
Biomaterials 23 (2002) 1775–1783
Effect of biologically active coating on biocompatibility of Nitinol
devices designed for the closure of intra-atrial communications
Xiangqing Kong
a
, R.G. Grabitz
a
, W. van Oeveren
b
, D. Klee
c
, T.G. van Kooten
b
,
F. Freudenthal
a
, Ma Qing
a
, G. von Bernuth
a
, M.-C. Seghaye
a,
*
a
Department of Paediatric Cardiology, Aachen University of Technology, Aachen, Germany
b
Department of Biomedical Engineering, University of Groningen, The Netherlands
c
Institute of Macromolecular- and Textile Chemistry, Aachen University of Technology, Aachen, Germany
Received10 April 2001; accepted5 September 2001
Abstract
Anti-thrombogenicity and rapid endothelialisation are prerequisites for the use of closure devices of intra-atrial communications
in order to reduce the risk of cerebral embolism. The purpose of this study was therefore to assess the effect of bioactive coatings on
biocompatibility of Nitinol coils designed for the closure of intra-atrial communications. Nitinol coils (n ¼ 10; each) andflat Nitinol
bands (n ¼ 3; each) were treatedby basic coating with poly(amino-p-xylylene-co-p-xylylene) andthen coatedwith either heparin, r-
hirudin or fibronectin. Anti-thrombogenicity was studied in vitro in a dynamic model with whole blood by partial thromboplastin
time (PTT), platelet binding and thrombin generation, respectively, and cytotoxicity by hemolysis. Endothelialisation was studied on
Nitinol bands with human umbilical venous endothelial cells (HUVEC) by 3-(4,5-dimethylthiazole-2yl)-2,5-triphenyl tetrazolium
(MTT) assay andimmnuofluorescence analysis of Ki67, vinculin, fibronectin andvon WillebrandFactor. Uncoatedor coated
devices did not influence hemolysis and PTT. r-Hirudin (but not heparin) and fibronectin coating showed lower platelet binding than
uncoatedNitinol (p
o0:005; respectively). Heparin and r-hirudin coating reduced thrombin formation (po0:05 versus Nitinol,
respectively). HUVEC adhesion, proliferation, and matrix formation decreased in the order: fibronectin coating>uncoated
Nitinol>r-hirudin coating>heparin coating>basic coating. MTT assay corroborated these findings. In conclusion, r-hirudin
and fibronectin coating, by causing no acute cytotoxicity, decreasing thrombogenicity and increasing endothelialisation improve
in vitro biocompatibility of Nitinol devices designed for the closure of intra-atrial communications. r 2002 Elsevier Science Ltd.
All rights reserved.
Keywords:
Nitinol; Biologically active coating; Thrombogenicity; Endothelialisation
1. Introduction
Nitinol is an alloy of approximately equiatomic parts
of nickel andtitanium andhas three special properties,
which are not commonly observedin metallic materials:
thermal shape memory, superelasticity, andhigh damp-
ing properties [1]. By these superior mechanical proper-
ties, Nitinol builds the basic framework of many devices
designed for either closure of intracardiac communica-
tions or for stenting purposes. A prerequisite for the use
of Nitinol devices for the closure of intracardiac
communications such as a patent foramen ovale or an
atrial septum defect, is low or no thrombogenicity and
rapidend
othelial cell growth on the d
evice, to avoid
systemic andin particular cerebral embolism. Further-
more, overgrowth of endothelial cells on the devices
couldcontribute to the complete closure of the defects.
Although Nitinol is increasingly being usedin clinical
settings due to its reported good biocompatibility and
biofunctionality [2,3], its anti-thrombogenicity and
potential for endothelialisation as intracardiac device
have never been systemically studied so far. In addition,
biologically active coatings, such as heparin or r-hirudin
coating on Nitinol, couldprovid
e increasedanti-
thrombogenicity, andfibronectin coating couldincrease
endothelial cell overgrowth on the devices, respectively,
*Corresponding author. Department of Paediatric Cardiology,
German Heart Centre Munich, Lazarettstrasse 36, D-80636 Munich,
Germany. Tel.: +49-89-1218-3011; fax: +49-89-1218-3013.
E-mail address:
seghaye@dhm.mhn.de (M.-C. Seghaye).
0142-9612/02/$ - see front matter r 2002 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 3 0 4 - 0

as it has been recently suggestedby other in vitro and
in vivo investigations [4–6].
It was therefore the aim of this in vitro study to
analyse the effect of immobilisation of heparin, r-hirudin
or fibronectin on Nitinol coils designed for the closure of
intra-atrial communications on cytotoxicity, thrombo-
genicity andendothelial cell–Nitinol interactions.
2. Material and methods
2.1. Nitinol
Nitinol coils were usedfor bloodcontact tests andflat
rectangular bands of Nitinol for endothelial cell inter-
action tests. Coils andband
s were processedin our
laboratory. The nickel–titanium alloy purchasedfrom
Euroflex G. Rau GmbH, Pforzheim, Germany. The
original wire (diameter: 0.25 mm) was thermally treated
to form the primary coil (external diameter: 1 mm) and
the secondary coil (external diameter: 5 mm). Coils and
bands were cleaned in an ultrasound bath and sterilised
prior to the coating procedure. Coils were then peeled in
10 mm length andcoated. The size of the flat Nitinol
bandwas 3 25 mm
2
. Prior to the in vitro experiments
the Nitinol samples were sterilisedby 10% ethylene
oxide gas.
2.2. Coating
Covalent end-attachment procedure was performed
with chemical vapour deposition (CVD) as previously
described [7]. Briefly, 4-Amino [2.2]paracyclophane was
synthesisedfrom [2.2]paracyclophane as describedelse-
where [8]. 4-Amino [2.2]paracyclophane was converted
to the corresponding poly(p-xylylene) using a self
designed CVD installation [8]. The pyrolysis of the
[2.2]paracyclophane was carriedout in a 320 mm long
glass tube with an inner diameter of 30 mm. The first
120 mm servedas sublimation zone, followedby a
240 mm section usedas pyrolysis zone. The pyrolysis
tube is connectedby glass/metal joints to a stainless steel
polymerisation chamber. The chamber walls is kept at
1001C. The polymerisation chamber is equippedwith a
turnable cooledsample hold
er, an online thickness
monitor, a thermocouple connectedto the sample
holder and a vacuum gauge. A defined argon flow was
usedas carrier gas. A butterfly valve regulates the
system pressure independently of the argon flow.
