Extracellular post-translational modifications of collagen are major
determinants of biomechanical properties of fetal bovine cortical bone
Patrick Garnero
a,b,
⁎
, Olivier Borel
a
, Evelyne Gineyts
a
, Francois Duboeuf
a
, Helene Solberg
d
,
Mary L. Bouxsein
c
, Claus Christiansen
d
, Pierre D. Delmas
a
a
INSERM Unit 403, Hôpital E Herriot, Pav F, 69437 Lyon cedex 03, France
b
Molecular Markers, Synarc, Lyon, France
c
Orthopedic Biomechanics Laboratory, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
d
Center for Clinical and Basic Research and Nordic Biosciences, Copenhagen, Denmark
Received 13 July 2005; revised 6 September 2005; accepted 9 September 2005
Available online 3 November 2005
Abstract
Mechanical behavior of bone depends on its mass and architecture, and on the material properties of the matrix, which is composed of a
mineral phase and an organic component mainly constituted of type I collagen. Mineral accounts largely for the stiffness of bone, whereas type I
collagen provides bone its ductility and toughness, i.e., its ability to undergo deformation and absorb energy after it begins to yield. The molecular
mechanisms underlying the effect of alterations in type I collagen on bone mechanical properties are unclear.
We used an in vitro model of fetal bovine cortical bone specimens (n = 44), where the extent of type I collagen cross-linking was modified by
incubation at 37°C for 0, 60, 90 and 120 days, keeping constant the architecture and the mineral content. At each incubation time, the following
parameters were determined: (1) the bone concentration of enzymatic (pyridinoline; PYD and deoxypyridinoline, DPD) and non-enzymatic
(pentosidine) crosslinks by HPLC, (2) the extent of aspartic acid isomerization of the type I collagen C-telopeptide (CTX) by ELISA of native
(alpha CTX) and isomerized (beta CTX) forms, (3) the mineral density by DXA, (4) the porosity by micro-computed tomography and (5) the
bending and compressive mechanical properties.
Incubation of bone specimens at 37°C for 60 days increased the level (per molecule of collagen) of PYD (+98%, P = 0.005), DPD (+42%,
P = 0.013), pentosidine (+55-fold, P = 0.005), and the degree of type I collagen C-telopeptide isomerization (+4.9-fold, P = 0.005). These
biochemical changes of collagen were associated with a 30% decrease in bending and compressive yield stress and a 2.5-fold increase in
compressive post-yield energy absorption (P < 0.02 for all), with no significant change of bone stiffness. In multivariate analyses, the level of
collagen cross-linking was associated with yield stress and post-yield energy absorption independently of bone mineral density, explaining up to
25% of their variance.
We conclude that the extent and nature of collagen cross-linking contribute to the mechanical properties of fetal bovine cortical bone
independently of bone mineral density.
© 2005 Elsevier Inc. All rights reserved.
Keywords: Collagen; Crosslink; Pentosidine; Osteoporosis; Biomechanics
Introduction
Osteoporosis is a common age-related disease characterized
by increased skeletal fragility leading to fracture. The strength
of bone depends on different parameters including its mass and
geometry, its microarchitecture, but also the material properties
of the bone matrix itself
. Bone matrix can be considered a
composite material, comprised of mineral and organic phases.
The mineral phase largely accounts for the stiffness of bone
, whereas the organic phase, mainly constituted of type I
collagen, provides bone its ductility and toughness, i.e., its
ability to undergo deformation and absorb energy after it begins
to yield
.
The molecular mechanisms that underlie the effects of
collagen on bone mechanical properties are unclear, although it
is suggested that post-translational modifications of the collagen
Bone 38 (2006) 300
–309
www.elsevier.com/locate/bone
⁎ Corresponding author. INSERM unit 403, Hôpital E Herriot, Pav F, 69437
Lyon cedex 03, France. Fax: +33 4 72 68 65 08.
E-mail address: patrick.garnero@synarc.com (P. Garnero).
8756-3282/$ - see front matter © 2005 Elsevier Inc. All rights reserved.
doi:10.1016/j.bone.2005.09.014

molecule, including the intra- and inter-molecular crosslinks
may play a role
. Biochemical studies comparing bone
specimens from osteoporotic patients and controls have shown
abnormalities in enzymatic post-translational modifications of
type I collagen molecules, including an over-hydroxylation of
lysine residues, an over-glycosylation of hydroxylysine or a
reduction in the concentration of reducible divalent crosslinks
such as dihydroxylysinonorleucine (DHLNL) and hydroxyly-
sinonorleucine (HLNL)
. Part of the divalent crosslinks
in bone tissue can further maturate into trivalent crosslinks
including pyridinoline (PYD), deoxypyridinoline (DPD) and
pyrolle. Recent data indicate that the ultimate compressive
strength of human vertebra is correlated
– independently of
bone mineral density (BMD)
– with the ratio PYD/DPD, but not
with PYD, DPD or pyrolle separately
. In addition to
enzymatic cross-linking, collagen also undergoes a series of
non-enzymatic transformations. These include the advanced
glycation end products (AGE), which result from the reaction of
sugars in the extracellular space with amino groups on proteins
to form complex products with characteristic fluorescence
AGEs have been shown to form in bone matrix and some in
vitro studies suggest that they may influence bone mechanical
properties
. Another non-enzymatic post-translational
modification of collagen, the isomerization of aspartic acid
residues, may also influence bone mechanical properties. In this
regard, we found that the degree of aspartate isomerization of
α1 (I) C-telopeptide – which can be detected by measuring the
ratio of native (
α) and isomerized (β) degradation products of
the C-telopeptides (CTX) in urine
– was associated with
fracture risk independently of BMD and bone resorption in
post-menopausal women
Although there is a large body of evidence both from in vitro
and in vivo studies indicating that collagen post-translational
modifications may impact the mechanical properties of bone, it
remains unclear whether they contribute significantly to bone
mechanical properties independently of the other determinants.
Most of previous studies were cross-sectional and did not
control for important contributors to bone strength including
BMD, making it challenging to identify the relative contribution
of each parameter. In addition, no study has concomitantly
assessed the contribution of enzymatic and non-enzymatic post-
translational modifications of collagen on both bending and
compressive mechanical properties of cortical bone.
The aim of our study was to analyze the role of collagen
enzymatic and non-enzymatic post-translational modifications
on the mechanical properties of fetal bovine cortical bone using
an in vitro model where the extent of collagen cross-linking can
be modified, keeping constant the size and the mineral content
of bone.
