
Biomaterials 22 (2001) 2475}2480
E!ect of nickel}titanium shape memory metal alloy
on bone formation
夽
Anita Kapanen
*,Jorma Ryha
K nen
,Anatoli Danilov ,Juha Tuukkanen
Biocenter Oulu and Department of Anatomy and Cell Biology, University of Oulu, P.O. Box 5000, FIN-90014 Oulu, Finland
Department of Surgery, University of Oulu, FIN-90014 Oulu, Finland
Received 7 June 2000; accepted 7 December 2000
Abstract
The aim of this study was to determine the biocompatibility of NiTi alloy on bone formation in vivo. For this purpose we used
ectopic bone formation assay which goes through all the events of bone formation and calci"cation. Comparisons were made between
Nitinol (NiTi),stainless steel (Stst) and titanium}aluminium (6%)}vanadium (4%) alloy (Ti}6Al}4V),which were implanted for
8 weeks under the fascia of the latissimus dorsi muscle in 3-month-old rats. A light-microscopic examination showed no chronic
in#ammatory or other pathological "ndings in the induced ossicle or its capsule. New bone replaced part of the decalci"ed matrix
with mineralized new cartilage and bone. The mineral density was measured with peripheral quantitative computed tomography
(pQCT). The total bone mineral density (BMD) values were nearly equal between the control and the NiTi samples,the Stst samples
and the Ti}6Al}4V samples had lower BMDs. Digital image analysis was used to measure the combined area of new "brotic tissue
and original implanted bone matrix powder around the implants. There were no signi"cant di!erences between the implanted
materials,although Ti}6Al}4V showed the largest matrix powder areas. The same method was used for measurements of
proportional cartilage and new bone areas in the ossicles. NiTi showed the largest cartilage area (p)0.05). Between implant groups
the new bone area was largest in NiTi. We conclude that NiTi has good biocompatibility,as its e!ects on ectopic bone formation are
similar to those of Stst,and that the ectopic bone formation assay developed here can be used for biocompatibility studies.
2001
Elsevier Science Ltd. All rights reserved.
Keywords: Nitinol; Biocompatibility; Ectopic bone formation assay; PQCT; Decalci"ed bone matrix
1. Introduction
Nitinol (NiTi) is a promising new implant material
which has a shape memory e!ect,superelasticity and
high damping properties. Furthermore,it has an elastic
modulus closer to that of bone than any other metal
[1}3]. These features might be very promising for long-
term
or
permanent
implantation.
Recently,NiTi
implants have been developed for cardiovascular and
gastrointestinal surgery [4}7]. The lack of knowledge of
夽
Part of this work has been presented in the 27th European Sympo-
sium on Calci"ed Tissues,Tampere,Finland,on 7}10 May 2000. This
work was supported by Technology Development Center of Finland
(TEKES).
* Corresponding author. Tel.: #358-8-537-5180; fax: #358-8-537-
5172.
E-mail address: anita.kapanen@oulu." (A. Kapanen).
the biocompatability of this alloy in long-term bone
implantation has hindered its orthopedic applications.
Implantation of decalci"ed matrix into an extra-
skeletal site induces the formation and calci"cation of
new bone [8]. This autoinduction of ectopic bone led to
the discovery of bone morphogenetic proteins (BMPs)
[9]. Since then,this method has been widely used for
studying the osteoinductivity of di!erent agents [10}16].
A vehicle is needed for BMPs to induce ossi"cation.
Collagen I is often used as a carrier,but the best carrier is
decalci"ed bone matrix,which contains a mixture of
morphogenetic proteins.
The aim of this study was to analyze the possible
interference of the implant material in the whole induc-
tion cascade from mesenchymal stem cells to cartilage
and endochondral bone. We used decalci"ed allogenic
bone matrix powder as the inducer,and it was packed
around the implant. Comparisons to matrix alone and to
stainless steel and Ti}6Al}4V were performed. The e!ects
0142-9612/01/$ - see front matter
2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 0 ) 0 0 4 3 5 - X

on bone formation and calci"cation were determined
histologically and by mineral density measurements.
