
Biomaterials 23 (2002) 3733–3740
Effect of metal alloy surface stresses on the viability of ROS-17/2.8
osteoblastic cells
Anita Kapanen
a,
*, Anatoli Danilov
a,c
, Petri Lehenkari
b
, Jorma Ryh
.anen
b
, Timo J
.ams.a
c
,
Juha Tuukkanen
a
a
Biocenter Oulu and Department of Anatomy and Cell Biology, University of Oulu, P.O. Box 5000 FIN-90014 Oulu, Finland
b
Department of Surgery, University of Oulu, P.O. Box 5000 FIN-90014 Oulu, Finland
c
Department of Medical Technology, University of Oulu, P.O. Box 5000 FIN-90014 Oulu, Finland
Received 2 July 2001; accepted 14 March 2002
Abstract
In this study we compared the effect of structural stresses and surface roughness on biocompatibility of NiTi- and Ti-alloy for
ROS-17/2.8 osteoblastic cells. We suggest here that cell viability and cell attachment are linear functions of internal (structural)
stress and subgrain size of the implant alloy. However, this is not the case with surface roughness. The two-phase state in these
materials is characterized by different mean values of structural stresses (s) in a-martensite and b-phase. We found a straight
correlation between cell viability and s
b
=s
a
ratio. Atomic force microscopy revealed that, even after equal surface polishing
treatments, roughness varied significantly between the different alloys. The effect of the surface structure of the alloy on the
osteoblastic ROS-17/2.8 cell survival rate was studied with combined calcein-ethidium-homodimer fluorescence labeling. The
possible effects on cell attachment to substrate were studied by staining the focal contacts with paxillin antibody. All the NiTi
surfaces were tolerated well and the cells attached most abundantly to the roughest NiTi surface but the smoothest Ti-alloy surface.
However, other parameters of the material state, such as the surface stresses created by hot rolling seem to be responsible for some
of the attachment and cell survival features observed in this study. r 2002 Elsevier Science Ltd. All rights reserved.
Keywords: NiTi; Titanium alloys; Surface stresses; AFM; ROS-17/2.8
1. Introduction
A material consisting of nearly equiatomic parts of
nickel and titanium (Nitinol) has superelasticity, ther-
mal shape memory and good damping properties, which
make it a promising surgical implant material, especially
in orthopedics [1–3]. Studies done with human fibro-
blasts and human osteoblasts show good in vitro
biocompatibility of NiTi [4,5].
There are several papers convincing that surface
roughness contributes to osteoblast attachment and
spreading. Rat calvaria-derived osteoblast attachment
increased as a function of surface roughness [6]. A study
on chemically pure Ti and Ti-alloy surfaces further
indicated increased attachment as a function of surface
roughness [7]. The recent study by Lohmann et al.
(2000) with three different cell lines showed that surface
roughness promotes osteogenic differentiation of less
mature cells. More mature cells exhibit a reduced
sensitivity to their substrate, but are still affected by
changes in surface roughness [8]. However, there are in
vitro studies that indicate attachment favoring smooth
surfaces [9–14]. Some in vivo studies done with dogs,
rabbits and pigs indicate that increasing surface rough-
ness is associated with enhanced bone formation at
implant surfaces [15–17]. But surface roughness, being a
characteristic of surface topography, could not describe
the whole spectrum of subtle differences in material
surface state caused by the differences in its structures
and phase composition. At the same time, it is well
known that the internal or structural stresses are very
sensitive characteristics of the state of the material. The
structural stresses reflect such structural parameters as
density and distribution of dislocations, twins and
stacking faults. The stresses react on phase composition
changes. In addition, the ratios of stresses in different
*Corresponding author. Tel.: +358-8-537-5188; fax: +358-8-537-
5172.
E-mail address:
anita.kapanen@oulu.fi (A. Kapanen).
0142-9612/02/$ - see front matter r 2002 Elsevier Science Ltd. All rights reserved.
PII: S 0 14 2 - 9 6 12 ( 0 2 ) 0 0 10 7 - 2

phases inform about probable consequences of phase
transformations on corrosion and electrochemical prop-
erties. Among the important causes of internal stresses
we may mention mechanical, chemical, heat and
radiation treatments. Therefore, we find it relevant to
apply definition of structural stress to biocompatibility
investigations.
The aim of the present study was to assess the effect of
structural stresses in NiTi- and two-phase (a þ b) Ti-
alloys on the viability of ROS-17/2.8 osteoblastic cells in
comparison with the effect of surface roughness.
Different NiTi surfaces were compared with those of a
Ti-alloy with two different hot-rolling treatments. An
atomic force microscope (AFM) and X-ray structural
analysis was used to examine the surface characteristics.