Methods
Bone specimens
Bone from 7- to 8-month-old bovine fetuses was used because it is
characterized by a low degree of extracellular collagen modifications, including
type I collagen isomerization
. Femurs were obtained from 11 different
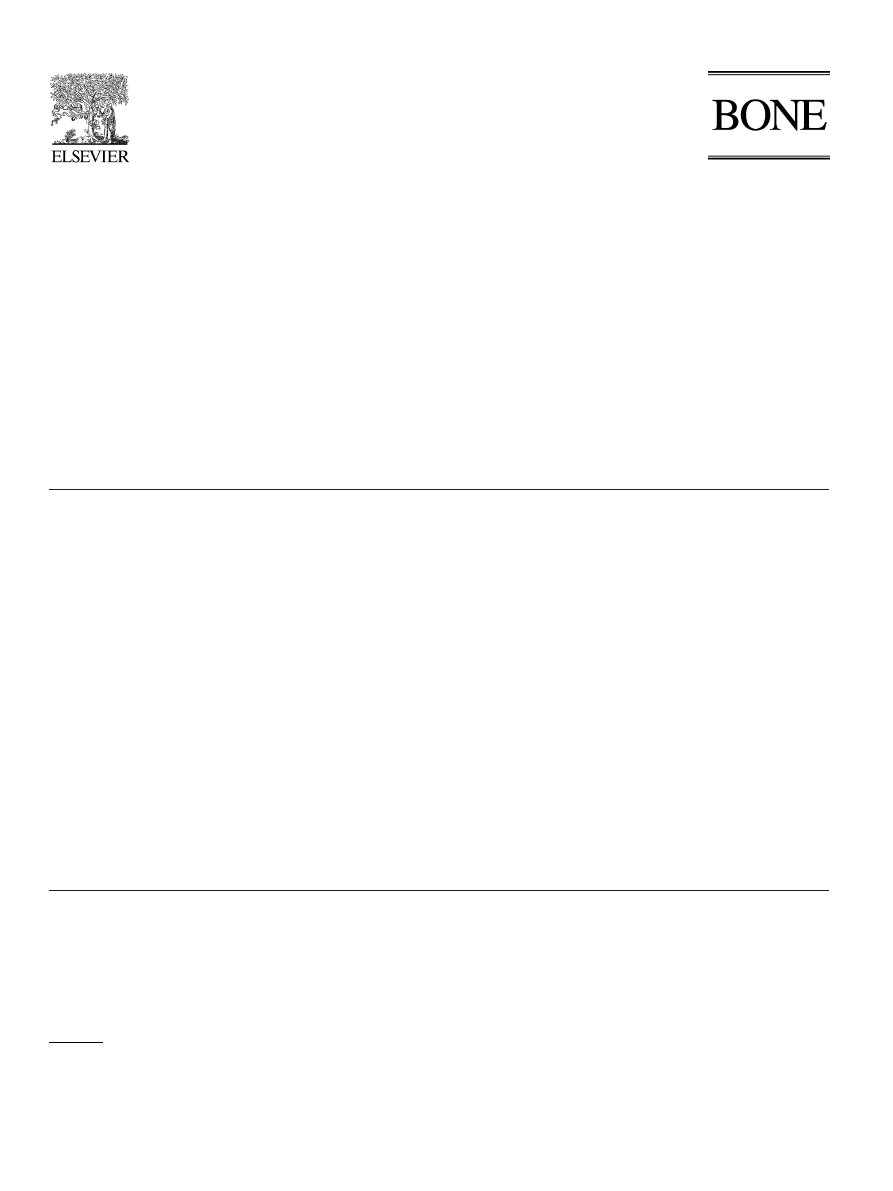
bovine fetuses. Mid-diaphyseal regions of the femur were cut into longitudinal
specimens using a diamond saw (Isomet low speed saw, Buehler, Lake Bluff,
Illinois, USA) to obtain 44 bone specimens (40 mm long, 4 mm width and 1
mm thick) which were used for mechanical tests (
a). Final dimensions
of each specimen were measured with a digital caliper. A bone sample
adjacent to each bone specimen was also taken to assess the amount of bone
collagen denaturation and the concentration of collagen post-translational
modifications. The 44 bone specimens were grouped into 11 different blocks,
each comprising 4 specimens from the femurs of the same animal. One
specimen from each bone was assigned to each of four incubation groups.
Specimens were incubated in phosphate buffered saline (PBS) 0.1 M, pH 7.4
in the presence of antibiotics at 37°C for 0, 60, 90 or 120 days to induce in
vitro collagen modifications. As a negative control, bone specimens from the
same animals and of identical dimensions as the one used in positive
experiments were also incubated for 0, 60 and 90 days at 4°C (n = 8 per
time point), to investigate whether the observed mechanical and biochemical
changes at 37°C could result only from incubation in PBS. At the end of
incubation, all bone specimens were kept hydrated at
−70°C in airtight
plastic containers wrapped in cloth soaked in PBS 0.1 M pH 7.4 until
biomechanical testing. Before testing, bone specimens were slowly thawed
over night at room temperature and also tested at room temperature.
Determination of mechanical properties
The non-destructive bending mechanical properties were determined by
loading bone specimens in a three-point bending configuration using a custom-
designed fixture with a support span of 30 mm. All the specimens were oriented
to have the external side of the bone facing the loading force (
a). A
material testing system (Schenck RSA-250) was used to apply a constant
displacement rate of 0.5 mm/min, for a maximum excursion of 4 mm. Load and
displacement data were converted to stress and strain, and used to compute the
bending elastic modulus (E
b
) and yield stress (
σ
yb
) according to American
Society for Testing and Materials standards
. During the bending test, the
specimen did not fracture, thus, the post-yield properties could not be accurately
assessed. Following non-destructive bending mechanical tests, destructive
compressive mechanical tests were performed on a bone specimen (4 × 4 × 1
mm; length × width × thickness) taken from one of the ends of the bone
specimen used in bending experiments (
a). The specimens were loaded
parallel to the direction of the longitudinal axis of the bone at a constant loading
rate of 0.5 mm/min until fracture. The following compressive mechanical
properties were derived from the stress
–strain curve: compressive elastic
modulus (E
c
), yield stress (
σ
yc
), ultimate stress (
σ
uc
), post-yield energy
absorption (EN
pc
) estimated as the area under the curve from yield to ultimate
stress and the post-yield strain (
ε
py
). The yield point was determined using the
0.02% strain offset method for both bending and compressive tests as previously
described
. Reproducibility of bending and compressive tests was
determined by analyzing 2 different bone specimens 3 times in the elastic
domain. The coefficients of variation of the elastic modulus were 1.2 and 1.5%
for bending and 1.3 and 1.6% for compressive tests. The definition of the
different biomechanical variables obtained from stress
–strain curves is given in
.
Bone mineral density (BMD) and porosity
The bone mineral content (BMC) of each bone specimen was measured by
dual-energy X-ray absorptiometry using the Hologic QDR 1000 device (Hologic
Inc., Bedford, MA). Bone samples were placed on a plastic layer during image
acquisition to decrease the baseline attenuation surrounding the specimens and
consequently improve the quality of the scans. Acquisition was performed using
specific high-resolution software (version 6.20D) dedicated to small animal
examination (line spacing = 0.254 mm, collimator diameter = 0.6 mm). The
average reproducibility of BMC determination assessed by duplicate
measurements of 15 bone specimens was 0.49%. Volumetric BMD was then
calculated by dividing the BMC of each bone specimen by the specimen volume
as measured using the digital calipers.
Porosity was assessed using high-resolution micro-computed tomography
(
μCT) imaging (μCT40, Scanco Medical AG, Basserdorf, Switzerland).
Tomographic slices were acquired at 12
μm isotropic resolution perpendicular to
the superior
–inferior (or longitudinal axis) of the bone specimen (
b). For
301
P. Garnero et al. / Bone 38 (2006) 300
–309
the specimens used for the bending tests, four sets of 25 slices each, equally
spaced along the length of the specimen, were acquired. For the specimens used
in compressive tests, a single set of 100 slices was acquired at the mid-point of
the specimen. Scan data were reconstructed, subjected to Gaussian filtration, and
porosity evaluated as 1
− (bone volume fraction) × 100.
Biochemical analyses of bone collagen
Bone specimens were finely ground in liquid nitrogen. The bone powder
was extracted with ice-cold acetone for 2 h, rinsed with ice-cold water, and then
demineralized in buffered (pH 7.4) 0.5 M EDTA, 4 M guanidine for 48 h. The
powder was then extensively washed with ice-cold water and freeze-dried.
Ninety-five percent of this powder consisted of collagen according to
hydroxyproline determination. On the bone powder, the following biochemical
determinations were performed:
– the amount of denaturated collagen expressed as the percentage of total
amount of collagen, was determined using a selective digestion technique as
previously described
. Briefly, 10 mg of demineralized bone powder
were digested at 37°C for 24 h in
α chymotrypsin (Sigma, Saint Louis,
MO) solution (1 mg/ml) which selectively dissolves denatured collagen,
whereas the intact collagen molecules remain in the insoluble fibrils. The
amount of denaturated collagen was estimated from the determination of
hydroxyproline in the supernatant and in the pellet after centrifugation.
