
Biomaterials 27 (2006) 256–261
In vivo behavior of acrylic bone cement in total hip arthroplasty
Michael D. Ries
a
, Ernest Young
b
, Laila Al-Marashi
b
, Philip Goldstein
b
,
Alexander Hetherington
b
, Timothy Petrie
b
, Lisa Pruitt
c,
a
University of California, 500 Parnassus Avenue (MU-320-W), San Francisco, CA 94143, USA
b
University of California, 2121 Etcheverry Hall, Berkeley, CA 94720, USA
c
University of California, 5134 Etcheverry Hall, Berkeley, CA 94720-1740, USA
Received 14 April 2005; accepted 31 May 2005
Available online 21 July 2005
Abstract
Polymethylmethacrylate (PMMA) bone cement serves as the primary fixation material between bone and the prosthetic
component in cemented total hip arthroplasty. In vivo degradation of bone cement may lead to a decrease in mechanical properties
of PMMA and result in aseptic loosening. However, other factors such as porosity and location of the cement relative to the bone
implant interface may also contribute to mechanical behavior in vivo. This study investigated the mechanical properties of Simplex
s
cement retrieved from 43 patients undergoing revision total hip arthroplasty. The time in vivo was between 1 month and 27 years.
The variables studied included fracture toughness (K
IC
), porosity, molecular weight, time in vivo of the cement, and relative in vivo
location of the cement with respect to the implant and bone. K
IC
did not correlate with time in vivo of the samples or with molecular
weight. This suggests that time in vivo may not be the limiting factor in the mechanical integrity of the bone cement, A significant
and inverse relationship was found between porosity and K
IC
. This implies that porosity is the most important factor in the
mechanical behavior of bone cement during in vivo use.
r
2005 Elsevier Ltd. All rights reserved.
Keywords: Bone cement; In vivo degradation; Fracture toughness; Porosity
1. Introduction
PMMA augments load transfer between the prosthe-
sis and the bone by forming a mechanical bond between
the two components. When used as the fixation method
in total hip arthroplasty, 10 year survivorship rates in
patients aged 60 years or more have been reported as
greater than 90%
. Since cemented total hip
implants undergo cyclic loads of up to five times body
weight
, the bone cement is vulnerable to failure by
tensile stresses
. Consequently, bone cement fracture
may contribute to loss of mechanical integrity and
aseptic loosening of cemented total hip implants
A number of different factors and properties have
been shown to affect the mechanical properties of
PMMA-based bone cement. For example, PMMA has
relatively low fracture toughness, which has been
correlated with low impact and fatigue strength
One factor that could affect the fracture toughness is
porosity, although some investigators have suggested
otherwise
. Since pores have been identified in vitro as
stress-risers and crack-initiators
, higher degrees of
porosity may contribute to microcracking
Microcracking may also lead to PMMA-particle release,
which can induce local inflammation and osteolysis
Molecular weight and porosity may be affected by
different mixing and sterilization methods
Sterilization techniques such as gamma irradiation can
cause chain scission in PMMA, leading to a reduction in
molecular weight
. The molecular weight of the
ARTICLE IN PRESS
www.elsevier.com/locate/biomaterials
0142-9612/$ - see front matter r 2005 Elsevier Ltd. All rights reserved.
doi:10.1016/j.biomaterials.2005.05.103
Corresponding author. Tel.: +1 510 642 2595;
fax: +1 510 643 5599.
E-mail address: lpruitt@me.berkeley.edu (L. Pruitt).
PMMA can further decrease over time during in vivo
use
. Since reduction in molecular weight can lead to
diminished fracture toughness, in vivo degradation of
PMMA may contribute to late mechanical failure of
total hip arthroplasty
. However, factors other than
length of time in vivo or molecular weight, such as
porosity, may also affect the mechanical performance of
PMMA.
This study investigated the effects of in vivo degrada-
tion on bone cement properties including fracture
toughness, porosity, and molecular weight. This analysis
utilized fracture toughness as a measure of the mechan-
ical integrity of the retrieved PMMA bone cement
The purpose of this study was to identify the most
important factors that affect the in vivo mechanical
behavior of PMMA.
2. Materials and methods
2.1. Sample preparation
Samples of Simplex
s
bone cement were retrieved from 43
patients who underwent revision total hip arthroplasty. The
size and amount of the retrievals varied among each patient.