Further evidence of the good biocompatibility of NiTi
with bone tissue was obtained.
2. Materials and methods
2.1. Decalcixed bone matrix
To obtain decalci"ed bone matrix,rat femurs were
crushed with an ultracentrifugal mill (Retsch ZM100,
F. Kurt Retsch GmbH & Co.,Germany) cooled with
liquid nitrogen to produce grains 0.5$0.1 mm in dia-
meter. Fat was extracted with 1:1 chloroform:methanol
for 1 h at room temperature with continuous stirring. The
particles were decalci"ed in 0.6
N
HCl for 24 h at #43C
with continuous stirring. Possible traces of HCl were
washed from the particles with sterile water by repeating
the washing step several times. The particles were
lyophilized and stored in sterile vials at !203C. For the
biocompatibility analysis,the matrix powder was placed
in gelatin capsules (size no. 4,Orion,Finland) with the
test materials.
2.2. Test materials
We tested and compared three alloys: vacuum-melted,
drawn and fully annealed NiTi (54% nickel by weight,
46% titanium by weight,NiTi Development Co.,USA),
AO/ASIF stainless steel,later referred to as Stst,(Synthes
GmbH,Switzerland) and AO/ASIF Ti}6Al}4V alloy
(90% titanium by weight,6% aluminum by weight,4%
vanadium by weight,Synthes GmbH,Switzerland). The
surface treatment of stainless steel consisted of electro-
lytic polishing,and the NiTi and Ti}6Al}4V samples
were supplied in a mechanically ground condition. Iden-
tical cylindrical implants 1.8 mm in diameter and 6 mm in
length were taken from a longer wire by mechanical
cutting. The implants were degreased with 70% ethanol,
washed with an ultrasonic vibrobath and autoclaved
(30 min,1213C). The implants were packaged with al-
logenic decalci"ed bone matrix into gelatin capsules.
Allogenic decalci"ed bone matrix without any im-
plants was used as control.
2.3. Animals
Three-month-old Sprague-Dawley male rats (10 con-
trol rats and 10 test rats) weighing 400$50 g were used
for the ectopic bone formation assay. The rats were
allowed standard laboratory rat food and water
ad libitum. The animal tests were performed after ap-
proval by the ethical committee of the University
of Oulu. All aspects of animal care complied with
the Animal Welfare Act and the recommendations of
the NIH-PHS Guide for the Care and Use of Laboratory
Animals.
2.4. Surgical procedure
Test implants of rat allogenic bone matrix with NiTi
(n"10),Ti}6Al}4V (n"8),and Stst (n"8) in gelatin
capsules were placed under the fascia of the latissimus
dorsi muscle. Ten rats had one NiTi capsule and 8 of the
10 rats also had two other alloy capsules inserted. The
control rats received only gelatin capsules containing
allogenic matrix without any metal implants through
a similar surgical operation.
2.5. Specimen processing
After 8 weeks of follow-up,the animals were eutha-
nized and the induced ossicles with the implants were
removed and "xed with PBS-bu!ered neutral formalin.
2.6. pQCT studies
The total bone mineral density (BMD) of the ossicles
was measured with pQCT (XCT920A,Stratec,Ger-
many). Pixel size was 0.145
m and section thickness
1.25 mm. pQCT scans were taken 1 mm apart from the
implant based on a scout view image.
2.7. Histological observations
After density measurements,the implants were embed-
ded in metacrylate (Technovit 7200),cut with a diamond
saw,and micro-ground (Exakt apparatebau GmbH) to
25
m. The ground samples were stained with the Mas-
son}Goldner-Trichrome method. Morphological and
histological observations were performed under a light
microscope (Nikon Optiphot II,Nikon,Japan) with
a 10
; objective (Nikon,Japan NA 0.04) and a confocal
LSM 510 microscope with a 63
; (NA 1.2/w) objective
(Zeiss,Germany).