– the degree of type I collagen isomerization was assessed by measuring the
native (
α) and isomerized (β) forms of CTX released after digestion of 10
mg of bone power with trypsin (EC 3.4.21.4, Sigma, 1 mg/ml) for 24 h at
37°C as previously described
. The native and isomerized forms were
measured by specific two sites ELISA using monoclonal antibodies raised
against the EKAHDGGR and the isomerized EKAH
βDGGR sequence,
respectively, from human type I collagen C-telopeptide
. The cross-
reactivity of the two assays toward the respective non-reactive peptide
was below 1.2% and the intra- and inter-variation of both tests were
below 10%
– the amount of PYD, DPD and pentosidine was measured after hydrolysis of
the bone powder. Briefly, aliquots of powdered tissue (20 mg wet weight/
ml), were hydrolyzed by 6 M HCl at 110°C during 20 h. Collagen crosslinks
were extracted from hydrolysates using cellulose CF11 partition column
chromatography. Separation of the different crosslinks was performed by
HPLC on a Beckman ultra sphere ODS (5
μm, 25 cm × 4.6 mm) protected
by a Brownlee RP-18 Guard cartridge (7
μm, 15 × 3 mm). For
determination of PYD and DPD, effluent was monitored for fluorescence
at an emission of 395 nm and an excitation of 297 nm using a highly
sensitive fluorescence detector (Jasco FP-920)
. For pentosidine, the
corresponding emission and excitation wavelengths were 335 nm and 385
nm. The amount of collagen crosslinks was expressed per molecule of
collagen estimated from hydroxyproline content or per mg of bone extract.
Statistical analyses
Data are presented as mean ± SEM, unless otherwise noted. The effect of
incubation time on mechanical parameters and collagen crosslinks was assessed
by ANOVA, followed by paired non-parametric Wilcoxon tests. Associations
between BMD or collagen parameters and bone mechanical properties were
investigated by both linear regression and exponential model analyses. Because
there was no apparent advantage of exponential models, linear regression
analysis was used. To investigate whether collagen post-translational modi-
fications predicted bone mechanical properties independently of BMD, stepwise
multivariate regression analyses were performed. Non-normally distributed
variables were log-transformed before being entered in the regression model.
Results
Effect of incubation on collagen cross-linking and BMD
There was no change during incubation in the amount of
hydroxyproline (135 ± 17; 143 ± 17; 142 ± 18 and 147 ± 16
Fig. 1. (a) Bone specimens were cut from the mid-diaphysis of fetal bovine femur. Three-point bending tests were performed with the load applied in the center of the
external side (e) of the bone specimen perpendicular to the longitudinal axis of the intact bone (
↔). For compressive tests, a 4-mm sample was cut from the end of the
specimen used in non-destructive bending tests and the load was applied parallel to the direction of the long axis of the intact bone; (b) micro-computed tomography
image of the fetal bovine cortical bone specimen showing high porosity; (c) polarized light microscopy of an histological section of fetal cortical bone after Goldner
staining (magnification factor: ×10) indicating the presence of a mixture of lamellar and woven bone.
302
P. Garnero et al. / Bone 38 (2006) 300
–309
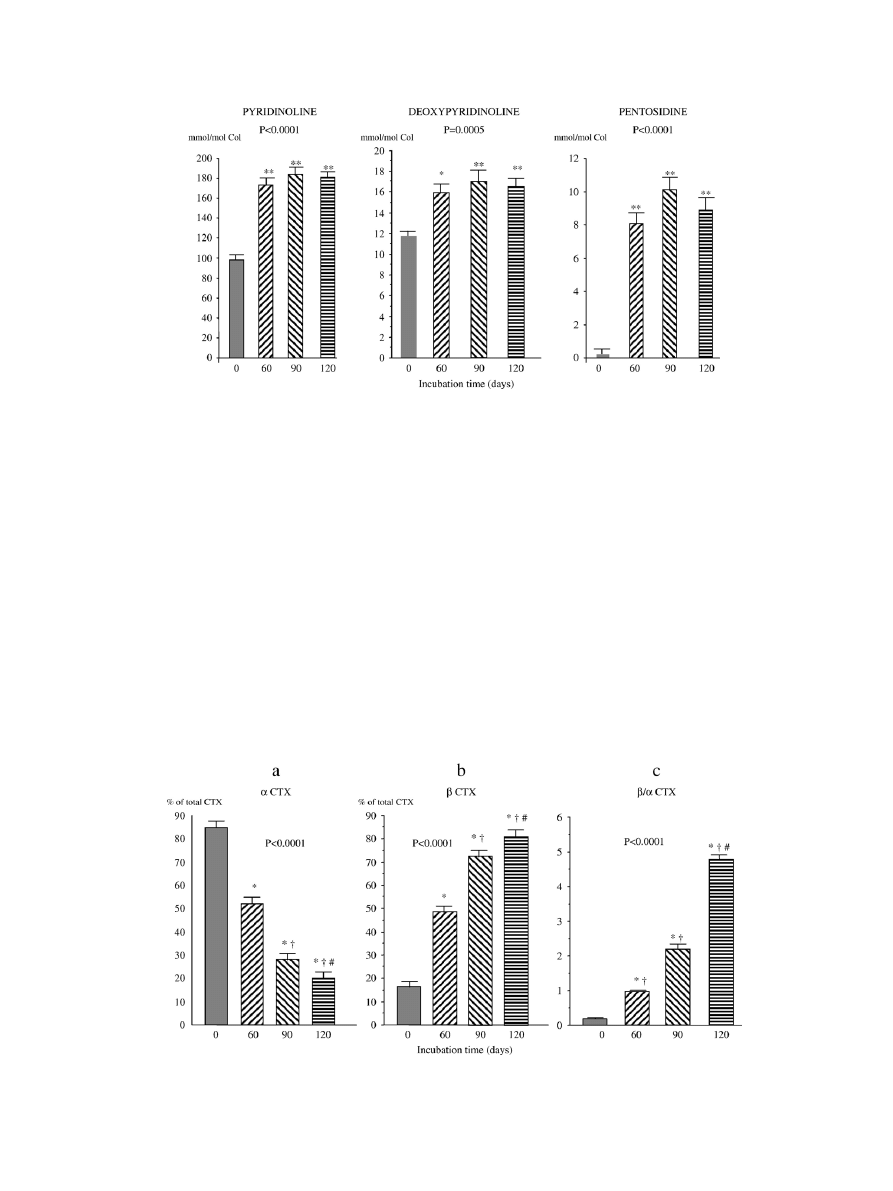
μg/mg of bone extract at time 0, 60, 90 and 120 days,
respectively, P = 0.42). After 60 days of incubation at 37°C,
there was a significant 1.8-, 1.4- and 56-fold increase in the
concentration (per mole of bone collagen) of PYD, DPD and
