Biomaterials 19 (1998) 1621 — 1639
Review
Titanium alloys in total joint replacement—a materials
science perspective
Marc Long, H.J. Rack*
School of Chemical and Materials Engineering, Clemson University, Clemson, SC 29634, USA
Received 26 May 1996; accepted 6 July 1997
Abstract
Increased use of titanium alloys as biomaterials is occurring due to their lower modulus, superior biocompatibility and enhanced
corrosion resistance when compared to more conventional stainless steels and cobalt-based alloys. These attractive properties were
a driving force for the early introduction of
a (cpTi) and a#b (Ti—6Al—4V) alloys as well as for the more recent development of new
Ti-alloy compositions and orthopaedic metastable
b titanium alloys. The later possess enhanced biocompatibility, reduced elastic
modulus, and superior strain-controlled and notch fatigue resistance. However, the poor shear strength and wear resistance of
titanium alloys have nevertheless limited their biomedical use. Although the wear resistance of
b-Ti alloys has shown some
improvement when compared to
a#b alloys, the ultimate utility of orthopaedic titanium alloys as wear components will require
a more complete fundamental understanding of the wear mechanisms involved. This review examines current information on the
physical and mechanical characteristics of titanium alloys used in artifical joint replacement prostheses, with a special focus on those
issues associated with the long-term prosthetic requirements, e.g., fatigue and wear.
( 1998 Published by Elsevier Science Ltd.
All rights reserved
Keywords: Titanium; Titanium alloys; Total joint replacement; Orthopaedics; Fatigue; Wear; Biocompatibility
1. Introduction
Natural synovial joints, e.g., hip, knee or shoulder
joints, are complex and delicate structures capable of
functioning under critical conditions. Their performance
is due to the optimized combination of articular carti-
lage, a load-bearing connective tissue covering the bones
involved in the joint, and synovial fluid, a nutrient fluid
secreted within the joint area [1, 2] (Fig. 1). Unfortunate-
ly, human joints are prone to degenerative and inflam-
matory diseases that result in pain and joint stiffness.
Primary or secondary osteoarthritis (osteoarthrosis), and
to a lesser extent rheumatoid arthritis (inflammation of
the synovial membrane) and chondromalacia (softening
of cartilage), are, apart from normal ageing of articular
cartilage, the most common degenerative processes af-
fecting synovial joints [3, 4]. In fact, 90% of the popula-
tion over the age of 40 suffers from some degree of
* Corresponding author.
degenerative joint disease [5]. Premature joint degener-
ation may arise from deficiencies in joint biomaterial
properties, from excessive loading conditions, or from
failure of normal repair processes, the explicit degen-
erative processes not yet being completely understood.
Degeneration of weight bearing joints often requires
surgery to relieve pain and increase mobility. Through
minimum invasive damage, arthroscopic surgery, most
frequently performed on knee joints, provides an efficient
surgical method for diagnosis and symptomatic relief of
painful joints. Ultimately replacement of diseased joint
surfaces by metal, plastic, or ceramic artificial materials is
accomplished through arthroplastic surgery when the
natural joint can no longer adequately perform.
Total joint replacement (TJR) arthroplasty is recog-
nized as a major achievement in orthopaedic surgery.
Successful replacement of the natural joints through ar-
throplastic surgery has been the long-time objective of
orthopaedic surgeons. Arthroplasty (Dorland’s Medical
Dictionary definition ‘plastic repair of a joint’) is a surgi-
cal technique which replaces all articulating degenerated
natural surfaces with artificial materials, hence achieving
0142-9612/98/$ — See front matter
( 1998 Published by Elsevier Science Ltd. All rights reserved.
PII S 0 1 4 2 - 9 6 1 2 ( 9 7 ) 0 0 1 4 6 - 4
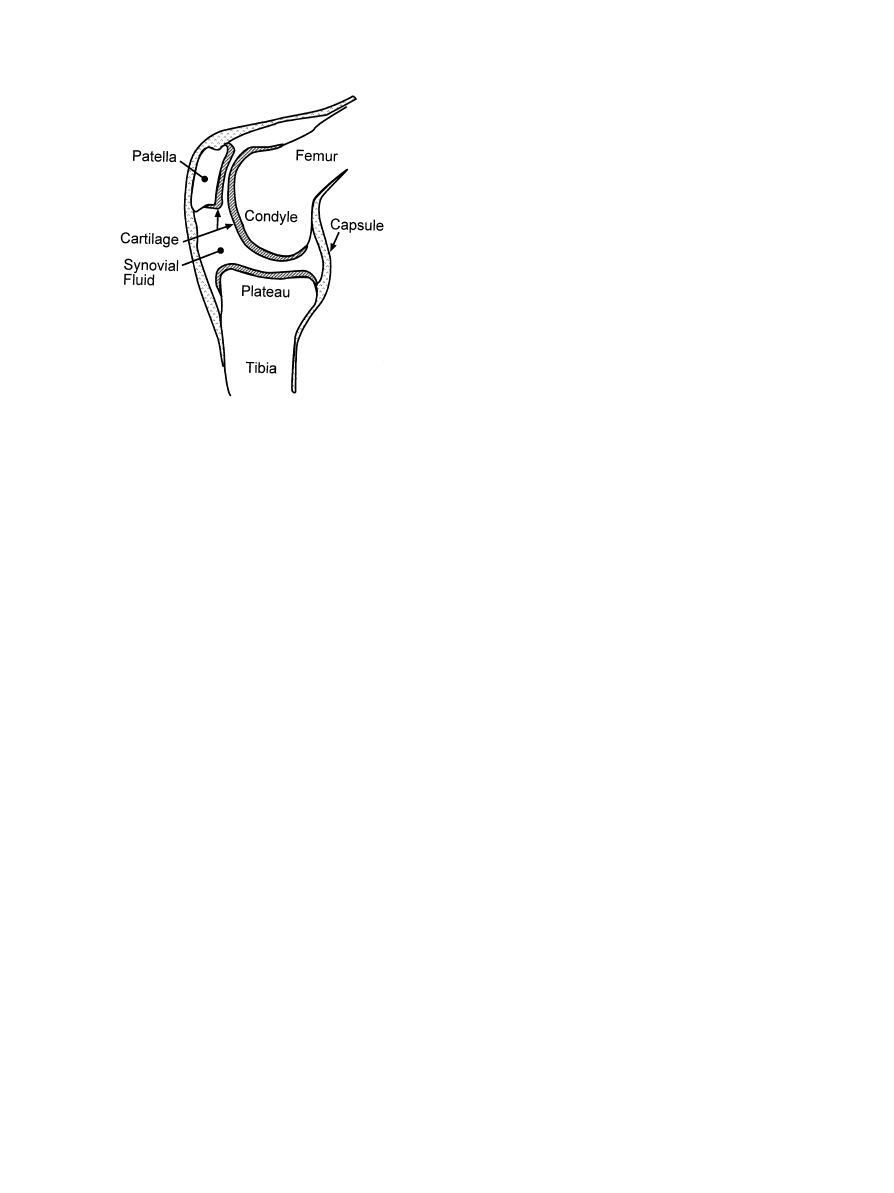
Fig. 1. Representation of human knee and hip joints during loading
[44].
relief of pain and improved joint mobility by creation of
a new prosthetic joint. From early excision through inter-
position to replacement arthroplasty, great progress has
been achieved over 170 years of orthopaedic surgery and
joint prostheses are now being considered for many
joints in the human body [6]. Total hip (THR) and total
knee (TKR) joint replacements, due to population needs
and their complex behavior, have nevertheless been the
principal focus of artificial joint studies. TJR is now
a fairly well established orthopaedic technique involving
the replacement of a growing number of hip and knee
joints, 275 000 hip and knee joints having been replaced
during 1995 in the United States [7]. Unfortunately
in vivo degradation, primarily as a result of the higher
wear rates associated with artificial implant materials,
and the consequent adverse biological effect of the gener-
ated wear debris on bone mass/density and implant fix-
ation, typically, however, results in a shorter lifetime for
these artificial joints when compared with natural
synovial joints. Further, when compared to the initial
TJR surgery, revision surgery of an implant is more
difficult, has a lower success rate, may induce additional
damage to the surrounding tissues and increases health
care costs by one third [7].
Replacement arthroplasty made important advance-
ments during the 1950s and 1960s, through the out-
standing contributions of G.K. McKee and Sir John
Charnley. McKee introduced metal-on-metal hip pros-
thesis in which components were originally made of
stainless steel which rapidly changed to a cobalt—chro-
mium—molybdenum alloy (Vitallium
TM) to mitigate the
excessive friction and rapid loosening of the stainless steel
pair [8, 9]. Moreover, the substitution of methyl-methac-
rylate cement for fixation screws increased the short-term
implantation success rate to'90%. It was further recog-
nized that the use of identical metals in the tribological
pair, though necessary to avoid galvanic corrosion, was
not an optimized tribology design. A high rate of loosen-
ing was encountered with early metal-on-metal artificial
joints due to non-optimum fit between the articulating
surfaces which produced high frictional moments and
excessive wear of the bearing surfaces [8—10]. These early
concerns limited the application of metal-on-metal ar-
ticulating devices, although follow-up examinations of
metal-on-metal hip protheses have shown very low wear
rates (a few
lm per year per component) for protheses
implanted for up to 20 years [11].
Sir John Charnley in the 1960s developed the concept
of low-friction arthroplasty by introducing a new design
consisting of a small-diameter metallic femoral head ar-
ticulating with a polymeric (originally PTFE to be later
replaced by ultra-high-molecular-weight polyethylene
(UHMWPE)) acetabular cup [12, 13]. The initial success
of UHMWPE as the cup material [14] has prevailed for
30 yrs, UHMWPE being the dominant orthopaedic ma-
terial in TJRs [6]. Wear of UHMWPE has, however,
been invariably observed when rubbing against metal
femoral heads or femoral condylar components of TJRs
[15].
Beside clinical factors and design considerations, the
latter to minimize contact stresses, choice of counterpart
material has been shown to be a critical factor in
UHMWPE wear behavior. Ti—6Al—4V has generally
been found to have a more detrimental impact on
UHMWPE wear than Co—Cr—Mo alloys [14, 15]. In
order to achieve minimum wear and maximum success
rate, subsequent studies have considered alternative ma-
terials combinations [16—23], Table 1, e.g., ceramic/
UHMWPE prostheses where the ceramic component
creates minimum damage to the UHMWPE counterpart
when compared to Co—Cr—Mo or Ti—6Al—4V. Wear of
joint prostheses materials unavoidably represents a long-
term limitation to the lifetime of a total joint replacement
as accumulation of UHMWPE, and to a lesser extent
metal or ceramic wear debris has been associated with
incidence of non-specific pain and prosthesis loosening.
The former is a result of adverse tissue reaction, while the
latter is a result of adverse reaction to wear debris of the
implant/bone fixation [24—36]. There is therefore an in-
creasing concern about the long-term use of UHMWPE,
underscored by recent recognition of the non-specificity
(variable MW and MW distribution, processing and fab-
rication history) of the material, reports on the possible
harmful effects of UHMWPE sterilization, and the inter-
action of UHMWPE debris with the body fluids and
tissues [37—41].
Simultaneously development in metal-on-metal tech-
nology [10], through optimization of CoCrMo alloys,
prosthesis geometry, and manufacturing practices, has
created a renaissance of metal-on-metal prostheses in
1622
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
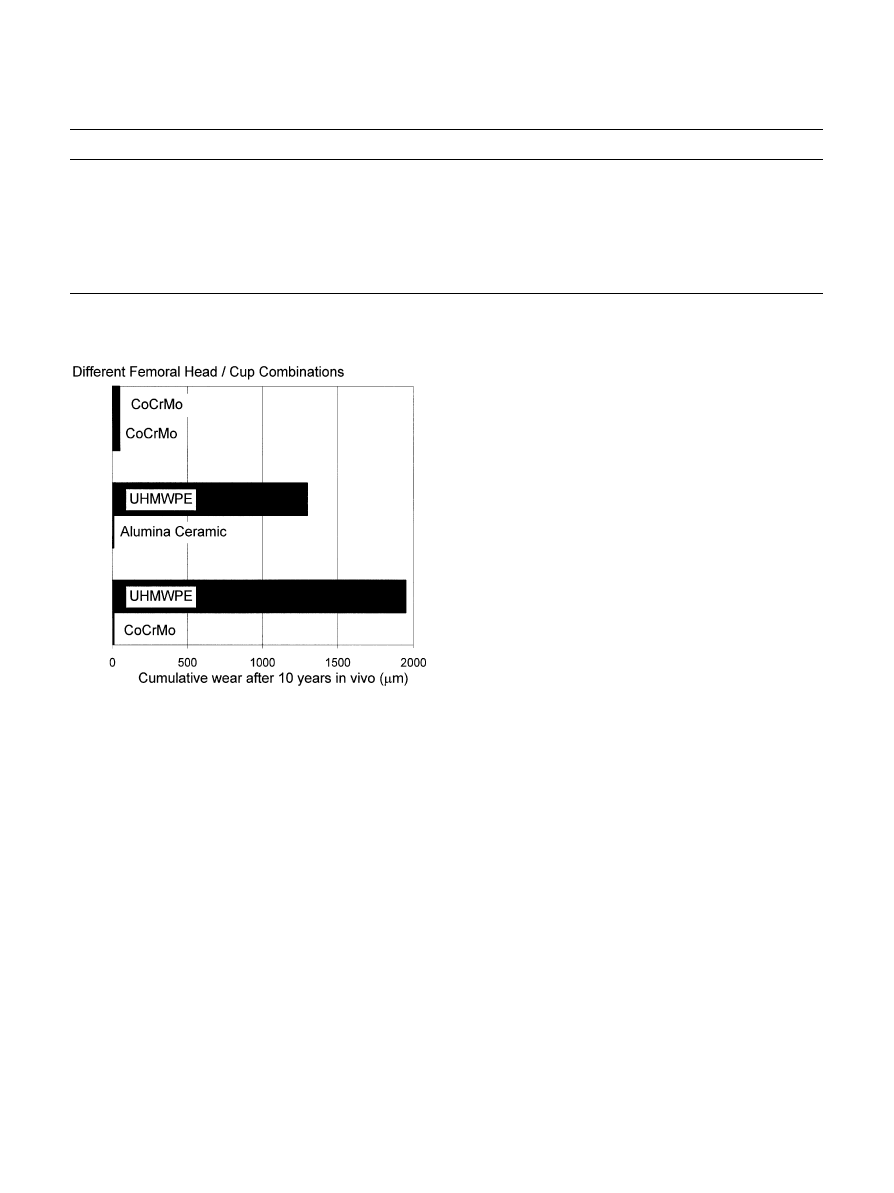
Table 1
Materials combinations used in TJR prostheses
Femoral component
Hip/tibial component
Results
Co—Cr—Mo
Co—Cr—Mo
Early high loosening rate and limited use. New developments show lowest
wear rate (THR only — in clinical use in Europe)
Co—Cr—Mo
UHMWPE
Widely employed; low wear
Alumina/zirconia
UHMWPE
Very low wear rate. Zirconia more impact resistant (not used in TKR but in
clinical evaluation in Japan)
Alumina
Alumina
Minimum wear rate (components matched) Pain—Not in clinical use in the US
Ti—6Al—4V
UHMWPE
Reports of high UHMW-PE wear due to breakdown of titanium surface
Surface coated Ti—6Al—4V
UHMWPE
Enhanced wear resistance to abrasion. Only thin treated layer achieved
Fig. 2. Comparison of wear behavior of different material combina-
tions (adapted from Ref. [10]).