2.8. Histomorphometric analysis
Polarized light microscopy was also used to distin-
guish between "brotic tissue and bone. The proportional
areas of "brotic tissue and non-resorbed bone matrix
powder compared to the implant area were histomor-
phometrically measured with a digital image analyzer
(MCID M4 v.3.0.rev.1.1,Imaging Research Inc.,Cana-
da). The target area was outlined by excluding the new
woven bone,but including "brotic tissue and non-resorb-
ed initial allogenic matrix. The proportional area of carti-
lage versus ossicle was measured with a digital image
analyzer. The third histomorphometric measure,the pro-
portional new bone area versus ossicle area,was also
determined with a digital image analyzer.
2476
A. Kapanen et al. / Biomaterials 22 (2001) 2475} 2480
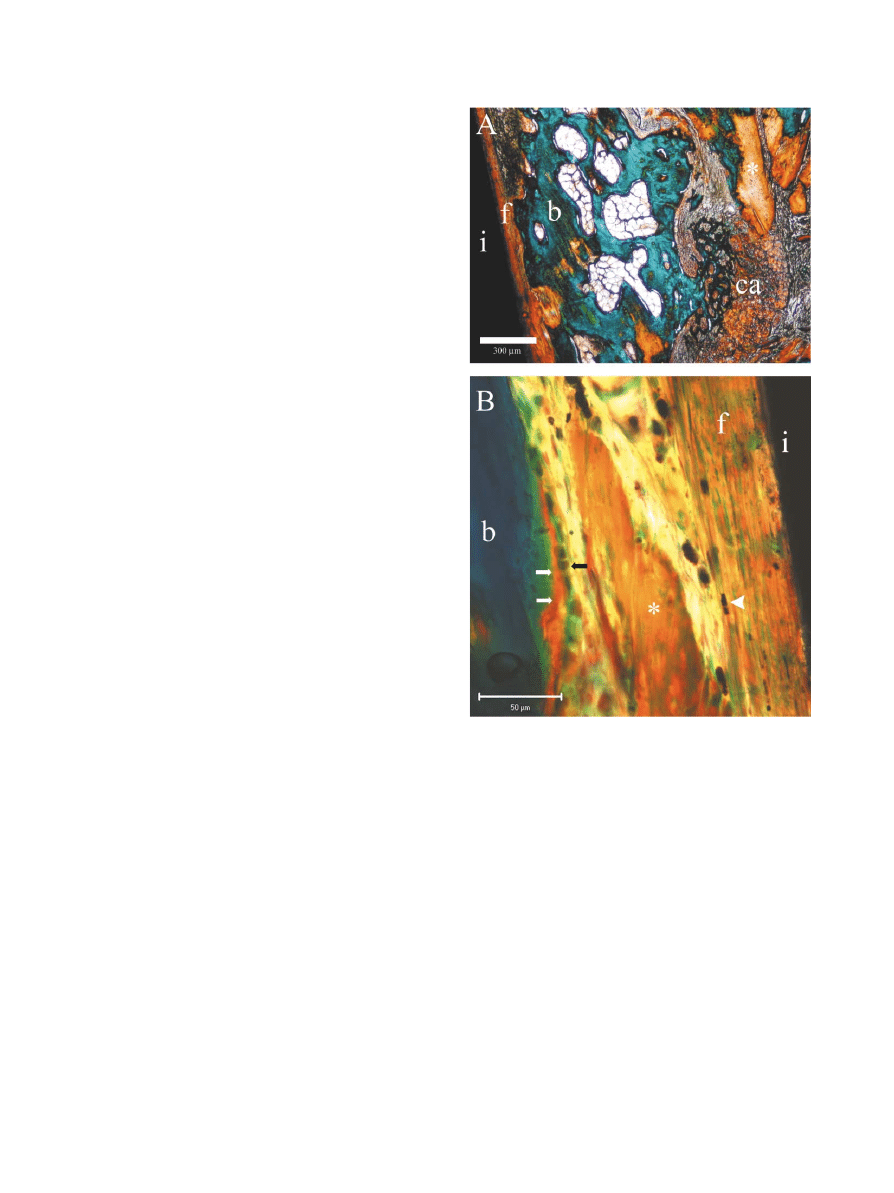
Fig. 1. (A) Light-microscopic view of a Masson}Goldner-Trichrome-
stained ossicle in the NiTi implant group after 8 weeks of implantation.