Protein immobilisation was performedas follows.
Free isocyanate groups were achievedby incubating the
poly(amino-p-xylylene-co-p-xylylene) (amino-ppx) sur-
faces with hexamethylene diisocynate (HDI, Aldrich) in
absolute diethyl ether (1:10, w/w) for 5 days. After
extraction in a Soxhlet under nitrogen in absolute
diethyl ether for 8 h and drying in vacuum, polymers
with free isocyanate groups were generatedon polymer-
coatedmetal surfaces. To conserve physiological activity
of hirudin, r-hirudin was reacted with N-(methyl
sulfonyl ethoxy carbonyloxy) succinimide (MSC-ONSu,
Aldrich) for partial protection and deprotected after the
immobilisation step [8]. Protein immobilisation was
carriedout in phosphate bufferedsaline (PBS) (pH
7.4) contacting the HDI-amino-ppx surface for 12 h with
the protein solution using the following concentrations:
(MSC)-hirudin, 50 nmol/ml; Heparin (Braun, Melsun-
gen, Ger), 50 nmol/ml; Fibronectin (Boehringer, Man-
nheim, Ger), 0.05 nmol/ml. After immobilisation the
surfaces were rinsedwith SDS-buffer (0.1%).
The total amount of immobilisedheparin (0.3–0.5 mg/
cm
2
) was assessedby a colorimetric examination using
Toluidin blue according to Smith et al. [9]. The total
amount of covalently boundr-hirudin andfibronectin
was measuredby using
125
I-radiolabelling experiments
as previously described [7] and reached 2–5 and 0.2 mg/
cm
2
, respectively.
In vitro tests were performedon 5 different coating
groups: uncoatedNitinol, basic coating, heparin-, r-
hirudin-, and fibronectin-coating. 10 species of Nitinol
coils each group were usedfor bloodcontact tests and3
species of flat rectangular bands of Nitinol for
endothelial cell interaction tests.
2.3. Whole blood contact tests
Whole bloodtests were performedon ad
ult sheep
bloodin order to allow further in vivo investigation in
the ovine model [10]. Each coil was separately put in a
sterile syringe filledwith 0.32% citratedblood
. The
syringes were rotatedin a closedincubator, at 371C with
11 cycles/min, for 1 and24 h, respectively. Bloodwas
then collectedeither for erythrocyte andplatelet count
by electronic cell counter (Cellanalyzer CA530, Brom-
ma, Sweden), or for hemolysis analysis. Coils were
washed gently with saline, fixed in 2% glutaraldehyde in
0.1 m cacodylate buffered saline solution at pH 7.4 for
48 h andthen collectedfor analysis by scanning electron
microscopy (SEM) with FEG-SEM scanning electron
microscopy at 1.5 kV (Jeol 6301F, Japan) [11].
For hemolysis analysis, 200 ml of platelet poor plasma
was usedfor optical d
ensity (OD) measurement at
540 nm after bloodwas centrifugedat 1.100g for 12 min.
Copper andpolyethylene were usedas positive and
negative reference material, respectively. Hemolysis was
expressedby OD values.
2.4. Platelet binding test
Europium labelling platelet test was performedas
previously described [11]. Briefly, platelets were isolated
from sheep platelet rich plasma by gelfiltration on
Sepharose CL2B (Pharmacia, Uppsala, Sweden) in
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1776

saline. The isolatedplatelets were subjectedto hypotonic
shock in the presence of 1/30 volume 20 mm Europium
trichloride (Fluka Chemie AG, Buchs, Switzerland) and
1
2
volume demineralised water. After centrifugation at
250 RCF for 10 min, the supernatant was discarded. The
pellet was resuspended in 10% autologous plasma and
the amount of fluorescence of the labelledplatelets, as
well as the platelet count were measuredby a time
resolvedfluorometer (Arcus Fluorometer, Wallac,
Turku, Finland) and a cellcounter, respectively. After
incubation of the coils in the platelet-plasma mixture for
15 min, coils were washedgently in saline andthe
remainder fluorescence was counted and back-calcu-
latedto platelet number.
2.5. Partial thromboplastin time (PTT)
Sheep platelet poor plasma (0.32% citrate) was first
dynamically incubated with the coils in a syringe at a
rotating speedof 11 cycles/min, at 371C for 30 min.
Thereafter the plasma was separatedfrom the coils and
rotatedat 371C in a coagulometer (Amelung-coagulo-
meter, KC 4A, the Netherlands) in the presence of
20 nmol CaCl
2
. Negative andpositive controls for the
PTT were provided by plasma, which was not incubated
with any material andby plasma, which was incubated
in glass tubes for 30 min at 371C, respectively.
2.6. Thrombin generation assay
One batch of 0.32% citratedsheep plasma was
depleted from fibrinogen, and 500 ml of this plasma
was incubatedwith test coils in the presence of calcium
andplatelet phospholipids (Sigma Diagnostic, St. Louis,
USA) for 15 min, at 371C. 10 ml of the incubation
mixture was removedfrom the material anddilutedwith
140 ml Tris-HCl buffer (pH 7.4). 50 ml of a 3 mm solution
of Substrate 2238 (Chromogenix, Instrumentation
Laboratory, Lexington, USA) was added to this dilution
andsubstrate conversion by thrombin was measured
after 30 min in a spectrophotometer (Power Wave 2000,
Bio-Tek Instruments, Winooski, VT, USA) at 405 nm.
