SPME App Note 13 Page 1
Screening Packaging
Materials with
Automated SPME
and GC/MS
Varian Application Note
Number 13
Zelda Penton
Varian Chromatography Systems
Key Words:
SPME, 8200CX, Polymers, Saturn
Solid phase microextraction (SPME) was used to compare various packaging materials to assess their suitability
for storing and shipping analytical materials. In a previous publication (SPME Application Note #7), polymeric
beads that had been subjected to various heat treatments were compared; in this note, finished sheets were
examined. The various materials showed specific repeatable contamination patterns. The technique was very
simple—approximately 1-cm
2
of the various samples were placed into 2-mL screw cap vials and the air in the
vials was sampled at ambient temperature.
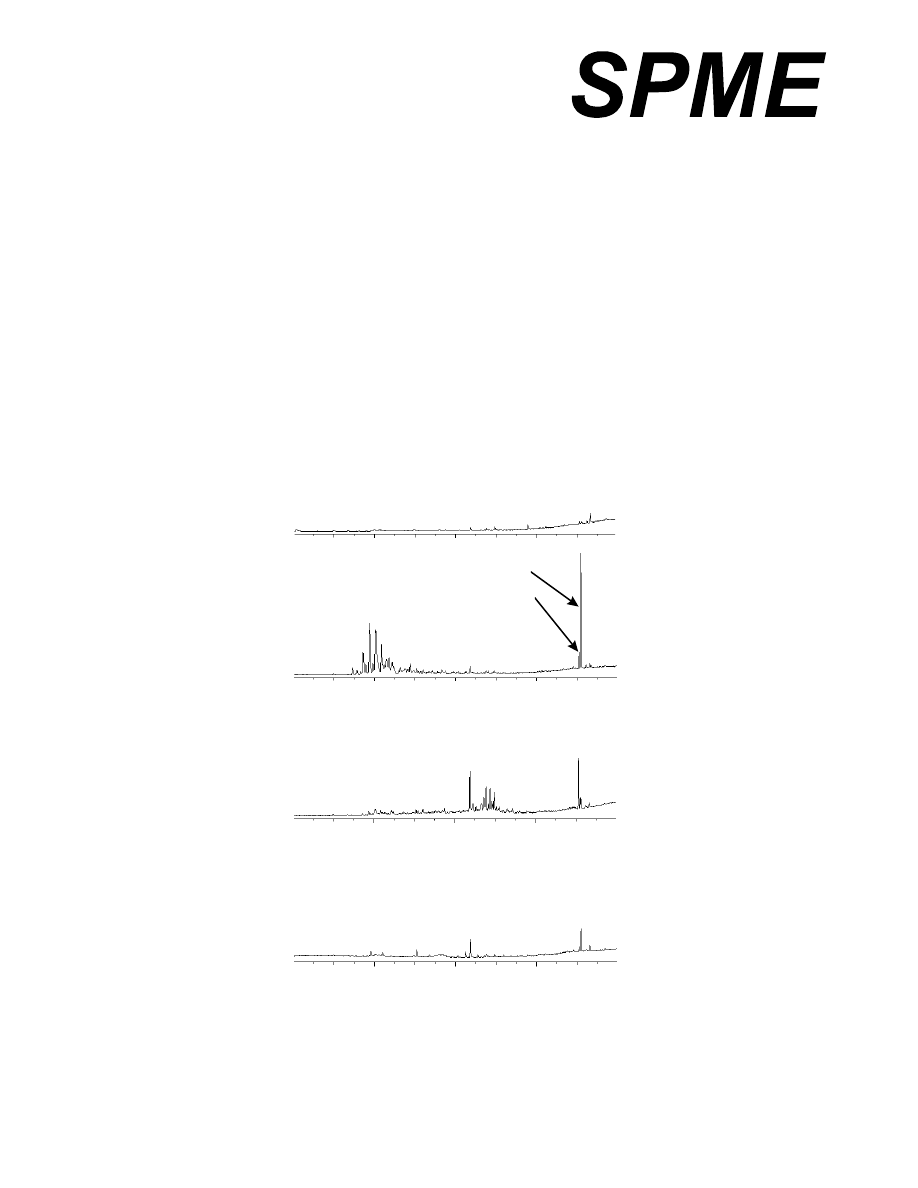
0 5 10 15 20
Retention time (min)
C
B
A
Empty vial
2
1
Figure 1: Total ion chromatograms of air sampled with a SPME fiber from a blank vial and vials
containing three different packaging materials. Peaks 1 and 2 were tentatively identified as butylated
hydroxytoluene and 2,6-bis (1,1-dimethylethyl)-4-ethylphenol.
