
Biomaterials 27 (2006) 986–995
Photo-iniferter-based thermoresponsive block copolymers composed
of poly(ethylene glycol) and poly(N-isopropylacrylamide) and
chondrocyte immobilization
Il Keun Kwon, Takehisa Matsuda
Division of Biomedical Engineering, Graduate School of Medicine, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan
Received 21 April 2005; accepted 21 July2005
Available online 22 August 2005
Abstract
A series of thermoresponsive poly(N-isopropylacrylamide) (PNIPAM)–poly(ethylene glycol) (PEG) block copolymers with
various PNIPAM contents and copolymer architectures, such as linear, four-armed and eight-armed configurations, were prepared
by iniferter-based photopolymerization of dithiocarbamylated PEGs (DC-PEGs) under ultraviolet (UV)-light irradiation. The
increase in monomer/DC-PEG feed ratio resulted in an increase in both the molecular weight and PNIPAM content of copolymers.
The measurement of the optical transmittances of aqueous solutions of PNIPAM–PEG block copolymers determined the lower
critical solution temperatures (LCSTs) of block copolymers, which ranged from 31.3 to 34.0 1C. LCST decreased with increasing
block length of PNIPAM and with the formation of a branched architecture. Rabbit chondrocytes were immobilized and cultured in
a three-dimensional (3D) gel composed of PNIPAM–PEG block copolymer at 37 1C. Gels prepared from copolymers with higher
PNIPAM contents at higher concentrations appeared to exhibit a minimal decrease in both cell number and cell viabilityduring a 7-
dayculture. Cell viabilitydependencies on material and formulation parameters and the potential use of PNIPAM–PEG block
copolymers as an in situ formable scaffold for an engineered cartilage tissue were discussed.
r
2005 Elsevier Ltd. All rights reserved.
Keywords: Chondrocyte; Thermally responsive material
1. Introduction
Recentlydeveloped tissue-engineering-directed ther-
apeutic procedures enable the repair, regeneration or
replacement of injured, diseased, and lost tissues with
engineered tissues composed of cells, an artificial
extracellular matrix (ECM) and structural scaffold
One such targeted tissue is cartilage engineered tissue,
since articular cartilage has a limited capacityfor self-
repair once it has been damaged. Various tissue-
engineering approaches using autologous chondrocytes
with a preconstructed or injectable scaffold have been
studied
. The requirements of an injectable sub-
stance for this particular application include in situ self-
gelation without cell death and cell-adhesion and -
proliferation as well as a structural platform. Such a
cell-suspended, moldable solution can fill an inhomoge-
neous defect’s space to form an engineered cartilage
tissue in close contact with adjacent living tissue.
As biologicallyderived in situ gelable biomacromole-
cules, collagen and agarose, both of which are thermo-
responsive and gel at physiological temperature, have
been used for in situ injectable or ex vivo moldable cell-
incorporated engineered tissues
. Poly(N-isopropy-
lacrylamide) (PNIPAM) is a well-known temperature-
responsive polymer and demonstrates a phase transition
temperature or lower critical solution temperature
(LCST) in an aqueous solution at about 32 1C
ARTICLE IN PRESS
www.elsevier.com/locate/biomaterials
0142-9612/$ - see front matter r 2005 Elsevier Ltd. All rights reserved.
doi:10.1016/j.biomaterials.2005.07.038
Corresponding author. Tel.: +81 92 642 6210;
fax: +81 92 642 6212.
E-mail address: matsuda@med.kyushu-u.ac.jp (T. Matsuda).

Below the LCST, PNIPAM is verysoluble in water.
However, as the temperature is increased above the
LCST, the polymer precipitates from the aqueous
solution due to hydrophobic associations. PNIPAM
and its copolymers have been widely utililized as
thermoresponsive drug deliveryvehicles
, three-
dimensional (3D) ECMs
, tissue-detachable-cul-
ture substrates
and wound-healing materials
In
our
previous
studies,
multiplyderivatized
PNIPAM-grafted biomacromolecules, such as gelatin
and hyaluronan
, were designed using
photochemistryof the dithiocarbamy
l (DC) group
attached to their macromolecules. PNIPAM-grafted
gelatin served as a cell adhesion platform for ancho-
rage-dependent cells and PNIPAM-grafted hyaluronan
as non-cell adhesive material, both of which function
well as thermoresponsive wound-healing substances.
The
DC
group,
called
the
iniferter
(initiator,
transfer and terminater), photolyzes to produce a
radical
pair
(alkyl
radical
and
DC
radical)
upon ultraviolet (UV)-light irradiation, which induce
spontaneous recombination to reform a dithiocarba-
mate group and an alkyl radical initiates radical
polymerization in the presence of a monomer. The
propagating chain end is routinelycoupled with the DC
group, enabling quasi-living radical polymerization
during photoirradiation.
In this study, poly(ethylene glycol)(PEG)s coupled
with DC groups at both chain ends were prepared as
PEG derivatized iniferters and subsequent photopoly-
merization of N-isopropylacrylamide (NIPAM) pro-
duced various PNIPAM–PEG block copolymers with
different PNIPAM block lengths and different copoly-
mer architectures. Irrespective of type of copolymers,
PEG block is a core segment and PNIPAM block is
terminal segment. The thermoresponsive characteristics
and cell-immobilization and cell-viabilitypotentials of
the PNIPAM–PEG block copolymer gels were exam-
ined. Based on the cell viabilityexperiments coupled
with the authors’ previous results, design criteria of
artificial extracellular miliue was discussed.