2. Materials and methods
2.1. Test materials
The tested materials were binary NiTi shape memory
alloy (Ti–44 wt%, Ni–56 wt%, Unitec) and two phase
(a þ b) Ti-based alloy (Ti–90.5 wt%, Al–6 wt%, Mo–
2.2 wt%, Cr–1.3 wt%, Institute of Light Metals, Mos-
cow, Russia) vacuum-melted and hot-rolled. The choice
of materials was governed by their good perspectives for
biomedical application: NiTi because of its well-known
biomechanical and corrosion properties, Ti6Al2.2-
Mo1.3Cr alloy seems to be a competitor to wide-spread
Ti6Al4V-alloy because of toxic vanadium substitution
for less toxic elements as Mo and Cr. To initiate
different phase composition and thus different structural
stresses, two rolling temperatures for two groups of Ti-
alloy were used: 8501C (TiI) and 10501C (TiII). The
rolling temperature of NiTi was 9501C.
To initiate further surface stress grades, test disks of
5 mm in diameter and 3 mm in thickness were ground by
grinding stone N80 (group 80, n ¼ 6) followed by
carbon silicon paper of decreasing coarseness 240, 320,
400, 600 (group 600, n ¼ 6), 800, 1200 and finally
polished by rubber wheel (only NiTi-alloy samples) and
cloth with chromium oxide paste (group 1200, n ¼ 6).
The rubber wheel was used for NiTi samples to achieve
the high polished state, which could not be achieved
only by polishing with cloth because of the high wear-
proofness of NiTi. The test disks were then washed in an
ultrasonic vibrobath, degreased with 70% ethanol for
10 min and autoclaved at 1201C for 20 min before use.
2.2. Surface roughness measurement
AFM measurements were performed with Explorer
system (Thermomicroscopes, Sunnyvale, USA) and
SPMLabNT software ver. 5.01Explorer AFM (Ther-
momicroscopes, Sunnyvale, USA). The sizes of scanned
area were 100 100 mm
2
.
The following roughness parameters were measured:
R
a
is the average roughness is the arithmetic average
deviation from the mean line. R
p
the maximum peak is
the maximum height or the highest peak of the
roughness profile above the mean line. R
t
the maximum
peak to valley is the sum total of the maximum peak and
maximum valley measurements of roughness within the
length assessed. R
tm
is the more representative mean
value of the entire profile.
Three disks in each test group were analyzed on three
randomly chosen lines, and the means of the parameters
were calculated. Table 1displays the surface roughness
parameters of the different alloys.
2.3. X-ray structural analysis
X-ray structural analysis of phase composition,
internal stresses and subgrain sizes (in NiTi and TiI
samples) was performed on the X-ray diffractometer
DRON-3.0 (X-ray equipment Co ‘‘Burevestnik’’, Saint-
Petersburg, Russia) with filtered Cu K
a
-radiation. The
mean values of lattice strains (e) that were used for stress
calculation, and the mean size of subgrains were
determined in accordance with the procedure described
in [18]. The necessity to separate the effect of stresses
and subgrain sizes on diffraction line broadening was
the initial reason to analyze both of these structural
Table 1
Roughness parameters of the tested alloys
Alloy
R
a
(nm)
R
p
(nm)
R
t
(nm)
R
tm
(nm)
NiTi 80
362.2
7209.2
636.6
7152.6
1434.3
7501.1
448.4
754.8
NiTi 600
156.0
720.5
632.7
7195.2
633.2
7443.5
506.9
741.4
NiTi 1200
95.2
741.4
158.0
738.9
425.2
784.11
98.4
721.5
TiI 80
1479.5
738.9
3120.0
7583.4
6847.0
7301.9
2568.0
712.7
TiI 600
1428.5
7173.2
1850.0
71064.2
5823.0
7707.1
1198.0
7142.8
TiI 1200
304.8
731.4
846.0
7548.7
1353.0
71061.2
539.8
7161.7
TiII 80
732.1
749.12285.07304.13740.0772.1948.1
749.9
TiII 600
436.3
710.4
1098.0
7210.2
2244.0
7318.9
669.0
756.1
TiII 1200
339.2
739.9
579.8
785.8
1381.0
711.3
418.2
721.4
A. Kapanen et al. / Biomaterials 23 (2002) 3733–3740
3734

parameters. The structural stresses s were calculated
from formula s ¼ Ee; where Young
’
s modulus E was
7 10
4
MPa for NiTi, 10.3 10
4
MPa for b-phase and
11.3 10
4
MPa for a-martensite in titanium alloy. The
values of structural stresses and subgrain sizes in
martensitic phase of NiTi were not studied because of
the small amounts of martensite (weak diffraction lines).
The analysis of internal stresses in TiII group samples
was not performed in the present study.