pentosidine, respectively, with no significant further change
between day 60 and day 120 (
). When data for collagen
crosslinks were expressed in mole per mg of bone extract,
similar findings were obtained with a 1.9-, 1.4- and 56-fold
increase of PYD, DPD and pentosidine, respectively, after 60
days of incubation. Incubation of bone specimens induced a
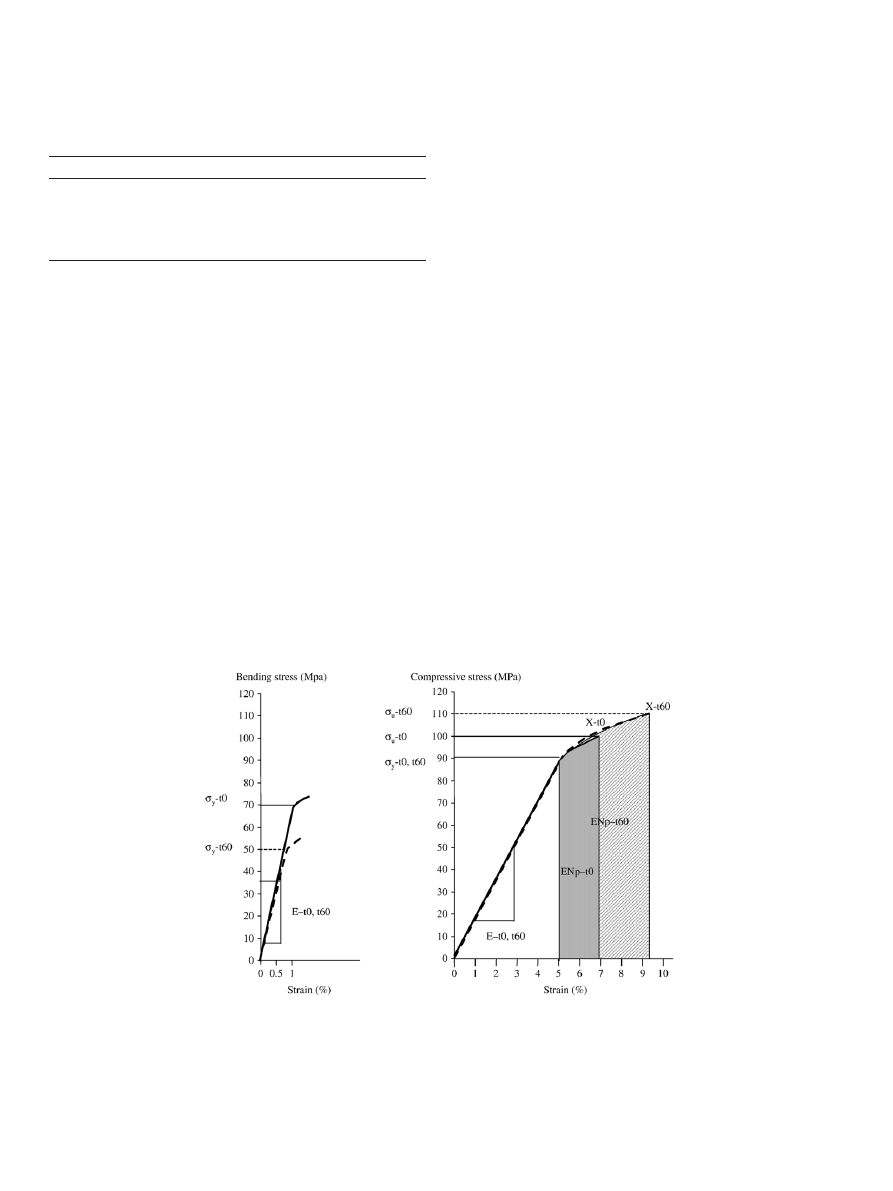
significant decrease in the proportion of type I collagen
molecules with C-telopeptides in the native form (
α CTX)
(
a) with a parallel increase in the proportion of collagen
molecules bearing isomerized (
β CTX) C-telopeptides (
b). This resulted in a 5-fold increase of the ratio of
isomerized to native CTX after 60 days, and a further increase
between day 60 and day 120 (
c). There was no
significant change in the proportion of denaturated collagen as
assessed by selective
α chymotrypsin digestion (10.0 ± 1.0;
12.5 ± 0.6; 11.6 ± 1.3; 12.2 ± 2.4% at 0, 60, 90 and 120 days,
respectively, P = 0.80). There was also no change in BMD and
porosity with incubation time (
For the bone specimens incubated at 4°C and used as
negative controls, there was no significant change in PYD
(mean ± SD: 50 ± 9.7; 48 ± 9.2 and 47 ± 8.9 mmol/mol collagen
at 0, 60 and 90 days, respectively), DPD (8.1 ± 1.1; 7.2 ± 1.3;
and 8.0 ± 1.2 mmol/mol collagen) and pentosidine (0.15 ± 0.13;
0.14 ± 0.14; and 0.15 ± 0.12 mmol/mol collagen). There was
also no significant change of BMD with time of incubation at
4°C (data not shown).
Fig. 2. Effects of incubation at 37°C of fetal cortical bone on the bone content of collagen crosslinks. Each bar represents the mean and SEM of 11 individual bone
specimens from 11 different animals. P values on the graph are derived from ANOVA testing changes of collagen crosslink concentration with time of incubation.
*P < 0.05, **P < 0.01 vs. time 0.
Fig. 3. Effects of incubation at 37°C of fetal cortical bone on type I collagen C-telopeptide isomerization. Each bar represents the mean and SEM of 11 individual bone
specimens from 11 different animals.
α CTX and β CTX represent the native and isomerized forms of type I collagen C-telopeptides, respectively, as measured by
specific ELISA after trypsin digestion of bone powder (see Materials and methods). P values on the graph are derived from ANOVA testing changes in proportion of
CTX forms with time of incubation. *P < 0.01 vs. time 0;
†
P < 0.01 vs. time 60 days;
#
P < 0.01 vs. time 90 days.
303
P. Garnero et al. / Bone 38 (2006) 300
–309
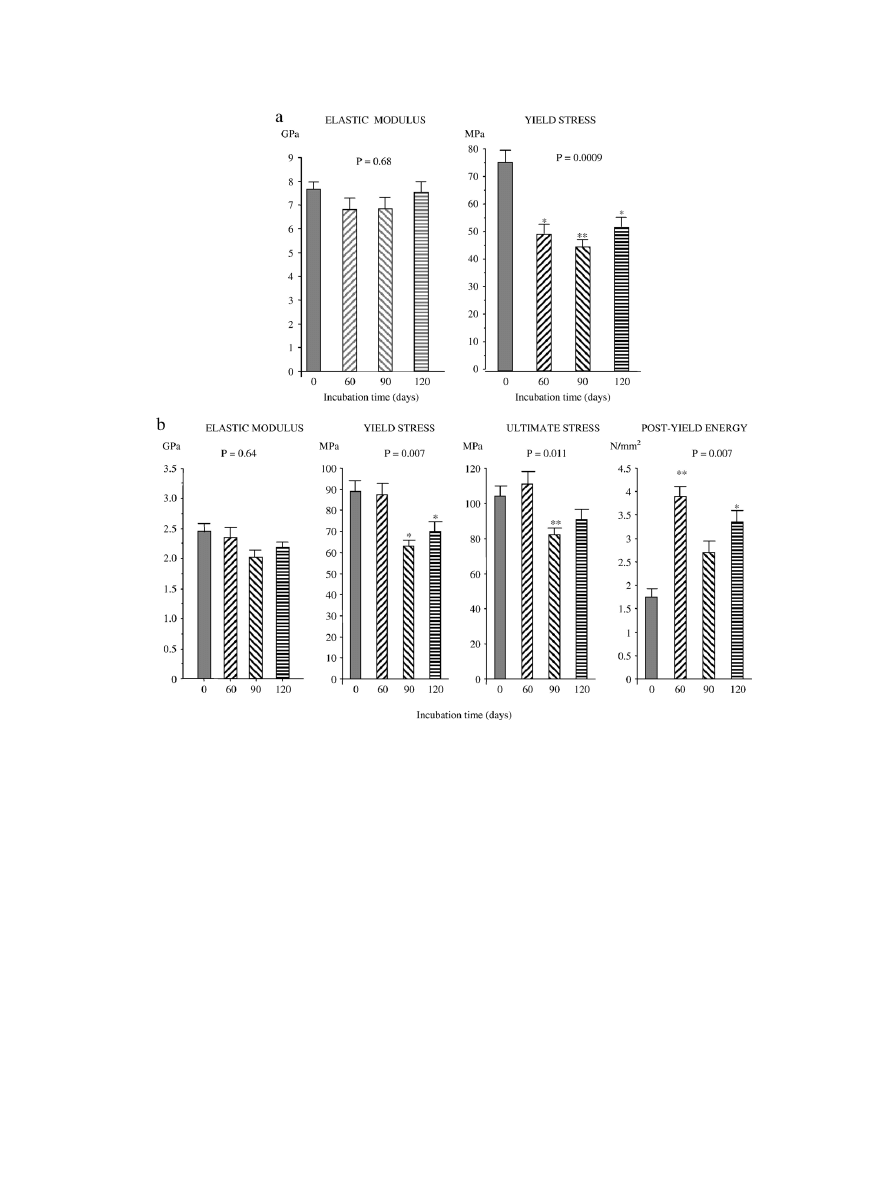
Effect of incubation on mechanical properties
Whereas there was no change in bending elastic modulus,
incubation of fetal bovine cortical bone at 37°C induced a
significant 30% decrease of bending yield stress after 60 days
with no significant further change with increasing incubation
time (
). Similarly, incubation at 37°C produced a
29% decrease of compressive yield stress which was significant
at day 90, with no effect on compressive elastic modulus (
). Moreover, incubation at 37°C induced a significant but
modest decrease of ultimate compressive stress which was
observed only at day 90 (
−20%, P = 0.02). In contrast,
incubation at 37°C produced a 2.5-fold increase in post-yield
energy absorption which was significant at day 60 and day 120
(
). This increase in post-yield energy absorption
resulted mainly from an increase in post-yield strain
(9.6 ± 0.56% vs. 6.7 ± 0.29%, 60 days after incubation
and before incubation, respectively, P = 0.01) (data not shown).
