
Building Collagen Molecules, Fibrils, and Suprafibrillar Structures
David J. S. Hulmes
Institut de Biologie et Chimie des Prote´ines, CNRS UMR 5086, Lyon, France
Received November 29, 2001
Fibril-forming collagens are synthesized in pre-
cursor form, procollagens, with N- and C-terminal
propeptide extensions. The C-propeptides direct
chain association during intracellular assembly of
the procollagen molecule from its three constituent
polypeptide chains. Following or during secretion
into the extracellular matrix, propeptides are cleaved
by specific procollagen proteinases, thereby trigger-
ing fibril formation. The recent determination of the
low-resolution structure of the C-propeptide trimer
gives insights into the mechanism of procollagen
chain association. In the extracellular matrix, the
procollagen C-propeptides ensure procollagen solu-
bility, while persistence of the N-propeptides controls
fibril shape. Mechanisms for the control of fibril diam-
eter are reviewed in terms of the radial packing
model for collagen fibril structure. Finally, procolla-
gen molecules have recently been shown to undergo
liquid crystalline ordering in solution, prior to fibril
assembly. This may provide an explanation for the
liquid crystal-like suprafibrillar architectures of dif-
ferent connective tissues.
© 2002 Elsevier Science (USA)
Key Words: collagen; procollagen; folding; assem-
bly; fibril; liquid crystal.
INTRODUCTION
Collagens are the main structural proteins re-
sponsible for the structural integrity of vertebrates
and many other multicellular organisms (Kadler,
1995; Brown and Timpl, 1995; Ricard-Blum et al.,
2000; Myllyharju and Kivirikko, 2001). In tissues
such as skin, tendons, bone, and cartilage, collagen
fibrils, each with a characteristic D (65– 67 nm) pe-
riodicity, provide resistance to tensile stress. De-
pending on the tissue, fibrils are arranged with dif-
ferent
suprafibrillar
architectures
and
with
diameters of up to 500 nm (Parry and Craig, 1984).
Small-diameter fibrils (
⬃20 nm) are found in carti-
lage and also in cornea, where in the latter the
highly ordered arrangement of fibrils within orthog-
onal lamellae is essential for optical transparency.
All fibrillar collagens are synthesized and secreted
into the extracellular matrix in the form of soluble
precursors, procollagens. Proteolytic processing of
N- and C-terminal propeptides by specific procolla-
gen N- and C-proteinases leads to the production of
mature collagen molecules which then spontane-
ously assemble into fibrils (Kadler et al., 1996).
Fibril-forming collagens (types I, II, III, V, and XI;
Fig. 1a) account for only 5 of more than 20 different
genetic types of collagen known to occur in humans
(Myllyharju and Kivirikko, 2001; Kadler, 1995). Other
collagens form networks (types IV, VIII, and X), asso-
ciate with fibril surfaces (types IX, XII, and XIV), occur
as transmembrane proteins (types XIII and XVII), or
form 11-nm periodic beaded filaments (type VI)
(Ricard-Blum et al., 2000). All collagens are modular
proteins consisting of three polypeptide chains with at
least one stretch of triple helix. Nontriple-helical re-
gions can be short (e.g., the N- and C-terminal telopep-
tides of the fibril-forming collagens) or can include
large structural domains (e.g., fibronectin type III re-
peats) to the extent that in some collagens the triple-
helical region is a relatively minor component (e.g.,
types XII and XIV). The collagen superfamily also
includes molecules involved in, for example, the im-
mune response (e.g., C1q, mannan binding protein,
and other collectins) and neurotransmission (acetyl-
cholinesterase) (Hulmes, 1992).
Here we take a structural view of the different
levels of collagen assembly, with particular atten-
tion to the fibrillar collagens. We discuss how the
C-propeptide domain might direct chain association
during intracellular assembly of the procollagen
molecule. We then discuss the role of N-propeptide
domains, as well as interactions between different
collagen types and with other components of the
extracellular matrix, in the control of extracellular
fibril formation. Finally we review recent data which
point to the novel hypothesis that the suprafibrillar
architecture of collagen-rich tissues is determined
by liquid crystalline association of procollagen mol-
ecules prior to fibril assembly.
Journal of Structural Biology 137, 2–10 (2002)
doi:10.1006/jsbi.2002.4450
2
1047-8477/02 $35.00
© 2002 Elsevier Science (USA)
All rights reserved.