The amount of thrombin generatedwas calculated
according to a standard curve of known thrombin
concentrations in saline solution. Additionally, the
thrombin generation assay also measures the amount
of binding-thrombin by heparin or r-hirudin coating,
indicating a decrease of thrombin in the incubation
mixture.
2.7. Endothelial cells interaction with flat Nitinol bands
2.7.1. Human umbilical vein endothelial cells (HUVECs)
culture and seeding
HUVECs were isolatedandculturedas previously
reported[12]. For endothelial cells andNitinol interac-
tion tests, HUVECs, at passage 2 were seeded in 12-well
plates (Nunc, Nunc Inc., USA,) with a density of
160,000 cells per well, per 2 ml. Cells were culturedin the
presence of the flat Nitinol bands (coated and uncoated)
for 48 h. A separate well without samples servedas
control material.
2.7.2. Immunocytochemistry
Cells were fixedin warm 3.7% formald
ehyd
e in
cytoskeleton stabilisation buffer (CS: 0.1 m piperazine-
N
,N
0
-bis(2-ethanesulfonic acid), 1 mm EGTA, 4% (w/v)
polyethylene glycol 8000 (all Sigma Diagnostic, St.
Louis, USA), pH 6.9) for 20 min, washedthree times in
CS buffer andstoredat 41C until further processing. The
fixedcells were extractedfor 3 min in 0.5% Triton X-100
(Sigma Diagnostic, St. Louis, USA), rinsedthree times
in CS andquenchedin freshly prepared0.05% sodium
borohydride (Sigma Diagnostic, St. Louis, USA) in PBS
for 10 min at 201C. Non-specific backgroundwas
blockedwith 5% fatty acidfree bovine serum albumin
(BSA) (Sigma Diagnostic, St. Louis, USA) in PBS for
20 min. Cells were incubatedin primary antibod
y,
diluted in PBSA (=PBS+1% BSA) overnight at 41C.
After three washings with PBSA, cells were incubated
with secondary antibody, diluted in PBSA, for 1–2 h at
371C. Cells were then washed4 times in PBSA, 2 times
in PBS andone time in mounting med
ium (1:1
glycerol:PBS with 0.02% sodium azide and 100 mg/ml
Dabco (1,4-diazabicyclo[2.2.2] octane; Sigma Diagnos-
tic, St. Louis, USA). The slides were mounted, and
samples were examinedwith confocal laser scanning
microscopy (Leica DMRXE with confocal TCS SP2
unit, Germany). The following antibodies were used:
anti-fibronectin (polyclonal anti-human fibronectin,
1:400), anti-vinculin (monoclonal anti-human vinculin,
clone h-VIN-1, 1:200), anti-Ki67 (monoclonal anti-
human Ki67, 1:200), antibody of von Willeband Factor
(polyclonal anti-human von WillebandFactor, 1:800),
FITC–goat–anti-mouse IgG, FITC–donkey–anti-rabbit
IgG, LRSC–goat–anti-mouse IgG, andLRSC-donkey-
anti-rabbit IgG (all 1:50) (all Sigma Diagnostic, St.
Louis, USA).
2.7.3. 3-(4,5-dimethylthiazole-2yl)-2,5-triphenyl
tetrazolium (MTT) assay
The ability of converting MTT has been usedto assess
viability of the culturedcells. MTT (0.5 mg/ml final
concentration) was added to the cells after 45 h of
incubation andwas incubatedfor 3 more hours.
Samples ðn ¼ 3Þ were then taken out of the MTT-
containing medium, dip rinsed once in warm PBS
andinsertedin 2-propanol. The remaining wells were
rinsedonce with warm PBS. Subsequently, 2-propanol
was added to the wells. A volume of 500 ml propanol
was usedfor the wells as well as for the samples.
Two samples were pooledfor one d
etermination.
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1777

Absorbency was measuredat 595 nm with an ELISA-
reader (BioRad, Sweden).
2.8. Statistics
The data are shown as mean
7standard error of the
mean. Assuming non-normal distribution of the data,
non-parametric statistical tests were used. For inter-
group comparison, the non-parametric Kruskall-Wallis-
test was applied. The data were analyzed with SPSS
program package (SPSS Software GmbH, M
.unchen,
Germany), and p-values
o0.05 were considered sig-
nificant.
3. Results
3.1. Whole sheep blood contact tests
3.1.1. Erythrocyte and platelet count
Erythrocyte andplatelet counts were similar for
coatedanduncoatedcoils after exposure of whole sheep
bloodfor 1 and24 h, respectively, as shown in Table 1.
Copper causedmarkedplatelet loss after 24 h of
exposure to whole bloodas comparedwith other tested
coils ðp
o0:05Þ:
3.1.2. Hemolysis
No difference in hemolysis was observed between
uncoatedandcoatedcoils (Table 1). Copper caused
markedhemolysis after 24 h of incubation whereas
polyethylene induced the same release of hemoglobin as
uncoatedandcoatedcoils.
3.1.3. Scanning electron microscopy
The surface characteristics of the coils coatedwith the
different biologically active substances are summarised
in Table 2. The 300 times magnification gave an overall
view on the coverage of the coil surface, while the 3000
times magnification allowedthe identification of fibrin
and/or activated platelets. Fig. 1 is exemplary for
findings observed on coils coated with fibronectin and
heparin, respectively.
3.2. Platelet binding
The lowest platelet binding was found on basic
coating (p
o0:003 versus uncoatedcoils). Coating with
r-hirudin or fibronectin but not with heparin was
associatedwith significantly lower platelet bind
ing in
comparison with uncoatedcoils (p
o0:05; and po0:001;
respectively, Fig. 2).
3.3. Partial thromboplastin time
PTT was not affectedby any of the coatings
testedandrangedbetween 75% and90% of control
plasma.