Incubation at 4°C of bone specimens used as negative
controls induced no significant change in bending young
modulus (P = 0.57), yield stress (mean ± SD: 93 ± 21; 107 ± 32
and 100 ± 26 MPa, at time 0, 60 and 90 days, respectively),
ultimate stress (122 ± 21; 143 ± 46; and 135 ± 36 MPa) and
post-yield energy absorption (1.82 ± 0.27; 2.13 ± 0.75;
2.17 ± 0.61 N/mm
2
).
Relationships among BMD, collagen characteristics and
mechanical properties
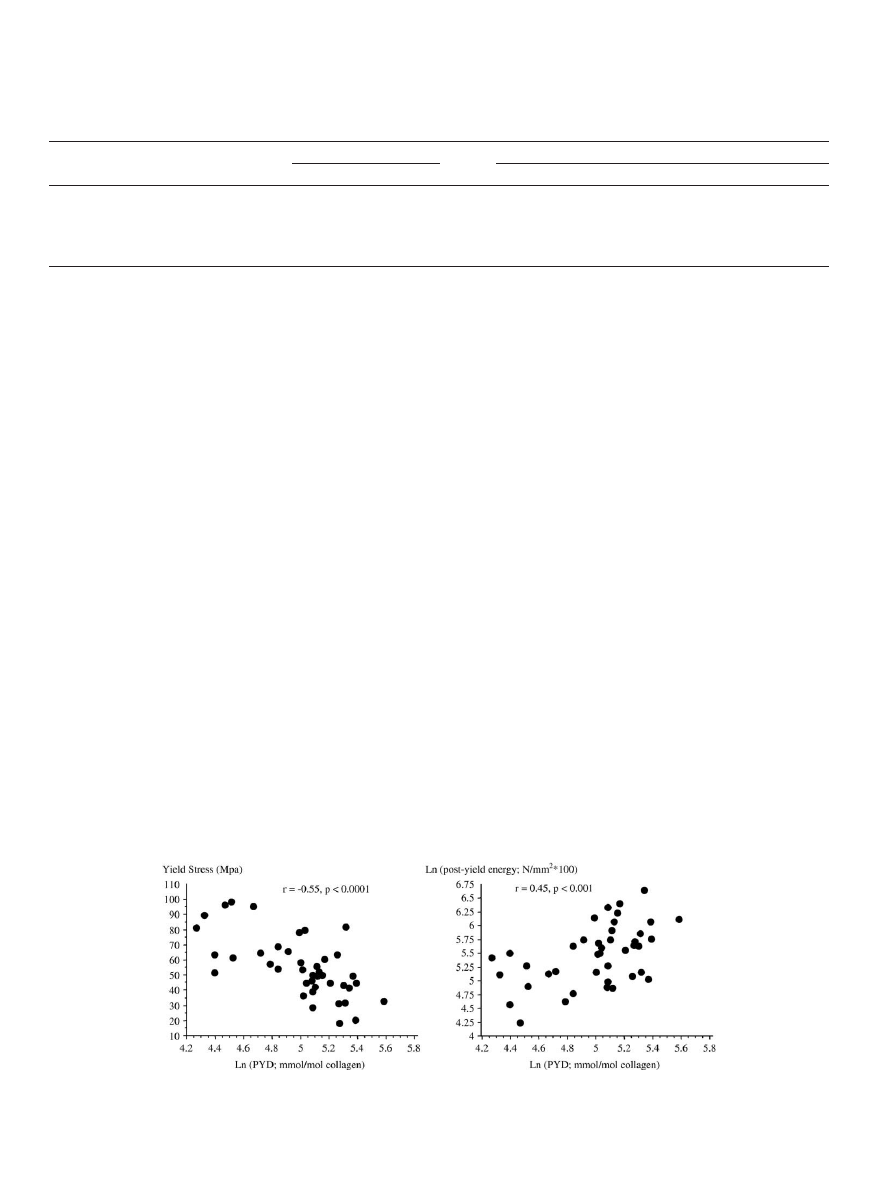
We then analyzed the relationships between BMD,
biochemical collagen properties and mechanical parameters
taking all bone specimens together (n = 44). As shown in
, there was a significant but modest negative association
between the bending and compressive elastic modulus and PYD
content. The associations between the extent of enzymatic
(PYD, DPD) and non-enzymatic (pentosidine, isomerization)
post-translational collagen modifications were stronger and
more consistent with the bending and compressive yield stress
and with the compressive post-yield energy absorption than
with the elastic modulus (
and
). BMD
significantly correlated with bending and compressive elastic
modulus, with bending and compressive yield stress and with
compressive ultimate stress, but not with compressive post-
yield energy (P = 0.84). Porosity correlated negatively with
BMD (r =
−0.66, P < 0.001), bending young modulus
(r =
−0.61, P < 0.0001), compressive yield (r = −0.45, P < 0.01)
and ultimate (r =
−0.56, P < 0.01) stress, but not with post-yield
energy. When the data of collagen crosslinks were expressed in
mol per mg of bone extract very similar associations were
observed (data not shown).
Table 1
Bone mineral density (BMD) by dual-energy X-ray absorptiometry and porosity
by micro-computed tomography of bone specimens with incubation time
Incubation time (day)
BMD (g/cm
3
)
Porosity (%)
0
1.251 ± 0.051
25.9 ± 4.1
60
1.166 ± 0.054
25.9 ± 4.1
90
1.158 ± 0.052
22.6 ± 3.5
120
1.186 ± 0.042
24.5 ± 3.7
P value
0.54
0.76
Results are shown as mean ± SE (n = 11 at each time point).
Fig. 4. Representative stress versus strain curve for bending (left panel) and compressive mechanical test (right panel) of fetal bovine cortical bone: Effect of incubation
at 37°C. The solid lines represent the average mechanical behavior of non-incubated bone specimen (t
0
), whereas the dotted lines represent the specimens incubated for
60 days at 37°C. For bending test, the deformation of the specimen did not go until failure (X) in contrast to compressive experiments. The following parameters were
derived from the stress
–strain curve: Elastic Modulus (E): Slope of the linear portion of the stress–strain curve; region where the deformation applied to the bone
specimen is reversible. Reflects bone stiffness. Yield stress (
σ
y
): stress at the yield point. Point where the curve becomes nonlinear and gives way to the plastic region.