Europe [6, 42, 43]. Metal-on-metal combinations may
now provide wear rates lower than metal-or ceramic-
on-UHMWPE combinations (Fig. 2), eliminating the
high-wear debris generation and long-term degradation
associated with UHMWPE.
While continued development of TJR materials has
increased the success of total joint replacements (with
success rates of 95% and higher at 10 years and 85—90%
at 15 years), longer human life expectancy and implanta-
tion in younger patients has driven bioengineers from
original implant concerns, e.g., materials strength, infec-
tion and short-term rejection, to consideration of long-
term materials limitations, e.g., wear, fatigue strength,
and long-term biocompatibility, Fig. 3. The ‘ideal’ mate-
rial or material combination for TJR prostheses should
therefore exhibit the following properties: a ‘biocompat-
ible’ chemical composition to avoid adverse tissue reac-
tions, an excellent resistance to degradation (corrosion)
in the human body environment, acceptable strength to
sustain the cyclic loading endured by the joint, a low
modulus to minimize bone resorption, and a high-wear
resistance to minimize debris generation.
Until recently, the mainstream approach taken for the
introduction of orthopaedic materials has involved ad-
aptation of existing materials, as exemplified by the use of
Ti—6Al—4V ELI, an alloy originally designed for aero-
space applications. Standard metallic orthopaedic mate-
rials include stainless steels, cobalt-base alloys, and
titanium-base alloys [44—46] (Table 2), with an increas-
ing number of devices being made of titanium and tita-
nium alloys. The latter alloys are generally preferred to
stainless steel and Co-alloys because of their lower
modulus, superior biocompatibility and corrosion resist-
ance [6, 44]. Recently, new titanium alloy compositions,
specifically tailored for biomedical applications, have
been developed. These first generation orthopaedic alloys
included Ti—6Al—7Nb [47] and Ti—5Al—2.5Fe [48, 49],
two alloys with properties similar to Ti—6Al—4V that
were developed in response to concerns relating V to
potential cytotoxicity [50, 51] and adverse reaction with
body tissues [52]. Further, biocompatibility enhance-
ment and lower modulus has been achieved through the
introduction of second generation titanium orthopaedic
alloys including Ti—12Mo—6Zr—2Fe ‘TMZF’ [53, 54]
Ti—15Mo—5Zr—3Al [55], Ti—15Mo—3Nb—3O (21SRx)
[56],
Ti—15Zr—4Nb—2Ta—0.2Pd
and
Ti—15Sn—4Nb—
2Ta—0.2Pd alloys [57], as well as the ‘completely biocom-
patible’ Ti—13Nb—13Zr alloy [58, 59]. Finally, minimum
elastic moduli have been achieved by ‘TNZT’ alloys
based on the Ti—Nb—Ta—Zr system [60], specifically by
the development of the ‘biocompatible’ Ti—35Nb—5Ta—
7Zr alloy.
This review presents the advances in the development
of orthopaedic titanium alloys. After a brief summary of
the physical metallurgy of titanium and titanium alloys,
their biocompatibility, corrosion behavior, and mechan-
ical properties will be discussed. Finally, their wear be-
havior will be examined.
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1623
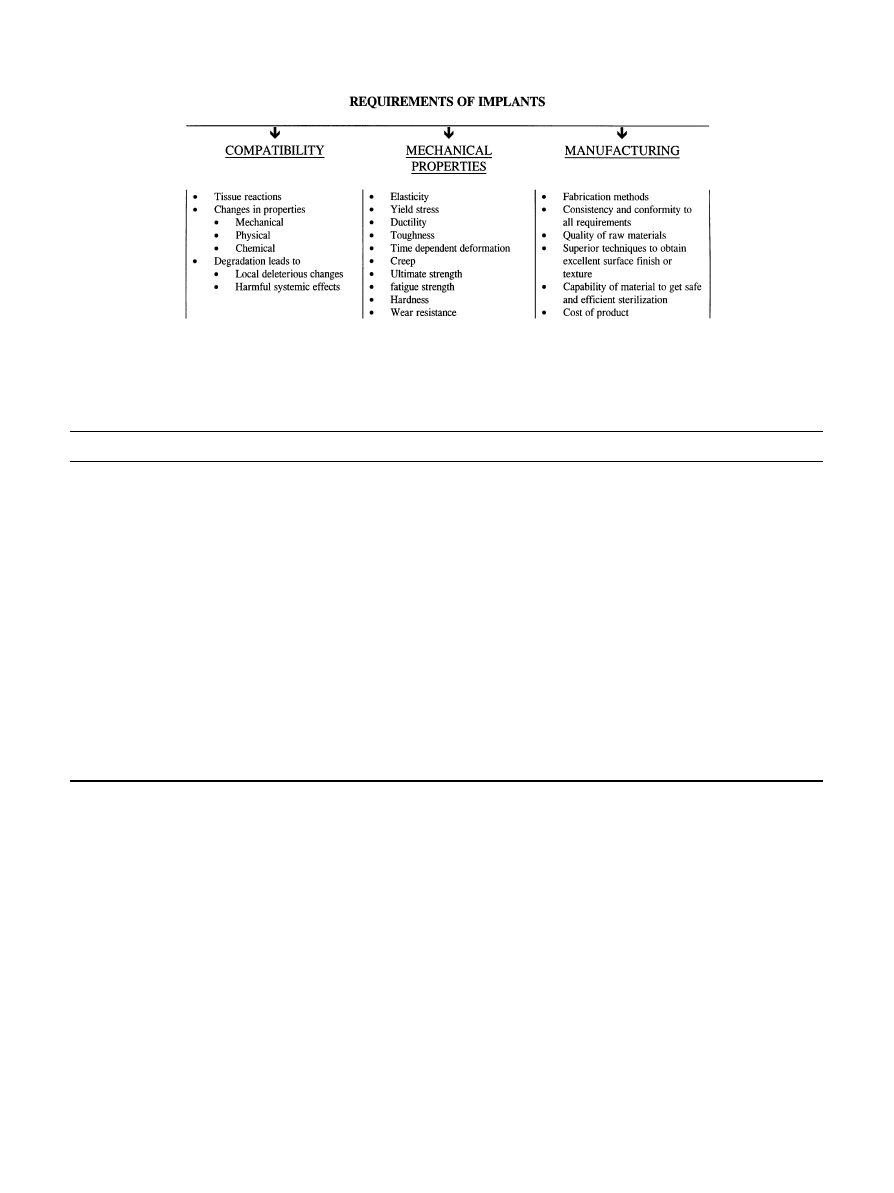
Fig. 3. Implant materials requirements in orthopaedic applications (adapted from Ref. [126]).
Table 2
Some characteristics of orthopaedic metallic implant materials
Stainless steels
Cobalt-base alloys
Ti & Ti-base alloys
Designation
ASTM F-138
ASTM F-75
ASTM F-67 (ISO 5832/II)
(‘316 LDVM’)
ASTM F-799
ASTM F-136 (ISO 5832/II)
ASTM F-1537
ASTM F-1295
(Cast and wrought)
(Cast and wrought)
Principal alloying
Fe(bal.)
Co(bal.)
Ti(bal.)
elements (wt%)
Cr(17—20)
Cr(19—30)
Al(6)
Ni(12—14)
Mo(0—10)
V(4)
Mo(2—4)
Ni(0—37)
Nb(7)
Advantages
f
cost, availability
f
wear resistance
f
biocompatibility
f
processing
f
corrosion resistance
f
corrosion
f
fatigue strength
f
minimum modulus
f
fatigue strength
Disadvantages
f
long term behavior
f
high modulus
f
power wear resistance
f
high modulus
f
biocompatibility
f
low shear strength
Primary utilisations
Temporary devices
Dentistry castings
Used in THRs with modular
(fracture plates, screws, hip nails)
Prostheses stems
(CoCrMo or ceramic) femoral heads
Used for THRs stems
Load-bearing components
Long-term, permanent
in UK (high Nitrogen)
in TJR (wrought alloys)
devices (nails, pacemakers)
2. Physical metallurgy of titanium alloys—a brief
overview
Titanium is a transition metal with an incomplete shell
in its electronic structure enables it to form solid solu-
tions with most substitutional elements having a size
factor within $20%. In its elemental form titanium has
a high melting point (1678°C), exhibiting an hexagonal
close packed crystal structure (hcp)
a up to the beta
transus (882.5°C), transforming to a body centered cubic
structure (bcc)
b above this temperature [61].
Titanium alloys may be classified as either
a, near-a,
a#b, metastable b or stable b depending upon their
room temperature microstructure [61, 62]. In this regard
alloying elements for titanium fall into three categories:
a-stabilizers, such as Al, O, N, C, b-stabilizers, such as
Mo, V, Nb, Ta, (isomorphous), Fe, W, Cr, Si, Ni, Co, Mn,
H (eutectoid), and neutral, such as Zr.
a and near-a
titanium alloys exhibit superior corrosion resistance with
their utility as biomedical materials being principally
limited by their low ambient temperature strength. In
contrast,
a#b alloys exhibit higher strength due to the
presence of both
a and b phases. Their properties depend
upon composition, the relative proportions of the
a/b
phases, and the alloy’s prior thermal treatment and
thermo-mechanical processing conditions.
b alloys (meta-
stable or stable) are titanium alloys with high strength,
good formability and high hardenability.
b alloys also
1624
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
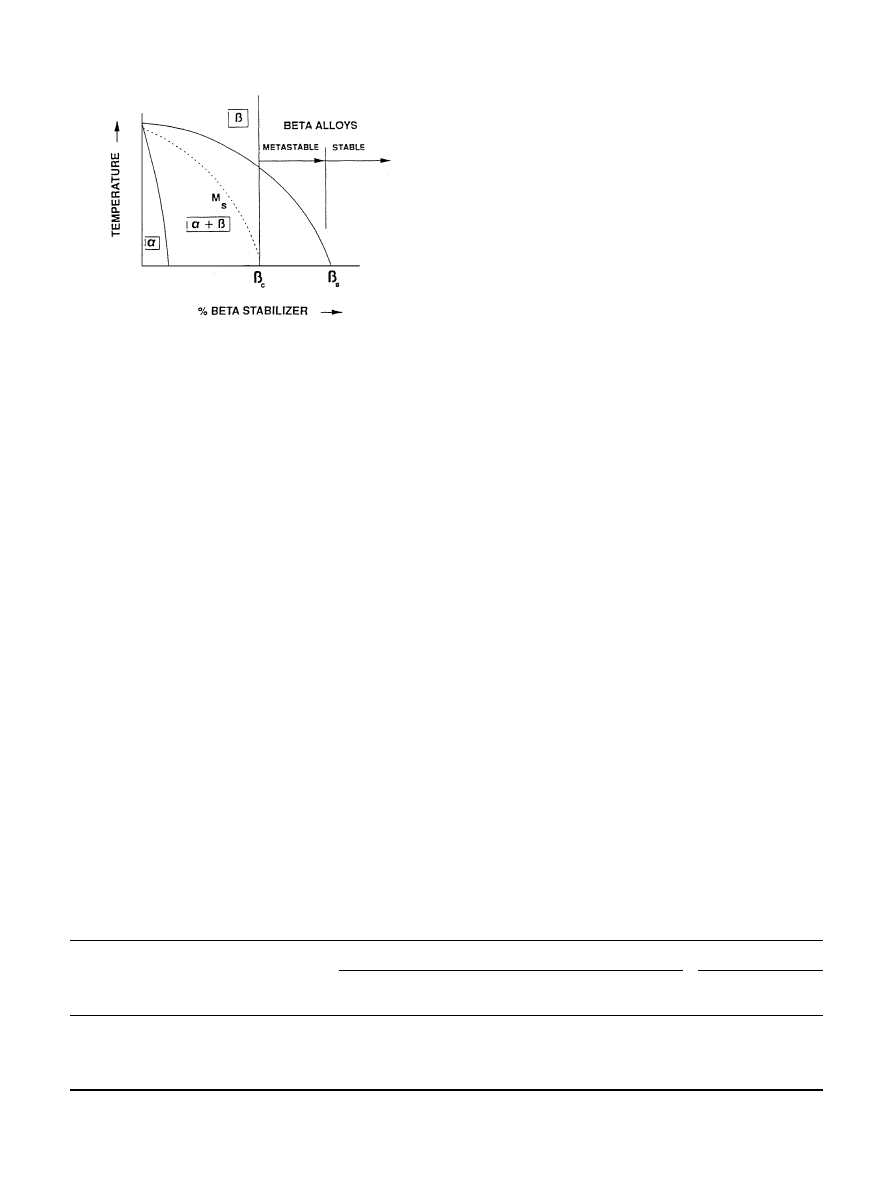
Fig. 4. Pseudo-binary phase diagram of Ti-
b stabilizer [63].
Table 3
Tissue reaction around metallic implants (adapted from [50, 51])
Classification by thicknesses of
Minor reaction
Severe reaction
pseudomembrane around implant
Titanium alloys
Fe, Co, Cr,Ni,
Stainless steels, CoCr alloys
Mo, V, Mn, Incoloy
Classification by type of reaction
‘Vital’
‘Capsule’
‘Toxic’
(cellular inflammatory, fibrous)
Ti, Zr, Nb, Ta,
Al, Fe, Mo, Ag, Au,
Co, Ni, Cu, V
Pt, Ti alloys
Stainless steels,
CoCr alloys
offer the unique possibility of combined low elastic
modulus and superior corrosion resistance [63, 64].
A
b-alloy is operationally defined as an alloy whose
chemical composition lies above
b#, (Fig. 4) that is, it
contains sufficient total
b stabilizer content to retain
100%
b upon quenching from above the b transus [63].
Alloys lying above this critical minimum level of
b-stabi-
lizer content may still lie within a two-phase region, with
the resulting as-quenched
b-phase being metastable with
the potential of precipitating a second phase upon aging.
Alloys with increasing alloying content ultimately ex-
ceeding a critical
b4 value are considered stable b alloys,
in which no precipitation takes place during practical
long-time thermal exposure.
Process variations are traditionally used to control the
alloy microstructure and therefore to optimize titanium
alloys properties, i.e. ductility, strength, fatigue resistance
or fracture toughness. The effects of various microstruc-
tures are then correlated with engineering properties,
with the most common microstructural features studied
in metastable
b alloys being b grain size and the size and
distribution of aged
a [65]. Apart from a phase, precipi-
tation of transient
b@ or u phases and/or intermetallic
compounds may be observed in metastable
b alloys de-
pending upon alloy composition, heat treatment, pro-
cessing history and service conditions [65, 66].