BUILDING MOLECULES
Each procollagen molecule assembles within the
rough endoplasmic reticulum from its three constit-
uent polypeptide chains (Lamande and Bateman,
1999; McLaughlin and Bulleid, 1998). Depending on
the collagen type, chains can be either identical (e.g.,
three
␣1(III) chains as in type III collagen) or differ-
ent (e.g., two
␣1(I) chains and one ␣2(I) chain as in
type I collagen). This leads to the question of how
correct chain stoichiometry is ensured, particularly
in cells producing more than one collagen type. Nu-
merous studies have shown that it is the C-propep-
tide of the procollagen molecule that determines
chain selection. Newly synthesized procollagen
chains associate into trimers via their C-propep-
tides, leading to nucleation and folding of the triple-
helical region in a zipper-like manner from the C- to
the N-terminus (Engel and Prockop, 1991). The C-
propeptide domains of the fibrillar procollagens
(each
⬃245 amino acid residues in length) are highly
conserved, but recently Bulleid and colleagues (see
Lees et al., 1997) have identified a discontinuous
variable sequence of 15 residues, known as the chain
recognition region, which appears to determine
chain stoichiometry (Fig. 1b). If, for example, the
chain recognition region from type III procollagen is
exchanged for the equivalent region of the pro
␣2(I)
chain,
the
resulting
hybrid
pro
␣2(I)/pro␣1(III)
chains can form homotrimers, while parent pro
␣2(I)
chains cannot. Pro
␣2(I) chains assemble only in the
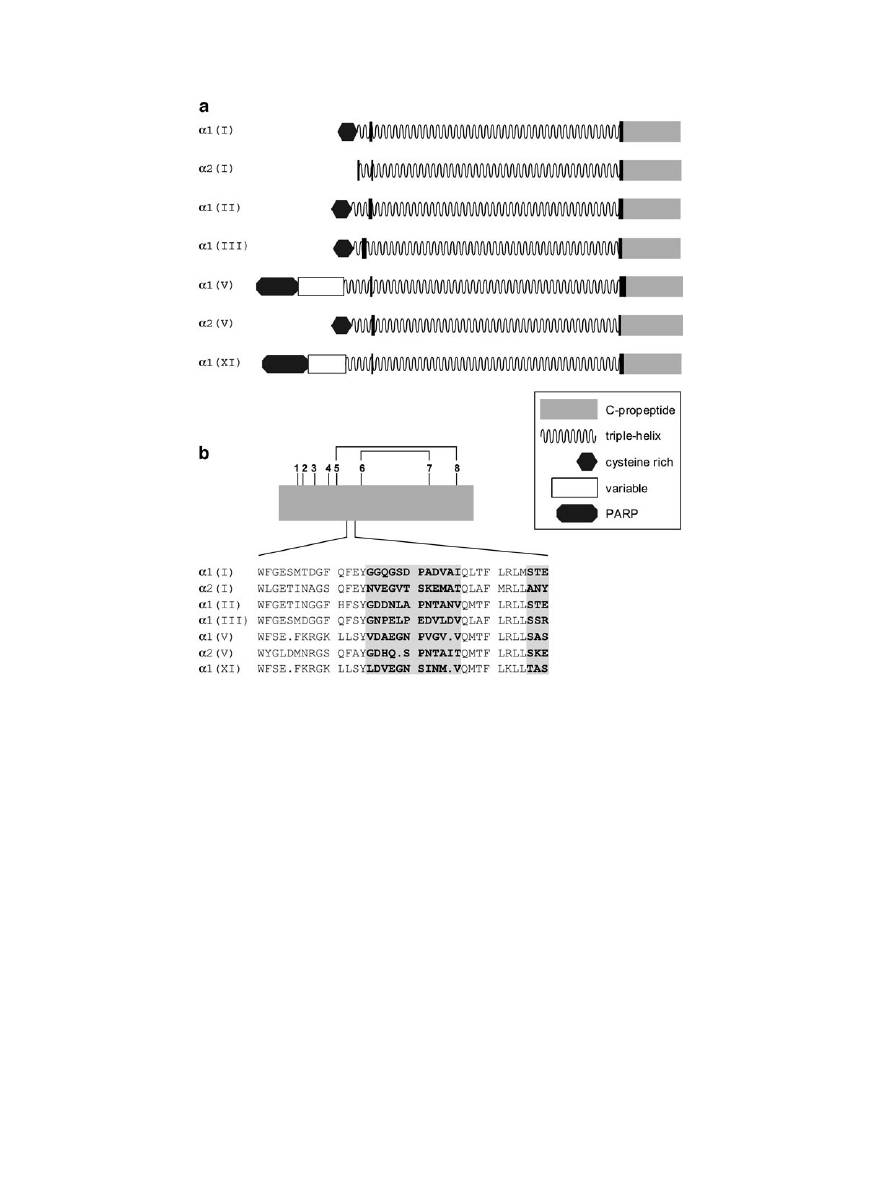
FIG. 1.
Fibrillar collagens and their C-propeptide domains. (a) Schematic representation of some of the
␣ polypeptide chains making
up the procollagen precursors of the fibrillar collagens (types I, II, III, V, and XI), showing the different structural domains (based on
Brown and Timpl, 1995). (b) Enlarged view of the C-propeptide domain of procollagen type III chains, showing the location of cysteine
residues 1 to 8, internal disulfide bonds, and a sequence comparison with other C-propeptide domains in the region of the discontinuous
chain recognition sequence (highlighted) (based on McLaughlin and Bulleid, 1998).
3
BUILDING COLLAGEN ASSEMBLIES
presence of two pro
␣1(I) chains to form heterotrim-
ers.
In order to obtain three-dimensional structural
information on the C-propeptide region of the pro-
collagen molecule, we have recently carried out a
biophysical characterization of the recombinant C-
propeptide trimer from human type III procollagen
(Bernocco et al., 2002). By analytical ultracentrifu-
gation, as well as static and dynamic light scatter-
ing, the trimer (90 kDa) behaves as an elongated
molecule. This is backed up by small-angle X-ray
scattering, where the radial distribution function
p(r) indicates a maximum interatomic distance ap-
proximately double that expected for a sphere of the
same molecular mass. Model fitting to the X-ray
scattering data using both spherical harmonics and
a recently devised genetic algorithm point to a low-
resolution cruciform structure with three large lobes
and one small lobe (Fig. 2a).
The structure of the C-propeptide trimer is readily
interpretable in terms of the subunit composition
and known positions of inter- and intrachain disul-
fide bonds. Among the 8 cysteines found in the C-
propeptide domains of the three polypeptide chains
of type III procollagen (Fig. 1b), cysteines 1 to 4 are
involved in interchain disulfide bonding, while cys-
teines 5 to 8 form intrachain disulfide bonds (Lees
and Bulleid, 1994). The simplest interpretation of
the model derived from small-angle X-ray scattering
is therefore that each of the three large lobes corre-
sponds to the intrachain disulfide bonded region of
each of the three polypeptide chains, while the small
lobe corresponds to the junction region containing
the interchain disulfide bonds and linking to the rest
of the procollagen molecule (Fig. 2b). This would
place the chain recognition region at the core of the
structure, well placed to determine chain– chain in-
teraction specificity. While conformation of this
model must await crystal structure data, it is a
striking example of the power of recently developed
algorithms for interpreting small-angle X-ray scat-
tering information.
Recent data from X-ray fiber diffraction on the
axial structure of the C-terminal telopeptide region
(the short nontriple-helical region that remains fol-
lowing cleavage of the C-propeptide) indicate a hair-
pin conformation with the C-terminus folded back
onto the triple-helix (Orgel et al., 2000). If so, this
would require another hairpin loop in the propep-
tide–telopeptide junction in order for the propeptide
to project beyond the end of the molecule. Alterna-
tively, the telopeptide might fold back on itself fol-
lowing cleavage from the propeptide. It will be im-
portant in the future to determine the structure of
the telopeptide–propeptide junction in order to un-
derstand the mechanism of action of procollagen
C-proteinase.