2.4. Cell culture
Rat osteosarcoma cell line ROS-17/2.8 (a generous
gift from G.A. Rodan, Merck Research Laboratories,
West Point, PA, USA) cultures were carried out in
minimal essential medium (MEM, Gibco) supplemented
with 10% fetal calf serum (Bioclear), antibiotics (100 U
of penicillin/ml, 100 mg of streptomycin/ml) and l-
glutamine (2 mm) at +371C (5% CO
2
, 95% air). The
cultures were allowed to reach confluency before
subculturing onto metal alloy disks. The cells were
washed with 371C phosphate-buffered saline (PBS), and
adherent cells were detached by using trypsin-EDTA.
Five thousand cells were seeded per disk (n ¼ 6) and
allowed to attach for 3 h. The cells were allowed to grow
for 48 h before staining with a cytotoxicity test kit or
fixation with 4% paraformaldehyde (PFA).
Because cells grown on disks cannot be seen in normal
light microscopy, the cells were also cultured on glass
cover slips 10 mm in diameter to assess the time of
subculture confluency.
2.5. Cytotoxicity test
The cells on the disks were washed twice with a warm
PBS solution and stained with a LIVE/DEAD
s
Via-
bility/Cytotoxicity kit (Molecular Probes, Oregon,
USA). The optimal concentration of the ethidium
homodimer-1(EthD-1) dye was 0.1mm and that of the
calcein dye 1 mm. The samples were incubated for 15 min
at 371C and viewed under a fluorescence microscope.
Dead cells (stained red) and live cells (stained green)
counted from six randomly chosen areas (0.849 mm
2
) on
each disk were viewed. The cells were counted visually
under a fluorescence microscope (Nikon Eclipse E600,
Nikon, Japan) with a 10 objective, NA 0.25 (Nikon,
Japan), and the ratio of dead to live cells was computed.
Approximately 350 cells were seen in each area. The
number of dead cells per image was expressed as per
1000 cells.
2.6. Immunofluoresence microscopy of focal contacts
The PFA-fixed cells were permeabilized with 0.1%
Triton-X-100 in PBS for 10 min on ice. The cells were
stained
by
using
monoclonal
paxillin
antibody
(ZYMED Laboratories, Inc., San Francisco, CA,
USA) at 1:100 in PBS for 45 min on ice. Staining was
carried out with rhodamine-conjugated rabbit anti-
mouse immunoglobulin secondary antibodies (DAKO,
Glostrup, Denmark) for 30 min on ice. To visualize the
nuclei, the cells were incubated with the DNA-binding
fluorochrome Hoechst 33258 (1:1000) for 10 min at
room temperature. The focal contacts were studied
under a confocal microscope LSM 510 equipped with an
inverted microscope Axiovert 100M and 63 objective
(NA 1.2/water, Zeiss, Germany). From each sample
disk, 6 frames were scanned with 1024 1024 frame size
(pixel size 0.14 0.14 mm
2
). The number of focal
contacts was measured with a digital image analyzer
(MCID M4 v.3.0.re.1.1, Imaging Research Inc., Cana-
da).
The
measured
region
of
interest
was
146.2 146.2 mm
2
. The confocal microscope images
were segmented based on red color intensity. The
interactively defined paxillin-containing focal contacts
were automatically counted from the region of interest.
2.7. Statistical analysis
Mean values and standard deviations were computed.
Analysis of variance (ANOVA) and Student’s t-test
were utilized to assess the level of significance of the
differences between the experimental groups. Bonferro-
nis corrections were applied to the t-tests. All statistical
analyses were performed with commercial software
(Origin 5.0, Microcal Software, Inc., USA).
3. Results
3.1. X-ray structural analysis
The results of X-ray experiments showed that the
above treatments of tested alloys resulted in different
phase state of samples. The initial hot-rolled state of
NiTi sample was austenite. The grinding by stone N80
did not change the phase composition but gave rise to
diffraction line broadening that is typical for cold-
worked metals because of increase in structural stresses.
Further grinding and polishing were accompanied first
by remarkable decrease of structural stresses (group
600) and then by their negligible increase (group 1200).
Another structural parameter that was parallel stu-
died—mean size of subgrains—changed in opposite
manner: group 80 was characterized by the smallest
sizes, in group 600 samples subgrain sizes were the
biggest and in group 1200 this parameter again became
smaller (Table 2). Simultaneously, small amounts of
martensitic phase appeared in the samples of groups 600
and 1200.
The same behavior of structural stresses was observed
in the basic martensitic a-phase of titanium alloy. The
A. Kapanen et al. / Biomaterials 23 (2002) 3733–3740
3735
highest values of s
a
were in samples of group 80, in
group 600 they were the smallest and then rose again in
group 1200. As a result of the described behavior, the
ratio s
b
=s
a
had the minimum value in group 80,
maximum in group 600, and again decreased in group
1200. Nevertheless, the mean values of stresses in b-
phase (s
b
) steadily increased as well as the subgrain sizes
in both b-phase and a-martensite (Table 2). The
mechanical treatment of titanium alloy was not accom-
panied by changes in phase composition, and initial b-
phase content (10–12 vol%) were kept in the samples
within investigated groups.