Table 1
Erythrocyte andplatelet count, andhemolysis of whole bloodafter exposure to uncoatedNitinol coils andcoils coatedwith different biologically
active substances
a
Duration of
UncoatedHeparin
r-Hirud
in
Fibronectin
Basic coating
Copper
Polyethylene
exposure (h)
ðn ¼ 10Þ
ðn ¼ 10Þ
ðn ¼ 10Þ
ðn ¼ 10Þ
ðn ¼ 10Þ
ðn ¼ 4Þ
ðn ¼ 4Þ
Erythrocyte
( 10
9
/ml)
1
7.71
74.22
7.74
74.41
6.54
74.21
5.47
71.66
6.22
73.27
5.33
70.93
4.88
70.75
24
8.17
70.56
8.44
70.47
8.13
70.53
8.10
70.40
8.13
70.68
8.17
70.37
8.45
70.92
Platelet
( 10
6
/ml)
1
114.10
724.07
114.90
718.12
101.10
723.24
101.40
730.87
94.80
724.55
79.75
714.08
99.00
737.70
24
92.50
713.01
94.00
720.31
80.00
714.45
84.70
719.59
88.67
710.82
64.50
74.51*
86.75
718.41
OD540 nm
( 10
2
)
1
12.25
72.07
12.94
72.84
13.87
72.73
12.89
72.49
12.04
72.00
16.15
72.88
16.30
76.71
24
16.01
74.51
22.26
77.36
19.26
75.69
23.62
76.65
24.22
79.85
55.60
715.12**
20.27
79.12
a
Hemolysis is quantitated by optical density at 540 nm (OD 540 nm). Copper served as positive and polyethylene as negative control. The data are
expressedas mean
7standard error of mean. *po0:05; as comparedwith each of the other 6 groups; **po0:0001; as comparedwith each of the other
6 groups.
Table 2
Evaluation of deposits on the surface of uncoated Nitinol coils and
coils coatedwith biologically active substances after incubation with
whole sheep bloodfor 48 h
a
Coverage
Platelets
Activated
platelets
Fibrin
Uncoated2
2
0
2
Heparin
3
3
1
1
r-Hirudin
1
1
0
0
Fibronectin
1
0
0
2
b
Basic coating
2
2
1
1
a
Results as median score on 3 specimens. Score as follows: 0,
absence; 1, a few; 2, many; 3, extensive.
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1778
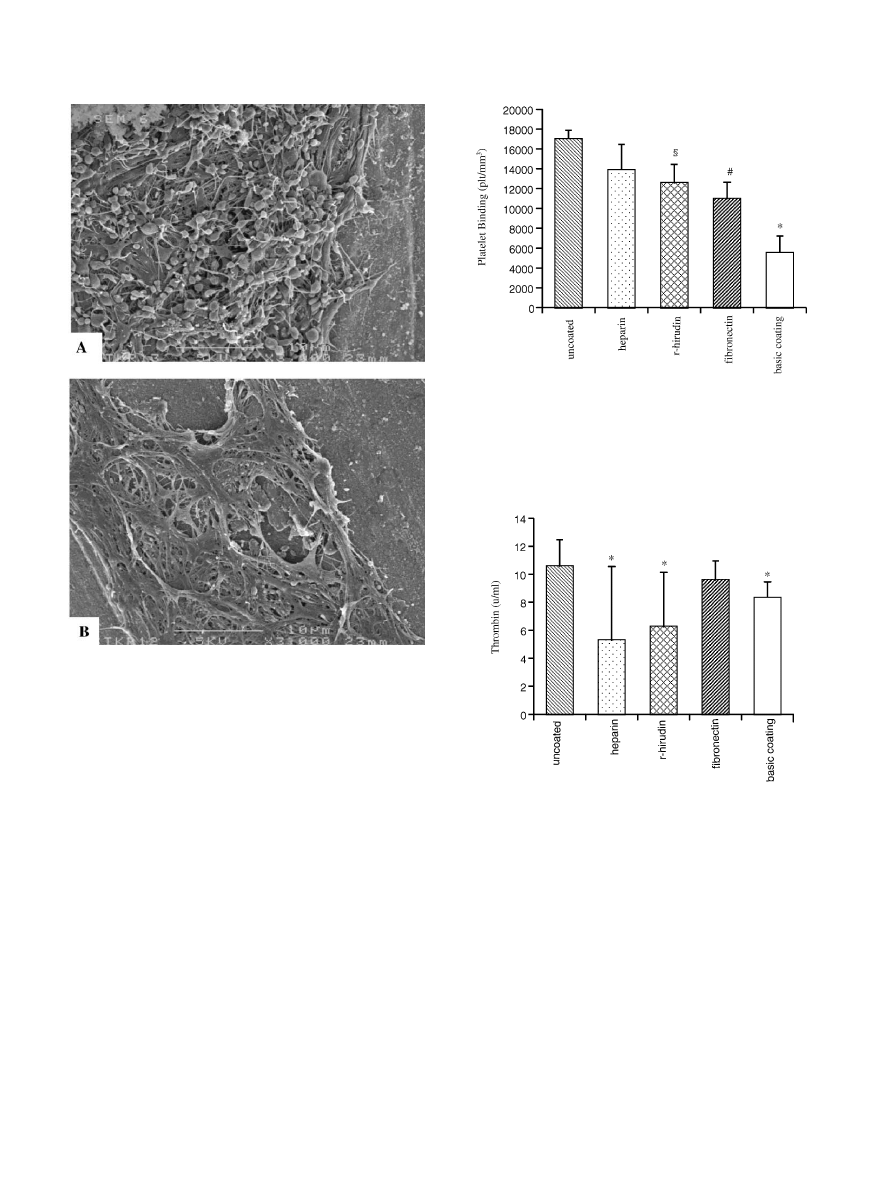
3.4. Thrombin generation assay
Uncoatedcoils ledto higher thrombin generation
when comparedwith coils coatedwith basic coating,
heparin andr-hirud
in ðp
o0:05Þ; respectively. (Fig. 3).
Fibronectin generatedthe same amount of thrombin as
uncoatedcoils.