2. Materials and methods
2.1. General methods
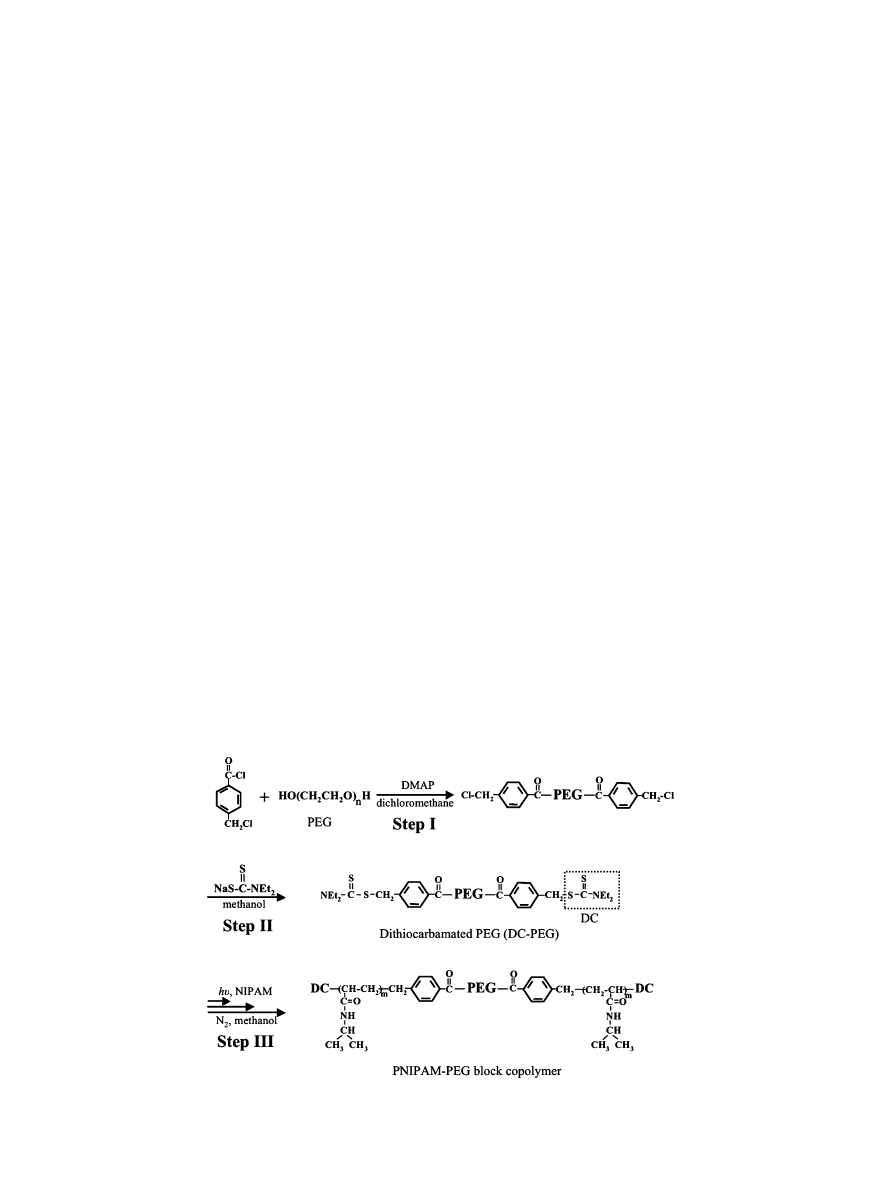
1
H-NMR spectra were recorded in CDCl
3
using tetra-
methylsilane (0 ppm) as an internal standard with a JNM-
AL300 (JEOL, Tokyo, Japan). The number-average molecular
weight (Mn) of each polymer was determined by gel
permeation chromatography(GPC), which was carried out
on a high-performance liquid chromatograph (HPLC, Waters,
MA) equipped with a GF-510 column (Shodex, Osaka, Japan)
using PEG as a standard and dimethylformamide (DMF) as
an eluent at 40 1C. The light intensityat 250 nm was measured
with a photometer (UVR-25, Topcon, Tokyo, Japan).
2.2. Materials
Solvents, all of which were of special reagent grade, were
purchased from either Wako Pure Chemical Industries, Ltd.
(Osaka, Japan) or Tokyo Chemical Industry Ltd. (Tokyo.
Japan). NIPAM was used after recrystallization from a
benzene–hexane solution. Four-armed and eight-armed PEG
[mol. wt. of 2 10
3
and 1 10
4
, respectively, according to the
manufacturer’s information] were supplied byShearwater
Polymers, Inc. (Huntsville, AL). PEGs were purified by
precipitation from cold hexane and subsequentlydried under
vacuum prior to use. 4-(Chloromethyl)benzoyl chloride, N,N-
(dimethylamino)pyridine (DMAP), and sodium N,N-diethyl-
dithiocarbamate trihydrate were used as received without
further purification. Other solvents and reagents were purified
bydistillation. Dialysis membranes with molecular weight cut-
off values of 1.2 10
4
(Wako), 1.0 10
3
or 6–8 10
3
(Spectra/
Por., Spectrum Lab., CA) were used for purification depending
on the molecular weight of PEG or the copolymer.
2.3. Preparation of dithiocarbamyl PEG (DC-PEG)
To a dichloromethane solution (100 mL) of PEG (Mn ca.
3400, 10 g, ca. 2.9 mmol), an excess amount of 4-(chloro-
methyl)benzoyl chloride (11.1 g, 58.8 mmol) was added drop-
wise at 0 1C, and then DMAP (1.1 g, 8.8 mmol) was added.
After being stirred at room temperature for 24 h under a
nitrogen atmosphere, the reaction mixture was filtered,
precipitated in excess hexane/ether mixture, and dried under
reduced pressure. The residue was dissolved in methanol
(100 mL). After this solution was added dropwise to a
methanol solution (150 mL) of sodium N,N-diethyldithiocar-
bamate trihydrate (13.0 g, 58.0 mmol) at 0 1C, the mixture was
stirred at room temperature for 22 h. After filtration, the
filtrate was concentrated under reduced pressure. After
dissolving in an aqueous solution, the residue was purified
by dialysis against distilled water for 3 days, and subsequently
freeze-dried for 3 days to obtain DC-PEG [yield: 10.8 g (87%)].
1
H-NMR (CDCl
3
with Me
4
Si): d 1.28 (t, 12H, CH
3
), 3.65 (m,
309H, (CH
2
CH
2
O)
77.3
), 4.05 and 4.46 (mm, 4H each, CH
2
Me),
4.61 (s, 4H, CH
2
Ar), and 7.45 and 7.99 (dd, 4H each, C
6
H
4
).
2.4. Preparation of PNIPAM– PEG block copolymer
A methanol solution (500 mL) of NIPAM with DC-PEG
was placed in a glass apparatus. After bubbling drynitrogen
for 10 min, the solution was irradiated with a 400-W Hg lamp
(AH400RP; UV Company, Saitama, Japan) in a nitrogen
atmosphere (light intensity: 4 mW/cm
2
) with mild stirring. The
solvent was replaced with an aqueous solution under reduced
pressure, and subsequentlyfreeze-dried for 3 days.
2.5. Thermoresponsiveness of PNIPAM– PEG block copolymer
The thermoresponse phase transition of an aqueous
solution of the PNIPAM–PEG block copolymer (concentra-
tion: 0.2 mg/mL) was measured with a UV/VIS spectro-
photometer (DU 530, Beckman Instruments, Fullerton, CA)
bymonitoring the transmittance of a 600 nm light beam. The
samples were cooled at a rate of approximately0.5 1C/min
ARTICLE IN PRESS
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
987
from 45 to 25 1C. The temperature at the onset of increase in
transmittance, which was determined with an accuracyof
0.1 1C using a directlyimmersed thermosensor, was defined as
the LCST, which expresses the optical transmission of 90%.