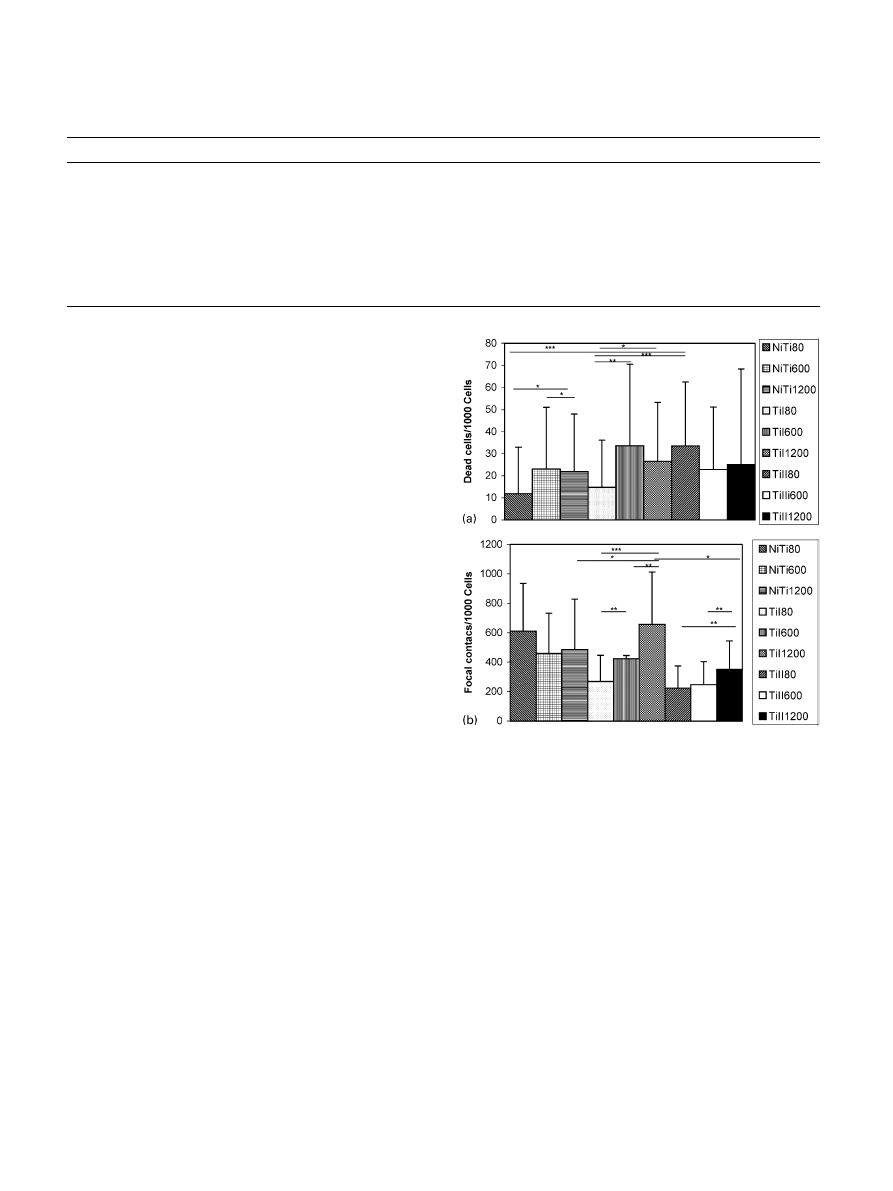
3.2. Cytotoxicity test
The cells cultured on sandpapered surfaces appeared
larger in a visual examination. On the roughest disks in
the TiII group, the cultures did not reach complete
confluency in 48 h, as did the cultures on NiTi. The
cytotoxicity test showed that the roughest NiTi and TiI
surfaces were significantly better in view of cell viability
than the other surfaces in the test groups. When we
compared the different alloys within the same roughness
group, it turned out that, in group 80, NiTi and TiI had
significantly fewer dead cells (12
721and 1
5
721,
respectively) than TiII (34
729), (pp0:001). The 600
and 1200 groups did not differ significantly. Within the
NiTi test group, the number of dead cells on the
roughest (80) surface (12
721) was significantly lower
compared to the 600 (23
728), (pp0:05) and 1200
(22
726), (pp0:05) surfaces. The results in the TiI group
were similar, with the 80 surface (15
721), showing a
significantly lower number of dead cells compared to the
600 (34
737), (pp0:01) and 1200 surfaces (27727),
(p
p0.05). The TiII group showed no significant
differences
between
the
three
roughness
groups
(34
729, 23729 and 25743, respectively) (Fig. 1A).
3.3. Attachment of cells
The focal adhesions of the cells grown on the test
materials seemed to locate parallel to the grinding
grooves (Fig. 2). This phenomenon was clearly seen on
the roughest surface, but was also observed on the other
surfaces.