3. Biocompatibility and corrosion behavior of orthopaedic
titanium alloys
Studies of the biological behavior of metallic elements
have shown that the composition of biomaterials should
be carefully tailored to minimize adverse body reactions
[50, 51]. Local adverse tissue reactions or elicit allergy
reactions caused by metallic implants originate from the
release of metal ions from the implant. This release of
ions depends upon the corrosion rate of the alloy and on
the solubility of the first formed corrosion products. In
an in vivo corrosion study, Steinemann [51] concluded
that V, Ni, and Co were toxic elements while Ti and its
alloys, stainless steels and CoCrMoNi alloys, and Ta, Zr,
Nb, and Pt composed the class of ‘resistant metallic
biomaterials’ based on corrosion rates. Consideration of
corrosion product stability in tissue further limits this
choice [79, 84], Ti and some of its alloys, Ta, Nb, and Zr,
producing essentially insoluble oxides, Table 3.
Response to these observations initially resulted in the
development of two alloys, Ti—6Al—7Nb [47] and
Ti—5Al—2.5Fe [67], where Nb and Fe were substituted for
V in Ti—6Al—4V, V having been reported to be toxic
[50, 51] and to show adverse tissue effects [52]. These
alloys still, however, contained Al which has been sugges-
ted to be causal in osteolysis and neural disorders
[68, 69]. Subsequent
b-titanium alloys based on the
Ti—Mo system were then developed: ‘TMZF’ Ti—12Mo—
6Zr—2Fe [53, 54], Ti—15Mo—5Zr—3Al [55], and Ti—
15Mo—3Nb—3O (21SRx) [56], although the large per-
centage of Mo may still be potentially detrimental, Mo
having been associated with severe tissue reactions in
animal studies [52]. Elimination of Mo was preferred in
Ti—15Zr—4Nb—2Ta—0.2Pd
and
Ti—15Sh—4Nb—2Ta—
0.2Pd alloys [57], although here again elemental Sn and
Pd do not show complete biocompatibility. Ultimately,
development of Ti—13Nb—13Zr [58, 59] may have an-
swered the issue of biocompatibility with the exclusive
addition of biocompatible elements, i.e. Zr and Nb. An-
other group [70] has investigated the possible use of
titanium—zirconium binary alloys. Finally, recently
synthesized Ti—Nb—Zr—Ta ‘TNZT’ alloys [60] offer
the opportunity of minimizing potentially adverse tissue
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1625
Table 4
Nature of oxides formed on titanium and its alloys (adapted from [71])
Material
Oxide
TiO2 Al2O3 Nb2O5 MoO3/MoO2 ZrO2
cp Ti
x
Ti—6Al—4V
x
x
Ti—5Al—2.5Fe
x
x
Ti—6Al—7Nb
x
x
x
Ti—15Mo—5Zr—3Al
x
x
x
x
Table 5
Orthopaedic alloys developed and/or utilized as orthopaedic implants and their mechanical properties (E"elastic modulus, YS"yield strength,
UTS"ultimate strength)
Alloy designation
Microstructure
E (GPa)
YS (MPa)
UTS (MPa)
cpTi
MaN
105
692
785
Ti—6Al—4V
Ma/bN
110
850—900
960—970
Ti—6Al—7Nb (protasul-100)
Ma/bN
105
921
1024
Ti—5Al—2.5Fe
Ma/bN
110
914
1033
Ti—12Mo—6Zr—2Fe (TMZF)
MMetastable bN
74—85
1000—1060
1060—1100
Ti—15Mo—5Zr—3Al
MMetastable bN
75
870—968
882—975
MAged b#aN
88—113
1087—1284
1099—1312
Ti—15Mo—2.8Nb—3Al
MMetastable bN
82
771
812
MAged b#aN
100
1215
1310
Ti—0/20Zr—0/20Sn-4/8Nb-2/4Ta#(Pd, N, O)
Ma/bN
N/A
726—990
750—1200
Ti—Zr
Cast
Ma@/bN
N/A
N/A
900
Ti—13Nb—13Zr
Ma@/bN
79
900
1030
Ti—15Mo—3Nb—0.3O (21SRx)
MMetastable bN#silicides
82
1020
1020
Ti—35Nb—5Ta—7Zr (TNZT)
MMetastable bN
55
530
590
Ti—35Nb—5Ta—7Zr—0.4O (TNZTO)
MMetastable bN
66
976
1010
CoCrMo
MAustenite(fcc)#hcpN
200—230
275—1585
600—1795
Stainless Steel 316 L
MAusteniteN
200
170—750
465—950
Bone
Viscoelastic composite
10—40
—
90—140
MOHAp#collagenN
150—400
!
! Compressive strength.
reaction through the restricted use of ‘biocompatible’ Nb,
Ta, and Zr.
As previously mentioned, the biocompatibility perfor-
mance of a metallic alloy is closely associated with
its corrosion resistance and the biocompatibility of its
corrosion products. Corrosion data show excellent resis-
tance
for
titanium
and
its
alloys
though
some
precautions should be taken in order to optimize their
composition [50, 51].
b-titanium alloys generally show
attractive corrosion behavior, their corrosion resistance
again depending on alloy composition and environment
[64]. For example, anodic polarization tests [53] in-
dicated that Ti—12Mo—6Zr—2Fe (TMZF)’s protective ox-
ide has a breakdown resistance equal to Ti—6Al—4V,
while corrosion current densities lower than that of cp
titanium were found for Ti—5Mo—5Zr—3Al alloy [55].
Electrochemical
measurements
of
Ti—13Nb—13Zr
[58, 59] also confirmed the potency of Ti, Nb, and Zr to
develop highly protective passive layers, resulting in
a much lower potential electrochemical interaction than
Ti—6Al—4V. Finally, Nb and Zr exhibit ideal passivity
and are not prone to chemical breakdown of the passive
layer, exhibiting minimum passive dissolution rates. In
fact, Nb and Zr contribute to the formation of a spontan-
eous highly protective passive film on titanium alloys and
are not, as are Al and V, released into the environment as
dissolved metal ions, but are rather incorporated into the
passive layer [58]. The latter report again emphasizes the
relationship between bulk alloy composition and the
nature of the surface oxides. Electropolishing studies
confirm this suggestion, Table 4, the authors noting that
when the alloying elements (except V and Fe) form an
oxide, those oxides occur as discrete clusters embedded in
a titanium oxide matrix [71]. Surface oxides composition
and/or distribution should be expected to affect the cor-
rosion behavior of orthopaedic alloys and detailed char-
acterizations of these surfaces are required in order to
adequately interpret and understand corrosion data to
optimize the ‘biocompatibility’ of titanium alloys.
4. Mechanical properties of orthopaedic titanium alloys
Alloy design and thermo-mechanical processing con-
trol of titanium alloys has allowed the production of
implant materials with enhanced properties. As shown in
Table 5, strength levels for orthopaedic alloys are gener-
ally acceptable with adequate ductility, as defined by
either the percent elongation or the percent reduction of
1626
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
Table 6
Typical modulus of elasticity of joints materials is use (adapted from
[2])
Joint material
Elastic modulus (GPa)
Articular cartilage
0.001—0.17
Natural rubber
0.0025—0.1
Silicone rubber
0.01
PTFE
0.5
UHMW-PE
0.5
Bone cement (PMMA)
3.0
Bone
10—30
TNZT alloys
55—66
‘New generation’ Ti-alloys
74—85
Ti—6Al—4V alloy
110
Zirconia
200
Stainless steel
205
Co—Cr—Mo alloy
230
Alumina
350
area in a standard tensile test, being retained at room
temperature. However there has been, and is still, con-
cern about the high elastic modulus of the alloys as
compared to bone, and the variable fatigue resistance of
the metallic implant. Both properties, if not optimized,
may eventually lead to prosthesis failure through loosen-
ing or fracture.
Long-term experience indicates that insufficient load
transfer from the artificial implant to the adjacent re-
modeling bone may result in bone resorption and event-
ual loosening of the prosthetic device [72, 73]. ‘¼olff ’s
¸
aw (‘The form being given, tissue adapts to best fulfill its
mechanical function’) suggests that the coupling of an
implant with a previously load bearing natural structure
may result in tissue loss. Indeed, it has been shown that
when the tension/compression load or bending moment
to which living bone is exposed is reduced, decreased
bone thickness, bone mass loss, and increased osteoporo-
sis ensue [74—76]. This phenomenon, termed ‘stress
shielding’, has been related to the difference in flexibility
or stiffness, dependent in part on elastic moduli, between
natural bone and the implant material [77]. Dowson [2]
appropriately pointed out that, as improvements in the
combinations of TJR sliding material pairs have been
recorded, the elastic modulus of the prosthetic materials
has been moving further away from those of the natural
joint they were intended to replace (Table 6). Any reduc-
tion in the stiffness of the implant, for example, through
substitution of present orthopaedic alloys with newer,
lower modulus materials, is expected to enhance stress
redistribution to the adjacent bone tissues, therefore min-
imizing stress shielding and eventually prolonging device
lifetime.
The problems related to implant stiffness-related-stress
shielding of bone have resulted in a number of proposed
solutions for more flexible designs and low modulus
materials. For example, carbon—carbon and carbon—
polymer composites, because of the ability to tailor their
elastic modulus closer to bone than metals [44, 47], have
been investigated as candidates for a new generation of
implants. However, they are far from being totally effec-
tive due to potential environmental degradation and
poor tribological behavior.
Alternatively, a first attempt at reducing the elastic
modulus of orthopaedic alloys was made by the intro-
duction of
a/b titanium alloys having elastic modulus
values approximately half that of stainless steels or
CoCrMo alloys (Fig. 5). However, the modulus of
Ti—6Al—4V and related
a/b alloys is still high (110 GPa),
approximately 4—10 times that of bone. Recent attempts
at further minimizing orthopaedic alloys moduli have led
to the introduction of metastable
b-titanium alloys,
Ti—15Mo—5Zr—3Al,
Ti—12Mo—6Zr—2Fe
(TMZF),
Ti—15Mo—3Nb—0.3O (21SRx) and Ti—13Nb—13Zr, hav-
ing minimum elastic modulus values ranging from 74 to
88 GPa (Fig. 5 and Table 5). The elastic modulus values
of these second generation
b-alloys are still 2—7 times
higher than E"0/%. Continued synthesis of minimum
modulus Ti—Nb—Zr—Ta alloys (TNZT) intended for or-
thopaedic applications has recently been demonstrated
[60], these alloys exhibiting moduli 20—25% lower than
other available alloys (Fig. 5).
Cyclic loading is applied to orthopaedic implants
during body motion, resulting in alternating plastic de-
formation of microscopically small zones of stress con-
centration produced by notches or microstructural
inhomogeneities. The interdependency between factors
such as implant shape, material, processing and type of
cyclic loading, makes the determination of the fatigue
resistance of a component an intricate, but critical, task.
Since testing an actual implant under simulated im-
plantation and load conditions is a difficult and expen-
sive process, standardized fatigue tests have been selected
for initial screening of orthopaedic material candidates,
joint simulator trials being generally reserved for a later
stage in the implant development process. ‘Standard’
fatigue tests include tension/compression, bending, tor-
sion, and rotating bending fatigue (RBF) testing, the
latter, a relatively simple test, being widely used to evalu-
ate orthopaedic metallic materials. Unfortunately, no
standard for fatigue evaluation of biomaterials testing
has yet been established, a variety of testing conditions
being encountered in reported fatigue studies of ortho-
paedic materials.
Nonetheless, Ti—6Al—4V is generally considered as
a ‘standard material’ when evaluating the fatigue resis-
tance of new orthopaedic titanium alloys. The mechan-
ical response of Ti—6Al—4V alloy is, however, extremely
sensitive to prior thermo-mechanical processing history,
e.g., prior
b grain size, the ratio of primary a to trans-
formed
b, the a grain size and the a/b morphologies, all
impacting performance, particularly high-cycle fatigue
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1627
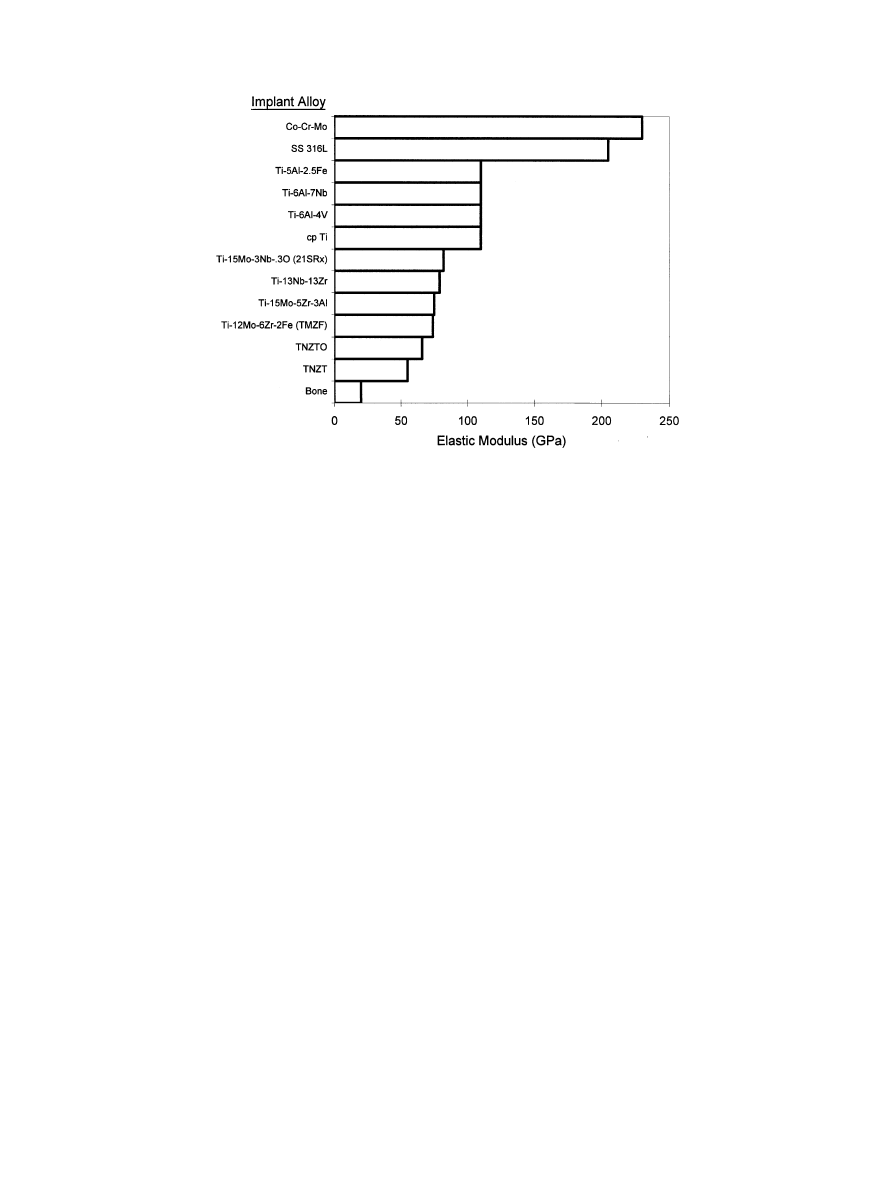
Fig. 5. Elastic modulus values of orthopaedic alloys.
lifetime (HCF) [78—80]. For example, maximum fracture
toughness and fatigue crack growth resistance is achieved
with Widmansta¨tten microstructures resulting from a
b
recrystallization anneal. However, this microstructure
results in inferior HCF performance, the development of
a bi-modal primary
a plus transformed b microstructure
being preferred [81] to prevent fatigue crack initiation.