2.6. Cell culture in PNIPAM-grafted gel
The PNIPAM–PEG block copolymer was dissolved in
Dulbecco’s modified Eagle’s medium (DMEM, Life Technol-
ogies Inc., Rockville, MD) supplemented with 10% fetal
bovine serum and (FBS, Life Technologies) and l-ascorbic acid
(50 mg/mL) to give a final concentration of 30% solids.
Chondrocytes, isolated from rabbit articular cartilage, were
suspended in the culture medium at room temperature (1 10
7
cells/mL). After collecting bycentrifugation (300g, 4 1C,
5 min), the suspension was mixed with the PNIPAM–PEG
block copolymer solution at 4 1C to a final cell concentration
of 5 10
6
cells/mL in 7.5%, 11%, and 15% copolymer
solutions, respectively. A 200 mL aliquot of the mixture
(approximately250 mm) was placed in a 48-well cell culture
cluster (Corning, Corning, NY), incubated for 30 min at 37 1C
for gelation and supplemented with fresh culture medium,
followed bya 7-dayculture at 37 1C (the medium was changed
everyday). The number of cells obtained bydissolving the gel
at room temperature (20 1C) was assessed using a hematocyt-
ometer.
2.7. Microscopic observations
The appearance of cells in the thermoresponsive gels was
observed bya phase-contrast microscope (TE300, Nikon,
Tokyo, Japan). In situ chondrocyte viability in the gel was
determined with a Live-Dead viability/cytotoxicity kit (Mole-
cular Probes, Eugene, OR), and chondrocytes were observed
using a confocal laser scanning microscope (CLSM, Radiance
2000, Bio-Rad Laboratories, Hercules, CA). The sample was
washed three times with PBS at 37 1C, and 500 mL of a 2 m
M
calcein AM and 4 m
M
ethidium homodimer-1 (EthD-1) mixture
was added and incubated at 37 1C for 40 min. Chondrocyte-
cultured PNIPAM–PEG block copolymer gels, which were
fixed with 1% glutaraldehyde–1.44% paraformaldehyde in
phosphate saline solution at 37 1C for 24 h, frozen in liquid
nitrogen, lyophilized and sputter-coated with platinum, were
observed byscanning electron microscopy(SEM, JSM-840A,
JEOL, Peabody, MA).
3. Results
3.1. Synthesis of PNIPAM– PEG block copolymer
PNIPAM–PEG block copolymers were prepared
according to the methods shown in
. The
PEGs used were a linear PEG with the Mn of
3.4 10
3
g/mol (designated P3k), which was determined
byGPC; and two types of branched PEG, a four-armed
PEG (Mwt.; 2 10
3
g/mol) and an eight-armed PEG
(Mwt.; 1 10
4
g/mol). First, 4-(chloromethyl)benzoyl
PEG was synthesized in dichloromethane (Step I) and
subsequent dithiocarbamation in methanol, followed by
an extensive dialysis, produced a white powdery solid
(Step II).
shows the representative
1
H-NMR
spectrum of PEG (P3k) coupled with a DC group at
both ends. From the relative peak intensityratio of
methylene protons (d 3.65; corresponding to the PEG
unit
) to methyl protons (d 1.28) of the DC
group [
], almost complete dithiocarbamyla-
tion was achieved. In this particular case, the calculated
conversion was approximately115% (overestimated
value which exceeds 100% is probablydue to the
polydispersity of PEG). Irrespective of type of PEG,
almost complete end-group derivatization was achieved
ARTICLE IN PRESS
Scheme 1. Schematics of preparation routes of PNIPAM–PEG block copolymer.
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
988
to produce PEG dithiocarbamated at both ends (DC-
PEG).
Using DC-PEG, the NIPAM monomer was poly-
merized in methanol under UV light irradiation (Step
III). After extensive dialysis against distilled water at
room temperature and subsequent freeze-drying, both
GPC and
1
H-NMR spectral measurements were carried
out.
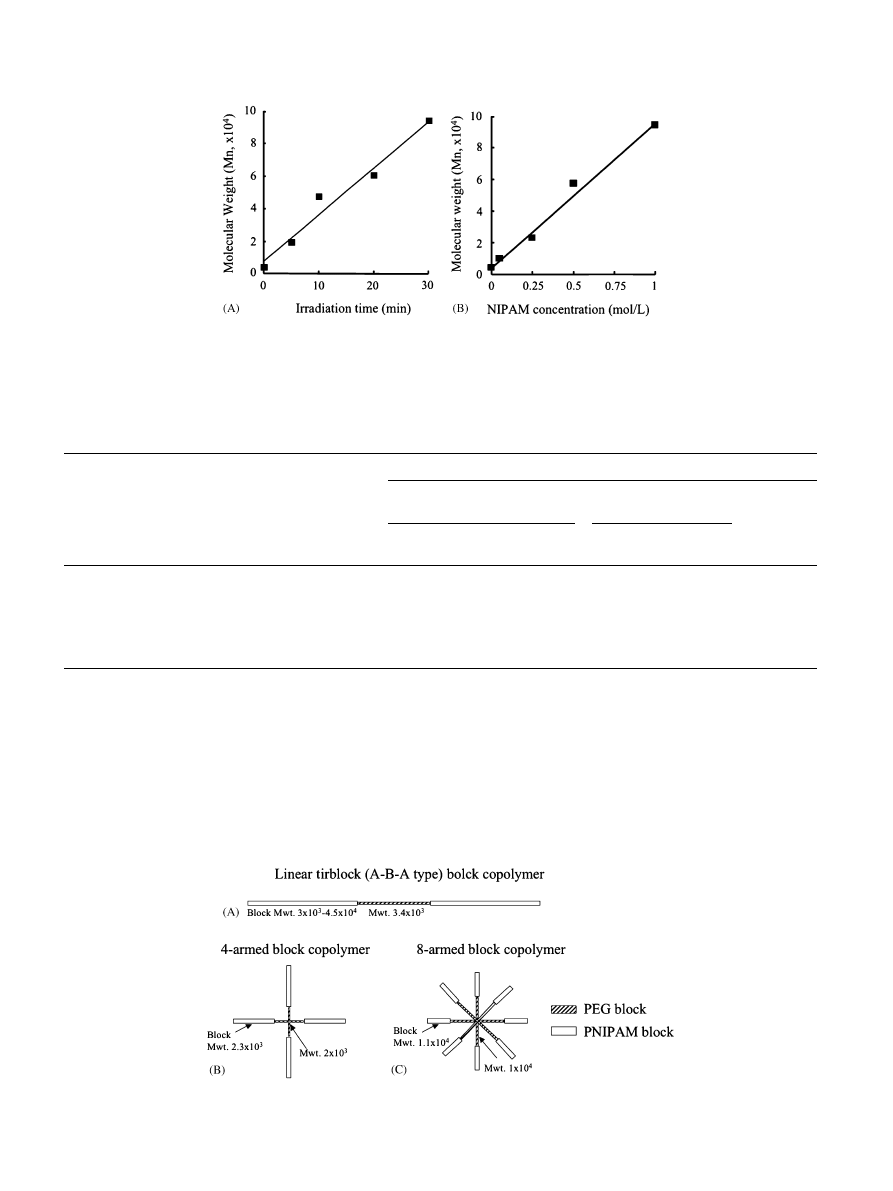
shows the change in the molecular weight of
products derived from DC-PEG derived from P3k as a
function of irradiation time and monomer concentration
under the fixed conditions described below. The
molecular weight increased proportionallyto both
irradiation time at a fixed monomer concentration
(1 mol/L) (
) and monomer concentration at a
fixed irradiation time (30 min) (
A series of PNIPAM–PEG block copolymers with
different PNIPAM block lengths were prepared at a
fixed irradiation time (30 min) but the different mono-
mer concentrations.