We determined the number of focal adhesions based
on the paxillin staining of the cells. The results showed
that NiTi 80 strongly stimulated the formation of focal
adhesions formation. Only TiI 1200 was equally efficient
as NiTi 80 as a promoter of cell attachment. Overall, TiI
was a better matrix for osteoblast attachment than TiII.
The different surface roughness grades of NiTi did not
significantly differ in the number of focal adhesions
(611
7325, 4607272 and 4857343). In the TiI group,
the number of focal adhesions was significantly lower on
the roughest surface (269
7177) compared to the 600
(423
7222), (pp0:01) and 1200 (6587355), (pp0:001)
Table 2
The structural stresses and mean values of subgrain size in austenite of NiTi, b-phase and a-martensite of titanium alloy and Ti-alloys s
b
=s
a
ratio
Sample group NiTi
Structural stresses (MPa)
Subgrain size (nm)
80
259
71 9
35
600
143
79
88
1200
162
79
43
Sample groupTiI
b-phase
a-martensite
Structural stresses (MPa)
Subgrain size (nm)
Structural stresses (MPa)
Subgrain size (nm)
s
b
=s
a
80
164
76
28
1
24
77
62
1.32
600
228
71 3
39
45
73
100
5.07
1200
306
722
110
79
75
235
3.87
Fig. 1. (A) Results of the cytotoxicity test. NiTi—nickel titanium
alloy, TiI—titanium alloy with 8501C hot rolling, TiII—titanium alloy
with 10501C hot rolling. ¼ p
p0:05; ¼ pp0:01; ¼ pp0:001:
(B) Number of focal adhesions. ¼ p
p0:05; ¼ pp0:01; ¼
p
p0:001:
A. Kapanen et al. / Biomaterials 23 (2002) 3733–3740
3736

surfaces. The TiI 1200 group had a significantly
higher number of focal adhesions than the 600 group
(p
p0:01). The TiII 1200 surface had a significantly
higher number of focal adhesions (351
7194) than the
surfaces of the other two roughness grades (223
7151 in
group 80 and 248
7156 in group 600), (pp0:01)
(Fig. 1B).
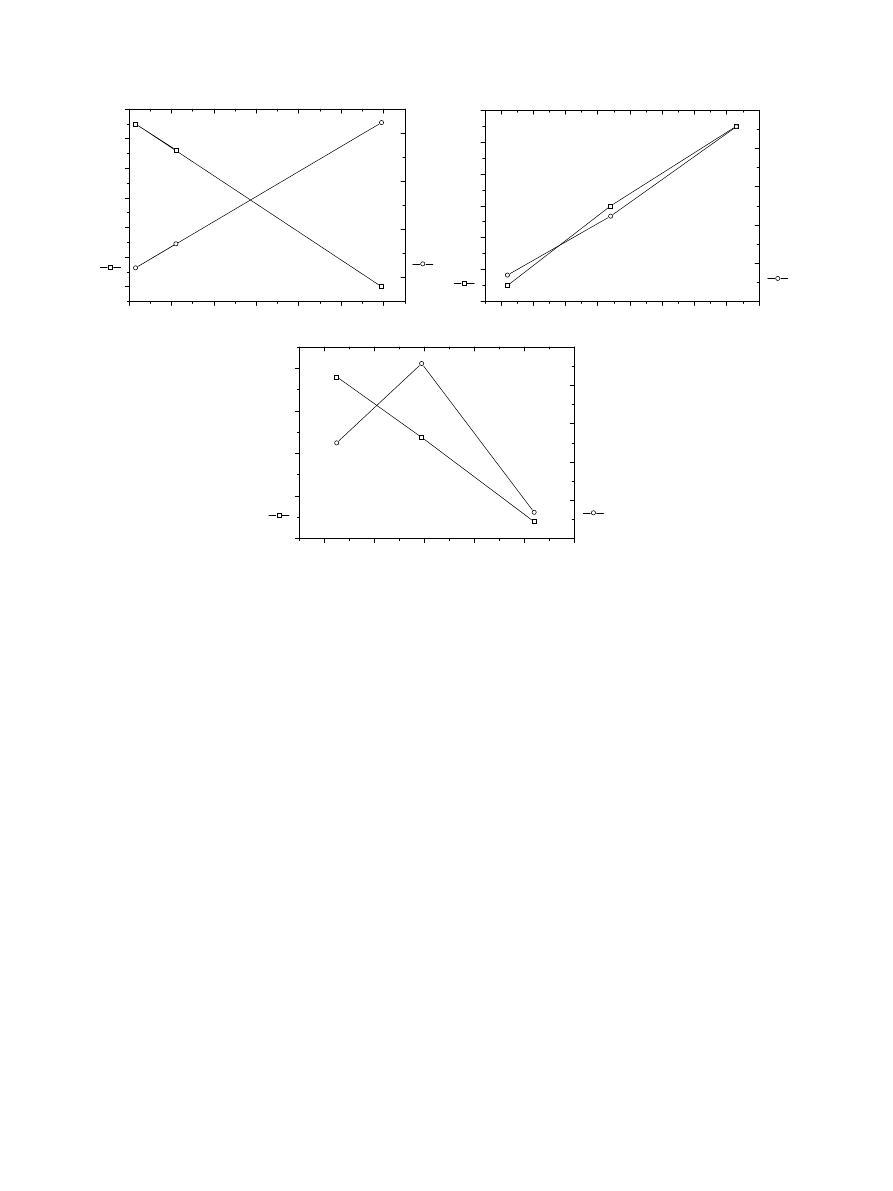
3.4. Effect of surface stress on biological parameters
The analysis of the results obtained from biological
tests and X-ray experiments demonstrate that mean
values of both biocompatibility parameters, cell survival
and cell attachment, seem to be linear functions of
internal stresses and subgrain sizes, respectively. In
the NiTi group, the increase in structural stress
increased the cell attachment but was in reverse
association to cell death rate (Fig. 3A). Interesting
finding was that in the b-phase of TiI alloy both the
cell attachment and the cell death rate increased with
increasing structural stress (Fig. 3B). In the a-martensite
phase of TiI alloy the effect of structural stress was
opposite (Fig. 3C).
4. Discussion
To detect common regularities of biocompatibility
relative to the surface of the implant, our study suggests
that it would be better to characterize surface stress than
roughness. A cyclic recovery effect (structural stress
decrease) during permanent deformation of pure metals
Fig. 2. Confocal microscope image of ROS-17/2.8 cultures on surfaces of different roughness. The small white arrows show the direction of the
grinding grooves, to which the focal contacts are parallel. The white arrowheads point out the diffusively stained cells without clear focal contacts.
Scale 20 mm.
A. Kapanen et al. / Biomaterials 23 (2002) 3733–3740
3737
has been long known [18] and is a result of dislocation