Indeed, the transition to fine equiaxed, fine lamellar,
coarse equiaxed, and coarse lamellar leads to progressive
reductions in lifetime [82].
Further enhancement of the HCF resistance of
Ti—6Al—4V may be achieved, under careful control, by
shot peening. Shot peening is a cold working process in
which the surface is bombarded with small, typically
spherical media plastically deforming the surface. The
resulting compressive residual stresses may provide in-
creased part life when surface-related failure mechanisms,
such as fatigue or corrosion, are involved. While shot
peening may increase the fatigue limit, a balance between
the high compressive surface residual stresses and the
increased surface roughness produced during shot peen-
ing is required for optimal fatigue performance. Wagner
et al. [83—85], have suggested that since the high-cycle
fatigue strength for a smooth surface is determined by the
resistance to fatigue crack nucleation, shot-peening im-
proves HCF mainly though the beneficial influence of
residual compressive stresses on microcrack initiation
and propagation in the surface region. For instance,
shot-peening prior to grit blasting can increase by 10%
the fatigue strength of Ti—6Al—4V over grit blasting alone
[86]. In contrast, the increase of surface damage and
surface roughness due to shot peening (as well as poor
polishing or surface preparation) may induce early crack
initiation causing a reduction in the fatigue limit. Indeed,
electropolishing after shot-peening results in the highest
fatigue limit achievable (30% higher than unshot-peened)
[85].
The sensitivity of Ti-alloy fatigue properties to surface
condition is associated with their high notch sensitivity,
as exemplified by Ti—6Al—4V whose smooth RBF
strength is reduced by 40% with notched samples [87].
Various surface preparation techniques and treatments
may result in even larger reductions (up to 80%) in
fatigue strength [88]. This is illustrated by the effect of
surface finishing techniques on the fatigue strength of
a and a/b titanium alloys, where a reduction in fatigue
limit of as much as 80% may be observed (Table 7). For
biomedical applications the notch sensitivity of
a/b tita-
nium alloys is a critical factor in the performance of
porous-coated implant for cementless prostheses, where
the application of a bead- or wire-coating produces pref-
erential crack initiation sites at the porous-coating/sub-
strate interface. Porous-coated Ti—5Al—2.5Fe [89] and
Ti—6Al—4V [90] hip stems both show a large reduction in
the fatigue limit as compared to the smooth condition,
resulting in an unacceptable low fatigue resistance, i.e.,
below the suggested 425 MPa minimum required for
prostheses [91]. An FEM model of Ti—6Al—4V implants,
correlating with actual results, showed that the porous-
coated condition exhibits a HCF strength approximately
one-third the strength of the uncoated condition [92] due
to the poor fatigue crack initiation resistance of the
Ti—6Al—4V substrate. The latter substrate has, because of
the coating sintering treatment, been transformed to
1628
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639

Table 7
Effect of surface preparation on the fatigue properties of
a and a/b titanium alloys
Titanium alloy
Test conditions
Fatigue limit
K
`
& "
(MPa)
!
cpTi
Mechanically polished [88]
234
—
Electrolytically polished
200
0.9
Ti—6Al—4V
Gentle surface grinding [88]
427
—
Gentle chemical machining
90
0.8
Abusive chemical machining
352
0.8
Abusive surface grinding
310
0.2
Polished (320—600 alumina grit) [86]
596
—
Belted and glass bead blasted
610
1.0
Belted, beaded, shot-peened, and grit blasted
505
0.9
Belted, beaded, and grit blasted
555
0.8
Ti—5Al—2.5Sn
Ultrasonic machined [88]
676
—
Shot peened
531
0.8
Ground
359
0.5
Electrical-discharge machined
145
0.2
! At 107 cycles.
" K& is a fatigue strength reduction factor defined as fatigue limit (surface treatment)/fatigue limit (smooth-control) under same test conditions.
Table 8
Smooth fatigue strength of orthopaedic titanium alloys
Alloy designation
Test conditions
Fatigue limit
!
Fatigue limit/
(MPa)
yield strength
cpTi
RBF(R"!1/100 Hz) [55]
430
0.6
Ti—6Al—4V
axial (R"!1/292 Hz) [87]
500
0.6
axial (R"0.1/292 Hz) [87]
330
0.4
RBF(R"!1/60 Hz)
610
0.7
Ti—6Al—7Nb
RBF(R"!1) [47]
500—600
0.7
Ti—5Al—2.5Fe
RBF(R"!1) [48]
580
0.8
Ti—15Mo—5Zr—3Al
RBF(R"!1/100 Hz) [55]
560—640
0.5
(aged
b#a condition)
Ti—13Nb—13Zr
axial (R"0.1/60 Hz) [59]
500
0.6
Ti—12Mo—6Zr—2Fe (TMZF)
RBF(R"!1/167 Hz) [53]
525
0.5
Ti—15Mo—3Nb—0.3O (21SRx)
RBF(R"!1/60 Hz)
490
0.5
TNZT
RBF(R"!1/60 Hz)
265
0.5
TNZTO
RBF(R"!1/60 Hz)
450
0.5
SS 316L
RBF(R"!1/100 Hz)
440
0.6
CoCrMo
RBF(R"!1) [91]
400—500
0.4—0.5
RBF(R"!1/100 Hz) [55]
500—580
—
! Fatigue limit at 107 cycles.
a low-crack-initiation-resistance
b-transformed coarse
lamellar microstructure, with associated high surface
stress concentrations at the coating/substrate interface
[92].
The introduction of new low modulus orthopaedic
titanium alloys has been accompanied with renewed real-
ization that the smooth fatigue resistance of
b-titanium
alloys is generally low [93, 94] when compared to
a/b
titanium alloys on an equivalent yield strength basis
(Table 8). Aging the SHT (Solution Heat Treated: heated
above
b-transus followed by rapid cooling) alloy below
the
b-transus will increase the fatigue resistance of meta-
stable
b-alloys by the transformation of the b-phase to
two-phase
a/b microstructures. For instance, the fatigue
limit of beta-C may be increased from 390 MPa in the
SHT condition to 650 MPa after a 16 h/530°C aging
treatment [94]. However, aging increases the elastic
modulus, therefore eliminating the benefit of modulus
reduction associated with
b-alloys. For instance, the elas-
tic modulus of Ti—15Mo—5Zr—3Al increases from 75 GPa
in SHT condition to 88—113 GPa after various aging
treatments [55].
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1629
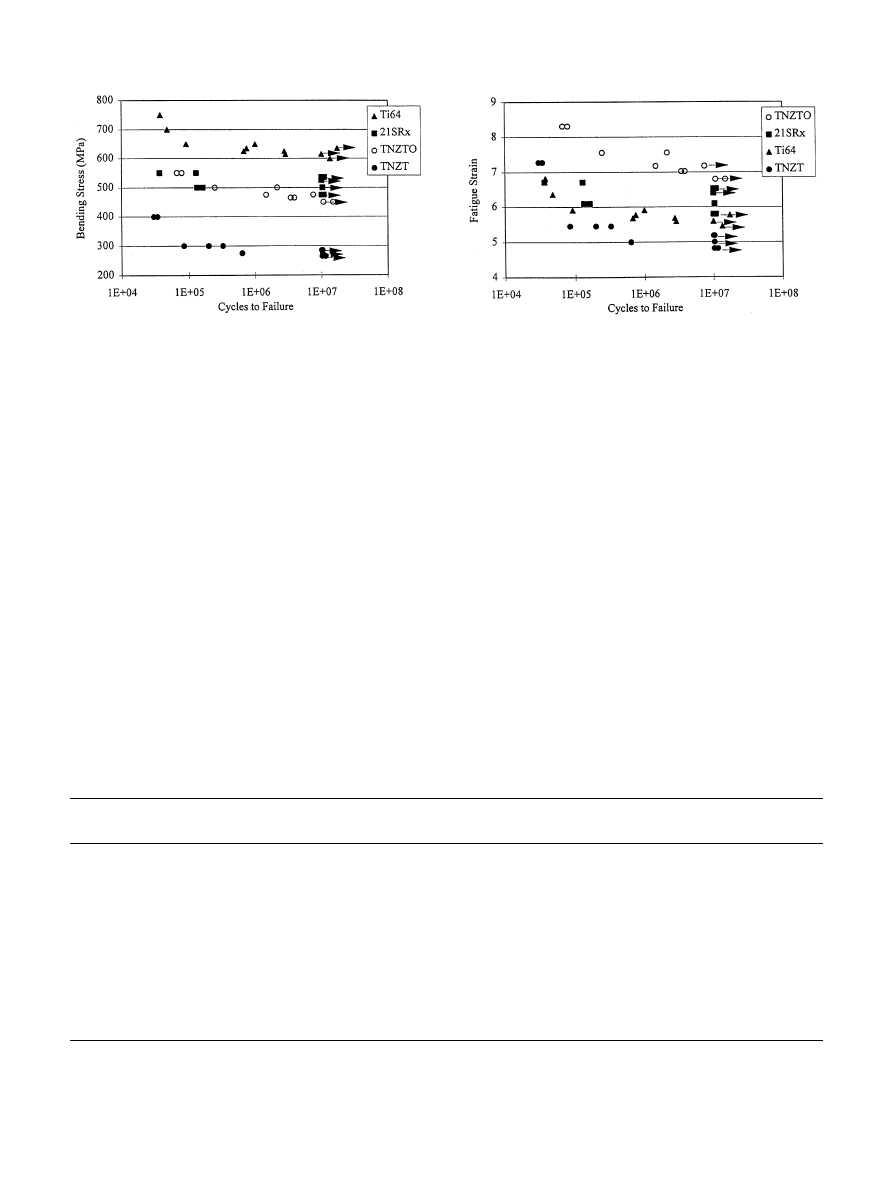
Fig. 6. Stress-controlled (RBF, R"!1, 60 Hz) fatigue response of new
metastable-
b Ti-alloys as compared to a#b Ti—6Al—4V.
Fig. 7. Total strain-controlled (RBF, R"!1, 60 Hz) fatigue response
of TNZT alloys and 21SRx as compared to Ti—6Al—4V.
Table 9
Notch fatigue strength of orthopaedic titanium alloys
Alloy designation
Smooth fatigue limit
Notch fatigue limit
K&"
K
Ti6Al4V
#
(MPa)
!
(MPa)
!
a/b alloys
Ti—6Al—4V
500
290 (K5"3.3)
0.6
—
290 (K5"3.3)
Ti—5Al—2.5Fe
580
300 (K5"3.6)
0.5
—
Ti—15Mo—5Zr—3Al
560—640
190 (K5"2.8)
0.3
1.0
(aged)
Martensitic
a@/b alloy
Ti—13Nb—13Zr
500
335 (K5"1.6)
0.7
1.0
215 (K5"3.0)
0.4
1.3
Metastable-
b alloy
Ti—12Mo—6Zr—2Fe
525
410 (K5"1.6)
0.8
1.4
! At 107 cycles.
" K& is a fatigue strength reduction factor defined as fatigue limit (notch)/fatigue limit (smooth control) under same test conditions.
# K
Ti6Al4V
is a fatigue strength factor relative to Ti—6Al—4V defined as fatigue limit (alloy)/fatigue limit (Ti6Al4V) under same test conditions.
Fatigue properties may also be improved by altering
the interstitial content (O, C, N, H) as illustrated by the
TNZT alloys (Fig. 6) [60]. Increasing the oxygen level in
TNZT resulted in an increase in strength and fatigue
limit for TNZTO, with some increase in modulus, the
latter value still, however, remaining below the presently
available orthopaedic alloys. A similar approach has
been undertaken in the development of TIMETAL
'
21SRx (21SRx), an orthopaedic grade of the TIME-
TAL
'
21S commercial alloy, where ‘toxic’ Al present in
the latter was eliminated in the former and compensated
by an increase in O content to 0.3 wt % in order to confer
additional strength to the Rx grade [56]. SHT-21SRx
show typical strength values for
b-titanium alloys with
a good fatigue behavior (only 15% lower than
Ti—6Al—4V) (Fig. 6).
Finally, the lower stress-controlled smooth fatigue
limit of
b-alloys may not be an appropriate characteriza-
tion for orthopaedic applications, where notch fatigue
behavior, more closely associated with strain-controlled
fatigue, may be more representative of in vivo conditions.
For example, hip stems rarely have a smooth surface but
are typically structured with wedges and coatings cre-
ating stress concentration sites. When considering fatigue
strain, i.e., the ratio between fatigue stress and elastic
modulus (Fig. 7), the strain-controlled fatigue behavior of
TNZT alloys is comparable to that of
a/b Ti—6Al—4V
alloy. Indeed a smaller reduction in fatigue limit occa-
sioned by the introduction of notches is typically ob-
served in
b-alloys when compared to Ti—6Al—4V, the
former exhibiting a comparable or higher notch fatigue
resistance than Ti—6Al—4V in all cases (Table 9).
5. Wear behavior of orthopaedic titanium alloys
Tribology, defined as the science and technology of
interacting surfaces in relative motion, and embracing
1630
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
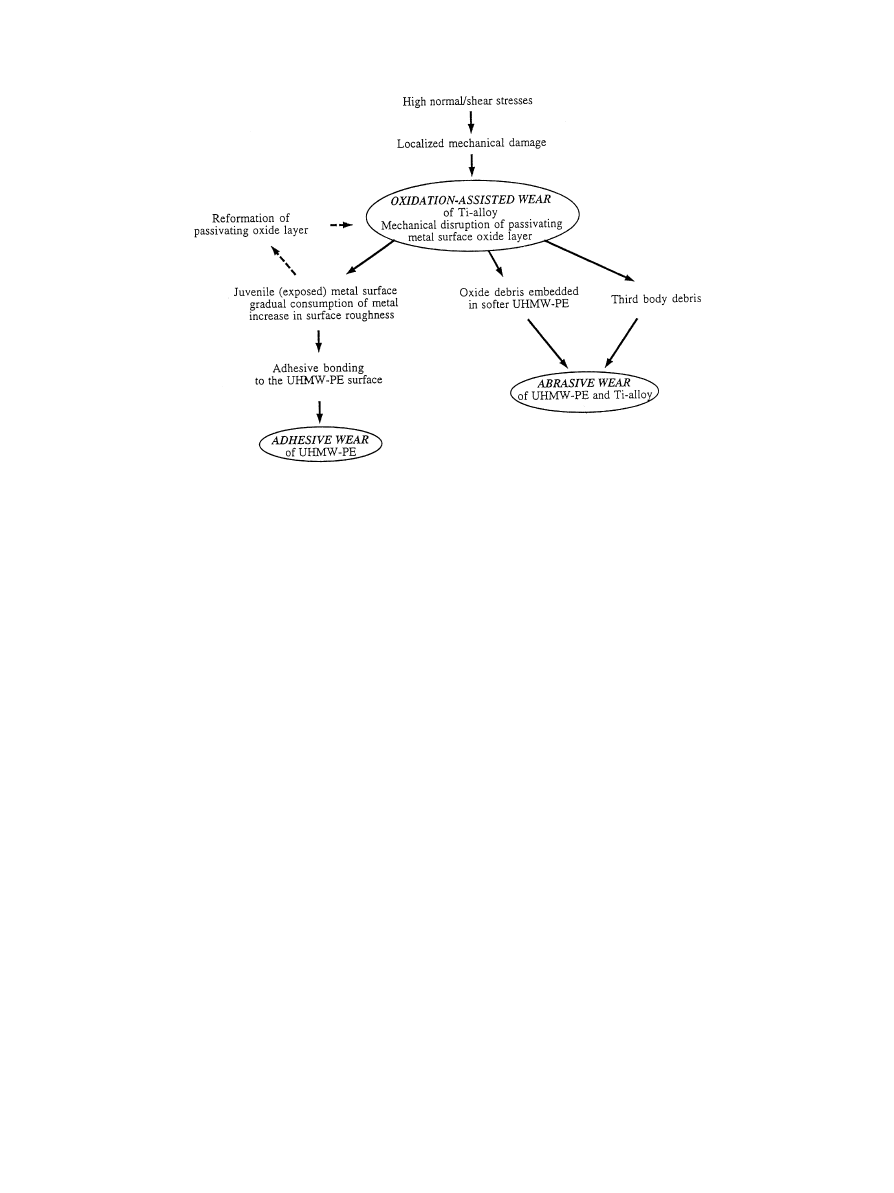
Fig. 8. Diagram illustrating the sequence of events during oxidative/abrasive/adhesive wear of the Ti—6Al—4V/UHMW-PE tribological pair (adapted
from [16]).
the study of friction, wear and lubrication [95, 96], has
emerged as a primary field in bioengineering. While
healthy natural joints exhibit remarkable tribological
characteristics, the latter being attributed to the intrinsic
properties of articular cartilage (high compliance) and
synovial fluid and the subsequent optimized lubrication
modes [1, 2, 97, 98], total replacement joints based upon
current available materials experience mixed/boundary
lubrication [6, 97]. This lower lubrication performance is
generally attributed to the high rigidity (low compliance)
of artificial materials. As some surface contact takes
place, friction between artificial materials is much higher
than in natural joints (
k"0.005—0.02) and non-recover-
able wear of the artificial joint materials takes place.