BUILDING FIBRILS
Following or during secretion of procollagen mol-
ecules into the extracellular matrix, propeptides are
removed by procollagen N- and C-proteinases,
thereby triggering spontaneous self-assembly of col-
lagen molecules into fibrils (Kadler et al., 1996;
Prockop and Hulmes, 1994). As long as the
C-propeptide remains attached to the rest of the
molecule, solubility remains high. Thus a major ex-
tracellular function of the C-propeptides is to pre-
vent fibril formation. In contrast, persistence of the
N-propeptide does not prevent fibril formation,
though it does influence fibril shape and diameter.
While the C-propeptide domains of the fibrillar
procollagens are highly conserved, much greater
variability is seen in the N-propeptides (Fig. 1a)
(Brown and Timpl, 1995). All N-propeptide regions
include a triple-helical-forming domain which pre-
cedes the N-terminal telopeptide (the short non-
triple-helical region that remains following cleavage
by procollagen N-proteinase). In addition, most N-
propeptides contain large nontriple-helical domains
at their N-termini. In the case of type V and XI
procollagens, the N-propeptides of the
␣1 chains are
particularly large and begin with a proline- and
arginine-rich (PARP) domain followed by a variable
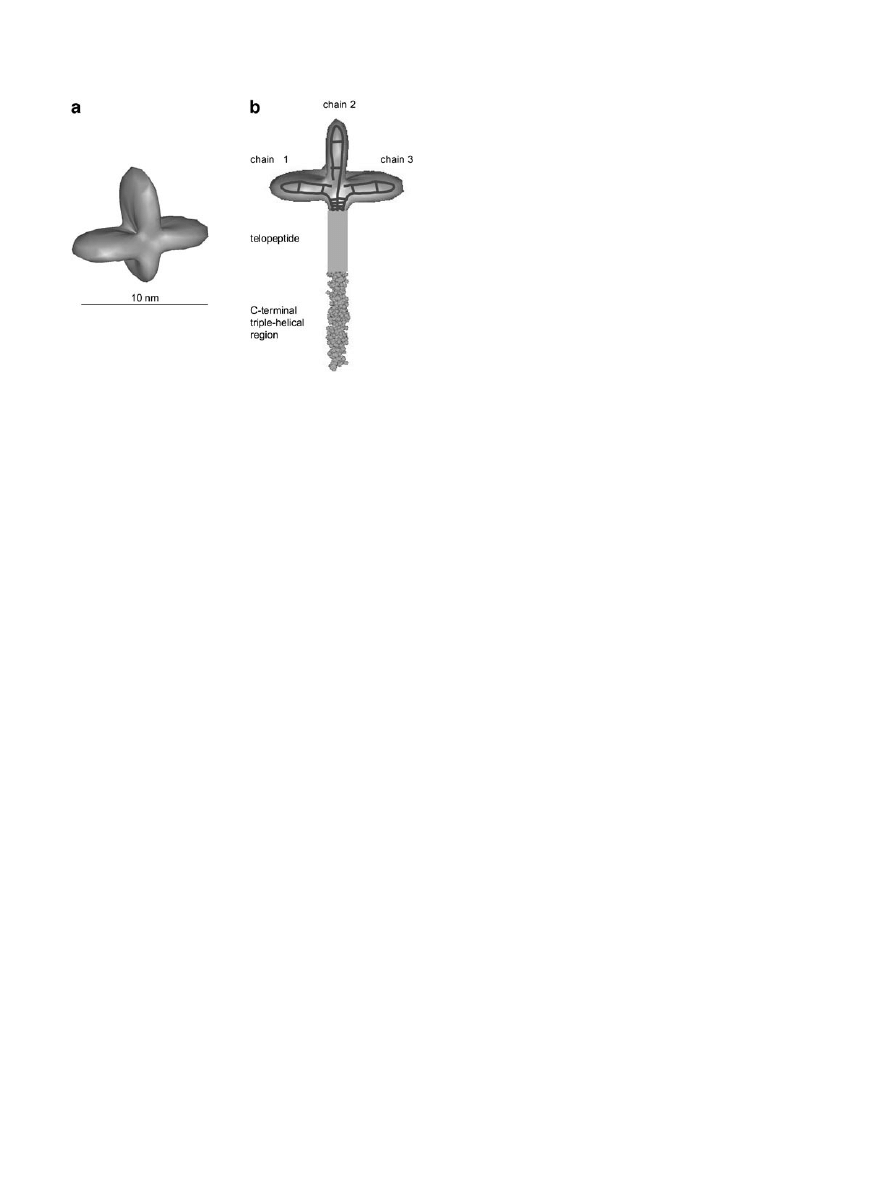
FIG. 2.
Three-dimensional structure of the procollagen III
C-propeptide trimer and its junction with the rest of the procol-
lagen molecule. (a) Low-resolution structure derived from small-
angle X-ray scattering of the recombinant human procollagen III
C-propeptide trimer. (b) Possible structure of the C-terminal re-
gion of the procollagen III molecule showing each major lobe of
the C-propeptide structure corresponding to one of the three
polypeptide chains. Superimposed on each lobe is a schematic
representation of the possible locations of both inter- and intra-
chain disulfide bonds (based on Bernocco et al., 2002).
4
DAVID J. S. HULMES
domain which in type XI can present different se-
quences as a result of alternative splicing (Fichard et
al., 1994; Oxford et al., 1995; Zhidkova et al., 1995).
Procollagens I, II, and III are cleaved at the junction
of the N propeptide triple-helix and the telopeptide,
by collagen type-specific procollagen N-proteinases
(Prockop et al., 1998; Cal et al., 2001). Normally,
N-terminal cleavage of types I and II procollagens is
rapid, while type III procollagen N-terminal process-
ing is relatively slow. Delayed N-terminal process-
ing leads to the accumulation of a partially pro-
cessed form of procollagen, called pN-collagen,
which lacks the C-propeptides but retains the N-
propeptides. N-terminal processing of procollagens
V and XI is more complex, involving a number of
different cleavage sites and leading to several pro-
cessing intermediates (Moradi-Ame´li et al., 1994,
1998; Rousseau et al., 1996). Frequently, processing
occurs at the end of the PARP region, leading to
mature collagen V and XI molecules that retain the
additional short triple-helix plus the variable region
(Fig. 1a).