redistribution, which is testified by subgrain sizes
changes. Further investigations showed that the same
effect takes place as a result of strain-induced phase
reactions. Obviously, both of these mechanisms deter-
mine stress behavior in NiTi alloy, but only the
dislocation redistribution is responsible for stress
behavior in titanium alloy, though its details in b-phase
and martensite are different because of their different
crystal lattices.
The initial softness or hardness of the metal alloy
affects surface roughness. Therefore, in our study, the
roughness of the sandpaper used did not correlate with
the measured surface roughness parameters in Table 1.
Our results showed the roughest NiTi surface to be
favorable for osteoblastic cells. Both the low number of
dead cells and the high number of focal contacts showed
that a rough NiTi surface is well tolerated by ROS-17/
2.8 cells. Despite the fact that attachment number does
not give data about attachment strength, inverse
correlation between attachment site number and cell
death rate proves that cells do not tolerate different
surfaces on the same manner. Our finding of the effect of
NiTi surface roughness is contradictory to some earlier
studies done with other metal alloys, such as Ti6Al4V,
cobalt–chromium,
titanium
and
hydroxyapatite
[19,20,14]. However, our results on Ti-alloy are in line
with these studies. In both Ti-alloy groups, focal
contacts were less numerous on rough surfaces than
on smooth ones.
In addition, we found that focal contacts seemed to
align with the grinding grooves of the rough NiTi and
TiI surfaces. In cell culture studies, Anselme et al. (2000)
observed that the rougher the surface, the more
disorganized was the cell layer [19]. In our experiments,
no such correlation was seen.
We further found that there was a change in cell size
related to surface roughness. Larger cells were more
numerous on the roughest surface, especially on NiTi
disks. Large cells were also seen on the second roughest
surface (600). However, only few of them were noticed
on the smoothest surface (1200) and none on any of the
TiII alloy specimens. A study done with human
osteoblastic MG-63 cells showed that the cells cultured
on the roughest surfaces had more cuboidal morphology
and were more differentiated [12].
The regularities of biocompatibility parameters as
functions of structural stresses in NiTi-alloy reveal
that higher stresses promote better biocompatibility
parameters. This result is in contradiction with the
140
160
180
200
220
240
260
12
14
16
18
20
22
24
Structural stress, MPa
Dead cells
/1000 cells
450
500
550
600
NiTi
Focal adhesions
160
180
200
220
240
260
280
300
320
16
18
20
22
24
26
28
Structural stress, MPa
Dead cells
/1000 cells
200
300
400
500
600
700
Ti I(
β
)
Focal adhesions
40
60
80
100
120
140
15
20
25
30
35
Structural stress, MPa
Dead cells/1000 cells
200
300
400
500
600
700
Ti I(
α
)
Focal adhesions
(a)
(b)
(c)
Fig. 3. (A) Biocompatibility parameters as functions of structural stresses in NiTi. (B) Biocompatibility parameters as functions of structural stresses
of TiI-alloy b-phase. (C) Biocompatibility parameters as functions of structural stresses of TiI-alloy a-martensite.