Clinical studies and retrieval examinations have shown
evidence that excessive wear of UHMWPE and/or metal
appears to be the principal mode of failure for the long
term use of TJRs [24—29, 30, 33]. Failure generally oc-
curs due to excessive wear of the components [99], wear
debris accumulation producing an adverse cellular re-
sponse leading to inflammation, release of damaging
enzymes, bone cell lysis, osteolysis, infection and pain,
implant loosening eventually ensuing [44, 45].
Early studies of Ti—6Al—4V wear performance in labo-
ratory tests has resulted in contradictory conclusions
[100]. Although the Ti—6Al—4V/UHMWPE combina-
tion seemed acceptable for use in total joint replacement
prosthesis, care should be taken as UHMWPE wear
rates for Ti—6Al—4V have been reported to 35% greater
than that for Co—Cr—Mo in hip simulator testing. Re-
trieval of implanted Ti—6Al—4V femoral components
have generally shown directional scratching and pitting/
delamination of bearing surfaces, those features being
non-uniformly distributed over the femoral head area
[27, 101, 102]. The high UHMWPE wear rates asso-
ciated with titanium alloy counterparts has been related
to the mechanical instability of the metal oxide layer
[16, 22, 103] (Fig. 8). It has been proposed that when
normal or shear stresses are high enough to induce
breakdown of the surface passive layer, the oxide will be
disrupted. The exposed metal surface may then either
reform a passive layer or adhesively bond to the polymer
surface. The latter situation leads to continuous removal
(material disruption) and reformation (oxidation) of the
passivating layer and results in gradual consumption of
alloy material. Concurrently, the surface roughness of the
metal surface will increase which results in yet higher
UHMWPE wear [104, 105]. Ultimately, the breakdown
of the oxide layer creates the potential for abrasive wear,
where the hard oxide debris act as third body abrasive
components (Fig. 9). Finally, it has also been observed
that excessive Ti—6Al—4V wear may be caused by the
presence of foreign bodies in the UHMWPE counterpart
component leading to severe abrasive wear of the
Ti—6Al—4V femoral head [27].
While wrought Co—Cr—Mo and ceramic (alumina and
zirconia) have been preferred to titanium alloys for bear-
ing surface UHMWPE counterpart implant materials,
UHMWPE wear and long-term degradation have gener-
ated renewed interest in metal-on-metal prostheses. In-
deed optimum friction and wear conditions can be
achieved and retained with metal-on-metal Co—Cr—Mo
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1631
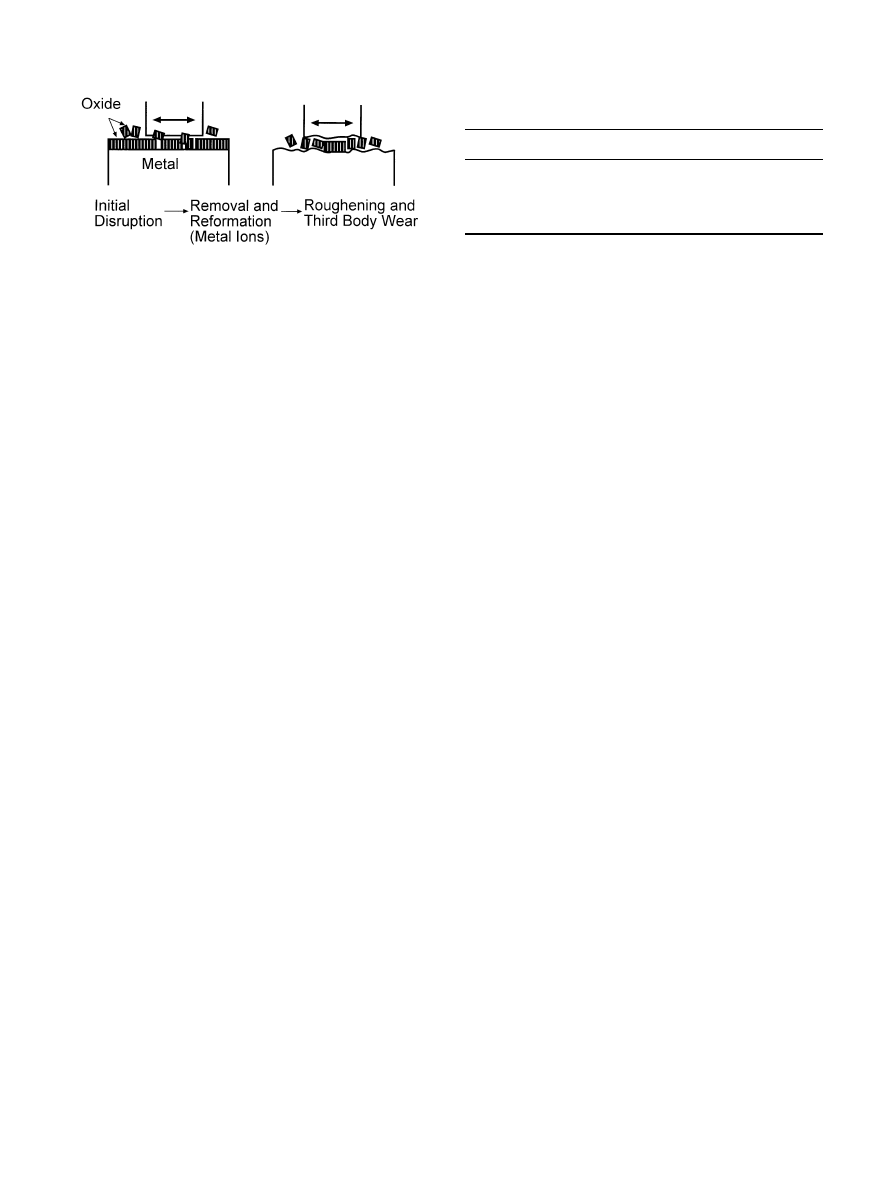
Fig. 9. Schematic illustration of the suggested oxidative/abrasive wear
process during articulation of metal on UHMWPE (adapted from Ref.
[16]).
Table 10
Oxides (dominant species) to be considered in aqueous solutions [113]
Alloying element
Oxide(s)
Ti
TiO, TiO2, Ti2O3, TiO3, Ti3O5
Nb
NbO, NbO2, Nb2O5
Ta
Ta2O5
Zr
ZrO2
pairs [10]. Notwithstanding this behavior the superior
biocompatibility and mechanical properties of titanium
alloys make them superior candidates for joint implants
should a better understanding of their tribological behav-
ior and an enhancement in their wear resistance be
achieved.
Detailed studies of titanium alloys friction and wear
performance are however sparse [106]. An original study
on friction and wear properties of
a-titanium has shown
poor wear characteristics for unalloyed Ti as well as
common titanium alloys [107]. This performance was
related to the properties of the oxide layer and the defor-
mation behavior of the subsurface regions.
a-titanium,
a relatively low shear strength hcp material, exhibited
higher
k values, but also greater material transfer, due to
its high reactivity, to non-metallic counterfaces, than
higher strength materials. Hence, titanium and titanium
alloys were considered to have poor oxidative wear res-
istance when ‘tribo-chemical’ reactions occur at the con-
tact area. In a fundamental tribological study of titanium
sliding against Al2O3 [108], the static formation of TiO2
was reported to decrease the wear and friction coefficient
of titanium. However, relatively high friction coefficients
(0.4—0.75) were observed at room temperature, these
values being in contradiction with the reported low fric-
tion coefficient of TiO2 (0.1—0.15) [109]. XRD analysis of
wear debris showed that TiO was a dominant oxide,
suggesting that the formation of TiO during tribo-oxida-
tion destroys the protective oxide layer and therefore
increases friction. It was proposed that the scaling layer
due to tribo-oxidation is composed, from surface to bulk
material, of a thin TiO2 layer, a thicker TiO layer, and
the Ti matrix. These findings were confirmed in another
study by analysis of wear debris revealing the cubic TiO
structure [110], this debris originating from regions
where critical wear was observed (‘smeared’ regions).
A non-continuous discrete layer of compacted wear frag-
ment was revealed, suggesting that the mechanical insta-
bility of this layer was responsible for the erratic and
high-friction behavior. Sufficient resistance of the under-
lying base material to plastic deformation is required for
mechanical integrity of the surface layer during rubbing
contact.
As the surface features of titanium alloys are of prime
importance of friction and wear resistance, the nature
and properties of the oxides present in the near-surface
region deserve special attention. Tribo-chemical reac-
tions during use, or even detailed surface treatments
before implantation, modify the surface characteristics of
the alloy [111, 112]. Further, bulk composition has been
shown to alter the composition of surface oxide layer, as
illustrated in Table 4, suggesting it may be possible to
optimize the mechanical response of this outermost layer
and improve its properties and integrity to the bulk
material through bulk chemical modification. For
example, the various ‘biocompatible’ oxides which could
possibly exist in a stable state in aqueous solution are
listed in Table 10 [113], where bold formulas correspond
to the surface oxide stable in potential-pH conditions
similar to human body fluids surrounding orthopaedic
implants [45].
However, care must be taken regarding the presence of
oxides at the surface and compositional effects cannot be
regarded as the only factor influencing the surface prop-
erties of oxides. The kinetics of repassivation (material-
electrolyte property) and the shear resistance of the oxide
layer (material property only) are two important para-
meters that will influence the behavior of the oxide layer
[114]. For instance, Ta tends to repassivate more rapidly
(96 ms) than Ti (172 ms) but more slowly than CoCrMo
(77 ms); Ta’s shear resistance (1.09 N mm
~2) is weaker
than Ti (3.7 N mm
~2) and CoCrMo (4.9 N mm~2), but
higher than Ti—6Al—4V (0.67 N mm
~2). Another study
[115] demonstrated that the presence of Ta and Nb
layers at the interface between Ti—30Ta and Al2O3 before
diffusion welding resulted in a reduction of the brittleness
of the interface and a decrease in the O and Al uptake of
the metal, and consequently a decrease in the brittleness
of the coupling. Oxide reaction at the interface might be
the cause of better bonding, reflecting the effect of com-
position on surface/interface properties.
In addition to the surface characteristics of titanium
alloys, a basic understanding of the mechanisms involved
in the friction and wear of titanium alloys is required with
particular attention on the subsurface deformation in-
duced during wear. High-strain deformation occurring in
the near-surface zone of a material undergoing wear is
1632
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
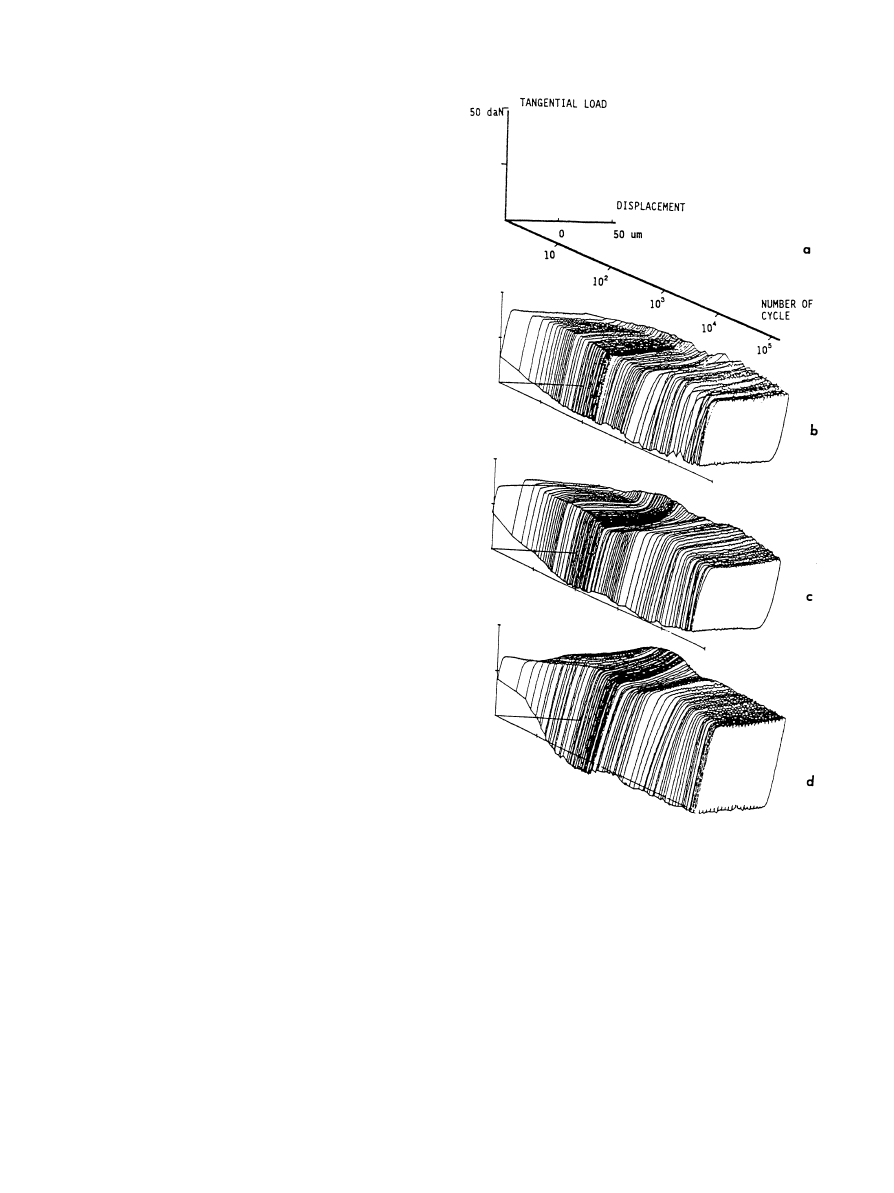
Fig. 10. ‘Friction log’ curves of titanium alloys: (a) definition of the
three axes, (b) aged ‘
a’-Ti—15V—3Cr—3Sn, (c) a#b-Ti—6Al—4V, (d) solu-
tion treated
b-Ti—15V—3Al—3Cr—3Sn [134].
a fundamental part of the wear process [116, 117] and
has been studied extensively in high stacking fault energy
materials such as copper alloys and aluminium [116—
119], but to a very limited extent in titanium alloys.