An implant (i) surrounded by "brotic tissue (f ) and bone matrix powder
(*). Cartilage (ca) and new bone (b). Bar"0.3 mm. (B) Higher magni"-
cation of implant interface. Implant (i), "brotic tissue (f ) with elongated
"broblasts (arrow head),bone matrix powder (
*),and new bone (b) with
osteoid (white arrows) synthesized by osteoblasts (black arrow).
Bar"0.05 mm.
2.9. Statistical analysis
All statistical analyses were performed with commer-
cial software (Origin5.0,Microcal Software Inc.,USA).
One-way ANOVA followed by two-sample t-test was
used. Probabilities of p)0.05 were considered signi"-
cant. Bonferroni corrections were applied to the t-test
comparisons.
3. Results
3.1. Soft tissue observations
We observed no skin irritation,infections or tumors in
any of the animals. In general,mature,#attened and
elongated "broblasts with wavy collagen "bers formed
a capsule around the implant (Fig. 1). A very close con-
tact between the implant and the new woven bone with-
out "brotic material was observed in some areas of two
Stst samples,two Ti}6Al}4V samples and one NiTi
sample. After 8 weeks,some of the decalci"ed allogenic
bone matrix particles were still unresorbed. In the con-
trol ossicles,advanced endochondral bone formation was
shown by the fact that there were very few decalci"ed
bone particles left. Most of the ossicles were "lled with
new woven bone.
3.2. pQCT studies
The BMD (Fig. 2A) of the Stst and the Ti}6Al}4V
groups were lower,even though not signi"cantly
(285$43 mg/cm
,293$73 mg/cm) compared with
the control group (350$69 mg/cm
). The BMD of NiTi
was equally high (360$87 mg/cm
) as in the control
group.
3.3. Histomorphometric analysis
Quantitation of the areas of "brotic tissue and bone
matrix powder around the alloy showed no di!erences
between the alloy groups (Fig. 2B). The mean proportion
of "brotic tissue and bone matrix powder was the same in
the Nitinol group as in the Stst group,but higher in the
Ti}6Al}4V group than in the other two groups. The
proportion of cartilage (Fig. 2C) was highest in the NiTi
group (0.16$0.09) compared to Ti}6Al}4V group
(0.05$0.04) (p)0.05). The proportion of cartilage in
the control group was low due to the advanced bone
formation compared to the alloy groups. The proportion
of new bone (Fig. 2D) in control group supports the
"nding
of cartilage measurement,since control group
showed highest amount of new bone. Instead,Stst group
had signi"cantly less new bone when compared to con-
trol (p)0.05). These three area parameters, "brotic tis-
sue#bone matrix powder/implant,cartilage/ossicle and
new bone/ossicle indicate the rate of endochondral ossi"-
cation
4. Discussion
Ectopic ossi"cation refers to bone induction at extra-
skeletal sites. This phenomenon can be seen in surgery,as
growth factors liberated during the operation may acti-
vate bone formation in the operative area. One impor-
tant question is whether the implant material and the
metal ions or wear particles released from its surface have
A. Kapanen et al. / Biomaterials 22 (2001) 2475} 2480
2477

Fig. 2. Histomorphometric measurements of ossicles formed in an
ectopic assay. (A) Total bone mineral density (BMD) of the ossicles.
Values do not di!er signi"cantly between alloy groups nor when
compared to control group. n"10 in the NiTi and control groups,
n"8 in the stainless steel and Ti}6Al}4V groups. The measurements
are means #1 SD. (B) Quantitation of the proportional area of "brotic
tissue and bone matrix powder around the implant was done by
utilizing the digital image analyzer connected to a normal light micro-
scope. There is no signi"cant di!erence between the alloy groups.