Further loading beyond this point causes permanent deformation to the bone specimen. Reflects yield strength. Ultimate stress (
σ
u
): Stress at the point of bone failure.
Reflects ultimate strength. Post-yield energy absorption (EN
p
): area under the curve between yield and failure points within the plastic domain. Reflects energy
absorption (toughness).
304
P. Garnero et al. / Bone 38 (2006) 300
–309
We then performed a multivariable stepwise regression
model to explain the variability of mechanical properties
including BMD and the collagen properties. The content of
PYD, DPD, pentosidine and
β/α CTX ratio parameters were
not included in the same model because they were highly
inter-related (r > 0.80, P < 0.0001). The association between
BMD and bending and compressive elastic modulus was not
improved by the addition of collagen properties to the model.
In contrast, the addition of PYD, pentosidine or the
β/α CTX
ratio contributed significantly to the prediction of bending
and compressive yield stress provided by BMD alone,
explaining up to 25% of the variance independently of BMD
(
). The content of PYD and
β/α CTX ratio – but not
pentosidine or DPD
– significantly contributed to the
prediction of ultimate compressive stress independently of
BMD (r
2
increasing from 0.23 for BMD alone to 0.33 and
0.36;
P
<
0.01
when
adding
PYD
and
β/α CTX,
respectively).
Discussion
Using an original in vitro model that induces changes in
collagen properties, while keeping constant other potential
determinants of bone mechanical properties, we found that the
extent of some enzymatic and non-enzymatic post-translational
modifications
of
collagen
contributed
to
bending
and
compressive mechanical properties of fetal bovine cortical
bone, independently of BMD.
Because we wanted to analyze the relationships between
changes in collagen cross-linking properties and mechanical
properties, we performed these experiments in fetal bone
characterized by low levels of mature enzymatic crosslinks
and of type I collagen isomerization
. Further, we used
bovine bone to perform these experiments because it is difficult
to collect fetal bone from humans. However, similarities
between human and bovine bones have been reported both in
terms of mechanical properties and collagen crosslinks
.
Fig. 5. Effects of incubation at 37°C of fetal cortical bone on biomechanical properties in bending (a) and compressive (b) tests. Each bar represents the mean and SEM
of 11 individual bone specimens from 11 different animals. P values on the graph are derived from ANOVA testing changes in mechanical properties with time of
incubation. *P < 0.02, **P < 0.01 vs. time 0.
305
P. Garnero et al. / Bone 38 (2006) 300
–309
Thus, our results obtained with fetal bovine bone are likely to be
representative of human fetal cortical bone, although this needs
to be confirmed.
Incubation of fetal bone specimens for 60 days at 37°C
induced a 2-fold increase of mature enzymatic crosslinks, a
more than 50-fold increase of pentosidine, and a greater than 5-
fold increase in the proportion of
β isomerized type I collagen,
changes which are all characteristic of the maturation of bone
collagen. Most of the changes were observed within 60 days of
incubation with only further modest modifications for type I
collagen isomerization with longer incubation times, suggesting
that the kinetics of maturation is relatively rapid and could be
observed within a shorter duration. The in vitro increase of
mature trivalent PYD and DPD crosslinks is likely to result
from the spontaneous maturation of divalent DHLNL and
HLNL molecules which are the major enzymatic crosslinks in
fetal and newborn tissues
. The increase of the
β/α CTX
ratio results from the spontaneous conversion of the native
α
CTX form to the isomerized
β CTX, with a kinetic and relative
concentration at equilibrium which are actually very similar to
those described previously for the corresponding CTX synthetic
peptides
. The concentration of pentosidine showed the
most dramatic increase because, as expected for an AGE, it was
present at a very low concentration in non-incubated fetal bone
specimens. The formation of pentosidine in vitro presumably
results from the spontaneous reaction of sugars with amino
groups of bone collagen. Because we did not add exogenous
sources of sugars, endogenous carbohydrates
likely
constitute the rate-limiting step and explain the absence of
further increase of pentosidine after 60 days of incubation.
The major finding of our study was that the changes in
collagen cross-linking properties induced in vitro by incubation
at 37°C were associated with alterations of some bending and
compressive mechanical parameters, which were independent
of the mineral content and porosity. Bending and compressive
mechanical tests gave consistent results, showing no effect of
the incubation at 37°C on the elastic modulus and a significant
decrease in the yield stress. When tests were continued beyond
yield, incubation was associated with a more than 2-fold
increase of the post-yield energy absorption. In multiple
variable models including BMD, the level of bone crosslinks
was independently associated with yield stress and post-yield
energy absorption, but not elastic modulus. All these findings
are in agreement with the concept that the mineral content of
bone tissue is the major contributor of the stiffness of bone,
whereas the collagen properties influence bone ductility and
Table 2
Bivariate association between collagen post-translational modifications, bone mineral density (BMD) and mechanical properties of fetal bovine cortical bone
Post-translational modification of bone
collagen and BMD
Bending mechanical properties
Compressive mechanical properties
E
σ
y
E
σ
y
σ
u
EN
p
PYD
−0.41⁎⁎
−0.55⁎⁎⁎
−0.40⁎⁎
−0.57⁎⁎⁎
−0.45⁎⁎
+0.45⁎⁎
DPD
−0.31⁎
−0.41⁎⁎
−0.14
−0.38⁎
−0.27
+0.37
Pentosidine
−0.30
−0.58⁎⁎⁎
−0.29
−0.47⁎⁎
−0.38⁎
+0.46⁎⁎
β/α CTX
−0.11
−0.39⁎⁎
−0.30
−0.55⁎⁎
−0.44⁎⁎
+0.44⁎⁎
BMD
0.69⁎⁎⁎
0.60⁎⁎⁎
0.41⁎⁎
0.43⁎⁎
0.48⁎⁎
0.032
The table shows the correlation coefficients between bending and compressive mechanical properties and the concentration of bone collagen parameters (expressed in
mmol per mole of collagen, except for
β/α CTX). PYD, DPD, pentosidine, β/α CTX and EN
p
were naturally log-transformed before entering in the model.
E: elastic modulus;
σ
y
: yield stress;
σ
u
: ultimate stress; EN
p
: post-yield energy absorption; PYD: pyridinoline; DPD: deoxypyridinoline;
β/α CTX: ratio between
isomerized (
β) and native (α) type I collagen C-telopeptides.
⁎ P < 0.05.
⁎⁎ P < 0.01.
⁎⁎⁎ P < 0.0001.
Fig. 6. Association between collagen post-translational modifications and biomechanical properties of cortical bone. Left panel: correlation between the bone
concentration of pyridinoline (PYD) and the yield stress determined from bending mechanical tests. Right panel: correlation between the PYD and the post-yield
energy absorption evaluated from compressive mechanical test.
306
P. Garnero et al. / Bone 38 (2006) 300
–309

toughness
, although the independent contribution
of these two matrix components has not been previously
demonstrated.
The values of elastic modulus and yield stress in this model
of fetal bovine cortical bone were lower than the data previously
reported for intact adult cortical bovine or human bone
, but still markedly higher than those observed in
demineralized adult cortical bone
. Consistent with
lower mechanical properties, the porosity we observed in fetal
bone was higher than that reported for adult cortical bone which
is in the range of 5 to 12%
. In our model, BMD
explained 20 to 50% of the variability of Young modulus and
yield stress, which is lower than the 60 to 80% previously
reported for adult cortical bone
. The fact that fetal bone is
still immature with a morphology and material composition
different from adult bone likely contributes to these findings.