Sub-surface wear damage of cp-Ti has been investigated,
cross sections of wear samples revealing a layer of com-
pacted debris at the worn surface, with plastic flow be-
neath. Subsurface plastic flow was characterized by
a highly deformed cellular microstructure with a prefer-
red crystallographic orientation, validating dislocation
theories of friction and wear [120, 121]. While limited
data is available for
a-Ti, none exists for b-Ti, as typified
by the new orthopaedic alloys. However, if the low strain
hardening characteristics of
a/b titanium alloys [122] are
responsible for their low resistance to deformation within
the subsurface were region, then
b-titanium alloys, which
exhibit some strain hardening [123], may offer the poten-
tial for enhanced wear resistance [124].
Cyclic deformation behavior, due to the reciprocating
motion/loading of implants, is a critical element of ortho-
paedic alloys. While in-depth reciprocating-sliding stud-
ies of titanium and titanium alloys are not available,
fretting wear [131—137], resulting from low amplitude
((:300
lm)-reciprocating sliding motion between two
materials, has been studied in titanium and titanium
alloys [137—138]. As the displacement amplitude in-
creases, reciprocating sliding must be considered, that is
when wear mechanisms and wear rates may be related to
those in unidirectional sliding [96]. An important dis-
tinction between fretting and reciprocating-sliding wear
rises from the ease with which the wear debris can escape
from the contact region. After only a few strokes or
passes, the contact situation changes from a two to
a three-body contact formed by the two rubbing speci-
mens and the interface [135]. The wear process, resulting
from velocity accommodation both in the bulk and at the
interface, may be divided into three steps, i.e., (i) particle
detachment (by adhesion, abrasion, corrosion, fatigue,
etc.), (ii) third body behavior (trapped particle in the
interface region, changing in both morphology and com-
position), and (ii) particle ejection.
Waterhouse and Taylor [125] concluded from a fret-
ting study on pure titanium that fretting debris were
produced by the spreading of sub-surface cracks leading
to the detachment of phatelike particles of oxide coated
metal, this observation being consistent with the de-
lamination theory of wear during sliding. Further studies
of fretting behavior in titanium alloys have focused on
defining the particle detachment process and the evolu-
tion of the superficial layers during wear (gross slip con-
dition) [133, 134]. Fretting maps or friction logs were
obtained (Fig. 10) in titanium—titanium friction condi-
tions such that gross slip at the interface was achieved.
These curves were divided into two parts: (1) tangential
load linearly increasing with the displacement, corres-
ponding to the elastic response of the system and the
elastic deformation of the samples and the device, (2)
tangential load nearly constant with increasing displace-
ment, actual sliding taking place at the interface. Particle
detachment was observed in every case, and compacted
debris particle were observed on the wear track. The
friction coefficient was very high ('1) and slightly higher
for the
b alloy (1.2) than for the other a/a#b alloys (1.1).
In all alloys, a superficial layer with a tribologically
transformed structure, named ‘TTS’ by the authors,
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1633

was observed. This TTS layer was formed of ultra fine
non-oriented grains of
a-titanium with no b-phase. The
thickness of this layer decreased with increasing
b con-
tent, from 100
lm (aged-Ti—15V—3Al—3Cr—3Sn) to 40 lm
(
a#b-Ti—6Al—4V) to 15 lm (b-Ti—15V—3Al—3Cr—3Sn).
Continuity between the TTS layer and the bulk alloy was
also reported, with cracks being observed in the TTS
region. The authors concluded that the thickness of the
debris layer, and thus that of the TTS, is a very critical
parameter, directly controlling the stress transmitted to
the surface and near-surface layers. The detachment of
wear particles was then associated with propagation of
the cracks in the TTS or at the interface between the TTS
and the bulk material. Here intense plastic deformation
occurred, wear debris particles, characterized as TiO and
TiO2 oxides, originating from the TTS layer. Formation
of this TTS was ascribed to deformation-induced trans-
formation, the transformation of the near-surface zones
leading to the formation of the more stable phase, with
the major controlling parameters being strains ampli-
tudes and rates. Two types of microstructural changes
were observed in the superficial layers of the titanium
alloys after friction: (i) transformation of
b-phase, and (ii)
formation of the ultra-fine grained
a-phase. While the
authors concluded through limited evidence that the
metastable
b-phase transformed to a, their X-ray data
can also be interpreted as the formation of stress-induced
orthorhombic martensite,
aA [66, 138]. Stress-induced
martensite and twinning around wear scratches were also
observed in Ti—6Al—4V. It was finally suggested that the
mechanisms of particle detachment are related to plastic
deformation of the superficial and subsurface layers, but
correlations with reciprocating/fretting sliding or con-
tinuous sliding observations were not fully established.
However, it can be agreed that the mechanisms of par-
ticle detachment during fretting wear are closely related
to those observed in continuous/reciprocating sliding,
i.e., formation of a highly deformed layer, transfer layer,
particle detachment/delamination, and third body (de-
bris, lubricant) contact. Introduction of the influence of
cyclic loading and consequent fatigue behavior still need
to be addressed.
The poor tribological properties of Ti—6Al—4V for im-
plant articulation surfaces has resulted in the develop-
ment of surface treatments to enhance the hardness and
the abrasive wear resistance of the alloy and thereby to
minimize UHMWPE wear debris generation [23, 103,
139, 140]. Various procedures including PVD coating
(TiN, TiC), ion implantation (N
`), thermal treatments
(nitriding, diffusion hardening) [141], or laser alloying
with TiC [142] have been examined. Ion-implantation
has been the most common treatment employed
[22, 140, 143], resulting in either little or substantial im-
provement in the sliding wear resistance of Ti—6Al—4V,
though there has been reported consistent improvement
in wear resistance to abrasion [139]. While surface treat-
ments producing a harder layer composed of various
oxides improve lubrication, no long term data are yet
available and the limitation of such surface treatments to
the modification of only a thin layer ((10
lm in best
cases) may promote catastrophic wear as the treated
surface wears away or become discontinuous.
Surface modification by oxygen diffusion hardening
(ODH) has been considered to enhance the wear resis-
tance of Ti—6Al—7Nb [144]. This treatment provides
a gradual increase in hardness through a relatively thick
50
lm transformed layer and a friction coefficient for
ODH—Ti—6Al—7Nb against UHMWPE lower than other
low wear materials (Table 11). A similar approach was
taken by Zwicker et al. [67] for enhanced friction behav-
ior of Ti—5Al—2.5Fe against UHMWPE (Table 11), using
oxide films formation by thermal oxidation. Properly
oxidized and oil quenched Ti—5Al—2.5Fe balls displayed
friction properties comparable to alumina balls based
on topography measurements made before and after
testing.
Sliding wear tests have also been conducted in order to
assess the wear properties of the newer titanium alloys
(Table 11). In general, improved friction and wear behav-
ior has been observed, with or without surface treatment,
relative to Ti—6Al—4V. In a pin-on-disk study against
PMMA cement in deionized water, the friction coeffic-
ient of TMZF was found to be less than half that of
Ti—6Al—4V [53, 54]. At low load and after 10
5 cycles,
TMZF exhibited no change in surface roughness and no
surface scratching. The ‘self-perpetuating’ wear asso-
ciated with Ti—6Al—4V, where the formation of third
body metallic and bone cement particles results in high
weight loss of both parts and black debris from the
titanium alloy, was not observed with the TMZF alloy.
When tested against UHMWPE, the friction coefficient
of the TMZF alloy was again half the value of Ti—6Al—4V
against UHMWPE.
Diffusion/oxidation surface hardening (DH) was very
beneficial in improving the abrasive wear of TI—13Nb—
13Zr to levels comparable to Co—Cr—Mo alloy and much
superior to TiN-coated Ti—6Al—4V [59] (Table 11). This
diffusion hardening process produced a hardened surface
by diffusion of oxygen into the substrate, and not by
deposition of an overlay coating on the substrate, as in
the case of N-implantation. A blue ceramic surface layer,
0.2
lm thick, composed of TiO2, TiO, and ZrO2, was
formed on the alloy, the depth of the diffusion hardened
layer being 2—3
lm. The presence of ZrO2 oxides in the
‘ceramic’ surface of diffusion hardened Ti—13Nb—13Zr
resulted in improved wear resistance to abrasion sugges-
ting again that the composition of the oxide layer can be
tailored through composition control of bulk composi-
tions in order to optimize the surface properties of ortho-
paedic alloys and improve their wear resistance.
Future improvements in the wear resistance of ortho-
paedic titanium alloys will eventually develop from a
1634
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639

Table 11
Summarized tribological studies of orthopaedic titanium alloys
Titanium alloy
Tribo-test conditins
!
Friction/wear data
[‘Reference’ material]
[load-stress/sliding velocity]
(same conditions)
Ti—6Al—7Nb [47, 48]
Pin-on-disk, Ringer’s#30% serum
k
k-value (
]10~7)
k
k-value
Abrasive PMMA pin [3.45 MPa/25 mm s
~1]
0.100
3.32 (N
`)
(
]10~7)
0.078
2.11 (TiN)
0.094
2.72 [CoCrMo]
0.051
1.35 (ODH
")
0.079
2.13 [Al2O3]
Ti—5Al—2.5Fe [67]
Pin-on-disk, 0.9% NaCl-solution
Depth (
km) of wear track on disk
UHMWPE disk [50N]
18—44 (polished)
N/A
#
12—24 (oxidized)
Ball-in-socket, 0.9% NaCl-solution
Friction moment (Nm)
Friction moment (Nm)
UHMWPE socket [100—2500 N]
0.5—1 (oxidized, induction hardened)
1.5 [Al2O3]
Ti—15Mo—5Zr—3Al [55]
NaCl-solution
k
wear
k
wear
Other parameters N/A
#
0.82(SHT
b) (SHTb)"3](SS316L)
0.43—0.53
cpTi"2
]SS
0.43—0.53
(
b#a)$"3](SS316L)
[SS, cpTi]
(
b#a)$
Ti—12Mo—6Zr—2Fe [53]
Pin-on-disk, deionized water
k
k
abrasive PMMA pin [100 g/74 mm s
~1]
0.30—0.44
:
0.80 [Ti—6Al—4V]
UHMWPE pin [500 g/73 mm s
~1]
0.04
0.08 [Ti—6Al—4V]
Ti—13Nb—13Zr [59]
Reciprocating pin-on-disk, Ringer’s
Depth (
km) of metal wear track
Depth (
km)
PMMA pin [107 MPa/74 mm s
~1
(2.5 Hz/15 mm)]
Ti—13Nb—13Zr
Ti—13Nb—13Zr(ODH
")
0.21
21 [Ti—6Al—4V]
0.15
7.8 [Ti—6Al—4V/TiN coated]
! Tribo-part in italics is titanium alloy.
" ODH: Oxygen Diffusion Hardening.
# Not Available.
$ Aged condition.
systematic approach based on achieving a basic under-
standing of their tribological properties. Though suc-
cessful in many cases, the ‘trial and comparison’ ap-
proach exemplified in Table 11 by the inconsistent proto-
cols followed, has limited progress in improving bi-
omaterials properties [145]. Because of the complexity of
tribology and wear problems, a systematic approach
aiming at understanding basic mechanisms, suitable to
a large number of non-specific applications, should
be implemented. More specifically, the separate invest-
igation of surface, subsurface, and third body (debris)
behaviors, the three wear ‘precursors’ as described
by Sannino and Rack [146] could ultimately identify
basic wear mechanisms while avoiding misleading extra-
polation when different experimental parameters are se-
lected.
6. Conclusions
1. Titanium alloys are generally preferred mateials for
orthopaedic applications due to their relatively low
modulus vis-a`-vis Co—Cr—Mo alloys, superior biocom-
patibility and corrosion resistance.
2. Enhanced biocompatibility and reduced elastic
modulus in titanium alloys have been achieved by the
recent development of biomedical alloys baed on
b-solu-
tion treatment (metastable
b or martensite a@#b) micro-
structures.
3. The wear resistance of
b-Ti alloys show some im-
provement when compared to
a#b alloys.
4. Overall alloy composition, which controls surface
oxide composition and subsurface deformation behavior,
is a critical factor in the wear behavior of
b-alloys.
5. Ultimately the use of orthopaedic titanium alloys as
wear components will require a more detailed under-
standing of the basic wear mechanisms involved.
Acknowledgements
The authors would like to acknowledge the partial
support of Osteonics, Inc. (Allendale, NJ), Teledyne-AL-
LVAC (Monro´e, NC), and TIMET (Henderson, NV).
The authors wish to thank Jonathan Black, Ph.D., FBSE,
for his review of this manuscript and his valuable com-
ments. Special thanks are due to Martine LaBerge,
Ph.D., for numerous beneficial discussions.
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1635

References
[1] Mow VC, Soslowsky LJ. Friction, lubrication, and wear of
dirthridial joints. In: Basic orthopaedic biomechanics. New
York: Raven Press Ltd., 1991:245—92.
[2] Dowson D. Bio-tribology of natural and replacement synovial
joints. In: Mow VC, Ratcliffe A, Woo SL-Y, editors. Bio-
mechanics of diarthrodial joints, vol. II. Chap. 29. New York:
Springer, 1992:305—45.
[3] Ardill J. What is orthopaedics? PhD Thesis. The Department of
Orthopaedic Surgery, The Queen’s University of Belfast, 1995.
[4] Schumacher HR. In: Schumacher HR, Klippel JH, Robinson
DR, editors. Primer on the Rheumatic Diseases, 9th edn. At-
lanta, GA: The Arthritis Foundation, 1988.
[5] Lowman EW. Osteoarthritis. J Amer Med Acad 1955;157:487.
[6] Dowson D. Friction and wear of medical implants and pros-
thetic devices. ASM Handbook, vol. 18. Gereland: Materials
Park, OH: ASM International, 1992:656—64.
[7] American Academy of Orthopaedic Surgeons. Survey Presented
at the Orthopaedic Research Society Annual Meeting. Orlando,
FL, February 1995.
[8] McKee GK, Watson-Farrar J. Replacement of arthritic hips by
the McKee-Farrar prosthesis. J Bone J Surg 1996;48-B(2):
245—59.
[9] Walker PS, Gold BL. The tribology (friction lubrication and
wear) of all-metal artificial hip joints. Wear 1971;17:285—99.
[10] Protek AG. METASUL
TM metal-on metal articulation. Tech-
nical brochure, Protek AG, Switzerland, Edition 2/94, 1994.