A. Kapanen et al. / Biomaterials 23 (2002) 3733–3740
3738

well-known negative effect of stresses on the corrosion
properties of a material and the straight correlation
between these properties and biocompatibility [18].
Small number of experiments does not allow discussion
of
the
structural
reasons
of
this
phenomenon.
Results obtained for biocompatibility parameters in
titanium alloy b-phase point out the existence of
common
structural
causes
for
such
behavior
in
homogeneous materials with b-phase structure. How-
ever, it is possible that unusual NiTi-alloys proper-
ties provide different biochemical interaction with
cells.
If taken into account that biocompatibility para-
meters are the characteristics of whole sample and that
the basic phase in titanium alloys sample was a-
martensite, the obtained regularities of biocompatibility
parameters behavior in a þ b titanium alloy reflect the
same regularities for basic a-martensite. But the
behavior of cell viability as function of structural
stresses in titanium alloy b-phase remains unexplained.
To explain this experimental fact the effect of s
b
=s
a
ratio was analyzed. It is known that sharp difference of
structural stresses in two pieces of the same metal may
generate the pronounced values of electromotive force
(between two wires of the same metal one being in cold-
drawn state and other annealed, the emf is of the order
10
7
V/1C [18]). This effect may be reinforced by
chemical composition difference, which undoubtedly
exists between b-phase and a-martensite, otherwise b-
phase had to transform to martensite.
The present study showed a straight dependence of
amount of dead cells upon values of s
b
=s
a
ratio. This
may explains the observed dependence of focal adhesion
number on structural stresses in titanium alloy b-phase
and allows presuming that difference in electrochemical
properties of phases in heterogeneous materials and
phase quantity ratio are the main factors which
determine the cell viability. The results obtained in TiII
group samples (25–27 vol% of b-phase) confirmed the
above argumentation. Obviously, the same mechanism
acts in homogeneous materials, like NiTi. In those,
grains and subgrains play the role of different phases
with different concentration of stress. The apparent
dependence
of
biocompatibility
parameters
upon
the mean values of subgrain size in NiTi-alloy may
be
commented
in
terms
of
more
homogeneous
stress distribution in structures with smaller subgrain
sizes.
As shown in our previous work, nickel is toxic to
these cells [21]. It is important to notice that the rough
surface of a NiTi implant might release a high
concentration of Ni
2+
ions. However, this postulation
is not supported by our findings of the better attachment
and lower cytotoxicity of rough NiTi. The question
about the mechanisms that prevent nickel ion release,
even on a rough surface, remains to be solved.
5. Conclusion
Our results indicate low cytotoxicity of NiTi, even
after very rough surface treatment. NiTi disks were well
tolerated by osteoblastic ROS-17/2.8 cells. Because the
initial softness or hardness of metal alloys has an impact
on surface roughness, the characterization of surface
stresses could be a better method for assessing the
surface state of the implant after equal surface
manipulation. The results of the present study showed
that definition of structural stresses might be a sensitive
instrument in biocompatibility investigations.
Acknowledgements
This study was supported by Technology Develop-
ment Center of Finland (TEKES).
References
[1] Buehler WJ, Wang FEA. Summary of recent research on the
Nitinol alloys and their potential application in ocean engineer-
ing. Ocean Eng 1968;1:105–20.
[2] Castleman LS, Motzkin SM, Alicandri FP, Bonawit VL.
Biocompatibility of nitinol alloy as an implant material. J Biomed
Mater Res 1976;10(5):695–731.
[3] Baumgart F, Bensmann G, Haasters J. Memory alloys—new
material for implantation in orthopedic surgery. In: Uthof HK,
editor. Current concepts of internal fixation of fractures, vol. 1.
Berlin: Springer, 1980. p. 122–7.
[4] Putters JL, Kaulesar Sukul DM, de Zeeuw GR, Bijma A,
Besselink PA. Comparative cell culture effects of shape memory
metal (Nitinol), nickel and titanium: a biocompatibility estima-
tion. Eur Surg Res 1992;24(6):378–82.
[5] Ryh
.anen J, Niemi E, Serlo W, Niemel.a E, Sandvik P, Pernu H,
Salo T. Biocompatibility of nickel–titanium shape memory metal
and its corrosion behavior in human cell cultures. J Biomed Mater
Res 1997;35(4):451–7.
[6] Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM.
Optimization of surface micromorphology for enhanced osteo-
blast responses in vitro. Int J Oral Maxillofac Implants
1992;7:302–10.