The
relatively
modest
correlation
between
BMD
and
mechanical properties may also have increased our power to
detect the independent contribution of post-translational
modifications of collagen to biomechanical properties.
We found very similar relationships between all types of
collagen post-translational modifications and bone mechanical
properties and because of their high inter-correlation, we could
not dissect out their relative contribution to bone strength.
Although, it seems reasonable that an increase in enzymatic
collagen crosslinks (PYD, DPD) could be associated with
increased ultimate stress as suggested by some studies (14, 15),
the positive association between higher concentration of
pentosidine and increased post-yield energy absorption initially
appears counter- intuitive due to the age-related nature of this
modification. To explain this apparent contradiction, one must
consider the differences in biochemical composition of adult
and fetal bone. The content of pentosidine in cortical bone
dramatically increases with age (18, 34) and is negatively
associated with some mechanical properties (18, 37), in contrast
to PYD and DPD levels which remained fairly constant with
age in adults (32, 34). After incubation, the content of
pentosidine in fetal bone did not reach the levels of bone from
elderly, but more closely approximate the levels found in young
adults (34). Thus, the relationships we observed may be
representative of those induced as bone collagen matures from
fetal to young adult, but may not be representative of the
transition from adult to old bone.
In our study, an increase in collagen crosslinks and
isomerization was associated with decreased yield stress and
increased in post-yield energy absorption. Bone is a brittle
micro-cracking material which derives its resistance against
fracture (toughness) by forming microcracks that absorb energy
and delay the propagation of a major crack
.
Consequently, an increase in yield stress often comes at the cost
of ductility and a greater propensity of a material to undergo a
fracture which is what we observed in our model but in the
opposite direction, i.e., a decrease in yield stress and an increase
in post-yield energy. Although hypothetical, it is tempting to
speculate that inter-fibrillar crosslinks when break may help
dissipate energy and consequently increase bone toughness.
However, it may also be possible that the concentration of
collagen crosslinks should not exceed a certain threshold to
render bone increasingly brittle and more prone to damage as
suggested by previous studies in bone from diabetic rats
.
As discussed above, this threshold is likely not to have been
reached in our model of fetal bone incubation.
Our study has strengths and some limitations. This is the first
study which investigated the specific effects of changes in
different enzymatic and non-enzymatic type I collagen
modifications and changes in both bending and compressive
mechanical properties. However, it also has limitations. We
used fetal bovine cortical bone which has a different structure
than adult cortical bone. To induce specific changes of collagen
properties, we incubated bone specimens in PBS at 37°C. This
experimental design offers the unique advantage of keeping
constant the other determinants of mechanical properties
including BMD, porosity and the concentration of non-
collagenous proteins, which could not be obtained by
comparing bones from different animals of various ages.
However, this in vitro method may not reflect adequately
normal in vivo collagen maturation and additional studies are
required to determine whether these results apply to age- and
disease-induced changes in adult bone. From this study, we
cannot infer that the changes in the post-translational
modifications we induced are directly involved in the alterations
of the mechanical properties. For example, pentosidine is only
one of the multiple senescent crosslinks occurring in aging
bone. We selected pentosidine because (1) this is one of the few
AGEs with a defined chemical structure and which can be
measured with adequate accuracy, (2) it has been commonly
used as an index of non-enzymatic glycation including in
biomechanical studies of bone
and cartilage
and (3) it
makes a covalent crosslink between adjacent molecules which
are likely to have, if any, more mechanical effects than non-
cross-linked AGEs. However because it is presently unknown
whether the changes in the concentration of pentosidine parallel
those of the other AGEs, the effects we observed may not fully
and/or adequately represent those of non-enzymatic crosslinks
Table 3
Multivariate prediction of bending and compressive mechanical properties of
cortical bone by bone mineral density (BMD) and post-translational
modifications of collagen
Model
a
Bending yield
stress (Mpa)
Compressive yield
stress (Mpa)
β
P value
r
2
β
P value
r
2
BMD (g/cm
3
)
0.43
0.0003
0.36
0.27
0.047
0.18
PYD (mmol/mol Col)
−0.52 <0.0001 0.25 −0.48 0.001
0.21
Model r
2
0.61
0.39
BMD (g/cm
3
)
0.45
0.0003
0.36
0.31
0.035
0.18
Pentosidine (mmol/mol Col)
−0.49 <0.0001 0.22 −0.37 0.012
0.13
Model r
2
0.58
0.31
BMD (g/cm
3
)
0.52
<0.0001
0.36
0.35
0.010
0.18
β/α CTX
−0.48 <0.0001 0.24 −0.49 0.0005 0.21
Model r
2
0.60
0.39
In all models, BMD was forced as the first variable. The table shows for each
model the standardized coefficient of the regression of each variable (
β), the
associated P value, and the square of the correlation coefficient (r
2
).
a
See
for abbreviations.
307
P. Garnero et al. / Bone 38 (2006) 300
–309

in general. It is also unlikely that the isomerization of the C-
telopeptide will introduce dramatic conformational changes in
the collagen molecules and have a direct influence on bone
strength. However, the degree of isomerization closely
correlated with the concentration of pentosidine in bovine bone,
and thus it may represent an indirect index of non-enzymatic
post-translational modifications of bone collagen. Interestingly,
changes in the degree of type I collagen isomerization in bone
tissues are adequately reflected by changes in the
β/α CTX ratio
measured by ELISA in urine samples
In conclusion, we found that changes in collagen post-
translational modifications induced in vitro were associated with
mechanical properties of fetal cortical bone, independently of
BMD. Altogether, these findings support the notion that the
extent and nature of collagen cross-linking are important
contributors to bone matrix quality and provide strong rationale
for additional studies to delineate the independent contributions
of collagen to bone mechanical properties and fracture risk.
Acknowledgments
We thank Mr. Patrice Clerc for performing biomechanical
tests and Mr. Yann Proust for helpful discussion and expert
technical support. This study was supported in part by a contract
INSERM-Lilly and Company.
References
[1] Boskey AL, Wright TM, Blank RD. Collagen and bone strength. J Bone
Miner Res 1999;14:330
–5.
[2] Burr DB. The contribution of the organic matrix to bone
’s material
properties. Bone 2002;31:8
–11.
[3] Currey JD. How well are bones designed to resist fracture? J Bone Miner
Res 2003;18:591
–8.
[4] Seeman E. Bone quality. Osteoporos Int Suppl 2003;5:3
–7.
[5] Currey JD. The mechanical consequences of variation in the mineral
content of bone. J Biochem 1969;2:1
–11.
[6] Currey JD. The effect of porosity and mineral content on the Young
’s
modulus of elasticity of compact bone. J Biochem 1988;21:131
–9.
[7] Wang X, Bank RA, TeKoppele JM, Agrawal CM. The role of collagen in
determining the bone mechanical properties and collagen denaturation. J
Orthop Res 2001;19:1021
–6.
[8] Zioupos P, Currey JD, Hamer AJ. The role of collagen in declining
mechanical properties of aging human cortical bone. J Biomed Mater Res
1999;45:108
–16.
[9] Currey JD. Role of collagen and other organics in the mechanical
properties of bone. Osteoporos Int 2003;14(Suppl 5):S29
–36.
[10] Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L.
Age-related changes in the biochemical properties of human cancellous
bone collagen: relationships to bone strength. Calcif Tissue Int 1999;65:
203
–10.
[11] Bailey AJ, Wotton SF, Sims TJ, Thompson PW. Biochemical changes in
the collagen of human osteoporotic bone matrix. Connect Tissue Res
1993;29:119
–32.
[12] Batge B, Deibold J, Stein H, Bodo M, Muller PK. Compositional analysis
of the collagenous bone matrix
—A study on adult normal and osteopenic
bone tissue. Eur J Clin Invest 1992;22:805
–12.