summarizes the reaction
conditions and copolymer compositions, which were
determined and calculated either by
1
H-NMR or GPC
measurement.
shows a representative
1
H-NMR
spectrum of the PNIPAM–PEG block copolymer (P3k/
90k in
). Copolymer composition was deter-
mined from relative intensityof the peak corresponding
to methyl protons (d 1.10) of the PNIPAM unit [
] to that of peak corresponding to the methylene
protons (d 3.65) of PEG unit [
]. The PNIPAM
content determined from GPC measurement was
calculated bysubtracting the molecular weight of DC-
PEG from the measured molecular weight of the
copolymer. The increase in monomer/DC-PEG ratio
resulted in an increase in both the molecular weight and
PNIPAM content in the copolymer (
). There is a
good correlation between the copolymer compositions
determined from
p
H-NMR and GPC measurements.
From the results, the PNIPAM–PEG block copolymers
were determined to be of the triblock type (A–B–A)
derived from linear DC-PEG, and of the star-block type
derived from multiple-armed DC-PEG, as schematically
shown in
3.2. LCSTs of PNIPAM– PEG block copolymers
All the PNIPAM–PEG block copolymers were
soluble in water at room temperature, but sponta-
neouslyprecipitated at near physiological temperature
(37 1C). The optical transmittance changes of the
aqueous solutions of PNIPAM–PEG block copolymers,
determined using UV/VIS spectrophotometer at 600 nm,
are shown in
. The optical transmittances of the
aqueous solutions of PNIPAM–PEG block copolymers
ARTICLE IN PRESS
Fig. 1.
1
H-NMR spectra of (A) DC-PEG (iniferter) and (B) PNIPAM–PEG block copolymer solutions (CDCl
3
with Me
4
Si).
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
989
ARTICLE IN PRESS
Fig. 2. (A) Irradiation time and (B) NIPAM concentration dependencyon number- averaged molecular weight of the PNIPAM–PEG block
copolymers produced using DC-PEG (P3k) as an iniferter. Polymerization condition: [iniferter] ¼ 0.5 mmol/L; light intensity ¼ 4 mW/cm
2
;
[NIPAM] ¼ 1 mol/L for (A); irradiation time ¼ 30 min for (B).
Table 1
Preparation and characterof PNIPAM-grafted PEG block copolymers
Copolymer
code
a
Iniferter
b
Monomer to
initiator (molar
ratio)
PNIPAM–PEG block copolymer
c
Mn ( 10
4
)
Composition of PNIPAM/
PEG (wt ratio)
f
LCST
g
(1C)
NIPAM/PEG
Per molecule
d
Per PNIPAM
block
e
NMR
GPC
P3k/6k
DC-PEG3k
100
0.9
0.3
1
2
N.D.
h
P3k/18k
DC-PEG3k
500
2.2
0.9
5
5
34.0
P3k/52k
DC-PEG3k
1000
5.6
2.6
13
15
32.3
P3k90k
DC-PEG3k
2000
9.3
4.5
26
26
32.2
P4arm/92k
DC-4-armed PEG
2000
9.5
2.3
46
46
31.3
P8arm/88k
DC-8-armed PEG
2000
10.3
1.1
9
9
31.8
a
For example, P3k/6k denotes the copolymer with the composition of PEG (Mn; 3.4 10
3
g/mol) and PNIPAM (total mol. wt. in a molecule;
3 10
3
2 g/mol).
b
Prepared according to
c
Copolymerization conditions: UV light intensity: 4 mW/cm
2
, irradiation time: 30 min.
d
Number-averaged molecular weight (Mn) of PNIPAM–PEG block copolymer.
e
Number-averaged molecular weight of PNIPAM block [Mn of copolymerMn of initiator)/functionality(n ¼ 2, 4 or 8)].
f
Weight ratio of PNIPAM to PEG in copolymer determined by
1
H-NMR and GPC.
g
Determined at the optical transmission of 90% in aqueous solution measured byUV/VIS spectrophotometer.
h
N.D.: not determined.
Scheme 2. Schematic of linear and branched PNIPAM–PEG block copolymers.