[7] Keller JC, Stanford CM, Wightman JP, Draughn RA, Zaharias
R. Characterizations of titanium implant surfaces. III. J Biomed
Mater Res 1994;28(8):939–46.
[8] Lohmann CH, Bonewald LF, Sisk M, Sylvia VL, Cochran DL,
Dean DD, Boyan BD, Schwartz Z. Maturation state determines
the response of osteogenic cells to surface roughness and 1,25-
dihydroxyvitamin D3. J Bone Miner Res 2000;15(6):1169–80.
[9] Malik MA, Puleo DO, Bizios R, Doremus RH. Osteoblasts on
hydroxyapatite, alumina and bone surface in vitro: morphology
during the first 2 h of attachment. Biomaterials 1992;13:123–8.
[10] Stanford CM, Keller JC, Solursh M. Bone cell expression on
titanium surfaces is altered by sterilization treatments. J Dent Res
1994;73(5):1061–71.
[11] Meyer U, Szulczewski DH, M
.oller K, Heide H, Jones DB.
Attachment kinetics and differentiation of osteoblasts on different
biomaterials. Cells Mater 1993;3:129–40.
[12] Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J,
Lankford Jr J, Dean DD, Cochran DL, Boyan BD. Effect of
A. Kapanen et al. / Biomaterials 23 (2002) 3733–3740
3739

titanium surface roughness on proliferation, differentiation, and
protein synthesis of human osteoblast-like cells (MG63). J
Biomed Mater Res 1995;29(3):389–401.
[13] Alliott-Licht B, Gregoire M, Orly I, Menanteau J. Cellular
activity of osteoblasts in the presence of hydroxyapatite: an in
vitro experiment. Biomaterials 1991;12:752–6.
[14] Desantis D, Guerriero C, Nocini PF, Ungerspock A, Richards G,
Gotte P, Armato U. Adult human bone cells from jaw bones
cultured on plasma-sprayed or polished surfaces of titanium or
hydroxyapatite discs. J Mater Sci 1996;7:21–8.
[15] Gotfredsen K, Nimb L, Hj
.orting-Hansen E, Jensen JS, Holm!en
A. Histomorphometric and removal torque analysis for TiO2-
blasted titanium implants. An experimental study on dogs. Clin
Oral Implants Res 1992;3:77–84.
[16] Wennerberg A, Albrektsson T, Lausmaa J. Torque and histo-
morphometric evaluation of c.p. titanium screws blasted with 25-
and 75-microns-sized particles of Al
2
O
3
. J Biomed Mater Res
1996;30(2):251–60.
[17] Buser D, Nydegger T, Hirt HP, Cochran DL, Nolte LP.
Removal torque values of titanium implants in the maxilla
of miniature pigs. Int J Oral Maxillofac Implants 1998;13(5):
611–9.
[18] Taylor A. X-ray metallography. New York, London: Wiley, 1961,
p. 993.
[19] Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A,
Hardouin P. Qualitative and quantitative study of human
osteoblast adhesion on materials with various surface roughness.
J Biomed Mater Res 2000;49(2):155–66.
[20] Naji A, Harmand MF. Study of the effect of the surface state on
the cytocompatibility of a Co–Cr alloy using human osteoblasts
and fibroblasts. J Biomed Mater Res 1990;24:861–71.
[21] Kapanen A, Ilvesaro J, Danilov A, Ryh
.anen J, Lehenkari P,
Tuukkanen J. Behaviour of Nitinol in osteoblast-like ROS-17 cell
cultures. Biomaterials 2002;23(3):645–50.
A. Kapanen et al. / Biomaterials 23 (2002) 3733–3740
3740
Document Outline
- Effect of metal alloy surface stresses on the viability of ROS-17/2.8 osteoblastic cells
Wyszukiwarka
Podobne podstrony:
Zieliński, Marek i inni Effects of Constant Magnetic Field on Electrodeposition of Co W Cu Alloy (2
Effect of long chain branching Nieznany
Effect of Kinesio taping on muscle strength in athletes
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
Effect of File Sharing on Record Sales March2004
31 411 423 Effect of EAF and ESR Technologies on the Yield of Alloying Elements
21 269 287 Effect of Niobium and Vanadium as an Alloying Elements in Tool Steels
(10)Bactericidal Effect of Silver Nanoparticles
Effect of?renaline on survival in out of hospital?rdiac arrest
Effects of the Great?pression on the U S and the World
4 effects of honed cylinder art Nieznany
Effects of the Atomic Bombs Dropped on Japan
Effect of aqueous extract
Effect of Active Muscle Forces Nieznany
Effects of Kinesio Tape to Reduce Hand Edema in Acute Stroke
1 Effect of Self Weight on a Cantilever Beam
effect of varying doses of caffeine on life span D melanogaster
Possible Effects of Strategy Instruction on L1 and L2 Reading
Pleiotropic Effects of Phytochemicals in AD
więcej podobnych podstron