Number of samples n"10 in the NiTi group and n"8 in the stainless
steel and Ti}6Al}4V groups. The columns depict the ratio of areas #1
SD in the di!erent material groups. (C) Amount of cartilage as an area
proportional to the ossicle area. The values of NiTi are signi"cantly
higher when compared to Ti}6Al}4V (p)0.05). The columns depict
the ratio of areas#1 SD in the di!erent material groups. *"p)0.05.
(D) Amount of new bone as an area proportional to the ossicle area.
The value of Stst group is signi"cantly lower when compared to control
(p)0.05). There is no signi"cant di!erence between alloy groups. The
columns depict the ratio of areas #1 SD in the di!erent material
groups. *"p)0.05.
negative e!ects on bone formation. Some implant alloy
components may disturb bone formation. For example,
aluminum has a harmful e!ect on bone formation by
interfering with nodule formation [17] and inhibiting the
formation of hydroxyl apatite [18]. Here,ectopic bone
formation assay was used to study the e!ect of NiTi
material on bone formation. The method we introduced
tests the whole sequence of the induction of ossi"cation.
The trauma of implantation to bone induces osteogenesis
around the implant. The disturbances in the induction of
mesenchymal cells into chondroblasts and osteoblasts
may be of utmost importance in the lack of biocompati-
bility of a speci"c material. To our knowledge,ectopic
bone formation assay has not been used before in bio-
compatibility tests.
To avoid any immunological reactions that matrix
from other species would cause [19],we chose rat al-
logenic decalci"ed bone matrix. Packaging of the matrix
and implant into a gelatin capsule made the surgical
operation faster and the samples easy to handle. The
operation and the implant cause minimum discomfort
for the animal post-operatively or later.
Nickel may have toxic e!ects in vitro and in vivo at
high concentrations [20]. The high nickel content of
NiTi (54% by weight) might cause biocompatibility
problems due to the dissolution of nickel ions or wear
particles from the alloy. The release of Ni from NiTi
correlates with its corrosion resistance. Surface treat-
ments may markedly a!ect corrosion properties. At the
early stages,post-implantation Nitinol without any sur-
face treatment may release Ni ions more than stainless
steel before the titanium oxide surface of NiTi becomes
dominant. This hypothesis is supported by the previous
in vitro studies of RyhaKnen et al. [21] and Wever et al.
[22].
In our study,the surface preparation di!ered between
the test material groups. Stainless steel was electrolyti-
cally polished,while NiTi and Ti}6Al}4V were polished
by mechanical water sanding. Despite the di!erent sur-
face properties of the implants,all ossicles showed similar
morphological features. Endochondral bone formation
was evident,as cartilage was found in all of the ossicles
and new woven bone was produced. Unresorbed decalci-
"ed bone matrix,which had not yet been replaced by new
bone,was also seen due to the relatively short follow-up
time (8 weeks). On the other hand,the control ossicles
showed fully developed woven bone,indicating that en-
dochondral bone formation can be completed in 8 weeks
in a rat model.
In our experiment,pQCT was used for the total bone
mineral density measurements of ossicles. pQCT has
been proved as an e$cient and precise tool in evaluating
the geometric and densitometric properties of rat and
mouse bones [23}25]. Since metal causes scattering of
X-rays,no metal implants could be included in the pQCT
scanning. The scan section was 1.25 mm wide and the
2478
A. Kapanen et al. / Biomaterials 22 (2001) 2475} 2480

scanning center was placed 1 mm away from the implant.
The data acquired in this way represent the degree of
ossi"cation and mineralization in the vicinity of the
implant.