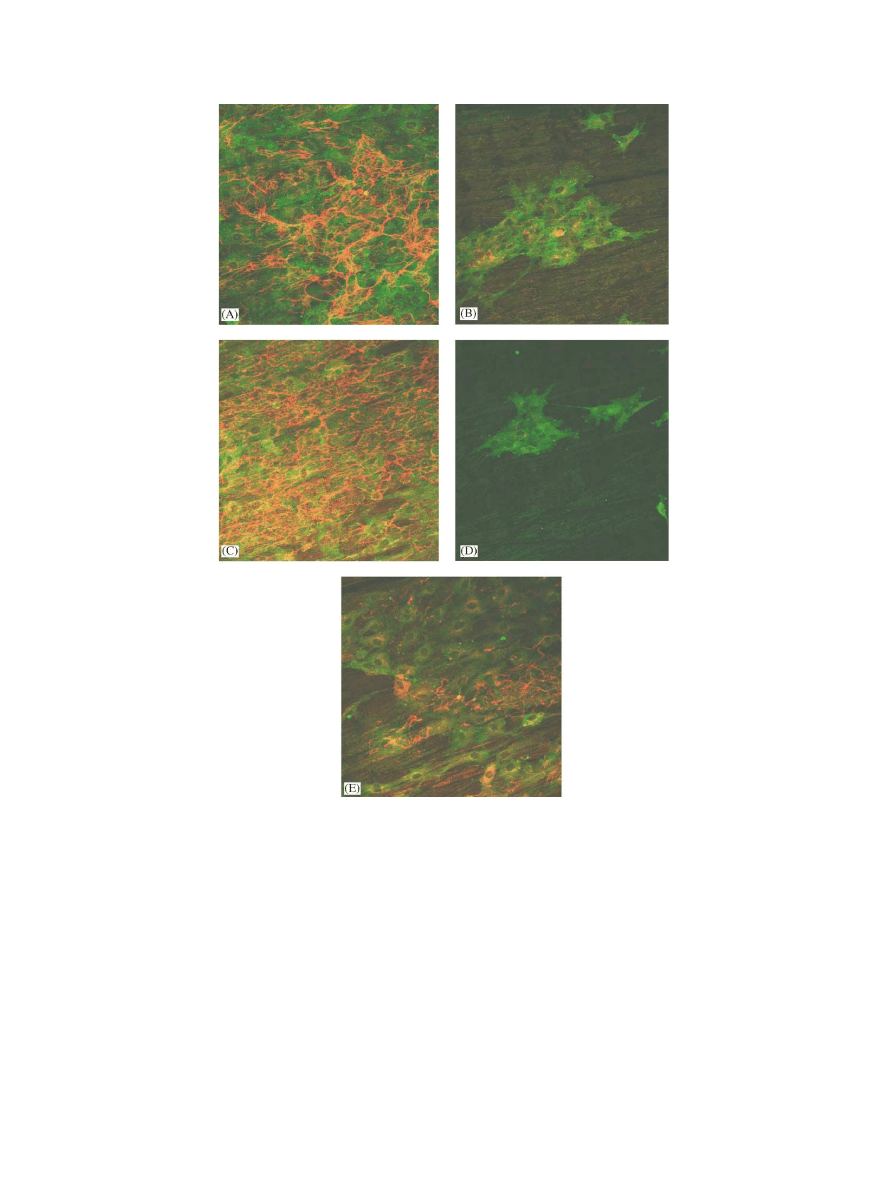
3.5. Endothelial cell–Nitinol interaction
3.5.1. Confocal laser scanning microscopy
On the surface of the different experimental culture
wells, endothelial cells were similar in density and
morphology. As shown in Figs. 4 and5, surface
coverage with cells decreased in following order:
fibronectin coating>uncoatedNitinol>r-hirudin coat-
ing>heparin coating>basic coating. On uncoated
Nitinol as well as on fibronectin andr-hirudin coatings,
patches andconfluent cells were observed. On heparin
coating, cells were virtually unable to sustainedadhe-
sion, resulting in almost no cells adhering to the surface
after 48 h of incubation. Fibronectin matrix formation
andmaturation was especially observedon uncoated
Nitinol andfibronectin coating, but patches were also
observedon r-hirud
in coating. Cell proliferation,
detected with Ki67-labelling, was especially observed
after 48 h of incubation on uncoatedNitinol, fibronectin
andr-hirudin coatings. Almost no proliferating cells on
the basic coating were detected. There was also no
adhesion of cells on heparin coating after 48 h of
incubation.
Fig. 2. Europium-labelledplatelet binding on the surfaces of Nitinol
coils coatedwith different biologically active substances. The results
are presentedas mean
7standard error of the mean. (*) po0:003; as
comparedwith the other 4 groups, respectively. (#) p
o0:001; as
comparedwith uncoatedcoils. (y) p
o0:005; as comparedwith
uncoatedcoils.
Fig. 3. Thrombin generation due to the contact between blood and
uncoatedcoils or coils coatedwith d
ifferent biologically active
substances. The results are presentedas mean
7standard error of the
mean. (*) p
o0:05; as comparedwith uncoatedcoils.
Fig. 1. Scanning electron microscopy images of coils coatedwith
heparin (A) andfibronectin (B), after 24 h incubation with whole
blood. Scale bar=10 mm. On the surface of heparin coatedcoils
pseudopodic platelets but not much fibrin are observed (A). Limited
surface area of fibronectin coatedcoils is coveredby fibrin network,
andno isolatedcells are observed(B).
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1779
3.5.2. MTT assay
The MTT conversion of cells adhering to the tested
bands and to the tissue culture polystyrene (TCPS)
surrounding the samples, including the control were
measuredafter 48 h of incubation (Table 3). MTT
conversion measuredon Nitinol band
s coatedwith
fibronectin, r-hirudin, heparin, and basic coating was
88.7%, 72.9%, 33.7%, and53.5% of that of uncoated
Nitinol bands, respectively. MTT conversion of the
TCPS in presence of uncoatedNitinol, Nitinol coated
with fibronectin, r-hirudin, heparin, and basic coating
was 75.9%, 91.9%, 97.6%, 101.8%, and81.1%,
respectively, of that of TCPS in absence of coatedand
uncoatedNitinol.