The time in vivo before revision ranged from 1 month to 27
years. The age of the patients was between 50 and 92 years. All
samples were obtained from revision total hip arthroplasty in
aseptic conditions. Samples were rinsed in 0.85% saline
solution, and any residual organic tissue and constituents
were removed with a saline wash. Specimens were then allowed
to dry and afterwards stored in 10% neutral-buffered
formaldehyde (formalin) solution at 5 1C to preclude any
further environmental effects on the cement. For mechanical
testing preparation, all the samples were removed from
formalin, rinsed in deionized water twice, blotted with wipes,
and air-dried for 24 h.
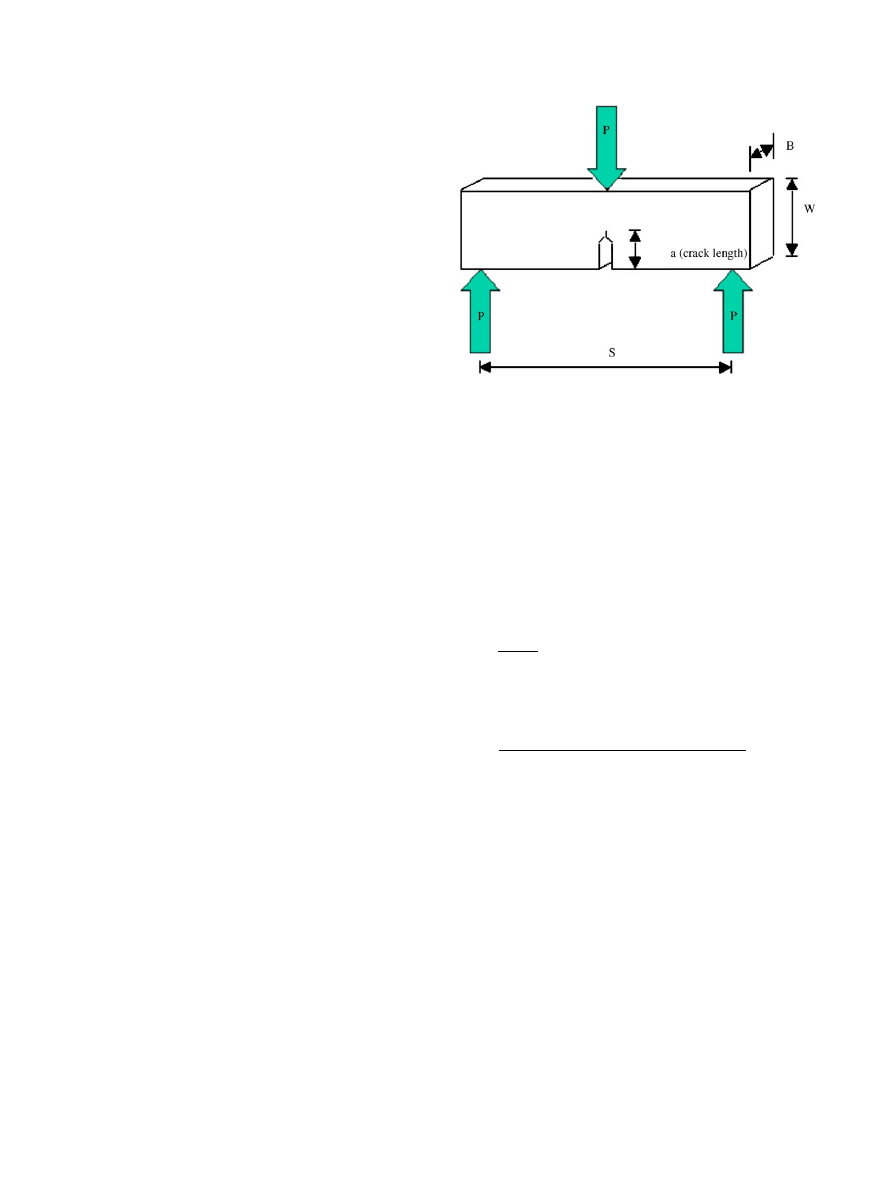
2.2. Fracture toughness testing
Fracture toughness, K
IC
, was determined for each specimen.
Retrieval samples were machined in accordance with ASTM
E399 standards for fracture toughness testing using a single
edge-notched bend (SENB) specimen, schematically illustrated
in
. Machining was performed with a diamond knife,
with beam shape and dimensions recommended by Lewis et al.
. The dimension of the specimens were as follows: span,
S ¼ 16 0:01 mm,
width
W ¼ 4:85 0:01 mm,
thickness
B ¼ 2:5 0:01 mm, initial crack length, a ¼ 1:45 0:01 mm.
