intermediate resonance stabilized carbocation restores
aromaticity to the product. This aromaticity imparts ~ 36 kcal/mole stabilization energy
compared to non-aromatic systems.
If the aromatic ring is already substituted prior to the reaction with an electrophile, the
nature of the substituent affects not only the rate of reaction, but the location of the
attacking electrophile. In this week’s laboratory exercise, the aromatic ester, methyl
benzoate, will be nitrated with nitric acid in the presence of concentrated sulfuric acid.
The sulfuric acid is necessary because nitric acid alone is not a strong enough electrophile
to react with most aromatic rings. The sulfuric acid protonates the nitric acid, and the
resulting cation dehydrates, forming the nitronium ion which is the active electrophile in
this reaction.
HNO
3
+
H
2
SO
4
N
+
O
-
OH
2
+
O
N
+
O
O
+
O
H
2
+
HSO
4
-
C
+
Br
H
H
path A
path B
Br
-
A
Br
-
B
Br
Br
Br
aromatic- lower energy
non-aromatic-higher energy
(does not occur)
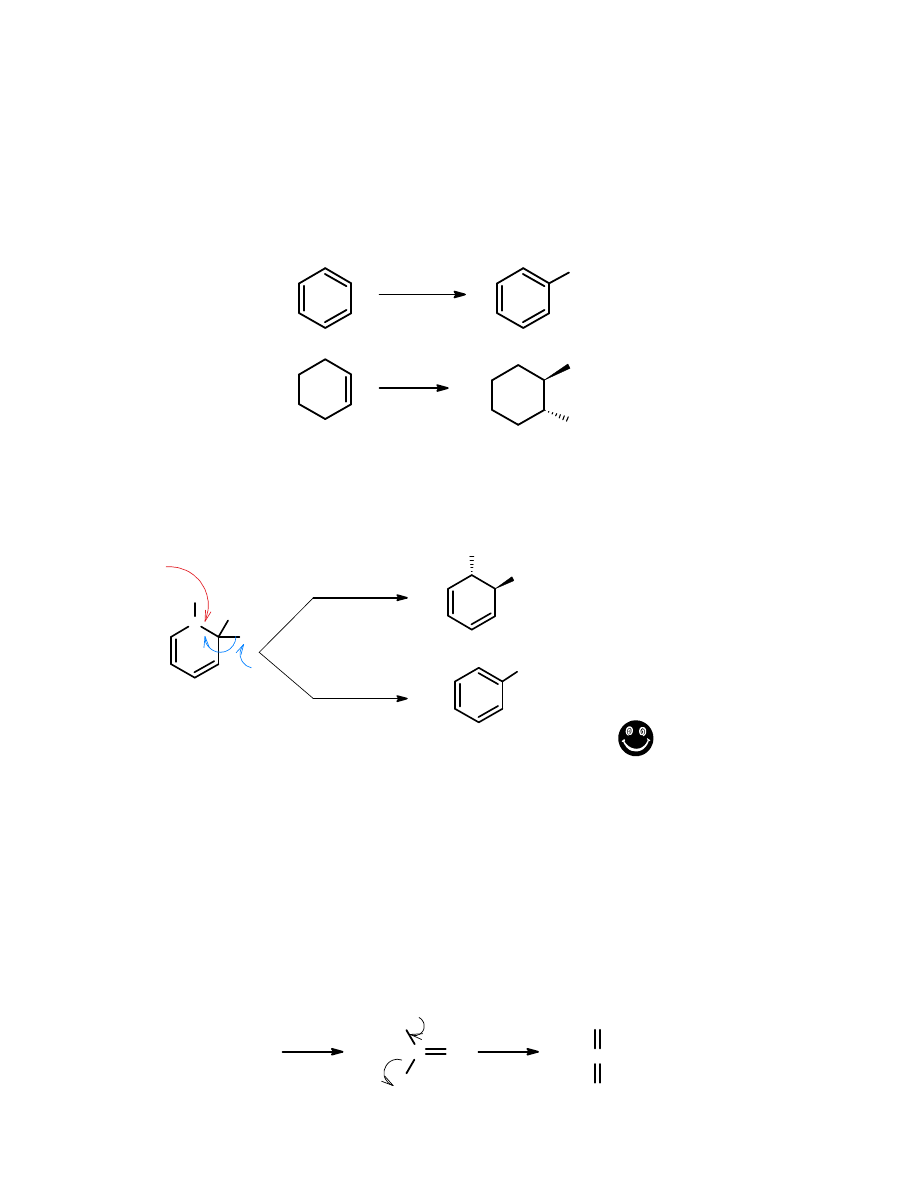
The nitronium ion reacts with methyl benzoate to produce the nitrated methyl benzoate as
shown below. Note that the position of the nitro group is unspecified in the drawing (the
nitro is shown attached to the aromatic ring, but the location of the bond is not specified).
O
OCH
3
NO
2
+
O
OCH
3
NO
2
Based on the stability of the resulting intermediate, draw the expected product of the
nitration of methyl benzoate as part of your pre-lab activity in the introduction to your lab
report. Also include the physical and chemical properties of methyl benzoate,
concentrated sulfuric acid, concentrated nitric acid and methanol.