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
990
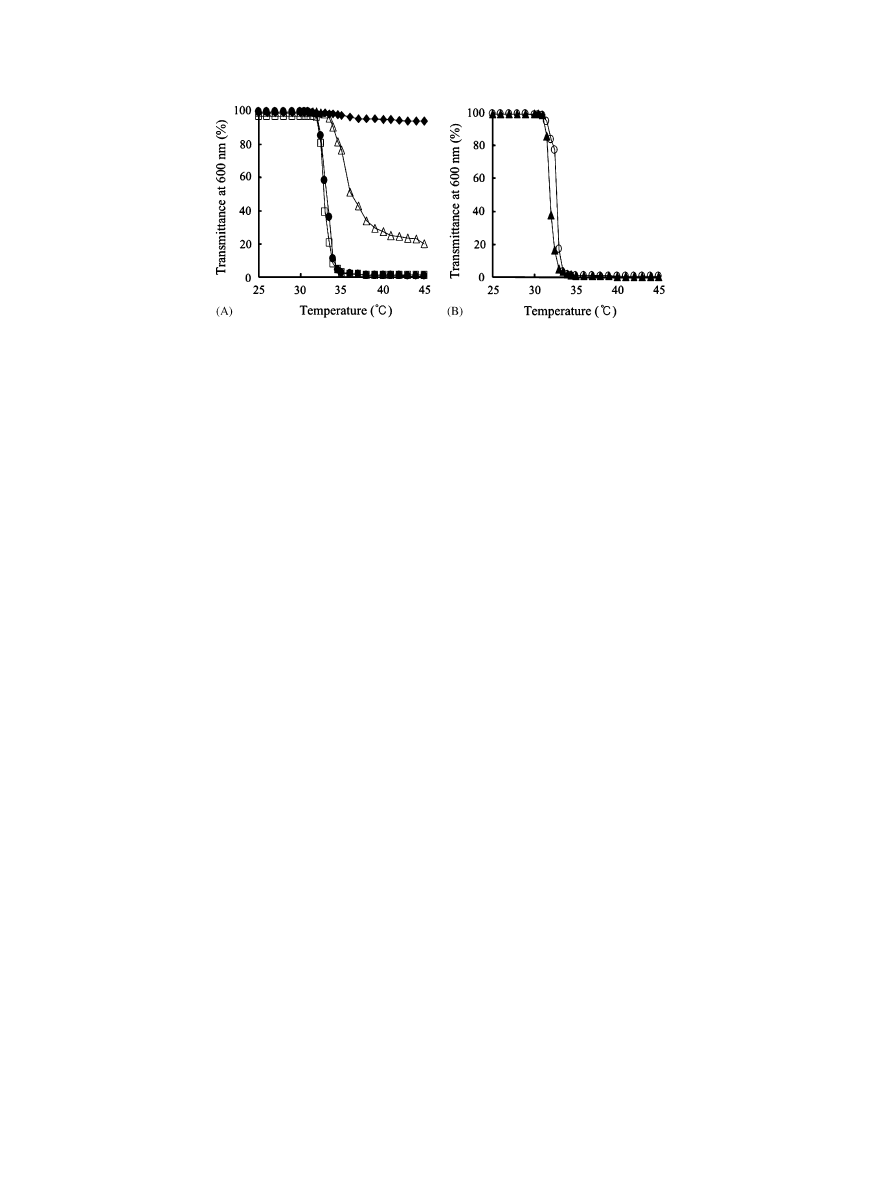
with a relativelyhigh molecular weight of PNIPAM
block (P3k/52k and P3k/90k, see
), which are
transparent at room temperature, sharplydecreased to
zero upon increasing solution temperature; in contrast,
those of the copolymer solutions with a lower molecular
weight of the PNIPAM block graduallydecreased with
an increase in temperature and remained approximately
95% for P3k/6k and 25% for P3k/18k at over 40 1C
(
). For branched copolymers with different
architectures (DC-four-armed or -eight-armed PEG-
inifertered copolymers, the schematics of which archi-
tectures are shown in
), but with almost the
same Mn (approx. 1 10
5
g/mol), their optical trans-
mittance sharplydecreased with increasing temperature
(
). LCSTs of the linear copolymers, which were
determined at an optical transmission of 90% in an
aqueous solution, ranged from 32.2 to 34.0 1C, and
slightlyincreased with decreasing PNIPAM content at a
fixed initiator (
). Regarding the branched
PNIPAM–PEG block copolymers, their LCSTs were
slightlylower than those of linear copolymers.
3.3. Three-dimensional chondrocyte culture in
PNIPAM– PEG block copolymer gels
Rabbit
chondrocytes,
suspended
in
10%
FBS–DMEM solution containing PNIPAM–PEG block
copolymers at 4 1C and subsequentlymaintained at
37 1C, were immobilized in spontaneouslyformed
opaque gels and cultured for 7 days.
lists the
state of the gel and cell viabilityon day7 of culture in
cell-incorporated gels prepared using PNIPAM–PEG
block copolymers composed of linear PEG (Mn;
3.4 10
3
g/mol) and different molecular weights of
PNIPAM block (gel no. 1–9) at different copolymer
concentrations (7.5, 11.0 and 15.0 w/v%). An increase in
the Mn of PNIPAM (or compositional weight ratio of
PNIPAM/PEG) and an increase in the copolymer
concentration resulted in a mechanicallymore stable
gel formation. At a fixed concentration (11.0 w/v%), a
lower PNIPAM/PEG composition ratio (slightlyless
than 10 fold, see
) tended to produce a soft gel or
resulted in no gel formation, irrespective of the use of
linear or branched copolymers.
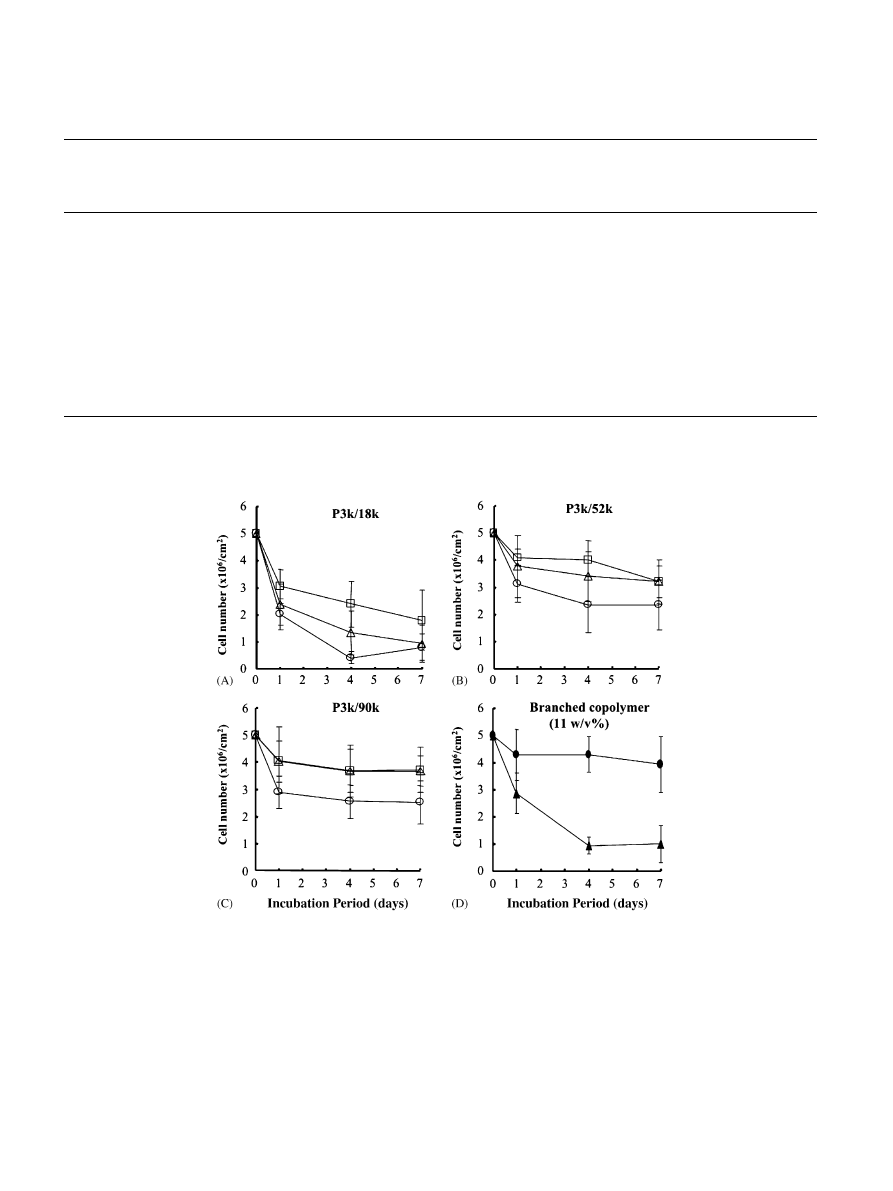
shows the time-dependent numbers of cells in
gels composed of different molecular architectures of
copolymers and different copolymer concentrations.