To understand the role of the procollagen
N-propeptide domains in fibril formation, we briefly
review the current status of our understanding of
molecular packing in collagen fibrils. Experimental
data come from X-ray diffraction of tendon fibers
and indicate the presence of three-dimensional crys-
tallinity admixed with liquid-like lateral disorder
(Hulmes et al., 1995a). The lateral unit cell, which
contains five molecules in cross section, gives rise to
row lines with a maximum spacing of 3.8 nm (Orgel
et al., 2001). Electron microscopy of a transverse
section of tendon fibrils reveals a similar periodicity
(
⬃4 nm) oriented radially with respect to the fibril
center (Hulmes et al., 1985). Combining these data
results in a concentric model for the molecular pack-
ing (Fig. 3), which, following energy minimization,
shows elements of both order and disorder in the
molecular packing (Hulmes et al., 1995).
A feature of the model is that molecules are tilted
obliquely in a plane oriented at 30° to the fibril
surface (Hulmes et al., 1981; Orgel et al., 2001). This
results in the helicoidal organization of collagen
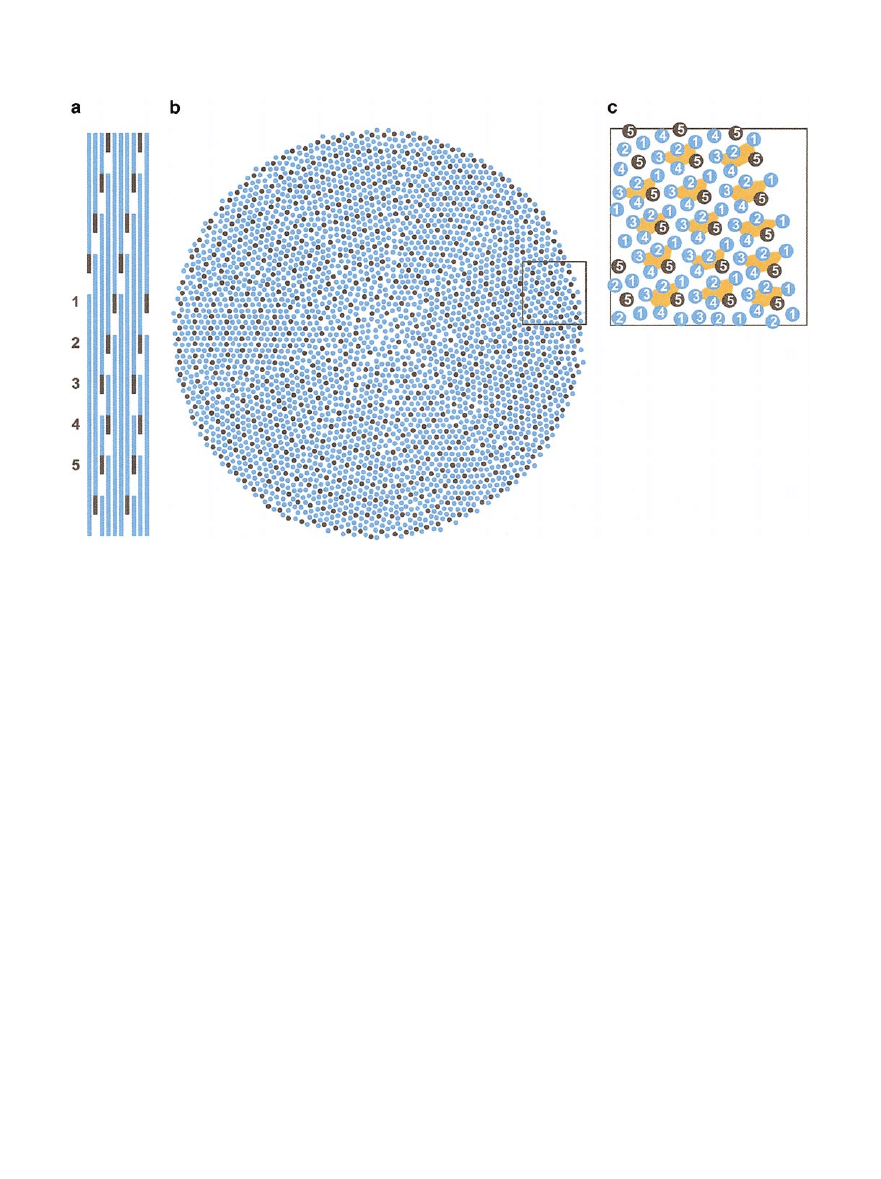
FIG. 3.
Molecular packing in collagen fibrils. (a) Longitudinal view of collagen molecules in D staggered array. Each molecule can be
considered as consisting of five molecular segments 1 to 5, of which the short section 5 is shown in black. (b) Transverse section of the
radial packing model (based on Hulmes et al., 1995) showing molecules in a section of thickness equal to the D repeat. Segments 5 (in
black) are arranged in concentric layers separated by a distance of
⬃4 nm. (c) Enlarged view of the boxed area in (b) showing molecules
grouped together in the form of microfibrils. Molecular segments are indicated in groups of five, corresponding to individual microfibrils
in transverse section (depicted in orange).
5
BUILDING COLLAGEN ASSEMBLIES
fibrils, which has been widely documented in the
literature (Ottani et al., 2001), particularly in recent
three-dimensional reconstructions of corneal colla-
gen fibrils (Holmes et al., 2001). A further feature of
the model (Fig. 3) is that the fibril surface is coated
in molecular ends (segments 1; Fig. 3a). This has
important consequences for fibril growth. For exam-
ple, persistence of the N-propeptide, as in type III
collagen, or limited processing, as in type V and XI
collagens, might prevent incorporation into the cen-
ter of the fibril, thereby forcing all N-termini to the
surface of the fibril and preventing further accretion
and limiting fibril diameter (Birk, 2001; Chapman,
1989; Linsenmayer et al., 1993). Since most collagen
fibrils are heterotypic (i.e., made up of different col-
lagen types), such as types I and III in skin, types I
and V in cornea, or types II and XI in cartilage, this
might provide a mechanism for diameter control by
heterotypic
collagen
interactions
(Birk,
2001;
Marchant et al., 1996; Blaschke et al., 2000; An-
drikopoulos et al., 1995; Li et al., 1995).