Methyl benzoate is found naturally and is known as niobe oil, cananga, or ‘harsh
wintergreen’. It occurs naturally in various plants, including clove and black current.
You may find the aroma familiar and pleasant, but the concentrated material can irritate
the mucous membranes and skin.
This experiment consists of four major parts:
1.
The nitration of methyl benzoate
2.
Isolation of crude methyl nitrobenzoate (you should provide the required number
indicating the position of the nitro group)
3.
Purification of the crude product
4.
Characterization of the purified product. (mp, IR)
Experimental Details
Safety:
1.
Concentrated
nitric acid
is a strong oxidizer as well as a strong acid. Therefore it is
corrosive to skin, eyes, and mucous membranes. Contact with skin will result in the
formation of a yellow or yellow-brown color on the affected part which will not be
removed by washing.
2.
Concentrated
sulfuric acid
is corrosive to skin, eyes, and mucous membranes
3.
Methanol
is toxic, both by ingestion and inhalation.
Procedure:
1.
Place 12mL concentrated sulfuric acid in a 125mL Erlenmeyer flask and cool the
contents in an ice bath until the temperature is <5
o
C.
2.
Add 6.1mL methyl benzoate (calculate the amount in grams) to the contents of the
flask described in step 1. Cool the contents to 5
o
C in an ice bath.
3.
Place 4.0mL of concentrated sulfuric acid in a 25mL Erlenmeyer flask and cool the
contents in a beaker of ice water. Add 4.0 mL of concentrated nitric acid to the
sulfuric acid and keep the mixture of acids in the ice bath.

4.
Using a Pasteur pipette, add the mixed acid reagent dropwise to the contents of the
125mL Erlenmeyer flask. Swirl the contents of the flask often to ensure good mixing.
Maintain the temperature during addition between 5
o
and 15
o
C. (The reaction is
exothermic and must be controlled by cooling and slow addition rate)
5.
After the addition of the nitric acid/sulfuric acid mixture is complete, remove the 125
mL Erlenmeyer flask from the ice bath and allow the reaction mixture to warm to
room temperature.
6.
After 15 minutes, pour the reaction mixture into a 250mL beaker containing ~50g ice
chips. After the ice has melted, isolate the solids by suction filtration. DO NOT
WASH THE PRODUCT UNTIL STEP 7 IS DONE.
7.
Transfer the acidic filtrate into the ‘waste acid’ container in the hood. Then replace
the filter on the filter flask and continue with step 8.
8.
Wash the isolated solid with 20 mL cold water; the clear filtrate can be disposed in
the sink with running water.
9.
Wash the filtrate with 10mL portions of cold methanol. Dispose of the filtrate in the
‘waste organic’ container in the hood. DO NOT COMBINE WITH THE WASTE
ACID.
10.
Save a small portion of ‘crude’ product for a melting point determination.
11.
Weigh the remainder of product and recrystallize from an equal weight of methanol.
Warm the methanol / product mixture on a hot plate until solution is achieved, then
remove from heat. Cool the solution in an ice bath.
12.
Isolate the purified product by suction filtration. Air dry for ~10 minutes, then place
the crude material in an oven at 60
o
C. Dispose of the filtrate in the ‘waste organic’
container.
13.
Weigh the dried material and obtain a melting point and IR.
14.
Calculate the per cent yield for the purified material.
Questions to be addressed as part of your conclusion:
• What evidence do you have that the purification procedure was effective in increasing
the purity of your product?
• Estimate the yield loss that occurred as a result of the purification steps. What caused
this loss?
• Does the spectral data support the proposed structure of your product?
• Would you expect the nitration of phenol to give the same substitution pattern? Why
or why not?
Created 1/2/06 by J. Neilan
Updated 3/11/07
Wyszukiwarka
Podobne podstrony:
Electrophilic Aromatic Substitution exam questions
cardiovascular disease and exercise
dictionary and exercises. ch 12 language focus 32-33, 02 law
Lectures 1, 2 and 3
SHSBC 358 TV?MO?SIC AUDITING LECTURE AND?MO
Academic Studies English Support Materials and Exercises for Grammar Part 1 Parts of Speech
Bearden Electret What it is and how it works (2005)
British Patent 6,481 Improvements relating to the Electrical Transmission of Power and to Apparatus
creatine supplementation and exercise performance
Dictionary of Weightlifting, Bodybuilding, and Exercise Terms and Techniques
Wack, Tantleff Dunn Relationship between Electronic Game Playing, Obesity and Psychological Functio
PROGRESS IN ESTABLISHING A CONNECTION BETWEEN THE ELECTROMAGNETIC ZERO POINT FIELD AND INERTIA
Rodrigues & Leo Quaternionic Electron Theory Geometry, Algebra and DiracÆs Spinors (1998)
Electron Densities, Electrostatic Potentials, and Reactivity Indices exercises
Lecture10 Medieval women and private sphere
lecture 15 Multivariate and mod Nieznany
Electronics 4 Systems and procedures S
więcej podobnych podstron