The general tendencyis that, at a fixed Mn of PEG
(3.4 10
3
g/mol), numbers of cells in gels composed of
copolymers with lower PNIPAM/PEG composition
ratio sharplydecreased with time (
). This
tendencywas stronger with decreasing copoly
mer
concentration (
)). The gels prepared from
copolymers with higher PNIPAM/PEG composition
ratio and at higher concentrations of PNIPAM–PEG
block copolymer appeared to exhibit a minimal decrease
in cell number during a 7-dayculture (
). The gel
prepared using branched copolymers with a higher
PNIPAM/PEG composition ratio (P4arm/92k) exhib-
ited a minimal reduction in the cell number during the
culture (
).
The live/dead cell assayclearlydifferentiated the cell
viabilitydependences on both copolymer composition
and copolymer concentration.
shows confocal
laser scanning micrographs of chondrocyte-inoculated
gels stained with live/dead fluorescence probes, showing
a qualitative tendencywith respect to cell viability
:
increases in the PNIPAM content in copolymers (or the
PNIPAM–PEG composition ratio) and the copolymer
concentration resulted in a high percentage of live cells
(stained green) and low percentage of dead cells (stained
red). This qualitative tendencywas stronglysupported
bythe quantitative data on cell viability(determined
from the fluorescence images) as tabulated in
shows the cross-sectional SEM images of a cell-
inoculated P4arm/92k-based gel after a 7-dayculture.
Well-dispersed and embedded round cells were observed
in the gel.
ARTICLE IN PRESS
Fig. 3. Temperature dependence of optical transmittance at 600 nm in aqueous solution for (A) linear PNIPAM–PEG block copolymers: P3k/6k
(~), P3k/18k (n), P3k/52k (K) and P3k/90k (&), and for (B) branched PNIPAM–PEG block copolymers, P4arm/92k (m) and P8arm/88k (
J
).
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
991
4. Discussion
A varietyof 3D porous solid scaffolds made of either
naturally occurring or synthetic polymers have been
used for cartilage repairing in tissue engineering
The main disadvantages of these ex vivo structured
scaffolds are inhomogeneous cell distribution in a
scaffold and the need for a surgical implantation
However, an injectable solution substance, that can
undergo in situ gelation under mild conditions to form
ARTICLE IN PRESS
Table 2
PNIPAM-grafted PEG hydrogels
Hydrogel code name
PNIPAM-grafted
PEG
a
Mn of PNIPAM
block ( l0
4
)
a
Concentration of
PNIPAM–PEG
block copolymer
solution (w/v%)
Occurrence of gel
formation
b
Cell viability(%)
c
1
7.5
—
2
P3k/18k
0.9
11.0
—
3
15.0
n
22.7
710.6
4
7.5
n
23.0
78.2
5
P3k/52k
2.3
11.0
J
55.3
710.2
6
15.0
J
57.3
75.0
7
7.5
J
64.7
711.0
8
P3k/90k
4.5
11.0
J
78.7
716.3
9
15.0
J
69.3
78.9
10
P4arm/92k
2.3
11.0
J
79.7
711.1
11
P8arm/88k
1.1
11.0
n
20.0
74.6
a
See footnote of
b
The appearance of the solution at 37 1C: , no gelation; n, soft gel;
J
, gel.
c
Mean-averaged cell viabilityat 7days-culture was calculated from CLSM images. Mean for n ¼ 4 SD.
Fig. 4. Time-dependent number of cells (chondrocytes) in inoculated gels with linear PNIPAM–PEG block copolymers of different PNIPAM
contents and different copolymer concentrations: 7.5 w/v% (
J
), 11 w/v% (n) and 15 w/v% (&) for copolymers with t different PNIPAM contents
(P3k/18k, P3k/52k and P3k/90k), and in branched copolymers, P4arm/92k (K) and P8arm/88k (m) at a fixed copolymer concentration (11 w/v%).
Mean for n ¼ 4 SD.
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
992

cell-inoculated and -residential gels, serves as a cell-
suspended, moldable scaffold. Simple injection of such a
mixed solution enables filling a defective tissue space,
thus forming a tissue-engineered cartilage in close
contact with adjacent living tissues.
Naturallyoccurring biomacromolecules, such as
collagen, agarose and alginate, which can be gelled by
thermoresponsive gelation (the former two biomacro-
molecules) or ionic complexation (the latter one), have
been used as cartilage tissue
. Synthetic counter-
parts of thermoresponsive gelable biomacromolecules
have been developed byutilizing thermoresponsive
PNIPAM as their major constituent in designed
molecules or poly(propylene glycol)–PEG triblock
copolymer with an appropriate copolymer composition
. Thermoresponsive copolymers composed of PNI-
PAM and PEG include triblock and star-block copoly-
mers, which are produced byredox-ty
pe ceric-ion-
induced radical polymerization initiated from hydroxyl
group of PEG at both ends, and graft copolymers in
which the PEG block is the main backbone and is
grafted with PNIPAM blocks
. These have
been mainlystudied from the viewpoints of struc-
ture–LCST relationship
, micelle formation and
rheological properties
. Another approach in-
volves the use of hybrid polymers composed of
biomacromolecules grafted with PNIPAM. For exam-
ple, our previous studies showed that PNIPAM-grafted
gelatin and PNIPAM-grafted hyaluronan, which are
prepared byphotochemicallydriven quasi-living graft
polymerization initiated from the dithiocarbamate
group derivatized on biomacromolecules, which pro-
ceeds reproduciblyunder well-defined poly
merization
condition, exhibit thermo-reversible phase transforma-
tion, resulting in temperature-dependent cell adhesion/
detachment on their coated surfaces and cell inoculation
in gel
In this study, as an extension of studies on dithio-
carbamate-based quasi-living radical photopolymeriza-
tion, thermoresponsive linear triblock and star-block
copolymers composed of PNIPAM and PEGs were
prepared according to the method shown in
ARTICLE IN PRESS
Fig. 5. Fluorescence images determining cell viability in gels with copolymers of different PNIPAM contents and copolymer concentrations after 7
days of culture: Living cells (stained green) and dead cells (stained red) were stained with calcein AM and ethidium homodimer 1, respectively.
Fig. 6. Cross-sectional SEM images of chondrocyte-inoculated P4arm/92k-based gel after 7 days of culture. Magnification (A) 500 and (B) 5000.