In order to test the steric blocking model for fibril
diameter control, the assembly of mixtures of colla-
gen and pN-collagen was studied using an in vitro
system in which the corresponding purified precur-
sors, pC-collagen and procollagen, respectively, were
cleaved with procollagen C-proteinase (Hulmes et
al., 1989) (Fig. 4). When the proportion of pN-colla-
gen was
⬃20% or less, cylindrical fibrils were formed
with diameters less than those formed by collagen
alone. As the proportion of pN-collagen increased to
⬃50%, however, fibrils took on an increasingly lob-
ular appearance in cross section, while retaining an
axial D (67 nm) periodicity. At
⬃75% pN-collagen,
fibril cross sections became stellate with frequent
branching and resembled those found in the skin of
sheep and cattle suffering from the genetic disorder
dermatosparaxis, where the skin becomes fragile
and hyperextensible as a result of a failure in N-
terminal procollagen processing. A similar condi-
tion, Ehlers-Danlos syndrome type VIIC, occurs in
humans (Colige et al., 1999). Finally, pN-collagen
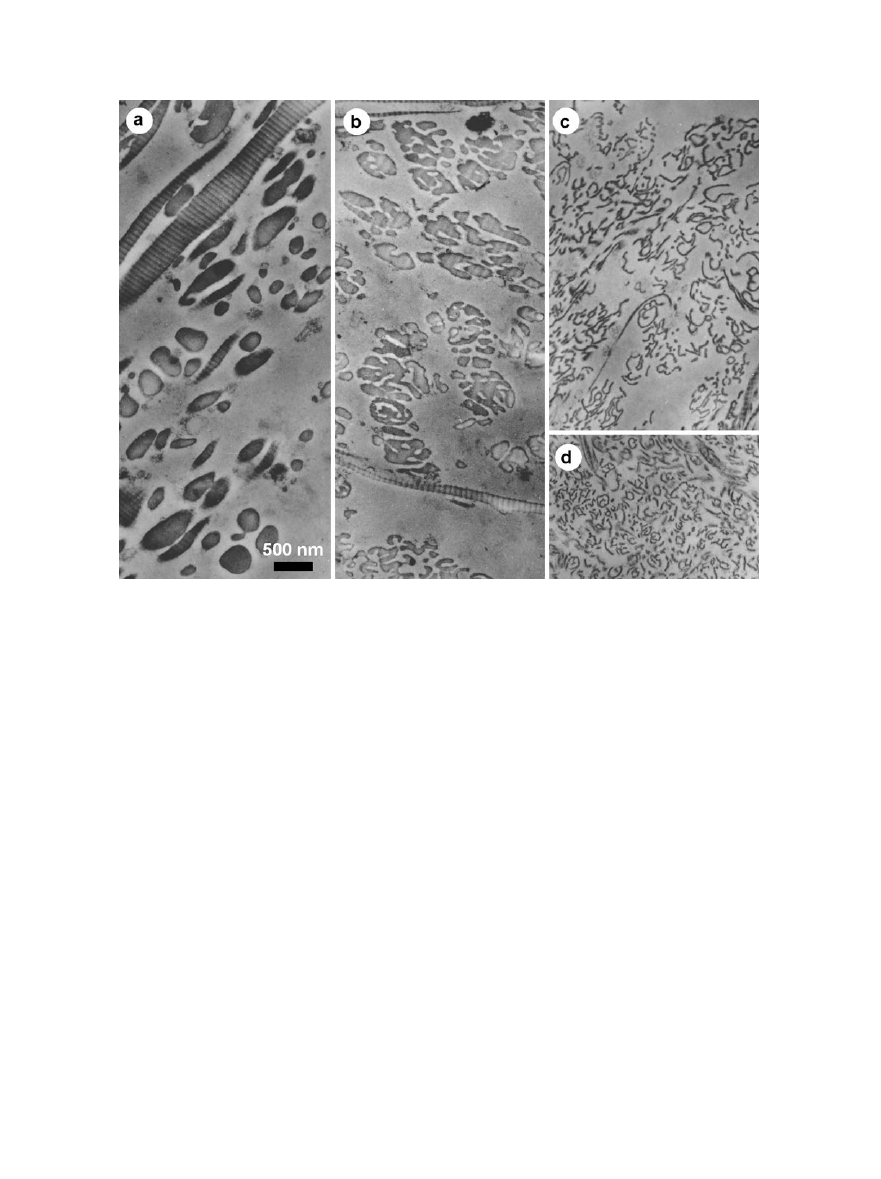
FIG. 4.
Pleomorphic forms of collagen fibrils produced in vitro and in vivo by persistence of the procollagen N-propeptide domain.
Mixtures of purified procollagen type 1 and pC-collagen type I (lacking the N-propeptide domain) were digested in vitro with procollagen
C-proteinase to produce mixtures of pN-collagen and collagen, respectively. Following spontaneous assembly, aggregated forms were
pelleted, sectioned and then observed by electron microscopy. (a) 18% pN-collagen; (b) 44% pN-collagen; (c) 68% pN-collagen; (d) section
of dermatosparactic skin showing abnormal fibrils as a result of procollagen N-proteinase deficiency. From Prockop and Hulmes (1994),
with permission.
6
DAVID J. S. HULMES

alone is capable of assembling in vitro to form D-
periodic structures, though in cross section these are
wide sheets (up to several micrometers) of thickness
11 nm (Hulmes et al., 1989). Thus, limited persis-
tence of the N-propeptide can limit fibril diameter,
while increasing the proportion of pN-collagen can
lead to a distortion of fibril shape from cylindrical to
sheet-like.