Care was taken to ensure that specimens maintained an initial
crack to width ratio, a
init
¼
a
init
=W ¼ 0:3 0:01. Initial crack
length is known to affect fracture toughness values so only
specimens with an a
init
¼
0:3 0:01 were used in this study.
The location and orientation of each machined beam relative
to the original cement retrieval was recorded on a sample map
for location-dependent analyses. The SENB specimens were
pre-cracked using a standard guillotine method
. Plain
strain fracture toughness testing was performed using a three-
point bend test with a span S between the top two loading
points 3.3 times the width of the beam. All fracture toughness
testing was performed in accordance with ASTM E399
standards. Beams were loaded to failure under load control
in an Instron 8871 (Instron Corporation, Canton, MA) at a
loading rate of 0.25 N/s. The Instron program SAX V 7.0
(Instron Corporation) was used to record the maximum load
at failure for each beam. A total of 242 beams from 43 patients
were tested.
K
IC
values were calculated as a function of the maximum
load P and beam geometry as
K
IC
¼
PSf ðaÞ
BW
3=2
,
where S is the span distance between support pins, B is the
thickness of the specimen, W is the specimen width, a is the
crack length, a ¼ a=W , and f(a) is
f ðaÞ ¼
3a
1=2
ð
1:99 að1 aÞð2:15 3:93a þ 2:7a
2
ÞÞ
2 þ ð1 þ 2aÞð1 aÞ
3=2
.
Fracture beams were classified according to location with
respect to the adjacent bone and prosthesis in order to analyze
location dependency on in vivo mechanical degradation of
bone cement. During machining, maps were prepared that
recorded the location of each beam relative to the sample from
which they were machined. Images of the samples taken before
machining were compared to these location maps in order to
qualitatively determine the relative transverse location of each
beam across the thickness of the bone cement sample. Beams
were classified into three relative groups based on their pre-
machined position within each retrieved cement sample. These
classes were comprised of beams nearest to the cement/device
interface, the interior portion, and the cement/bone interface.
2.3. Porosity analysis
Fracture surfaces from the fracture toughness samples were
sonic-cleaned with ethanol and blow dried with cool air.
Cleaned surfaces were coated with a layer of gold and
photographs of fracture surfaces were acquired using a
ARTICLE IN PRESS
Fig. 1. Schematic illustration of single edge notch bend specimen used
for fracture toughness testing.
M.D. Ries et al. / Biomaterials 27 (2006) 256–261
257
scanning electron microscope (SEM). The photograph magni-
fication was normalized among all images captured to allow
comparable planar surface porosity values to be calculated.
Analysis of planar porosity was restricted only to the fracture
surfaces of imaged beams. The maximum pore size at the front
of initial crack was measured to see if it played a role in crack
inception. Photographs were scanned into a computer and
porosity quantified using the Scion Image Beta 4.0 (Scion
Corp, Frederick, MD) imaging program. Pores were qualita-
tively identified according to defined pore standards, such as
the number of pores, surface area, and the mean, standard
deviation, minimum, maximum, major, and minor pore
diameters. This data was used to calculate the total fracture
area, percent porosity, average area porosity, and maximum
and minimum pore areas. The correlation of percent planar
porosity to fracture toughness, location, and time in vivo was
examined.
2.4. Molecular weight analysis
Gel permeation chromatography (GPC) analysis was used
to determine the average molecular weight and molecular
weight distribution of the retrieved cements. Molecular weight
calibration was established based on polystyrene standards.
Samples were dissolved in tetrahydrofuran (THF) to a
concentration of 3 mg/mol at 30 1C, and then filtered in 2 mm
cup filters. Each sample was injected three times into two
American Polymer Standards Corporation GPC Gel linear
columns, 7.8 mm inner diameter 30 cm, packed with THF
with a pore size of 500 A˚. Injection size was 100 mL and the
flow rate was 1 mL/min at 30 1C. A Waters 150C system, which
uses a differential refractive index detector, was used to
monitor the changes in concentration of the sample. The
molecular weight distributions of the samples relative to
polystyrene were found in terms of the number-averaged
molecular weight M
n
, the weight-averaged molecular weight
M
w
, the z-averaged molecular weight M
z
, and the polydis-
persity index. Molecular weight was plotted versus K
IC
and in
vivo location of retrieved PMMA samples to determine if a
significant correlation existed.
2.5. Statistical analysis
Data from each of the tests were compared using one-way
analysis of variance (ANOVA) tests (Stata, College station,
TX) to determine the overall significance of data trends. For
this statistical system, p
o0:05 was considered significant.