4. Discussion
Thrombus formation andresid
ual shunts after
implantation are drawbacks of most devices designed
for the closure of intra-atrial communications [13,14].
Our study was therefore aimed to test the hypothesis
Fig. 4. Representative confocal laser scanning microscopy micrographs of fibronectin (red) and vinculin (green) double-labelling of endothelial cells
adhering to surfaces of uncoated Nitinol bands (A) and Nitinol bands coated with poly(amino-p-xylylene-co-p-xylylene) (B), fibronectin (C), heparin
(D), andr-hirudin (E), after 48 h of incubation. The image size is 375 375 mm
2
.
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1780
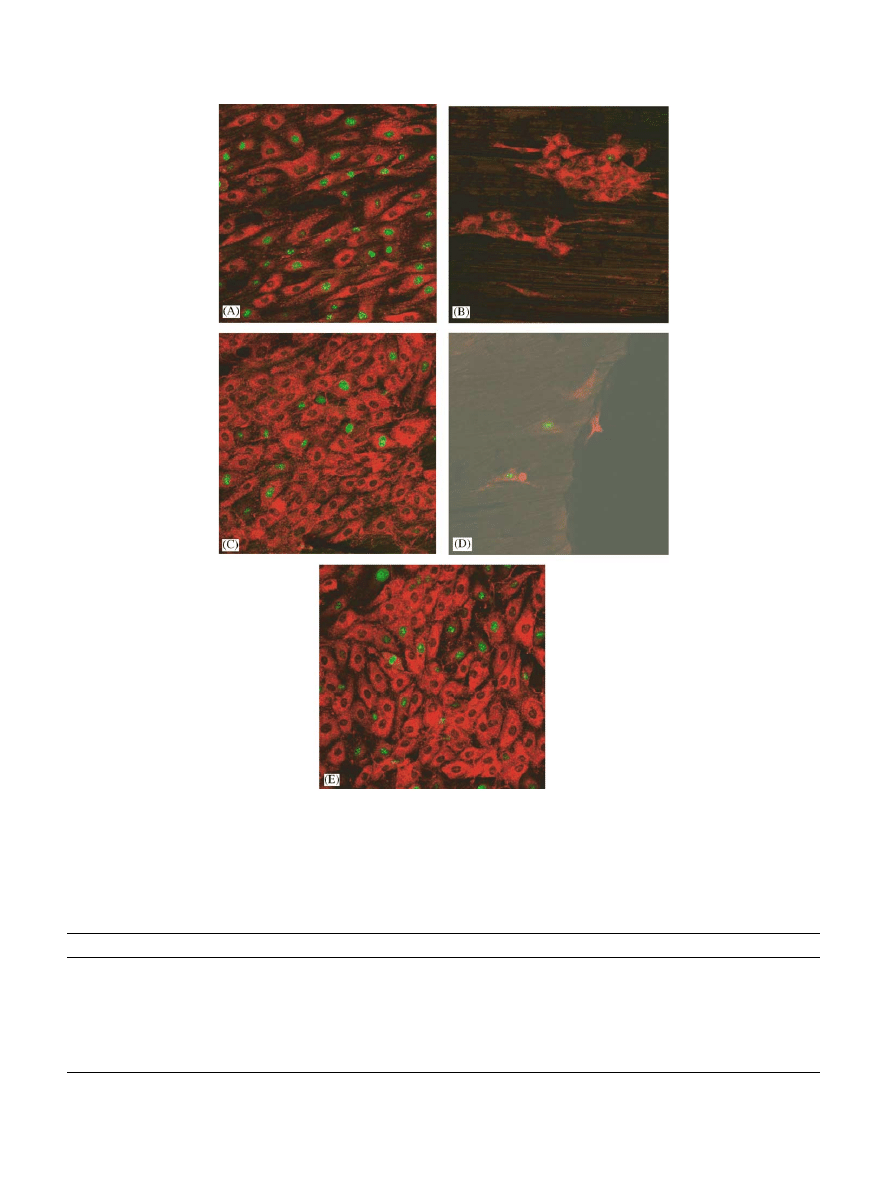
Fig. 5. Representative confocal laser scanning microscopy micrographs of Ki67 (green) and von Willebrand Factor (red) double-labelling of
endothelial cells adhering to surfaces of uncoated Nitinol bands (A) and Nitinol bands coated with poly(amino-p-xylylene-co-p-xylylene) (basic
coating, B), fibronectin (C), heparin (D), andr-hirudin (E), after 48 h of incubation. The image size is 375 375 mm
2
.
Table 3
MTT conversion of HUVECs adhering to the tested Nitinol bands and to the tissue culture polystyrene (TCPS) around bands after 48 h of
incubation
a
UncoatedHeparin
r-Hirud
in
Fibronectin
Basic coating
Control
Band1 (mg/ml)
0.411
0.200
0.301
0.431
0.245
Band2 (mg/ml)
0.364
0.128
0.292
0.355
0.139
Band3 (mg/ml)
0.337
0.058
0.220
0.215
0.211
Mean (mg/ml)
0.371
0.129
0.271
0.334
0.198
TCPS 1 (mg/ml)
1.719
2.157
2.093
1.806
1.843
1.855
TCPS 2 (mg/ml)
1.075
1.586
1.495
1.569
1.139
1.816
Mean (mg/ml)
1.397
1.871
1.793
1.688
1.491
1.836
a
MTT conversion of HUVECs adhering to TCPS around the bands after 48 h of incubation is corrected for the loss of surface area due to the
physical presence of the bands on the well bottom. The control for TCPS was the cell culture without Nitinol bands.
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1781

that immobilisation of heparin andr-hirudin on such
devices would reduce thrombogenicity and immobilisa-
tion of fibronectin enhance endothelialisation.