The large N-terminal extensions present in colla-
gens V and XI might play roles similar to that of the
N-propeptide in pN-collagen I. Collagens V and XI
are quantitatively minor collagens which occur in
heterotypic fibrils in association with collagens I and
II, respectively, where the proportion of the minor
collagen is about 10 –20%. By immunolocalization
using antibodies raised against different regions of
the type V molecule, for example, it has been shown
that while the central triple-helix is buried and re-
quires fibril disruption to expose hidden epitopes,
the large N-terminal region which remains after
procollagen processing is located on the surface (Lin-
senmayer et al., 1993). In contrast to pN-collagen I,
however, neither purified collagen V nor XI forms
sheet-like structures on its own (Blaschke et al.,
2000). Instead, these collagens aggregate into thin
(20 nm diameter) filaments, where the D periodicity
is difficult to discern. In both cases, thin filaments
and sheets, the surface to volume ratio is high, thus
allowing the N-terminal extensions to be located on
the surface. Why sheets should be favored over fila-
ments, or vice versa, however, is not clear.
The persistence of N-terminal extensions is not
the only way in which fibril diameters might be
controlled. Several in vitro and in vivo studies have
shown the importance of the leucine-rich repeat pro-
teoglycans, such as lumican (Chakravarti et al.,
1998), decorin (Danielson et al., 1997), and fibro-
modulin (Svensson et al., 1999), in limiting fibril
diameter, as well as fibril fusion (Graham et al.,
2000). Again the mechanisms involved are not clear,
but these are thought to involve specific binding
sites on collagen molecules exposed on the fibril
surface (Weber et al., 1996). Further factors, in the
case of heterotypic collagen fibrils, are properties
intrinsic to the triple-helical regions. This has been
shown in vitro with collagen V, for example, where
even after removal of the nontriple-helical regions
by pepsin treatment, the diameters of the hetero-
typic fibrils formed by collagen I:V mixtures de-
crease as the proportion of collagen V increases
(Adachi and Hayashi, 1986; Chanut-Delalande et
al., 2001). It is likely that at least part of the mech-
anism whereby collagen–proteoglycan interactions,
heterotypic collagen interactions, or interactions
with other extracellular matrix components can con-
trol fibril diameter is at the level of the initial nu-
cleation event in the early stages of fibril formation
(MacBeath et al., 1993).
Finally, this discussion of collagen fibril structure
would not be complete without considering the pos-
sible existence of the five-stranded Smith microfi-
bril. The microfibril is the minimum filamentous
structure (diameter approximately 4 nm) that pos-
sesses an axial D repeat. Attempts to visualize iso-
lated microfibrils have not been totally convincing,
though there is evidence of the existence of such a
structure in native fibrils by model fitting to X-ray
fiber diffraction data (Orgel et al., 2001). Further-
more, very recently three-dimensional image recon-
structions of 25-nm-diameter corneal collagen fibrils
show evidence of a 4-nm repeat in transverse sec-
tion, which might correspond to ordered arrays of
FIG. 5.
Liquid crystalline-like textures in connective tissues.
Polarized light microscopy of various connective tissues showing
cholesteric liquid crystalline-like textures of collagen fibrils in (a)
fish scale (from Giraud et al. (1978), with permission) and (b) bone
osteon (from Giraud-Guille (1994), with permission); (c) precho-
lesteric-like ordering in tendon (crimp structure); and (d) a com-
plex interweaving in the dermis of skin. Unpublished figures
courtesy of L. Bessau, R. Martin, and M. M. Giraud-Guille.
7
BUILDING COLLAGEN ASSEMBLIES

microfibrils, particularly at the level of the gap–
overlap junctions (Holmes et al., 2001). This is an
important observation which argues against other
models for the molecular packing in 25-nm fibrils
(Chapman, 1989; Blaschke et al., 2000) in which the
4-nm repeat is lacking. Thus a microfibrillar sub-
structure appears to be a common feature of both
large- and small-diameter fibrils.
BUILDING TISSUES
Different connective tissues are characterized not
only by differences in fibril diameter, but also by
tissue-specific suprafibrillar architectures, as seen
by polarized light microscopy (Giraud-Guille, 1996)
(Fig. 5). In fish scales or cornea, for example, fibrils
are arranged in multiple layers or lamellae. Within
each lamella, all fibrils are parallel (or anti-parallel).
Between adjacent lamellae, however, there is a con-
stant angular twist, often about 90°, which results
in a plywood-like arrangement. When viewed in
oblique section, fibrils in alternate lamellae map out
a series of ares. In bone osteons, again a lamellar
organization is seen, but here lamellae are arranged
in the form of concentric cylinders. In tendons,
fibrils are parallel or anti-parallel but subject to a
planar undulation, or crimp, on a scale of several
micrometers. Finally, in the dermis of skin, fibrils
take on a complex three-dimensional weave. It
should be noted that all these different suprafibril-
lar architectures are characterized by distances on
the scale of several micrometers, compared to 0.3
m for the length of a single collagen molecule.
As pointed out by Bouligand and Giraud-Guille
(see Giraud-Guille, 1996), the suprafibrillar archi-
tectures shown in Fig. 5 bear a striking resemblance
to different forms of liquid crystals. The lamellar
structure of fish scale or cornea, for example, corre-
sponds to a cholesteric organization, while the crimp
seen in tendons is analogous to a precholesteric ar-
rangement. However, connective tissues consist of
insoluble collagen fibrils and other matrix compo-
nents; they are not liquid-like as in true liquid crys-
tals. Thus the question arises of whether this resem-
blance to liquid crystals is significant and whether it
tells us something about the mechanisms by which
such suprafibrillar order might be generated.
Giraud-Guille (1996) has shown that collagen mol-
ecules in acetic acid solution at very high concentra-
tions (
⬎20 mg/ml) are capable of forming true liquid
crystals, both precholesteric and cholesteric, on a
scale of micrometers (Fig. 6). This organization is
liquid-like and can easily be disrupted by gentle
pressure. When these highly concentrated solutions
are exposed to ammonia vapors, however, in order to
neutralize the pH, collagen molecules spontaneously
assemble into 67-nm fibrils, while retaining the cho-
lesteric organization that existed prior to fibril as-
sembly. This suggests the hypothesis that the supra-
FIG. 6.