[13] Kowitz J, Knippel M, Schuhr T, Mach J. Alteration in the extent of
collagen I hydroxylation, isolated from femoral heads of women with a
femoral neck fracture caused by osteoporosis. Calcif Tissue Int
1997;60:501
–5.
[14] Oxlund H, Moselkilde L, Ortoft G. Reduced concentration of collagen
reducible cross-links in human trabecular bone with respect to age and
osteoporosis. Bone 1996;19:479
–84.
[15] Bailey AJ, Wotton SF, Sims TJ, Thompson PW. Post-translational
modifications in the collagen of human osteoporotic femoral head.
Biochem Biophys Res Commun 1992;185:801
–5.
[16] Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral
cancellous bone: correlation with collagen intermolecular crosslinks. J
Bone Miner Res 2002;17:1621
–8.
[17] Sell DR, Monnier VM. Structure elucidation of a senescence crosslink
from human extracellular matrix: implication of pentoses in the aging
process. J Biol Chem 1989;264:21597
–602.
[18] Vashishth D, Gibson GJ, Khoury JI, Shaffler MB, Kimira J, Fyhrie DP.
Influence of nonenzymatic glycation on biomechanical properties of
cortical bone. Bone 2001;28:195
–201.
[19] Wang X, Shen X, Li X, Agrawal C. Age-related changes in collagen
network and toughness of bone. Bone 2002;31:1
–7.
[20] Cloos PAC, Fledelius C. Collagen fragments in urine derived from
bone resorption are highly racemized and isomerized: a biological
clock of protein aging with clinical potential. Biochem J 2000;345:
473
–80.
[21] Garnero P, Cloos P, Sornay-Rendu E, Qvist P, Delmas PD. Type I collagen
racemization and isomerization and the risk of fracture in postmenopausal
women: the Ofely Study. J Bone Miner Res 2002;17:826
–33.
[22] ASTM Standard and D790-86 Standard test methods for flexural
properties of unreinforced and reinforced plastics and electrical
insulating materials. Philadelphia: American society for testing and
materials 1986
[23] Bank RA, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber FP, et
al. A simplified measurement of degraded collagen in tissues: application
in healthy fibrillated and osteoarthritic cartilage. Matrix Biol 1997;5:
233
–43.
[24] Gineyts E, Cloos P, Borel O, Grimaud L, Delmas PD, Garnero P.
Racemization and isomerization of type I collagen C-telopeptides in
human bone and soft tissues: assessment of bone turnover. Biochem J
2000;345:481
–5.
[25] Cloos PA, Lyubimova N, Solberg H, Qvist P, Christiansen C, Byrjalsen I,
et al. An immunoassay for measuring fragments of newly synthesized
collagen type I produced during metastatic invasion of bone. Clin Lab
2004;50:279
–89.
[26] Carter DR, Hayes WC. Bone compressive strength: the influence of
density and strain rate. Science 1976;194:1174
–6.
[27] Fyhrie DP, Vashishth D. Bone stiffness predicts strength similarly for
human vertebral cancellous bone in compression and for cortical bone in
tension. Bone 2000;26:169
–73.
[28] Catanese III J, Iverson EP, Ng RK, Keaveny TM. Heterogeneity of the
mechanical properties of demineralized bone. J Biomech 1999;32:
1365
–9.
[29] Haddock SM, Yeh OC, Mummaneni PV, Rosenberg WS, Keaveny TM.
Similarity in the fatigue behavior of trabecular bone across site and species.
J Biomech 2004;37:181
–7.
[30] Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu
Rev Biochem 1984;53:717
–48.
[31] Eyre D. Collagen cross-linking amino acids. Methods Enzymol 1987;144:
115
–39.
[32] Eyre D, Dickson IR, Van Ness K. Collagen crosslinks in human bone and
articular cartilage: age-related changes in the content of mature hydro-
xypyridinium residues. Biochem J 1988;252:495
–500.
[33] Otsubo K, Katz EP, Mechanic GL, Yamauchi M. Cross-linking
connectivity in bone collagen fibrils: the COOH-terminal locus of free
aldehyde. Biochemistry 1992;31:396
–402.
[34] Saito M, Marumo K, Fujii K, Ishioka N. Single-column high performance
liquid chromatographic fluorescence detection of immature, mature and
senescent crosslinks of collagen. Anal Biochem 1997;253:26
–32.
[35] Grant ME, Jackson DS. Carbohydrate content of bovine collagen
preparations. Biochem J 1968;108:587
–91.
[36] Currey JD, Foreman J, Laketic I, Mitchell J, Pegg DE, Reilly GC. Effects
of ionizing radiation on the mechanical properties of human bone. J Orthop
Res 1997;15:111
–7.
308
P. Garnero et al. / Bone 38 (2006) 300
–309

[37] Tang SY, Sharan AD, Novak EA, Ford TC, Vashishth D. Nonenzymatic
glycation causes loss of toughening mechanisms in human cancellous
bone. ORS Trans 2005;30:678 [Abstract].
[38] Broz JJ, Simske SJ, Greenberg AR. Material and compositional
properties of selectively demineralized cortical bone. J Biomech 1995;
28:1357
–68.
[39] Schaffer M, Burr D. Stiffness of compact bone: effects of porosity and
density. J Biomech 1988;21:13
–6.
[40] Vashishth D, Behiri JC, Bonfield W. Crack growth resistance in cortical
bone: concept of micro crack toughening. J Biochem 1997;30:763
–9.
[41] Zioupos P, Curey JD. The extent of microcracking and the morphology of
microcracks in damaged bone. J Mater Sci 1994;29:978
–86.
[42] Zioupos P. Recent developments in the study of failure of solid
biomaterials and bone:
“fracture” and “pre-fracture” toughness. Mater
Sci Eng 1998;C6:33
–40.
[43] Tomasek JJ, Meyers SW, Basinger JB, Green DT, Shew RL. Diabetic and
age-related enhancement of collagen-linked fluorescence in cortical bones
of rats. Life Sci 1994;55:855
–61.
[44] Bank RA, Bayliss MT, Maroudas A, Lafeber FP, te Koppele JM. Ageing
and zonal variation in post-translational modification of collagen in normal
human articular cartilage. The age-related increase in non-enzymatic
glycation affects biomechanical properties of cartilage. Biochem J 1998;
330:345
–51.
[45] Garnero P, Fledelius C, Gineyts E, Serre CM, Vignot E, Delmas PD.
Decreased
β Isomerization of the C-terminal telopeptide of type I collagen
1 chain in Paget
’s disease of bone. J Bone Miner Res 1997;12:1407–15.
309
P. Garnero et al. / Bone 38 (2006) 300
–309
Document Outline
- Extracellular post-translational modifications of collagen are major determinants of biomechani.....
Wyszukiwarka
Podobne podstrony:
Post translational processing of b D xylanases and changes
Post collisional melting of crustal sources constraints
Fibrillar Structure and Mechanical Properties of Collagen
Design Guide 12 Modification of Existing Steel Welded Moment Frame
Alex Thomson ITV Money and a hatred of foreigners are motivating a new generation of Afghan Fighte
Concept of God in Major Religions
S Belavenets Basic principles of game are in middlegame (RUS, 1963) w doc(1)
Home Power Magazine Issue 109 Extract pg22 Making Sense of Solar Electricity Costs
Marina Post The impact of Jose Ortega y Gassets on European integration
Laser surface modification of hydroxyapatite and glass
Modification of Intestinal Microbiota and Its Consequences for Innate Immune Response in the Pathoge
O E Deutsch The discovery of Schubert s Great C major symphony
Stimulation of Collagen Production in Human Fibroblasts
Thermal and chemical modification of titanium
Heavenly Shades of Night Are Fa Stephen King
Are the google translations of the sentences in the left column correct
Assessment of cytotoxicity exerted by leaf extracts
więcej podobnych podstron