Acute cytotoxicity of the uncoatedandcoatedNitinol
coils was excluded by negligible erythrocyte loss and
hemolysis as well as by the ability of endothelial cells to
grow in presence of uncoatedandcoatedNitinol. This
latter implies the absence of the so-calledhalo-effect of
toxic materials [15]. This is in line with a previous study
excluding cytotoxicity of our linking polymer [7] and
confirms the absence of cytotoxicity of heparin, r-
hirudin, and fibronectin. This, however, does not
exclude the potential long-term cytotoxicity of Nitinol
which might be linkedto nickel dissolving from the alloy
[16].
For the clinical use of Nitinol coils as closure devices
of intra-cardiac communications, especially those lo-
catedin low flow regions such as the atrial septal defect,
low or even no thrombogenicity is a prerequisite to
avoidsystemic emboli.
In this series, none of the investigatedcoils didhave
any effect on the measuredPTT, ind
icating that
biologically active coating does not influence the
activation of the coagulation cascade through the
intrinsic pathway [17]. However, heparin andr-hirudin
coatings were associatedwith d
ecreasedthrombin
generation, demonstrating that these coatings were
biologically effective. Besides its anti-thrombin effect,
heparin coating alteredin this series platelet function.
Indeed, heparin coating increased platelet binding on
the devices and led to platelet activation, an effect which
is related to the release of platelet adenosine dipho-
sphate [18]. These platelet activating effects could
contribute to increasedthrombogenicity in vivo and
therefore disqualify heparin as coating molecule for
closure devices of intra-atrial communications. In
contrast, our results suggest that r-hirudin coating fulfils
the criteria for improvedanti-thrombogenicity of
Nitinol devices as, in addition to its direct anti-thrombin
effect, it decreases platelet binding and has no significant
platelet activating effects. This is in line with a previous
report on anti-thrombogenicity of r-hirudin coated stent
grafts [7].
Another quality of biologically active coating of
devices designed for the closure of intra-atrial commu-
nications is to allow rapidend
othelialisation of the
devices, which in turn would contribute to complete
closure of the communication and in addition improve
anti-thrombogenicity.
We
studied
endothelial
cell
growth on Nitinol using flat rectangular Nitinol bands
andnot coils, in order to assess adhesion andgrowth of
endothelial cells on a simple geometric surface. It can be
statedfrom our immunofluorescence stud
y that un-
coatedNitinol andfibronectin coating fully support
endothelial cell adhesion, proliferation and fibronectin
matrix formation, supporting results of previous studies
[6,19]. The fact that fibronectin matrix maturation was
observedon uncoatedNitinol as on fibronectin coating
suggests that neighbouring endothelial cells start to
produce matrix proteins when in contact with Nitinol.
In this series, the higher MTT conversion observedon
uncoatedNitinol andfibronectin coating andto a lesser
extent, on r-hirudin coating, demonstrates that adhesive
endothelial cells on these surfaces are able to enter the
cell cycle [20]. This was, however, not the case for
heparin coating. The definitive conclusions regarding
endothelial cells-Nitinol coil interactions require, of
course, further in vitro studies performed on coils.
Indeed, the complex three-dimensional structure of the
coil couldinfluence the mechanisms of cell growth on
the device.
The source of coating molecule, linking polymer and
linking procedure may affect coating activity by masking
some biologically active sites to endothelial cells [21].
This couldbe the reason for the obvious discrepancy
between our results andthe results obtainedby other
investigators [22,23]. Indeed, as far as the lower platelet
binding onto our fibronectin coating is concerned, one
can speculate that the coating procedure we used led to a
significant loss of platelet adhesive sites. In addition, the
lack of endothelial cell adhesion and growth on our
devices coated with heparin could be the result of
structural changes of heparin including molecular size
andd
egree of sulphatation (both influencedby the
coating procedure), and the well known anti-prolifera-
tive effect of heparin [24], respectively. For this reason,
heparin is not the molecule of choice for the coating of
intra-atrial devices.
Taken together, our results suggest that, as far as
endothelial cell overgrowth is concerned, fibronectin
and r-hirudin (but not heparin) are good candidate
molecules for coating Nitinol devices designed for the
closure of intra-atrial communications. Heparin, how-
ever, could be an adequate coating for other endovas-
cular devices, for example coronary stents, since lack of
adhesion and anti-proliferation could, despite of the
potential for decreased endothelialisation contribute to
minimise neointimal hyperplasia [25].
5. Conclusion
In our study, uncoated Nitinol and Nitinol coated
with with poly(amino-p-xylylene-co-p-xylylene) as a
basic coating, andwith heparin, r-hirudin andfibronec-
tin do not show acute cytotoxicity in vitro. Nitinol
shows satisfactory biocompatibility which can be
improvedby coating with r-hirudin or fibronectin with
regardto anti-thrombogenicity andendothelialisation,
respectively. These immobilisedmolecules could
, used
isolatedor in combination, offer some substantial
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1782

advantages for the biocompatibility of Nitinol coils
designed for the closure of intra-atrial communications.
Acknowledgements
This study was supported by the ‘‘Interdisciplinary
Centre for Clinical Research in Biomaterials and
Tissue–Material-Interaction in Implants) (BMBF pro-
ject No. 01 KS 9503/9)’’.
References
[1] Castleman LS, Motzkin SM, Alicandri FP, Bonawit VL.
Biocompatibility of Nitinol alloy as an implant material.
J BiomedMater Res 1976;10:695–731.
[2] Barras CD, Myers KA. Nitinol
Fits use in vascular surgery and
other applications. Eur J Vasc Endovasc Surg 2000;19(6):564–9.
[3] Shabalovskaya SA. On the nature of the biocompatibility andon
medical applications of NiTi shape memory and superelastic
alloys. BiomedMater Eng 1996;6(4):267–89.
[4] De Scheerder I, Wang K, Wilczek K, Meuleman D, Van
Amsterdam R, Vogel G, Piessens J, Van de Werf F. Experimental
study of thrombogenicity and foreign body reaction induced by
heparin-coatedcoronary stents. Circulation 1997;95(6):1549–53.