Liquid crystalline ordering of collagen and procollagen in vitro. (a) Solutions of collagen type I molecules at high concentra-
tions (
⬎20 mg/ml) in acetic acid spontaneously form cholesteric liquid crystals, as seen by polarized light microscopy (courtesy of R. Martin
and M. M. Giraud-Guille). (b) After exposure to ammonia vapors to increase the pH and trigger fibril assembly, molecules in (a)
spontaneously assemble into banded fibrils, observed by electron microscopy, while preserving the cholesteric order that existed prior to
fibril formation. From Bessau and Giraud-Guille (1995), with permission. (c– e) Precholesteric ordering of procollagen solutions at
physiological pH and ionic strength, observed by polarized light microscopy. The planar crimp-like arrangement is characterized by
periodic pairs of bands which fuse as the crossed polar (P) and analyzer (A) are rotated. From Martin et al. (2000), with permission.
8
DAVID J. S. HULMES

fibrillar architecture of connective tissues might
indeed be a result of liquid crystalline ordering of
soluble precursors. Collagen molecules are of course
not soluble under physiological buffer conditions,
and a criticism of the experiments done in acetic acid
is their relevance to the in vivo situation. Therefore,
since in vivo procollagen molecules are the soluble
precursors of fibrils, we recently examined the be-
havior of procollagen molecules in solution at high
concentrations in a physiological buffer (Martin et
al., 2000). At concentrations of
⬃10 mg/ml, we ob-
served that procollagen molecules can also form liq-
uid crystals, of a precholesteric type, corresponding
to the crimp seen in tendons (Fig. 6). Other forms of
precholesteric order were also observed, approach-
ing true cholesteric liquid crystals. These results
support the hypothesis that liquid crystalline order
in connective tissues might take place prior to enzy-
matic procollagen processing and deposition of insol-
uble fibrils. Once liquid crystalline order is estab-
lished, perhaps in the immediate vicinity of the cell
surface, procollagen processing would trigger fibril
formation to stabilize the preexisting suprafibrillar
architecture.
CONCLUSION
In conclusion, we have seen that procollagen mol-
ecules, and their various structural domains, have a
remarkable capacity to control all stages of collagen
assembly, from intracellular assembly of the procol-
lagen molecule, through control of fibril diameter
and shape (along with heterotypic collagen interac-
tions and interactions with other matrix compo-
nents), right up to suprafibrillar ordering on the
scale of micrometers. In vivo, of course, the situation
will be more complex, and we have some way to go
before we can claim to understand the mechanisms
that control the different levels of self-assembly in
tissues.
I thank the many friends and colleagues, too numerous to
mention, who have contributed to the original work reviewed
here. Research in the author’s laboratory is currently supported
by the Centre National de la Recherche Scientifique, the Univer-
site´ Claude Bernard Lyon I, and the Fondation pour la Recherche
Me´dicale.
REFERENCES
Adachi, E., and Hayashi, T. (1986) In vitro formation of hybrid
fibrils of type V collagen and type I collagen. Connect. Tissue
Res. 14, 257–266.
Andrikopoulos, K., Liu, X., Keene, D. R., Jaenisch, R., and
Ramirez, F. (1995) Targeted mutation in the col5a2 gene re-
veals a regulatory role for type V collagen during matrix as-
sembly. Nat. Genet. 9, 31–36.
Bernocco, S., Finet, S., Ebel, C., Eichenberger, D., Mazzorana, M.,
Farjanel, J., and Hulmes, D. J. S. (2001) Biophysical charac-
terization of the C-propeptide trimer from human procollagen
III reveals a tri-lobed structure. J. Biol. Chem. 276, 48930 –
48936.
Besseau, L., and Giraud-Guille, M. M. (1995) Stabilization of
cholesteric phases of collagen to ordered gelated matrices. J.
Mol. Biol. 251, 197–202.
Birk, D. E. (2001) Type V collagen: Heterotypic type I/V interac-
tions in the regulation of fibril assembly. Micron 32, 223–237.
Blaschke, U. K., Eikenberry, E. F., Hulmes, D. J. S., Galla, H. J.,
and Bruckner, P. (2000) Collagen XI nucleates self-assembly
and limits lateral growth of cartilage fibrils. J. Biol. Chem. 275,
10370 –10378.
Brown, J. C., and Timpl, R. (1995) The collagen superfamily. Int.
Arch. Allergy Immunol. 107, 484 – 490.
Cal, S., Arguelles, J. M., Fernandez, P. L., and Lopez-Otin, C.
(2001) Identification, characterization, and intracellular pro-
cessing of ADAM-TS12, a novel human disintegrin with a com-
plex
structural
organization
involving
multiple
throm-
bospondin-1 repeats. J. Biol. Chem. 276, 17932–17940.
Chakravarti, S., Magnuson, T., Lass, J. H., Jepsen, K. J., LaMan-
tia, C., and Carroll, H. (1998) Lumican regulates collagen fibril
assembly: Skin fragility and corneal opacity in the absence of
lumican. J. Cell Biol. 141, 1277–1286.
Chanut-Delalande, H., Fichard, A., Bernocco, S., Garrone, R.,
Hulmes, D. J., and Ruggiero, F. (2001) Control of heterotypic
fibril formation by collagen V is determined by chain stoichi-
ometry. J. Biol. Chem. 276, 24352–24359.
Chapman, J. A. (1989) The regulation of size and from in the
assembly of collagen fibrils in vivo. Biopolymers 28, 1367–1382.
Colige, A., Sieron, A. L., Li, S. W., Schwarze, U., Petty, E., Wer-
telecki, W., Wilcox, W., Krakow, D., Cohn, D. H., Reardon, W.,
Byers, P. H., Lapie`re, C. M., Prockop, D. J., and Nusgens, B. V.
(1999) Human Ehlers-Danlos syndrome type VIIC and bovine
dermatosparaxis are caused by mutations in the procollagen I
N-proteinase gene. Am. J. Hum. Genet. 65, 308 –317.