varian
SPME App Note 13 Page 2
Instrumentation and Conditions
Instrument:
Varian Saturn 2000 GCMS equipped with an automated SPME III system.
Column:
30 m x 0.25 mm coated with 0.50-µm Supelcowax 10
TM
, 50°C, 1 minute, 10°C/min to 210°, hold 8 min.
Carrier gas: helium, 41 cm/s at 60°C.
Injector:
SPI with SPME insert at 210°C, isothermal.
Ion trap:
Electron impact ionization mode, mass range 50-250 m/z, ion trap temperature, 200°C.
Automated
SPME
Conditions:
Fibers (Supelco, Inc.) were coated with 100-µm Polydimethylsiloxane.
Headspace sampling without agitation in 2-mL vials, 30 minutes absorption, 2 minutes desorption, one
sampling per vial.
Samples:
Three different packaging materials.
Results and Discussion
The samples were cut into one-cm squares and placed in the vials (one piece per vial). Samples were run in
duplicate, with an empty vial at the beginning and end of the series. The total ion chromatograms were inspected
at comparable attenuation. Figure 1 clearly shows the differences in the packaging materials. Note that
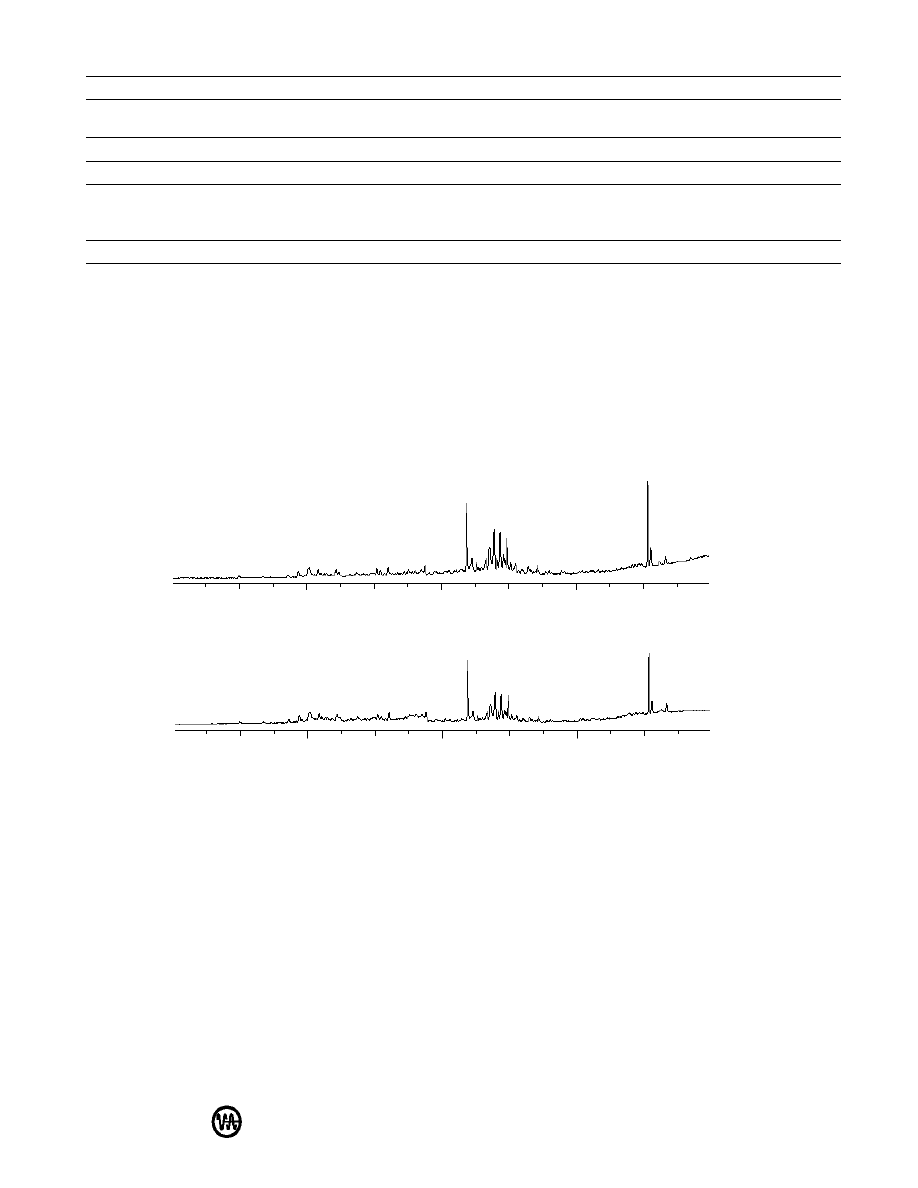
duplicates of the same sample were virtually identical (Figure 2). The method is not quantitative, as one would
expect the quantities of the various compounds released from the packaging material to be proportional to the
surface area, but if similar-sized pieces of the materials are placed in the sampling vials, the relative cleanliness
of the different samples becomes obvious.
Figure 2: Total ion chromatograms of two samples of packaging material “B”.
Conclusions