[11] Streicher RM, Scho¨n R, Semlitsch M. Investigation of the
tribological behavior of metal-on-metal combinations for artifi-
cial hip joints. Biomedizinische Technik 1990;35(5):3—7.
[12] Charnley J. Arthroplasty of the hip. Lancet 27 May 1961:1129—32.
[13] Charnley J. Total hip replacement by low-friction arthroplasty.
Clin Orthop Relat Res 1970;72:7—21.
[14] Galante JO, Rostoker W. Wear in total hip protheses. Acta
Orthp Scand Suppl 1973;145:1—46.
[15] Dumbleton JH. Wear and prosthetic joints. In: Morrey BF,
editor. Joint replacement arthroplasty, Chap 6. New York:
Churchill Livingstone, 1991:47—9.
[16] Poggie RA, Wert JJ, Mishra AK, Davidson JA. Friction and
wear characterization of UHMWPE in reciprocating sliding
contact with Co—Cr, Ti—6Al—4V, and zirconia implant bearing
surfaces In: Denton R, Keshavan MK, editors. In: Wear and
friction of elastomers. ASTM STP 1145, Philadelphia, PA:
American Society for Testing and Materials, 1992:65—81.
[17] McKellop H, Clarke IC, Markolf KL, Amstutz HC, Friction and
wear characteristics of polymer, metal, and ceramic prosthetic
joint materials evaluated on a multichannel screening device.
J Biomed Res 1981;15:619—53.
[18] Oonishi H, Tsuji E, Mizukoshi T, Fujisawa A, Murata N,
Kushitani S, Aono M, Meguro Y. Wear of polyethylene and
alumina in clinical cases of alumina total knee prosthesis. In:
Hulbert J, Hulbert SF, editors. Bioceramics 3. Terra Haute, IN:
Rose-Hulman Institute of Technology, 1992:137—45.
[19] Oonishi H, Aono M. Clinical results of total knee arthroplasty in
combination with alumina against polyethylene—a five to eight
year follow up study. In: Hulbert J, Hulbert SF, editors. Bio-
ceramics 3. Terra Haute, IN: Rose-Hulman Institute of Techno-
logy, 1992:147—56.
[20] Saikko V. Wear and friction properties of prosthetic joint mate-
rials evaluated on a reciprocating pin-on-flat apparatus. Wear
1993;166:169—78.
[21] Saikko V. Wear of polyethylene acetabular cups against zirconia
femoral heads studied with a hip joint simulator. Wear
1994;176:207—12.
[22] Buchanan RA, Rigney ED Jr, Williams JM. Wear-accelerated
corrosion of Ti—6Al—4V and nitrogen-ion-implanted Ti—6Al—4V:
mechanisms and influence of fixed-stress magnitude. J Biomed
Mater Res 1987;21:367—77.
[23] Peterson CD, Hillberry BM, Heck DA. Component wear of total
knee prostheses using Ti—6Al—4V, titanium nitride coated
Ti—6Al—4V, and cobalt-chromium-molybdenum femoral com-
ponents. J Biomed Mater Res 1988:22.
[24] Agins HJ, Alcock NC, Bansal M, Salvati EA, Wilson PD, Pel-
licci PM, Bullough PG. Metallic wear in failed titanium-alloy
total hip replacements. J Bone J Surg 1988;70-A(3):347—56.
[25] Argenson J-N, O’Connor JJ. Polyethylene wear in meniscal knee
replacement. J Bone J Surg 1992;74-B(2):228—32.
[26] Berry DJ, Wold LE, Rand JA. Extensive osteolysis around an
aseptic, stable, uncemented total knee replacement. Clin Orthop
Relat Res 1993;293:204—7.
[27] Black J, Sherk H, Bonini J, Rostoker WR, Schajowicz F, Galante
JO. Metallosis associated with a stable titanium-alloy femoral
component in total hip replacement. J Bone J Surg 1990;72-A(1):
126—30.
[28] Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene
wear in cemented metal-backed acetabular cups. J Bone J Surg
1993;75-B(2):249—53.
[29] Engh GA, Dwyer KA, Hanes CK. Polyethylene wear of metal-
backed tibial components in total and uncompartimental knee
prostheses. J Bone J Surg 1992;74-B(1):9—17.
[30] Winter M, Griss P, Scheller G, Moser T. 10—14 years results of
ceramic-metal-composite hip prostheses with a cementless
socket. In: Heimke G, editor. Bioceramics, vol. 2. Cologne,
Germany:
Deutsche
Keramische
Gesellschaft
eV,
1990:
436—44.
[31] Jones SMG, Pinder IM, Moran CG, Malcom AJ. Polyethylene
wear in uncemented knee replacements. J Bone J Surg 1992:
74-B:18—22.
[32] Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of
polyethylene acetabular components. J Bone J Surg 1993;75-B:
254—58.
[33] Nolan JF, Bucknill TM. Aggressive granulomatosis from poly-
ethylene failure in an uncemented knee replacement. J Bone
J Surg 1992;74-B(1):23—4.
[34] Santavirta S, Nordstro¨m D, Metsa¨rinne K, Kontinnen YT. Bio-
compatibility of polyethylene and host response to loosening of
cementless total hip replacement. Clin Orthop Relat Res 1993;
297:100—10.
[35] Shanbhag AS, Jacobs JJ, Glant TT, Gilbert JL, Black J, Galante
JO. Composition and morphology of wear debris in failed un-
cemented total hip replacement. J Bone J Surg 1994;76-B(1):
60—7.
[36] Jacobs JJ, Shanbag A, Glant TT, Black J, Galante JO. Wear
debris in total joint replacements. J Amer Acad Orthop Surg
1994;2(4):212—20.
[37] Dumbleton JH. The clinical significance of wear in total hip anf
knee prostheses. J Biomater Appl 1988;3:3—32.
[38] Ries MD, Rose RM, Greer J, Weaver KD, Sauer WL. Steriliza-
tion-induced effects on UHMWPE performance properties. Sci-
entific Exhibition Presented at the 62nd Annual Meeting of the
American Academy of Orthopaedic Surgeons. Orlando, FL,
16—21 February 1995.
[39] Sutula LC, Sperling DK, Collier JP, Saum KA, Williams IR.
Clinical wear of UHMWPE acetabular and tibial compo-
nents—impact of gamma sterilization in air and material con-
solidation. Scientific exhibition Presented at the 62nd Annual
Meeting of the American Academy of Orthopaedic Surgeons.
Orlando, FL, 16—21 Februay 1995.
[40] White SE, Whiteside LA, Poggie RA, Farrar D, Tanner MG.
In-vivo wear and material properties of UHMWPE tribial com-
ponents. Scientific Exhibition Presented at the 62nd Annual
Meeting of the American Academy of Orthopaedic Surgeons.
Orlando, FL, 16—21 February 1995.
1636
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639

[41] Wright Medical Technology Inc. Effects of sterilization methods
on UHMWPE. Technical Monograph Presented at the 62nd
Annual Meeting of the American Academy of Orthopaedic
Surgeon. Orlando, FL, 16—21 February 1995.
[42] Streicher RM, Semlitsch MF, Weber H, Schon R. Metal-on-
metal articulation: a new generation of wear resistant implants.
Transactions of the 20th Annual Meeting of the Society for
Biomaterials. Boston, MA, April 1994:323.
[43] Medley JB, Krygier JJ, Bobyn JD, Chan FW, Tanzer M.
Metal—metal bearing surfaces in the hip: early wear results from a
simulator apparatus. Transactions of the 21st Annual Meeting of
the Society for Biomaterials. San Francisco, CA, April 1995:47.
[44] Park JB, Lakes RS. Biomaterials—An introduction, 2nd edn.
New York, London: Plenum Press, 1992.
[45] Black J. Biological performance of materials—fundamentals of
biocompatibility, 2nd edn. New York: Marcel Dekker Inc., 1992.
[46] Freese H, Teledyne-Allvac, Monroe NC. Private communica-
tion, 1995.
[47] Semlitsch MF, Weber H, Streicher RM, Scho¨n R. Joint replace-
ment components made of hot-forged and surface-treated
Ti—6Al—7Nb alloy. Biomater 1992;13(11):781—8.
[48] Borowy K-H, Kramer K-H. On the properties of a new titanium
alloy (TiAl5Fe2.5) as implant material. In: Titanium’84 Science
and Technology, vol. 2. Munich, Deutsche Gesellschaft Fu¨r
Metallkunde EV, 1995:1381—6.
[49] Zwicker R, Bu¨hler K, Mu¨ller R, Beck H, Schmid H-J, Ferstl J. In:
Kimura H, Izumi O, editors. Titanium’80 Science and Techno-
logy. Warrendale, PA, TMS-AIME 1980:505—14.
[50] Steinemann SG. Corrosion of surgical implants-in vivo and in
vitro tests In: Winter GD, Leray JL, de Groot K, editors.
Evaluation of Biomaterials. New York: Wiley, 1980:1.
[51] Steinemann SG. Corrosion of titanium and titanium alloys
for surgical implants. Titanium’84 Science and Technology,
vol. 2. Munich, Deutsche Gesellschaft Fu¨r Metallkunde EV
1985;2:1373—9.
[52] Laing PG, Fergosun AB Jr, Hodge ES. Tissue reaction in rabbit
muscle exposed to metallic implants. J Biomed Mater Res
1967;1:135—49.
[53] Wang K, Gustavson L, Dumbleton J. The characterization of
Ti—12Mo—6Zr—2Fe. A new biocompatible titanium alloy de-
veloped for surgical implants. In: Beta Titanium in the 1990’s.
Warrendale: The Minerals, Metals & Materials Society, 1993:
49—60.
[54] Wang K, Gustavson L, Dumbleton J. Low modulus, high
strength, biocompatible titanium alloy for medical implants. In:
Titanium’92 Science and Technology. Warrendale: The Min-
erals, Metals & Materials Society, 1993:2697—704.
[55] Steinemann SG, Ma¨usli P-A, Szmukler-Moncler S, Semlitsch M,
Pohler O, Hintermann H-E, Perren SM. Beta-titanium alloy for
surgical implants. In: Titanium’92 Science and Technology.
Warrendale: The Minerals, Metals & Materials Society,
1993:2689—96.
[56] Fanning JC. TIMET Technical Laboratories. Henderson NV,
Private communication, 1995.
[57] Okazaki Y, Ito Y, Ito A, Tateishi T. Effect of alloying elements
on mechanical properties of titanium alloys for medical im-
plants. Mater Trans JIM 34(12):1217—22 (1993); J Japan Inst
Metals 1993;57(3):332—7.
[58] Kovacs P, Davidson JA. The electrochemical behavior of a new
titanium alloy with superior biocompatibility. In: Titanium’92
Science and Technology, Warrendale: The Minerals, Metals &
Materials Society, 1993:2705—12.
[59] Mishra
AK,
Davidson
JA,
Kovacs
P,
Poggie
RA.
Ti—13Nb—13Zr: a new low modulus, high strength, corrosion
resistant near-beta alloy for orthopaedic implants. In: Beta Tita-
nium in the 1990’s. Warrendale: The Minerals, Metals & Mate-
rials Society, 1993:61—72.
[60] Ahmed T, Long M, Silvestri J, Ruiz C, Rack HJ. A new low
modulus, biocompatible titanium alloy. Presented at the
8th World Titanium Conference. Birmingham, UK, October
1995.
[61] Collings EW. The physical metallurgy of titanium alloys, ASM
Series in Metal Processing. Gegel HL, editor. Cleveland, Metals
Park, OH: American Society for Metals, 1984.
[62] Polmear JJ. Titanium alloys. In: Light alloys, Chapter 6. Lon-
don, UK: Edward Arnold Publ, 1981.
[63] Bania PJ. Beta titanium alloys and their role in the titanium
industry In: Eylon D, Boyer RR, Koss DA, editors. Titanium
Alloys in the 1990’s. Warrendale: The Mineral, Metals & Mate-
rials Society, 1993:3—14.
[64] Schutz RW. An overview of beta titanium alloy environmental
behavior. In: Eylon D, Boyer RR, Koss DA, editors. Beta Tita-
nium Alloys in the 1990’s. Warrendale, PA: The Mineral, Metals
& Materials Society, 1993:75—91.
[65] Boyer RR, Hall JA. Microstructure-property relationships in
titanium alloys (critical review). In: Froes FH, Caplan I, editors.
Titanium’92 Science and Technology, Warrendale, PA: The
Mineral, Metals & Materials Society, 1993:77—88.
[66] Vassel A. Microstructural instabilities in beta titanium alloys. In:
Eylon D, Boyer RR, Koss DA, editors. Beta Titanium Alloys in
the 1990’s. The
Mineral, Metals & Materials Society,
1993:173—85.
[67] Zwicker J, Etzold U, Moser Th. Abrasive properties of oxide
layers on TiAl15Fe2.5 in contact with high density polyethylene.
In: Titanium’84 Science and Technology, vol. 2. Munich,
Deutsche Gesellschaft Fu¨r Metallkunde EV 1985;2:1343—50.
[68] Perl DP, Brody AR. Alzeihmer’s disease: X-ray spectrometric
evidence of aluminum accumulation in neurofibrillary tangle-
bearing neurons. Science 1980;208:297—99.
[69] Crapper DR, McLachlan DR, Farnell B, Galin H, Karlik S,
Eichhorn G, De Boni U. Aluminum in human brain disease. In:
Sarkar B, editor. Biological Aspects of Metals and Metals-Re-
lated Diseases. New York: Raven Press, 1993:209—18.
[70] Kobayashi E, Matsumoto S, Doi H, Yoneyama T, Hamanaka
H. Mechanical properties of the binary titanium-zirconium
alloys and their potential for biomedical materials. J Biomed
Mater Res 1995;29:943—50.
[71] Ma¨usli P-A, Steineman, SG, Simpson JP. Properties of surface
oxides on titanium and some titanium alloys. 6th World Confer-
ence on Titanium, les e´ditions de physique, Les Ulis Cedex,
France, part IV, 1988:1759—64.
[72] Dujovne AR, Bobyn JD, Krygier JJ, Miller JE, Brooks CE.
Mechanical compatibility of noncemented hip prostheses with
the human femur. J Arthroplasty 1993;8(1):7—22.
[73] Engh CA, Bobyn JD. The influence of stem size and extent of
porous coating on femoral bone resorption after primary cement-
less hip arthroplasty. Clin Orthop Relat Res 1988;231:7—28.
[74] Cheal EJ, Spector M, Hayes WC. Role of loads and prosthesis
material properties on the mechanics of the proximal femur after
total hip arthroplasty. J Orthop Res 1992;10:405—22.
[75] Prendergast P, Taylor D. J Biomed Eng 1990;12:379.
[76] Huiskes R, Weinans H, van Rietbergen B. The relationship
between stress shielding and bone resorption around total hip
stems and the effects of flexible materials. Clin Orthop Relat Res
1992;274:124—34.
[77] Sumner DR, Galante JO. Determinants of stress shielding: de-
sign versus materials versus interface. Clin Orthop Relat Res
1992:274:202—12.
[78] Chesnutt JC, Thompson AW, Williams JC. Influence of metal-
lurgical factors on the fatigue crack growth rate in alpha—beta
titanium alloys. Rockwell International Science Center, Thou-
sand Oaks, CA. Report
d AFML-TR-78-68, Air Force Ma-
terials Laboratory, Air Force Systems Command, Wright-Pat-
terson Air Force Base, OH, May 1978.