Our ectopic assay showed that ectopic bone formation
has better characteristics with NiTi implants than with
Stst or Ti}6Al}4V implants. The NiTi samples showed
equal proportions of combined "brotic tissue and bone
matrix powder area compared to the stainless-steel sam-
ples. The proportional cartilage and new bone areas were
even higher than in the stainless steel and Ti}6Al}4V
samples,indicating faster endochondral ossi"cation in
the NiTi group compared to the two other alloys. The
total bone mineral density of NiTi was as high as in the
control group,while in the Stst group it was the lowest. It
is possible that NiTi has lesser e!ects on mineralization
than the two other alloys tested here. Close bone contacts
were seen in some ossicles with all the tested materials.
However,no osteointegration occurred with any of these.
The closest contacts between bone and NiTi were under
10
m. In our case,the number of close contacts depends
on the follow-up time,since there was still some unresor-
bed matrix left after 8 weeks. It is known that ectopic
ossicles of this kind will form permanent remodeled bone
at the implantation site [26].
We do not believe that placing three di!erent types of
implants into the same animal would cause signi"cant
changes in the intra-individual responses to the di!erent
implants. It has been demonstrated that NiTi had
no negative e!ects on total new bone formation or
the normal regional acceleratory phenomenon (RAP)
after periosteal implantation during a 26-week follow-up
[27].
In vivo studies of NiTi implanted in soft tissues have
shown good biocompatibility [28,2]. In a study by
RyhaKnen et al.,NiTi had no negative e!ect on osteotomy
healing after 60 weeks of implantation [29]. However,
some controversial results have been reported after bone
implantation [30,31].
Recent in vitro studies also support the good biocom-
patibility of NiTi [21,32,33]. NiTi may not have equally
good corrosion resistance as Ti}6Al}4V [21],and NiTi
may initially release signi"cantly more nickel than stain-
less steel [34],but the amount of released nickel is
lower than the concentrations necessary to elicit
cytotoxic reactions.
5. Conclusion
Our results provided further evidence that NiTi has
good biocompatibility in bone tissue. NiTi showed signif-
icantly higher BMD around the implant than Stst. The
area of "brotic tissue around the implant was the same in
the NiTi and Stst groups. The amount of cartilage and
new bone were much greater in NiTi than in Stst or
Ti}6Al}4V,indicating faster endochondral bone forma-
tion with NiTi than with the other two implants. This
study also shows that the ectopic bone induction assay is
useful for testing the biocompatibility of biomaterials
and their in#uence on bone formation.
Acknowledgements
The authors thank Mrs. Minna Vanhala for her tech-
nical assistance. This study was supported by the Tech-
nology Development Center of Finland (TEKES).
References
[1] Buehler WJ,Wang FE. A summary of recent research on the
Nitinol* alloys and their potential application in ocean engineer-
ing. Ocean Engng 1968;1:105}20.
[2] Castleman LS,Motzkin SM,Alicandri FP,Bonawit VL. Biocom-
patibility of Nitinol alloy as an implant material. J Biomed Mater
Res 1976;10(5):695}731.
[3] Baumgart F,Bensmann G,Haasters J. Memory alloys * new
material for implantation in orthopedic surgery. In: Uthof HK,
editor. Current concepts of internal "xation of fractures. Berlin:
Springer,1980. p. 122}7.
[4] Cuschieri A. Variable curvature shape-memory spatula for
laparoscopic surgery. Surg Endoscopy 1991;5:179}81.
[5] Blum U,Voshage G,Lammer J,Beyersdorf F,Tollner D,Kret-
schmer G,Spillner G,Polterauer P,Nagel G,Holzenbein T,et al.
Endoluminal
stent-grafts
for
infrarenal
abdominal
aortic
aneurysms. N Engl J Med 1997;336:13}20.
[6] Chan KC,Godman MJ,Walsh K,Wilson N,Redington A,Gibbs
JL. Transcatheter closure of atrial septal defect and interatrial
communications with a new self expanding nitinol double disc
device (Amplatzer septal occluder): multicentre UK experience.
Heart 1999;82(3):300}6.