SPME is a very simple and effective technique for rapidly evaluating the cleanliness of packaging materials.
A simple GC-FID system may be used for fingerprinting, or if identification of the contaminants is required,
GC/MS should be utilized.
Document Outline
- I
- Instrumentation and Conditions
- Conclusions
- SPME is a very simple and effective technique for rapidly evaluating the cleanliness of packaging materials.
- A simple GC-FID system may be used for fingerprinting, or if identification of the contaminants is required, GC/MS should be utilized.
Wyszukiwarka
Podobne podstrony:
HS SPME in packaging materials
in vitro, materiały
Food analysis Packaging Materials
Barczyk i in Mikroekonomia Materiały do ćwiczeń (2)
Kultury in vitro materiał
zestawienie materiału do matury, in italiano, LICEUM
16 197 208 Material Behaviour of Powder Metall Tool Steels in Tensile
A picnic table is a project you?n buy all the material for and build in a?y
Wytrzymałość materiałów, Zginanie proste -wyznaczanie granicznej nośności belki zginanej, Wy?sza Szk
Pytania na zasady, POLITECHNIKA ŚLĄSKA Wydział Mechaniczny-Technologiczny - MiBM POLSL, Semestr 5, S
Legg Calvé Perthes Disease in Czech Archaeological Material
ca a chemia, Mol jest jednostk? liczno?ci substancji (materii), kt?ra zawiera tyle samo atom?w; jon?
materiały z niedzielnych wykładów i in, Terapia Pedagogiczna
rozmnazanie in vitro roslin, ogrodnictwo VII semestr, Od Mateusza S, materiały sggw, SGGW materiały
Wyklady In, Studia, I o, rok II, semestr III, inżynieria materiałowa, od Pauliny
lab, MATER1 C, Krzysztof Bry˙a Sprawozdanie z In˙.
więcej podobnych podstron