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1637

[79] Peters M, Gysler A, Lu¨tjering G. Influence of microstructure on
the fatigue behavior of Ti—6Al—4V. In: Kimura H, Izumi O,
editors. Titanium’80—Science and technology, 1981:1777—86.
[80] Ru¨dinger K, Fischer D. Relationship between primary alpha
content, tensile properties and high cycle fatigue behavior of
Ti—6Al—4V. In: Lu¨tjering G, Zwicker U, Bunk W, editors. Tita-
nium’84—Science and technology, vol. 4. Munich, Deutsche
Gesellschaft Fur Metallkunde EV, 1985:2123—30.
[81] Boyer RR, Bajoraitis R, Spurr WF. The effects of thermal pro-
cessing variations on the properties of Ti—6Al—4V. In: Micro-
structure, fracture toughness and fatigue crack growth rate in
titanium alloys. The Minerals, Metals & Materials Society,
Warrendale, PA, 1987:149—70.
[82] Lu¨tjering G, Gysler A. Fatigue. In: Lu¨tjering G, Zwicker U,
Bunk W editors. Titanium’84—Science and Technology, vol. 4.
Munich, Deutsche Gesellschaft Fur Metallkunde EV 1985:
2065—83.
[83] Wagner L, Gerdes C, Lu¨tjering G. Influence of surface treatment
on fatigue strength of Ti—6Al—4V. In: Lu¨tjering G, Zwicker U,
Bunk W, editors. Titanium’84 — Science and Technology, vol. 4.
Munich,
Deutsche
Gesellschaft
fu¨r
Metallkunde
EV,
1985:2147—54.
[84] Wagner L, Lu¨tjering G, Jaffee RI. Optimization of bi-modal
microstructure and texture in Ti—6Al—4V. In: Kim Y-W, Boyer
RR, editors. Microstructure/property relationships in titanium
aluminides and alloys. Warrendale: The Minerals, Metals &
Materials Society, 1991:521—31.
[85] Wagner L, Lu¨tjering G. Influence of surface condition on fatigue
strength. In: Kitagawa J, Tanaka T, editors. Fatigue 90. Birmin-
gham, UK: Materials and Component Engineering Publications
Ltd, 1991:323—8.
[86] Eberhardt AW, Kim BS, Rigney ED, Kutner GL, Harte CR.
Effects of precoating surface temperatures on fatigue of
Ti—6Al—4V. J Appl Biomat 1995;6:171—4.
[87] Military Handbook—Metallic materials and elements for aero-
space vehicle structures. US Department of Defense, Washing-
ton DC, MIL-HDBK-5C, 1976:5/78.
[88] Williams DN, Wood RA. Effects of surface condition on the
mechanical properties of titanium and its alloys. MCIC-71-01
Report. Metals and Ceramics Information Center, Battelle Col-
umbus Laboratories, OH, August 1971.
[89] Merget M, Aldinger F. Influence of technological parameters on
the fatigue strength of Ti5Al2.5Fe — a new material for endop-
rostheses. In: Lu¨tjering G, Zwicker U, Bunk W, editors. Tita-
nium’84—Science and Technology, vol. 4. Munich, Deutsche
Gesellschaft Fur Metallkunde EV 1985:1393—400.
[90] Cook SD, Georgette FS, Skinner HB, Haddad RJ Jr, Fatigue
properties of carbon- and porous-coated Ti—6Al—4V alloy.
J Biomed Mater Res 1984;18:497—512.
[91] Lorenz M, Semlitsch M, Panic B, Weber H, Willert HG. Fatigue
strength of cobalt-bae alloys with high corrosion resistance for
artificial hip joints. Eng Med 1978;7(4):241—50.
[92] Wolfarth D, Filiaggi M, Ducheyne P. Parametric analysis of
interfacial stress concentrations in porous coated implants.
J Appl Biomat 1990;1:3—12.
[93] Fanning JC. In: Eylon D, Boyer RR, Koss DA, editors. Beta
Titanium Alloys in the 1990’s Warrendale, PA: The Minerals,
Metals & Materials Society, 1993:411—62.
[94] Gregory JK, Wagner L. Heat treatment and mechanical behav-
ior in Beta C
TM. Presented at the VII International Meeting on
Titanium, 15 November 1991, Torino, Italy. Organised by GTT,
Ginatta Torino Titanium S.p.a. Torino.
[95] Dumbleton JH. Tribology of natural and artificial joints. Tribol-
ogy Series 3. Amsterdam: Elsevier Scientific Publishing Com-
pany, 1981.
[96] Hutchings IM. Tribology: friction and wear of engineering ma-
terials. Boca Raton, FL: CRC Press, 1992.
[97] Fisher J, Dowson D. Tribology of total artificial joints. Proc
Instn Mechanical Engineers—IMechE 1991;205:73—9.
[98] Unsworth A. Tribology of human and artifical joints. Proc Inst
Mechnical Engineers—IMechE 1991;205:163—72.
[99] Jasty M. Clinical reviews: particulate debris and failure of total
hip replacements. J Appl Biomater 1993;4:273—6.
[100] Clarke IC, McKellop HA, McGuire P, Okuda R, Sarmiento A.
Wear of Ti—6Al—4V implant alloy and ultrahigh molecular
weight polyethylene combinations. In: Luckey HA, Kubli F,
editors. Titanium alloys in surgical implants. ASTM STP 796,
Philadelphia, PA: American Society for Testing and Materials,
1983:136—47.
[101] Dobbs HS, Scales T. Behavior of commercially pure titanium
and Ti-318 (Ti—6Al—4V) in orthopedic implants. In: Luckey HA,
Kubli F, editors. Titanium alloys in surgical implants. ASTM
STP 796. Philadelphia, PA: American Society for Testing and
Materials, 1983:173—86.
[102] Easter TL, Graham RM, Jacobs JJ, Black J, LaBerge M. Clinical
performance of Ti6Al4V femoral components: wear mechanisms
vs. surface profile. Proc 20th Annual meeting of the Society For
Biomaterials, Boston, MA, 5—9 April 1994:185.
[103] Rostoker W, Galante JO. The influence of titanium surface
treatments on the wear of medical grade polyethylene. Biomater
1981;2(10):221—4.
[104] Dowson D, El-Hady Diab MM, Gillis BJ, Atkinson R. Influence
of counterface topography on the wear of ultra high molecular
weight polyethylene under wet or dry conditions. In: Lee L-H,
editor. Polymer wear and its control. Washington DC: Ameri-
can Chemical Society, 1985:171—87.
[105] Fisher J, Dowson D, Hamdzah H, Lee HL. The effect of sliding
velocity on the friction and wear of UHMW-PE for use in total
artificial joints. Wear 1994;175:219—25.
[106] Budinski KG. Tribological properties of titanium alloys. Wear
1991;151:203—17.
[107] Miller PD, Holladay JW. Friction and wear properties of tita-
nium. Wear 1958/59;2:133—40.
[108] Hong H, Winer WO. A fundamental tribological study of Ti/Al
2
O
3
contact in sliding wear. J Tribol—Trans ASME 1989;111:504—9.
[109] Steijn RP. Friction and wear of rutile single crystals. ASLE
Trans 1969;12:21—33.
[110] Nutt SR. A study of the friction and wear behavior of titanium
under dry sliding conditions. In: Wear of Materials 1983. New
York: The American Society of Mechanical Engineers,
1983:426—33.
[111] Herna´ndez de Gatica NL, Jones GL, Gardella JA Jr. Surface
characterization of titanium alloys sterilized for biomedical ap-
plications. Appl Surface Sci 1993;68:107—21.
[112] Raikar NG, Gregory J, Ong JL, Lucas LC, Lemons JE,
Kawahara D, Nakamura M. Surface characterization of tita-
nium implants. J Vac Sci Technol 1995;A 13(5):2633—7.
[113] Pourbaix M. Atlas of electrochemical equilibria in aqueous
solutions. New York: Pergamon Press, 1966.
[114] Tu¨mmler HP, Thull R. Surface properties of titanium and its
alloys — mechanical and electrochemical investigation. In: Tita-
nium’84 Science and Technology, vol. 2. Munich, Deutsche
Gesellschaft Fu¨r Metallkunde EV 1985;2:1335—42.
[115] Gibbesh B, Elssner G, Petzow G. Investigation of Ti/Al2O3
joints with intermediate tantalum and niobium layers. Biomater
1992;13(7):455—61.
[116] Rice SL, Nowotny H, Wayne SF. A survey of the development of
subsurface zones in the wear of materials. Key Eng Mater
1989;33:77—100.
[117] Wert JJ. The role of microstructure in subsurface damage in-
duced by sliding contact. Key Eng Mater 1989;33:101—34.
[118] Heilmann P, Clark WAT, Rigney DA. Orientation determina-
tion of subsurface cells generated by sliding. Acta Metall
1983;31(8):1293—305.
1638
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639

[119] Kuo SM, Rigney DA. Sliding behavior of aluminum. Mater Sci
Engng 1992;A157:131—43.
[120] Kulhman-Wilsdorf D. Dislocation concepts in friction and wear.
In: Rigney DA, editors. Fundamentals of friction and wear of
materials. Metals Park, OH: American Society for Metals,
1980:119—86.
[121] Hirth JP, Rigney DA. Microstructural models for friction and
wear. In: Haasen P, Gerold V, Kostorz G, editors. Strength of
Metals and Alloys, vol. 3. Oxford: Pergamon Press, 1980:
1483—502.
[122] Kuhlman GW. A critical appraisal of thermomechanical pro-
cessing of structural titanium alloys. In: Kim Y-W, Boyer RD,
editors. Microstructure/property relationships in titanium
alumindes and alloys. Warrendale, PA: The Minerals, Metals &
Materials Society, 1991:465—91.
[123] Ling F-W, Starke EA, Lafevre BG. Deformation behavior and
texture development in beta Ti—V alloys. Metall Trans
1974;15:179—87.
[124] Moore MA, Douthwaite RM. Plastic deformation below worn
surfaces. Metall Trans A 1976;7A:1833—9.
[125] Waterhouse RB, Taylor DE. Fretting debris and the delamina-
tion theory of wear. Wear 1974;29:337—44.
[126] Hoeppner DW, Chandrasekarn V. Fretting in orthopaedic im-
plants: a review. Wear 1994;173:189—97.
[127] Waterhouse RB, Dutta MK. The fretting fatigue of titanium and
some titanium alloys in a corrosive environment. Wear
1973;25:171—5.
[128] Waterhouse RB, Lamb M. Fretting corrosion of orthopaedic
implant materials by bone cement. Wear 1980;60:357—68.
[129] Fricker DC, Shivanath R. Fretting corrosion studies of universal
femoral head protheses and cone taper ingots. Biomater
1990;11:495—500.
[130] Sandstrom PW, Sridharan K, Conrad J. A machine for fretting
wear testing of plasma surface modified materials. Wear 1993;
166:163—8.
[131] Bill
RC.
Selected
fretting-wear-resistant
coatings
for
Ti—6%Al—4%V alloy. Wear 1985;106:283—301.
[132] Cronin J, Warburton J. Amplitude-controlled transitions in fret-
ting: the comparative behavior of six materials. Tribol Int
1988;21(6):309—15.
[133] Blanchard P, Colombie C, Pellerin V, Fayeulle S, Vincent L.
Material effects in fretting wear: application to iron, titanium,
and aluminum alloys. Metall Trans A 1991;2A:1535—44.
[134] Fayeulle S, Blanchard P, Vincent L. Fretting behavior of tita-
nium alloys. Tribol Trans 1993;36(2):267—75.
[135] Berthier Y, Vincent L, Godet M. Fretting fatigue and fretting
wear. Tribol Int 1989;22(4):235—42.
[136] Colombie C, Berthier Y, Vincent L, Godet M. Fretting wear and
fretting fatigue damage. Fatigue’87, Warley, England, 1987:567—75.
[137] Vincent L. Usure et frottement: une approach pluri-disciplinaire
‘me´canique-mate´riaux’. In: Traitements de surface et protection
contre la corrosion. Audisio S, Caillet M, Galerie A, Mazille H,
editors. CNRS, Paris, Chap. 2.6, 1987.
[138] Flower HM. The plastic deformation of metastable
b titanium
alloys. In: Lu¨tjering G, Zwicker U, Bunk W, editors. Tita-
nium’84—Science and Technology, vol. 4. Munich, Deutsche
Gesellschaft Fur Metallkunde EV 1985:1651—8.
[139] McKellop HA, Ro¨stlund TV. The wear behavior of ino-om-
planted Ti—6Al—4V against UHMW polyethylene. J Biomed
Mater Res 1990;24:1413—25.
[140] Rieu et al. J. Ion implantation effects on friction and wear of joint
prosthesis materials. Biomater 1991;12:139—43.
[141] Morton PH, Bell T. Surface engineering of titanium. Me´moires
et E¨tudes Scientifiques Revue de Me´tallurgie 1989; 86(10):639—46.
[142] Chengwei C, Zhiming Z, Xitang T, Wang Y, Sun XT. Influence
of rapidly solidified structures on wear behavior of Ti—6Al—4V
laser alloyed with TiC. Tribol Trans 1995;38(4):875—8.
[143] Tateishi T, Terui A, Yunoki H. Friction and wear properties of
biomaterials for artificial joint. In: Heimke G, editor. Bio-
ceramics, vol. 2. Cologne, Germany: German Ceramic Society,
1992:145—51.
[144] Streicher RM, Weber H, Scho¨n R, Semlitsch M. New surface
modification for Ti—6Al—7Nb alloy: oxygen diffusion hardening
(ODH). Biomater 1991;12:125—9.
[145] Berthier Y. Tribologie: science carrefour. Collection of Papers
and dabates, European Conference on Braking. Lille, France,
3—4 December 1992:1—26.
[146] Sannino AP, Rack HJ. Dry sliding wear of discontinuously
reinforced aluminum composites: review and discussion. Wear
1995;189:1—19.
M. Long, H.J. Rack / Biomaterials 19 (1998) 1621—1639
1639
Wyszukiwarka
Podobne podstrony:
My Heart will go on (Titanic) inst in B
Hydrogen absorption behavior of beta titanium alloy in acid
Electrochemical characterization of cast titanium alloys
Buddhism Learning In A Total Way (Lama Ole Nydahl)
Functional improvements desired by patients before and in the first year after total hip arthroplast
Effects of preoperative physiotherapy in hip osteoarthritis patients awaiting total hip replacement
Functional improvements desired by patients before and in the first year after total hip arthroplast
Diverted Total Synthesis in Medicinal Chemistry Research
In vitro corrosion resistance of titanium made using differe
In vitro biological effects of titanium rough surface obtain
Titanium in medicine
Analysis of total propionic acid in feed using headspace sol
Total number of students in tertiary education, as a percentage of the population aged 20–24, by EU
Minimum Quantity Lubrication Drilling of Lightweight Aluminum and Magnesium Alloys Used in A
Crystal Jordan [In the Heat of the Night 01] Total Eclipse of the Heart [pdf](1)
In vitro corrosion of titanium
Total Quality Management (TQM)
Education in Poland
więcej podobnych podstron