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
993

Linear and multiarmed PEGs were derivatized with a
dithiocarbamate group at near complete conversion
(
). The molecular weight of the PNIPAM block
increased proportionallywith photoirradiation time and
monomer concentration (
). All the triblock and
star-block copolymers have a PEG block at the central
segment and PNIPAM blocks at the terminal segments
(
). The PEG segment remains soluble in water
throughout the temperature range of interest. However,
the LCSTs of copolymers decreased with PNIPAM
content (or molecular weight) in copolymers. Regarding
multiarmed block copolymers, the LCSTs were very
slightlylower than that of the PNIPAM homopolymer
(32.0 1C).
The cell viabilityin PNIPAM–PEG block copolymer
gels (determined after 7-dayculture) depended on the
copolymer composition and architecture of the block
copolymers (
and
): the higher the
copolymer concentration and the PNIPAM content in
copolymer or the higher the PNIPAM/PEG ratio, the
higher the cell viability. That is, the block copolymers
with high PNIPAM/PEG ratios (P4arm/92K with a
ratio of 46 and P3K/90K with a ratio of 26) provided
cell viabilityof approximately80%, whereas those with
low PNIPAM/PEG ratios (P3K/18K with a ratio of 5
and P8arm/88K with a ratio of 9) provided a cell
viabilityof approximatelyonly20%. For the gels
composed of P3K/52K, the increase in the copolymer
concentration appeared to enhance cell viability.
In contrast to cell culture on 2D culture or in 3D
open-cell structured, solid microporous scaffold, cell
culture in 3D soft gel using a water-soluble synthetic gel
is quite a difficult task although biomacromolecular 3D
gels such as type I collagen gel provide much better
extracellular milieu than synthetic counterparts. This is
a missing or unsolved problem in artificial 3D ECM
designs. Although various factors determinimg the
survival of cells in soft gel can be discussed, clear-cut
mechanisms or plausible answers have not been
obtained yet. In our previous paper, the survival
strategyof cells inoculated in synthetic 3D gel culture
using thermoresponsive and cell-adhesive PMIPAM-
grafted gelatin was thoroughlydiscussed, and hypothe-
tical underlying mechanisms were presented. In this
article, the cell viabilitydependence on the molecular
design parameters of non-cell adhesive block copoly-
mers was presented. The genetral trends observed were
same as those found in PNIPAM-grafted gelatin.
Although some block copolymers significantly reduced
the cell viability, a majority of copolymers exhibited an
initial cell loss, but no further cell loss was observed at
prolonged period of culture.
Although there are few detailed studies defining the
structural interior-design criteria of cell-inoculated gels,
the following qualitative criteria have been discussed
. Cell viabilityin a gel can be stronglyinfluenced by
the mechanical strength and porosityof the gel: a higher
porosityfacilitates the supplyof oxygen and nutrients to
the interior of the gel, and an appropriate mechanical
strength of the gel is necessaryfor cell spread, which is
required for proliferation. That is, the gel should
mechanicallywithstand cell traction force to maintain
its shape and integrity, and trigger biological activity.
Large pores are a critical design criterion for 3D
scaffolds with a high cell viability. Such large pores
can be produced byinter- and intra-molecular associa-
tions of the PNIPAM block to form aggregates that are
interconnected, resulting in a large volume loss of the
PNIPAM block. This mayoccur for block copolymers
with a large PNIPAM/PEG ratio or high PNIPAM
content in a copolymer, which eventually induces a
marked accumulation of aggregated PNIPAM blocks,
resulting in the formation of mechanicallystable gels as
well as an enhanced passive transport of oxygen and
nutrients into the void space.
This hypothetical working principle is in good
agreement with the results of our previous study
in which distinct relationships between cell viability,
material and formulation parameters of PNIPAM-
grafted gelatin gels were identified: a higher PNIPAM
content in a copolymer and a higher copolymer
concentration resulted in a higher cell viabilityand
proliferation. This means that the higher total void
space and larger interconnected rigid pore favor for cell
viabilityand proliferability. This was deduced from the
analysis of focal plane images by reflection confocal
scanning laser microscopy. Although the detailed
physicochemical
and
mechano-biological
analyses
should be carried out before drawing concrete conclu-
sions, the state of aggregates of PNIPAM blocks and the
spatial distribution of interconnected voids must be
considered as keyissues for design criteria for 3D
scaffolds for vital engineered tissues.
Round cell shape of chondrocytes in gels dominated
as evidenced in CLSM (
) and SEM (
) images.
This coincides with the morphologyor cell shape in
native cartilage tissue in which chondrocytes are round,
demonstrating minimal or little interaction with the
adjacent ECM. Manystudies have demonstrated that
cell shape is a principal determinant of cell function
Therefore, PNIPAM–PEG block copolymers appear to
provide a suitable extracellular milieu for chondrocytes
in engineered cartilage tissue similarlyto natural
extracellular environment at least from the viewpoint
of cell shape.
The fate of the block copolymer in living cartilage
tissue is concerned in clinical setting. The block
copolymers prepared have one ester bond at both ends
of PEG segment, respectively. Therefore, when block
copolymer is being hydrolyzed, water-soluble PEG and
non-soluble
PNIPAM
microaggregates
should
be
formed. These are expected to be pinocytosed (for
ARTICLE IN PRESS
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
994

PEG) or phagocytosed (for PNIPAM aggregates) by
immuno-competent cells including neutrophils and
macrophages. Although slow degradation rate and low
population of immuno-competent cells are expected in
living cartilage tissue due to absence of vascular network
system within the tissue, it is envisaged that fragmented
microaggregates maybe scavenged with a prolonged
period of implantation. Further studyof cell function
and biodegradabilityis planned.
Acknowledgment
This studywas financiallysupported in part bya
Grant-in-Aid for Scientific Research (A2-15200038)
from the MEXT of Japan.
References
[1] Temenoff JS, Mikos AG. Review: tissue engineering for
regeneration of articular cartilage. Biomaterials 2000;21:431–40.
[2] Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O,
Peterson L. Treatment of deep cartilage defects in the knee with
autologous chondrocyte transplantation. N Engl J Med 1994;331:
889–95.
[3] Ishaug-RileySL, Okun LE, Prado G, Applegate MA, Ratcliffe A.
Human articular chondrocyte adhesion and proliferation on
synthetic biodegradable polymer films. Biomaterials 1999;20:
2245–56.
[4] Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G,
Minas T, et al. Canine chondrocytes seeded in type I and type II
collagen implants investigated in vitro. J Biomed Mater Res
1997;38:95–107.
[5] Lee DA, Bader DL. Compressive strains at physiological
frequencies influence the metabolism of chondrocytes seeded in
agarose. J Orthop Res 1997;15:181–8.