[7] Rickers C,Hamm C,Stern H,Hofmann T,Franzen O,SchraKder
R,Sievert H,Schranz D,Michel-Behnke I,Vogt J,et al. Per-
cutaneous closure of secundum atrial septal defect with a new self
centring device (
`angel wingsa). Heart 1998;80:517}21.
[8] Urist
M.
Bone:
formation
by
autoinduction.
Science
1965;150(698):893}9.
[9] Wozney JM,Rosen V,Celeste AJ,Mitsock LM,Whitters MJ,
Kriz RW,Hewick RM,Wang EA. Novel regulators of bone
formation: molecular clones and activities. Science 1988;242:
1528}34.
[10] Urist M,Strates BS. Bone morphogenetic protein. J Dent Res
1971;50(6):1392}406.
[11] Khouri RK,Koudsi B,Reddi AH. Tissue transformation into
bone
in
vivo.
A
potential
practical application.
JAMA
1991;266(14):1953}5.
[12] Lindholm TS,Urist M. A quantitative analysis of new bone
formation by induction in composite grafts of bone marrow and
bone matrix. Clin Orthop 1980;(150):288}300.
[13] Ono MD,Tateshita MD,Nakajima T. Porous hydroxyapatite
ceramics and their ability to be "xed by commercially available
screws. Biomaterials 1999;20(17):1595}602.
[14] Ogawa Y,Schmidt DK,Nathan RM,Armstrong RM,Miller KL,
Sawamura SJ,Ziman JM,Erickson KL,de Leon ER,Rosen DM.
Bovine bone activin enhances bone morphogenetic protein-
induced ectopic bone formation. J Biol Chem 1992;267(20):
14233}7.
A. Kapanen et al. / Biomaterials 22 (2001) 2475} 2480
2479

[15] Pinholt EM,Solheim E,Bang G,Sudmann E. Bone induction by
composites of bioresorbable carriers and demineralized bone in
rats: a comparative study of "brin}collagen paste, "brin sealant,
and polyorthoester with gentamicin. J Oral Maxillofacial Surg
1992;50(12):1300}4.
[16] Anderson HC. The role of cells versus matrix in bone induction.
[Review] Connect Tissue Res 1990;24(1):3}12.
[17] Sprague SM,Krieger NSK,Bushinsky DA. Aluminum inhibits
bone nodule formation and calci"cation in vitro. Am J Physiol
1993;264:F882}90.
[18] Blumenthal N,Posner A. In vitro model of aluminum-induced
osteomalacia: inhibition of hydroxyapatite formation and growth.
Calcif Tissue Int 1984;36(4):439}41.
[19] Nilsson OS,Urist MR. Immune inhibition of repair of canine
skull trephine defects implanted with partially puri"ed bovine
morphogenetic protein. Int Orthop 1991;15(3):257}63.
[20] Gerber H,Perren SM. Evaluation of tissue compatibility of
in vitro cultures of embryonic bone. In: Winter GD,Leray JL,de
Groot K,editors. Evaluation of biomaterials. New York: Wiley,
1980. p. 307}14.
[21] RyhaKnen J,Niemi E,Serlo W,NiemelaK E,Sandvik P,Pernu H,
Salo T. Biocompatibility of nickel}titanium shape memory metal
and its corrosion behavior in human cell cultures. J Biomed
Mater Res 1997;35(4):451}7.
[22] Wever DJ,Veldhuizen AG,de Vries J,Busscher HJ,Uges DR,
van Horn JR. Electrochemical and surface characterization of
a nickel}titanium alloy. Biomaterials 1998;19(7-9):761}9.
[23] Augat P,Merk J,Genant HK,Claes L. Quantitative assessment
of experimental fracture repair by peripheral computed tomogra-
phy. Calcif Tissue Int 1997;60(2):194}9.
[24] Takada M,Engelke K,Hagiwara S,Grampp S,Genant HK.
Accuracy and precision study in vitro for peripheral quantitative
computed tomography. Osteoporosis Int 1996;6(3):207}12.
[25] JaKmsaK T,Jalovaara P,Peng Z,VaKaKnaKnen HK,Tuukkanen J.