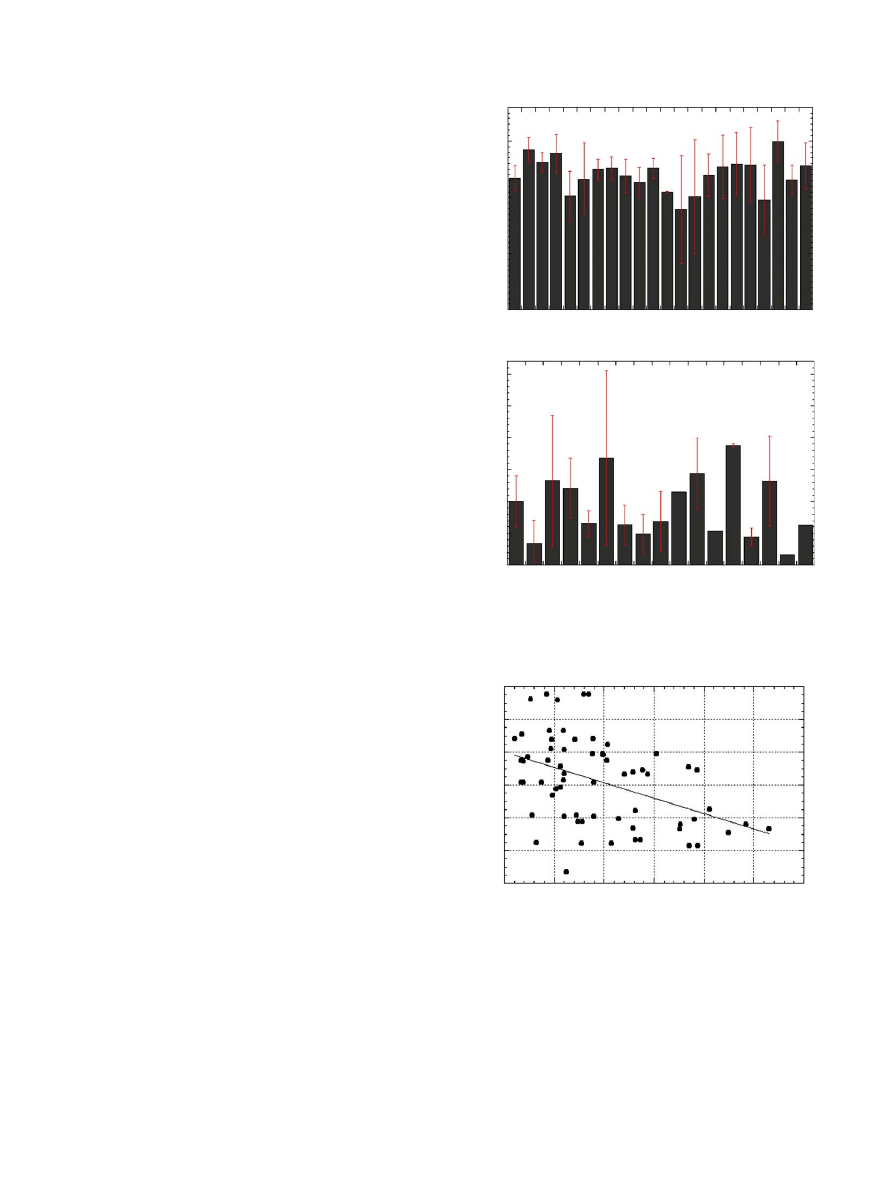
3. Results
Fracture toughness is known to depend on steriliza-
tion method, bone cement chemistry, and mixing
method
. In this study, a statistical correlation
between fracture toughness and time in vivo was not
observed (
). In addition, porosity did not vary
significantly with time in vivo (
). However, when
fracture toughness and porosity were directly compared,
the higher porosity values corresponded with signifi-
cantly lower K
IC
values ðp
o0:05Þ (
). This indicates
that porosity and K
IC
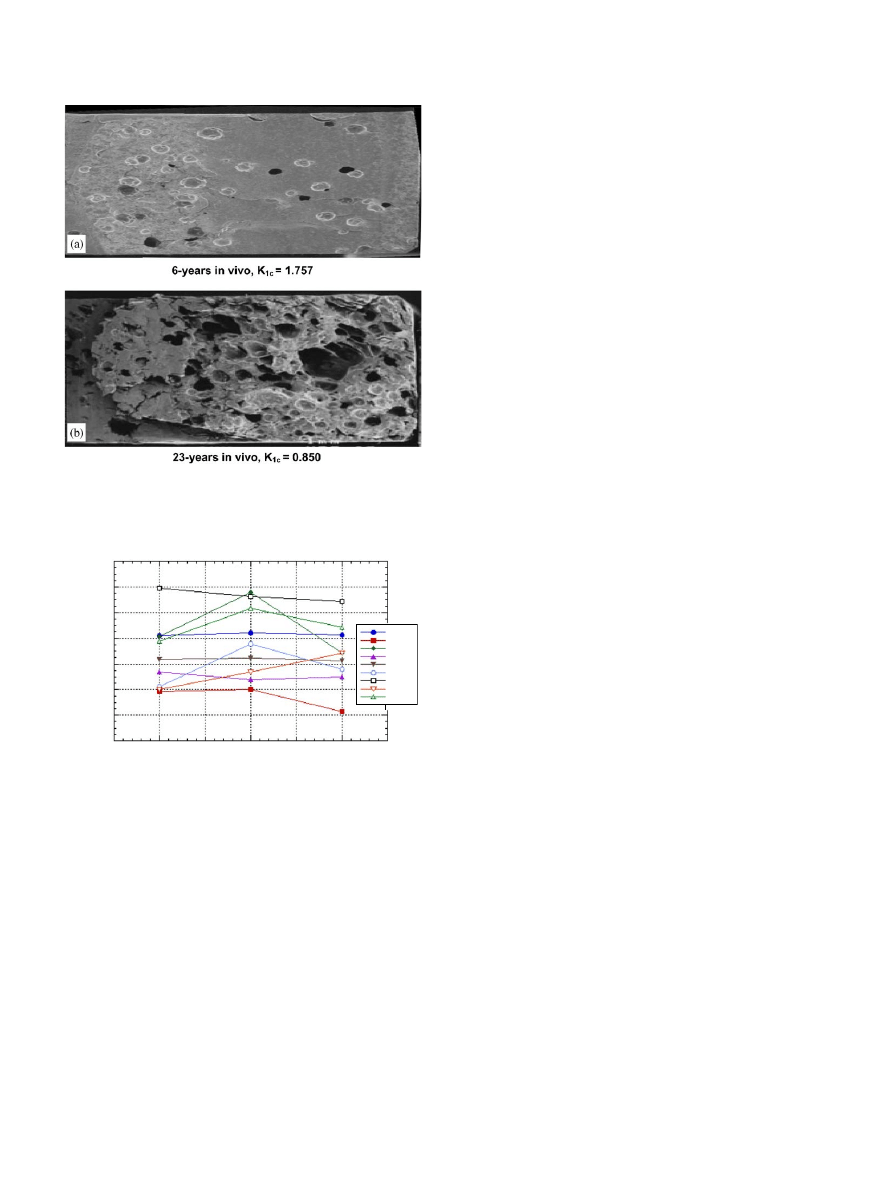
are inversely related. An example
of this correlation can be seen in representative SEM
photographs of fracture surfaces for samples retrieved
after 6 and 23 years in vivo (
). There was
no correlation of fracture toughness with pore size at
ARTICLE IN PRESS
0 1 3 4 5 6 7 9 10 11 12 13 14 15 17 18 19 20 22 23 25 27
Time In Vivo (Years)
0
1
4
5
6
9 10 11 12 15 17 18 20 22 23 25 27
Time In Vivo (Years)
Planar Porosity (%)
1.5
1
0.5
0
Fracture Toughness K
IC
(MPa m
0.5
)
30
25
20
15
10
5
0
(a)
(b)
Fig. 2. Plot showing (a) fracture toughness as a function of time in
vivo and (b) porosity as a function of time in vivo.
1.8
1.6
1.4
1.2
0.8
0.6
0
5
10
15
20
25
30
1
Planar Porosity (%)
Fracture Toughness K
IC
(MPa m
0.5
)
Fig. 3. Plot showing fracture toughness as a function of porosity.
M.D. Ries et al. / Biomaterials 27 (2006) 256–261
258
initial crack front. This finding is consistent with
previous studies
Sample location analysis results are presented in
. Sample K
IC
values were compared to relative
location across the transverse axis of the implant and no
significant trend was found ðp40:05Þ. Roughly half the
patients displayed larger K
IC
values in the interior of the
cement mantle and lower values at the interfaces.
Similarly, no significant relationship ðp40:05Þ between
relative cement beam location and porosity was
observed.
Moreover, molecular weight did not show a relation-
ship ðp40:05Þ with transverse location. In addition, a
significant correlation was not observed ðp40:05Þ
between molecular weight and K
IC
. Molecular weight
values for the samples tested ranged from 170,000 to
210,000 g/mol and K
IC
values ranged from 1.0 to
1.7 MPa
Om.