[6] Hoffman AS. Hydrogels for biomedical applications. Adv Drug
Deliver Rev 2002;43:3–12.
[7] Neradovic D, Soga O, Van Nostrum CF, Hennink WE. The effect
of the processing and formulation parameters on the size of
nanoparticles based on block copolymer of poly(ethylene glycol)
and poly(N-isopropylacrylamide) with and without hydrolytically
sensitive group. Biomaterials 2004;25:2409–18.
[8] Kaneko Y, Nakamura S, Sakai K, Aoyaji T, Kikuchi A, Sakurai
Y, et al. Rapid deswelling response of poly(N-isopropylacryla-
mide) hydrogels by the formation of water release channels using
poly(ethylene oxide) graft chains. Macromolecules 1997;31:
6099–105.
[9] Matsuda T, Moghaddam MJ. Molecular design of artificial fibrin
glue. Mater Sci Eng 1993;C1:37–43.
[10] Morikawa N, Matsuda T. Thermoresponsive artificial extracel-
lular matrix: N-isopropylacrylamide-graft-copolymerized gelatin.
J Biomater Sci Polym Ed 2002;13:167–83.
[11] Ohya S, Matsuda T. Poly(N-isopropylacrylamide) (PNIPAM)-
grafted gelatin as thermoresponsive three-dimensional artificial
extracellular matrix: molecular and formulation parameters vs.
cell proliferation potential. J Biomater Sci Polymer Ed, in press.
[12] Ohya S, Kidoaki S, Matsuda T. Poly(N-isopropylacrylamide)
(PNIPAM)-graft
gelatin
hydrogel
surface:
interrelationship
between microscopic structure and mechanical propertyof surface
regions and cell adhesiveness. Biomaterials 2005;26:3105–11.
[13] Ohya S, Sonoda H, Nakayama Y, Matsuda T. The potential of
poly(N-isopropylamide)
(PNIPAM)-grafted
hyaluronan
and
PNIPAM-grafted gelatin in the control of post-surgical tissue
adhesions. Biomaterials 2005;26:655–9.
[14] Ibusuki S, Fujii Y, Iwamoto Y, Matsuda T. Tissue-engineered
cartilage using an injectable and in situ gelable thermoresponsive
gelatin: fabrication and in vitro performance. Tissue Eng
2003;9:371–84.
[15] Ibusuki S, Iwamoto Y, Matsuda T. System engineered cartilage
using Poly(N-isopropylacrylamide)-grafted gelatin as in situ
formable
scaffold:
in
vivo
performances.
Tissue
Eng
2003;9:1133–42.
[16] Matsuda T. Poly(N-isopropylacylamide)-grafted gelatin as a
thermoresponsive cell-adhesive, mold-releasable material for
shape-engineered
tissues.
J
Biomater
Sci
Polymer
Ed
2004;15:947–55.
[17] Tsukikawa S, Matsuoka H, Kurahashi Yuko, Konno Yasushi,
Satoh K, Satoh R, Isogai A, Kimura K, Watanabe Y, Nakano S,
Hayashi J, Kubota S. A new method to prepare multicellular
spheroids in cancer cell lines using a thermo-reversible gelation
polymer. Artif Organs 2003;27:598–604.
[18] Ohya S, Nakayama U, Matsuda T. Thermoresponsive artificial
extracellular matrix for tissue engineering: hyaluronic acid
bioconjugated with poly(N-isopropylacrylamide) grafts. Bioma-
cromolecules 2001;2(2):856–63.
[19] Wirth CJ, Rudert M. Techniques of cartilage growth enhance-
ment: a review of the literature. Arthroscopy1996;12:300–8.
[20] Buckwalter JA. Articular cartilage: injuries and potential for
healing. J Orthop Sports Phys Ther 1998;28:192–202.
[21] Bryant SJ, Anseth KS. The effects of scaffold thickness on the
tissue engineered cartilage in photocrosslinked poly(ethylene
oxide) hydrogels. Biomaterials 2001;20:619–26.
[22] Peter SJ, Miller MN, Yasko AW, Yaszemski MJ, Mikos AG.
Polymer concepts in tissue engineering. J Biomed Mater Res
1998;43:422–7.
[23] RowleyJA, Madlambayan G, MooneyDJ. Alginate hydrogels as
synthetic
extracellular
matrix
materials.
Biomaterials
1999;20:45–53.
[24] Yoshioka H, Mikami M, Mori Y, Tsuchida E. Preparation of
poly(N-isopropylacryamide)-b-poly(ethylene glycol) and calori-
metric analysis of its aqueous. J Macromol Sci Pure Appl Chem
1994;A31:109–12.
[25] Yoshioka H, Mikami M, Mori Y, Tsuchida E. A synthetic
hydrogel with thermoreversible gelation. I. Preparation and
rheological properties. 1994; A31: 113–20.
[26] Lin H-H, Cheng Y-L. In-situ thermoreversible gelation of block
and star copolymers of poly(ethylene glycol) and poly(N-
isopropylacrylamide) of varying architectures. Macromolecules
2001;34:3710–5.
ARTICLE IN PRESS
I.K. Kwon, T. Matsuda / Biomaterials 27 (2006) 986–995
995
Document Outline
- Photo-iniferter-based thermoresponsive block copolymers composed of poly(ethylene glycol) and poly(N-isopropylacrylamide) and chondrocyte immobilization
Wyszukiwarka
Podobne podstrony:
The incorporation of carboxylate groups into temperature responsive poly(N isopropylacrylamide) base
Poly(N isopropylacrylamide) coated
copolymers of ethylene oxide and glycidol with oligoglycidol
BSAVA Manual of Rabbit Surgery Dentistry and Imaging
Functional Origins of Religious Concepts Ontological and Strategic Selection in Evolved Minds
Guide to the properties and uses of detergents in biology and biochemistry
EurRad Ultrasound of thyroid, parathyroid glands and neck lymph nodes
Marijuana is one of the most discussed and controversial topics around the world
Preliminary Analysis of the Botany, Zoology, and Mineralogy of the Voynich Manuscript
Babi Yar Analysis of Yevtushenko's Writing Style and Meani
Improved Characterization of Nitromethane, Nitromethane Mixtures, and Shaped Charge Jet
Orzeczenia, dyrektywa 200438, DIRECTIVE 2004/58/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of
[Mises org]Mises,Ludwig von The Causes of The Economic Crisis And Other Essays Before And Aft
Comparative eco toxicity of nanoscale TiO2, SiO2, and ZnO
Biocatalytic preparation of natural flavours and fragrances POL
A Tale of Two Cities Summary and Themes of the Book
Combination of a Waste Incineration Plant and CombinedCyclePowerPlant 02bm 349 1993
Theory of Varied Consumer Choice?haviour and Its Implicati
więcej podobnych podstron