Comparison of three-point bending test and peripheral quantita-
tive computed tomography analysis in the evaluation of the
strength of mouse femur and tibia. Bone 1998;23(2):155}61.
[26] Viljanen VV,Lindholm TC,Gao TJ,Lindholm TS. Low dosage
of native allogeneic bone morphogenetic protein in repair of sheep
calvarial defects. Int J Oral Maxillofacial Surg 1997;26:389}93.
[27] RyhaKnen J,Kallioinen M,Tuukkanen J,Lehenkari P,Junila J,
NiemelaK E,Sandvik P,Serlo W. Bone modeling and cell}material
interface responses induced by nickel}titanium shape memory
alloy after periosteal implantation. Biomaterials 1999;20(14):
1309}17.
[28] RyhaKnen J,Kallioinen M,Tuukkanen J,Junila J,NiemelaK E,
Sandvik P,Serlo W. In vivo biocompatibility evaluation of
nickel}titanium shape memory metal alloy: muscle and perineural
tissue responses and encapsule membrane thickness. J Biomed
Mater Res 1998;41:481}8.
[29] RyhaKnen J,Kallioinen M,Serlo W,PeraKmaKki P,Junila J,Sandvik
P,NiemelaK E,Tuukkanen J. Bone healing and mineralization,
implant corrosion and trace metals after nickel}titanium shape
memory metal intramedullary "xation. J Biomed Mater Res
1999;47(4):472}80.
[30] Takeshita F,Takata H,Ayukawa Y,Suetsugu T. Histomor-
phometric analysis of the response of rat tibiae to shape memory
alloy (nitinol). Biomaterials 1997;18:21}5.
[31] Berger-Gorbet M,Broxup B,Rivard C,Yahia LH. Biocompati-
bility testing of NiTi screws using immunohistochemistry on
sections containing metallic implants. J Biomed Mater Res 1996;
32(2):243}8.
[32] Assad M,Lemieux N,Rivard CH,Yahia LH. Comparative in
vitro biocompatibility of nickel}titanium,pure nickel,pure tita-
nium,and stainless steel: genotoxicity and atomic absorption
evalution. Bio-Med Mater Engng 1999;9:1}12.
[33] Putters JL,Kaulesar Sukul DM,de Zeeuw GR,Bijma A,Be-
sselink PA. Comparative cell culture e!ects of shape memory
metal (Nitinol),nickel and titanium: a biocompatibility estima-
tion. Eur Surg Res 1992;24(6):378}82.
[34] Wy J,Beatty MW,Reinhardt RA,Petro TM,Cohen DM,Maze
CR,Strom EA,Ho!man M. Nickel release from orthodontic arch
wires and cellular immune response to various nickel concentra-
tions. J Biomed Mater Res 1999;48(4):488}95.
2480
A. Kapanen et al. / Biomaterials 22 (2001) 2475} 2480
Wyszukiwarka
Podobne podstrony:
In vitro biological effects of titanium rough surface obtain
Effects of topography and composition of titanium surface ox
Effect of long chain branching Nieznany
Effect of Kinesio taping on muscle strength in athletes
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
Effect of File Sharing on Record Sales March2004
31 411 423 Effect of EAF and ESR Technologies on the Yield of Alloying Elements
21 269 287 Effect of Niobium and Vanadium as an Alloying Elements in Tool Steels
(10)Bactericidal Effect of Silver Nanoparticles
Effect of?renaline on survival in out of hospital?rdiac arrest
Effects of the Great?pression on the U S and the World
4 effects of honed cylinder art Nieznany
Effects of the Atomic Bombs Dropped on Japan
Effect of aqueous extract
Effect of Active Muscle Forces Nieznany
Effects of Kinesio Tape to Reduce Hand Edema in Acute Stroke
1 Effect of Self Weight on a Cantilever Beam
effect of varying doses of caffeine on life span D melanogaster
Possible Effects of Strategy Instruction on L1 and L2 Reading
więcej podobnych podstron