4. Discussion
Biological factors contribute to aseptic loosening of
total hip implants through a number of mechanisms.
Particulate debris can trigger a localized immune
response that leads to bone resorption
. UHMWPE
and PMMA particles cause macrophage activation and
subsequent secretion of proteolytic enzymes and cyto-
kines
. Degradation of PMMA bone cement may
also activate elements of the localized inflammatory
response and lead to a greater susceptibility of the
cement to fracture.
Hydrolysis of the bone cement may cause a reduction
in molecular weight of PMMA during in vivo use
. A
reduction in molecular weight can lead to a decrease in
mechanical properties. However, our results, utilizing
fracture toughness as the critical measurement of
resistance to failure, indicate that the in vivo time of
the bone cement does not have a significant correlation
with cement fracture toughness. Hence, the amount of
time that the bone cement spends in the implant
environment may not be the limiting factor in its in
vivo mechanical behavior. This result does not necessa-
rily imply that exposure to this degrading environment
does not have an effect. However, it does suggest that
other parameters may have a greater influence on the
resistance of the cement to this induced degradation.
For example, the cement mixing method used may
have a more significant impact on the long-term
integrity of the cement than biodegradation in vivo.
Vacuum mixing reduces the number and size of pores in
bone cement and improves fatigue resistance
. Pores
may act as stress-risers and crack-initiating sites that
reduce crack growth resistance. However, a correlation
between lower porosity and increased fracture toughness
in the literature has not been obvious. Topoleski et al.
hypothesized that pores may serve to blunt propa-
gating cracks, serving to enhance the fracture toughness
of a material. Rimnac et al.
postulated that existing
cracks and surface imperfections counteract any bene-
ficial stabilizing effects of pores since these existing flaws
are substantially larger than most internal pores in the
material. Vila et al.
suggested that the stress intensity
of the crack tip predominates over the stress concentra-
tion from porosity, in effect neglecting porosity effects
on fracture toughness.
Our results indicate that reduced porosity does
enhance the fracture toughness in implanted acrylic
bone cement. Factors affecting porosity include mixing
methods, intraoperative bleeding, bone preparation, and
cement implantation technique. An interesting finding in
ARTICLE IN PRESS
Fig. 4. Representative SEM photographs of fracture surfaces for
samples retrieved after (a) 6 years in vivo and (b) 23 years in vivo.
1 year
5 year
6 year
10 year
11 year
17 year
18 year
19 year
22 year
Cement/Device
Cement/Bone
Interior
2
1.8
1.6
1.4
1.2
0.8
0.6
1
Fracture Toughness K
IC
(MPa m
0.5
)
Fig. 5. Plot showing fracture toughness as a function of retrieval
location.
M.D. Ries et al. / Biomaterials 27 (2006) 256–261
259

this study is that although mixing methods have
drastically improved in the past 10 years with a trend
towards vacuum mixing, there was no clear reduction in
porosity with smaller aging times. This suggests that
that other factors such as surgical technique and
exothermal heating likely contribute to in vivo porosity.
A statistically significant inverse relationship between
porosity and fracture toughness was found in this
analysis. This finding correlates well with the findings
of Graham et al.
indication that reduced porosity
enhances fatigue resistance in PMMA bone cement.
In this study there was no relationship observed
between fracture toughness and molecular weight. The
findings revealed negligible variation in molecular
weight with a range of K
IC
values from 0.96 to
1.76 MPa
Om. Hence, no significant correlation was
inferred between MW and K
IC
of the retrieved bone
cement analyzed in this study. Hughes et al.
examined the effect of physiological aging on molecular
weight of PMMA and showed that molecular weight
degrades over time in a simulated oxidative and acidic
environment. Their results pointed at the existence of an
in vivo degradation mechanism in which the biological
environment becomes oxidative and pH is reduced
possibly due to particulate debris induced osteolysis.
However, studies by Kim et al.
deduced that many
mechanical properties, particularly fracture toughness
of PMMA, might be insensitive to MW values over
2 10
5
g/mol. Graham et al.
showed markedly
reduced changes in fracture toughness for PMMA
samples of MW over 1 10
5
g/mol. Our results appear
consistent with these studies. At equivalent MW levels
to those suggested, neither the porosity nor the fracture
toughness is significantly affected.
The findings in this study are also consistent with the
theory of ‘‘defect crazing’’
, which stipulates that
crazes are formed ahead of the crack tip, creating an
energy dissipation zone surrounding the crack
. This
zone contributes to ‘‘ligaments’’
of polymer chains
bridging these regions and the crack itself, enhancing the
load-bearing capacity of the cement microstructure.