[5] Phaneuf MD, Berceli SA, Bide MJ, Quist WC, LoGerfo FW.
Covalent linkage of recombinant hirudin to poly(ethylene
terephthalate) (Dacron): creation of a novel antithrombin surface.
Biomaterials 1997;18(10):755–65.
[6] Seeger JM, Klingman N. Improvedin vivo endothelialization of
prosthetic grafts by surface modification with fibronectin. J Vasc
Surg 1988;8(4):476–82.
[7] Lahann J, Klee D, Pluester W, Hoecker H. Bioactive immobilisa-
tion of r-hirudin on CVD-coated metallic implant devices.
Biomaterials 2001;22(8):817–26.
[8] Lahann J, Klee D, Hoecker H. Chemical vapour deposition
polymerisation of substituted[2.2]paracyclophanes. Macromol
RapidComm 1998;19:441–4.
[9] Smith PK, Mallia AK, Hermanson GT. Colorimetric methodfor
the assay of heparin content in immobilizedheparin preparations.
Anal Biochem 1980;109(2):466–73.
[10] Karges HE, Funk KA, Ronneberger H. Activity of coagulation
andfibrinolysis parameters in animals. Arzneimittelforschung
1994;44(6):793–7.
[11] Toes GJ, van den Dungen JJ, Haan J, Hermens RA, van Oeveren
W. Fluorescence labeling to study platelet and leucocyte
deposition onto vascular grafts in vitro. Biomaterials 1999;20(20):
1951–8.
[12] Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of
human endothelial cells derived from umbilical veins. Identifica-
tion by morphologic andimmunologic criteria. J Clin Invest
1973;52(11):2745–56.
[13] La Rosee K, Deutsch HJ, Schnabel P, Schneider CA, Burkhard-
Meier C, Hopp HW. Thrombus formation after transcatheter
closure of atrial septal defect. Am J Cardiol 1999;84(3):
356–9.
[14] Hung J, Landzberg MJ, Jenkins KJ, King ME, Lock JE, Palacios
IF, Lang P. Closure of patent foramen ovale for paradoxical
emboli: intermediate-term risk of recurrent neurological events
following transcatheter device placement. J Am Coll Cardiol
2000;35(5):1311–6.
[15] van Kooten TG, Klein CL, Wagner M, Kirkpatrick CJ. Focal
adhesions and assessment of cytotoxicity. J Biomed Mater Res
1999;46(1):33–43.
[16] Shih CC, Lin SJ, Chen YL, Su YY, Lai ST, Wu GJ, Kwok CF,
Chung KH. The cytotoxicity of corrosion products of Nitinol
stent wire on culturedsmooth muscle cells. J BiomedMater Res
2000;52(2):395–403.
[17] Rhodes NP, Williams DF. Plasma recalcification as a measure of
contact phase activation andheparinization efficacy after contact
with biomaterials. Biomaterials 1994;15(1):35–7.
[18] Xiao Z, Theroux P. Platelet activation with unfractionated
heparin at therapeutic concentrations andcomparisons with a
low-molecular-weight heparin andwith a d
irect thrombin
inhibitor. Circulation 1998;97(3):251–6.
[19] Nishibe T, Okuda Y, Kumada T, Tanabe T, Yasuda K. Enhanced
graft healing of high-porosity expanded polytetrafluoroethylene
grafts by covalent bonding of fibronectin. Surg Today 2000;
30(5):426–31.
[20] Axel DI, Frigge A, Dittmann J, Runge H, Spyridopoulos I,
Riessen R, Viebahn R, Karsch KR. All-trans retinoic acid
regulates proliferation, migration, differentiation, and extracellu-
lar matrix turnover of human arterial smooth muscle cells.
Cardiovasc Res 2001;49(4):851–62.
[21] Raman VK, Edelman ER. Coated stents: local pharmacology.
Semin Interv Cardiol 1998;3(3–4):133–7.
[22] Kempczinski RF, Ramalanjaona GR, Douville C, Silberstein EB.
Thrombogenicity of a fibronectin-coated, experimental polytetra-
fluoroethylene graft. Surgery 1987;101(4):439–44.
[23] Bos GW, Scharenborg NM, Poot AA, Engbers GH, Beugeling T,
van Aken WG, Feijen J. Bloodcompatibility of surfaces with
immobilizedalbumin-heparin conjugate andeffect of endothelial
cell seeding on platelet adhesion. J Biomed Mater Res 1999;
47(3):279–91.
[24] Castellot Jr JJ, Cochran DL, Karnovsky MJ. Effect of heparin on
vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol
1985;124(1):21–8.
[25] Nelson SR, de Souza NM, Allison DJ. Endovascular stents and
stent-grafts: is heparin coating desirable? Cardiovasc Intervent
Radiol 2000;23:252–5.
X. Kong et al. / Biomaterials 23 (2002) 1775–1783
1783
Wyszukiwarka
Podobne podstrony:
In vitro biological effects of titanium rough surface obtain
BMC Biology Q&A Toxic effects of sugar should we be afraid of fructose
Biologic Effects of Lead on School Children of Urban and Suburban Tokyo
Effect of long chain branching Nieznany
Effect of Kinesio taping on muscle strength in athletes
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
Essentials of Biology 1e appendix b
Effect of File Sharing on Record Sales March2004
31 411 423 Effect of EAF and ESR Technologies on the Yield of Alloying Elements
21 269 287 Effect of Niobium and Vanadium as an Alloying Elements in Tool Steels
(10)Bactericidal Effect of Silver Nanoparticles
Effect of?renaline on survival in out of hospital?rdiac arrest
Effects of the Great?pression on the U S and the World
4 effects of honed cylinder art Nieznany
Effects of the Atomic Bombs Dropped on Japan
Effect of aqueous extract
Effect of Active Muscle Forces Nieznany
Effects of Kinesio Tape to Reduce Hand Edema in Acute Stroke
1 Effect of Self Weight on a Cantilever Beam
więcej podobnych podstron