Increasing the length of these ligaments by increasing
molecular weight will improve the resistance to fracture
of the cement
. However, once the polymer ligament
is able to bridge the crack, a further increase in MW
does little to enhance fracture and fatigue character-
istics. This may partially explain why fracture toughness
displayed no correlation with MW at such high MW
levels (2 10
5
g/mol). The retrieved PMMA bone
cements retained MW levels that were higher than the
threshold (1 10
5
g/mol)—hence, fracture properties
were not significantly affected with MW differences.
One aim of this study was to investigate the possibility
of a diffusion-limited degradation mechanism that may
act at the cement interface with the bone and with the
prosthesis
. In this proposed mechanism, hydro-
lytic biological fluids may seep into the interfaces
between both the cement/bone and cement/prosthesis
over time. These fluids may then diffuse into the bone
cement and degrade the cement. Therefore, it would be
expected that the areas of the bone cement at the
interfaces would display reduced fracture properties or
molecular weight compared to cement in the ‘‘interior’’
of the mantle farthest away from these two diffusive
interfaces. However, no correlation between location of
PMMA samples and intrinsic MW, porosity, or fracture
toughness ðp40:05Þ was found.
5. Conclusions
This study examines the effects of long-term implan-
tation of acrylic bone cement in femoral components of
total hip prostheses. The main result of this study is that
there exists an inverse relationship between fracture
toughness and pore size.
Acknowledgment
This work was supported by the Orthopedic Research
and Education Foundation.
References
[1] James ETR, Hunter GA, Cameron HU. Total hip revision
arthroplasty: does septis influence the results. Clin Orthop 1982;
170:88–94.
[2] Topoleski LD, Ducheyne P, Cuckler JM. A fractographic analysis
of in vivo poly (methyl methacrylate) bone cement failure
mechanisms. J Biomed Mater Res 1990;24:135–59.
[3] Nordin M, Frankel VH. Basic biomechanics of the musculoske-
letal system. 2nd ed. Philadelphia: Lea and Fibiger; 1989. p.
22–30.
[4] James SP, Jasty M, Davies J, Piehler H, Harris WH. A
fractographic investigation of PMMA bone cement focusing on
the relationship between porosity reduction and increased fatigue
life. J Biomed Mater Res 1992;26(5):651–62.
[5] Hertzberg RW, Manson JA. Fatigue of engineering plastics.
London: Academic Press; 1980. p. 74–121.
[6] Malchau H, Herberts P, Ahnfelt L. Prognosis of total hip
replacement in Sweden. Follow up of 92,675 operations
performed 1978–1990. Acta Orthop Scand 1993;64:497–506.
[7] Lewis G, Mladsi S. Relationship between fracture toughness and
impact strength of acrylic bone cement. Crit Rev Biomed Eng
2000;28(3–4):451–5.
[8] Vila MM, Ginebra MP, Gil FJ, Planell JA. Effect of porosity and
environment on the mechanical behavior of acrylic bone cement
modified with acrylonitrile-butadiene-styrene particles: I. Fracture
toughness. J Biomed Mater Res 1999;48(2):121–7.
[9] Topoleski LD, Ducheyne P, Cuckler JM. Microstructural path-
way of fracture in poly(methyl methacrylate) bone cement.
Biomaterials 1993;14(15):1165–72.
[10] Bhambri SK, Gilbertson LN. Micromechanisms of fatigue crack
initiation and propagation in bone cements. J Biomed Mater Res
1995;29:233–7.
ARTICLE IN PRESS
M.D. Ries et al. / Biomaterials 27 (2006) 256–261
260

[11] McCormack BAO, Prendergast PJ, Gallagher DJ. An experi-
mental study of damage accumulation in cemented hip prostheses.
Clin Biomech 1996;11(4):214–9.
[12] Murphy BP, Prendergast PJ. The relationship between stress,
porosity, and nonlinear damage accumulation in acrylic bone
cement. J Biomed Mater Res 2002;59(4):646–54.
[13] Horowitz SM, Doty SB, Lane JM, Burstein AH. Studies
of the mechanism by which the mechanical failure of poly-
methylmethacrylate leads to bone resorption. J Bone Jt Surg
1993;75A:803–13.
[14] Hughes KF, Ries MD, Pruitt LA. Structural degradation of
acrylic bone cements due to in vivo and simulated aging. J Biomed
Mater Res 2003;65A(2):126–35.
[15] Dunne NJ, Orr JF. Influence of mixing techniques on the
physical properties of bone cement. Biomaterials 2001;22(13):
1819–26.
[16] Graham J, Pruitt LA, Ries MD, Gundiah N. Fracture and fatigue
properties of acrylic bone cement: the effects of mixing method,
sterilization treatment, and molecular weight. J Arthroplasty 2000;
15(8):1028–35.
[17] Chapiro A. Radiation chemistry of polymeric systems. New York:
Wiley-Interscience; 1962.
[18] Harper EP, Braden M, Bonfield W, et al. Influence of sterilization
upon a range of properties of experimental bone cements. J Mater
Sci Mater Med 1997;8:849.
[19] Lewis G, Mladsi S. Effect of sterilization method on properties of
Palacos Racrylic bone cement. Biomaterials 1998;19(1-3):117–24.
[20] Hertzberg RW. Deformation and fracture mechanics of engineer-
ing materials. 2nd ed. New York: Wiley; 1983 [chapters 7–8].
[21] Lewis G. Properties of acrylic bone cement: State-of-the-art
review. J Biomed Mater Res (Appl Biomater) 1997;38:155–82.
[22] Lewis G. Apparent fracture toughness of acrylic bone cement:
effect of test specimen configuration and sterilization method.
Biomaterials 1999;20:69–78.
[23] Atkins AG, Mai YW. On the guillotining of materials. J Mater Sci
1979;14:2747–54.
[24] Jasty M, Smith E. Wear particles of total joint replacements and
their role in periprosthetic osteolysis. Curr Opin Rheumatol
1992;4(2):204–9.
[25] Jasty M, Jiranek W, Harris WH. Acrylic fragmentation in total
hip replacements and its biological consequences. Clin Orthop
1992;285:116–28.
[26] Miyaguchi M, Kobayashi A, Iwaki H, et al. Human monocyte
response to retrieved polymethylmethacrylate particles. J Biomed
Mater Res 2002;62(3):331–7.
[27] Shardlow DL, Stone MH, Ingham E, Fisher J. Cement particles
containing radio-pacifiers stimulate pro-osteolytic cytokine pro-
duction from a human monocytic cell line. J Bone Jt Surg Br
2003;85(6):900–5.
[28] Rimnac CM, Wright TM, Mcgill DL. The effect of centrifugation
on the fracture properties of acrylic bone cements. J Bone Jt Surg
Am 1986;66:281–7.
[29] Kim SL, Skibo M, Manson JA. Fatigue crack propagation in
poly(methyl methacrylate): effect of molecular weight and internal
plasticization. Polym Eng Sci 1977;17:194.
[30] Hertzberg RW. Deformation and fracture mechanics of engineer-
ing materials, 4th ed. New York: Wiley; 1996. p. 786–800.
[31] Bishop NE, Ferguson S, Tepic S. Porosity reduction in bone
cement at the cement-stem interface. J Bone Jt Surg Br 1996;
78(3):356–9.
[32] Hamilton HW, Cooper DF, Fels M. Shrinkage of centrifuged
cement. Orthop Rev 1988;17(1):48–54.
ARTICLE IN PRESS
M.D. Ries et al. / Biomaterials 27 (2006) 256–261
261
Document Outline
Wyszukiwarka
Podobne podstrony:
In vivo behaviour
In vivo MR spectroscopy in diagnosis and research of
Fluorescent proteins as a toolkit for in vivo imaging 2005 Trends in Biotechnology
2008 5 SEP Practical Applications and New Perspectives in Veterinary Behavior
Badania in vivo we współczesnej kosmetologii, Kosmetologia, inne
Metodyka?dań in vivo obieralny dr A Piastowska Ciesielska
Anty aging czy nawilżający co o skuteczności kwasu hialuronowego mówią testy in vivo
Testy umozliwiajace zbadanie uszkodzeń materiału genetycznego komórek ssaków in vivo i in vitro
An in vivo Proton MRS study in schizohrenia patients
In vivo MR spectroscopy in diagnosis and research of
In vivo dissolution
Antioxidant activity of tea polyphenols in vivo evidence from animal studies
In vivo absorption of aluminium containing vaccine adjuvants using 26Al
Emotion and Reason in Consumer Behavior Arjun Chaudhuri
Stages of change in dialectical behaviour therapy for BPD
The in vivo
The pathogenesis of Sh flexneri infection lessons from in vitro and in vivo studies
Wpływ preparatów hormonalnych na przemiany metaboliczne in vivo
In vitro behavior
więcej podobnych podstron