Danielson, K. G., Baribault, H., Holmes, D. F., Graham, H.,
Kadler, K. E., and Iozzo, R. V. (1997) Targeted disruption of
decorin leads to abnormal collagen fibril morphology and skin
fragility. J. Cell Biol. 136, 729 –743.
Engel, J., and Prockop, D. J. (1991) The zipper-like folding of
collagen triple helices and the effects of mutations that disrupt
the zipper. Annu. Rev. Biophys. Biophys. Chem. 20, 137–152.
Fichard, A., Kleman, J.-P., and Ruggiero, F. (1994) Another look
at collagen V and XI molecules. Matrix Biol. 14, 515–531.
Giraud, M. M., Castanet, J., Meunier, F. J., and Bouligand, Y.
(1978) The fibrous structure of coelacanth scales: A twisted
‘plywood’. Tissue & Cell 10, 671– 686.
Giraud-Guille, M. M. (1994) Liquid crystalline order of biopoly-
mers in cuticles and bones. Microsc. Res. Tech. 27, 420 – 428.
Giraud-Guille, M.-M. (1996) Twisted liquid crystalline supramo-
lecular arrangements in morphogenesis. Int. Rev. Cytol. 166,
59 –101.
Graham, H. K., Holmes, D. F., Watson, R. B., and Kadler, K. E.
(2000) Identification of collagen fibril fusion during vertebrate
tendon morphogenesis. The process relies on unipolar fibrils
and is regulated by collagen–proteoglycan interaction. J. Mol.
Biol. 295, 891–902.
Holmes, D. F., Gilpin, C. J., Baldock, C., Ziese, U., Koster, A. J.,
and Kadler, K. E. (2001) Corneal collagen fibril structure in
three dimensions: Structural insights into fibril assembly, me-
chanical properties, and tissue organization. Proc. Natl. Acad.
Sci. USA 98, 7307–7312.
Hulmes, D. J. S. (1992) The collagen superfamily—Diverse struc-
tures and assemblies. Essays Biochem. 27, 49 – 67.
9
BUILDING COLLAGEN ASSEMBLIES

Hulmes, D. J. S., Holmes, D. F., and Cummings, C. (1985) Crys-
talline regions in collagen fibrils. J. Mol. Biol. 184, 473– 477.
Hulmes, D. J. S., Jesior, J. C., Miller, A., Berthet-Colominas, C.,
and Wolff, C. (1981) Electron microscopy shows periodic struc-
ture in collagen fibril cross sections. Proc. Natl. Acad. Sci. USA
78, 3567–3571.
Hulmes, D. J. S., Kadler, K. E., Mould, A. P., Hojima, Y., Holmes,
D. F., Cummings, C., Chapman, J. A., and Prockop, D. J. (1989)
Pleomorphism in type I collagen fibrils produced by persistence
of the procollagen N-propeptide. J. Mol. Biol. 210, 337–345.
Hulmes, D. J. S., Wess, T. J., Prockop, D. J., and Fratzl, P. (1995)
Radial packing, order, and disorder in collagen fibrils. Biophys.
J. 68, 1661–1670.
Kadler, K. E. (1995) Extracellular matrix 1: Fibril-forming colla-
gens. Protein Profile 2, 491– 619.
Kadler, K. E., Holmes, D. F., Trotter, J. A., and Chapman, J. A.
(1996) Collagen fibril formation. Biochem. J. 316, 1–11.
Lamande, S. R., and Bateman, J. F. (1999) Procollagen folding
and assembly: The role of endoplasmic reticulum enzymes and
molecular chaperones. Semin. Cell Dev. Biol. 10, 455– 464.
Lees, J. F., and Bulleid, N. J. (1994) The role of cysteine residues
in the folding and association of the COOH-terminal propeptide
of types I and III procollagen. J. Biol. Chem. 269, 24354 –24360.
Lees, J. F., Tasab, M., and Bulleid, N. J. (1997) Identification of
the molecular recognition sequence which determines the type-
specific assembly of procollagen. EMBO J. 16, 908 –916.
Li, Y., Lacerda, D. A., Warman, M. L., Beier, D. R., Yoshioka, H.,
Ninomiya, Y., Oxford, J. T., Morris, N. P., Andrikopoulos, K.,
Ramirez, F., Wardell, B. B., Lifferth, G. D., Teuscher, C., Wood-
ward, S. R., Taylor, B. A., Seegmiller, R. E., and Olsen, B. R.
(1995) A fibrillar collagen gene, Colllal, is essential for skeletal
morphogenesis. Cell 80, 423– 430.
Linsenmayer, T. F., Gibney, E., Igoe, F., Gordon, M. K., Fitch,
J. M., Fessler, L. I., and Birk, D. E. (1993) Type-V collagen—
Molecular structure and fibrillar organization of the chicken
alpha-1(V) NH
2
-terminal domain, a putative regulator of cor-
neal fibrillogenesis. J. Cell Biol. 121, 1181–1189.
MacBeath, J. R., Shackleton, D. R., and Hulmes, D. J. S. (1993)
Tyrosine-rich acidic matrix protein (TRAMP) accelerates colla-
gen fibril formation in vitro. J. Biol. Chem. 268, 19826 –19832.
Marchant, J. K., Hahn, R. A., Linsenmayer, T. F., and Birk, D. E.
(1996) Reduction of type V collagen using a dominant-negative
strategy alters the regulation of fibrillogenesis and results in
the loss of corneal-specific fibril morphology. J. Cell Biol. 135,
1415–1426.
Martin, R., Farjanel, J., Eichenberger, D., Colige, A., Kessler, E.,
Hulmes, D. J. S., and Giraud-Guille, M. M. (2000) Liquid crys-
talline ordering of procollagen as a determinant of three-dimen-
sional extracellular matrix architecture. J. Mol. Biol. 301, 11–
17.
McLaughlin, S. H., and Bulleid, N. J. (1998) Molecular recogni-
tion in procollagen chain assembly. Matrix Biol. 16, 369 –377.
Moradi-Ame´li, M., De Chassey, B., Farjanel, J., and van der Rest,
M. (1998) Different splice variants of cartilage alpha1(XI) col-
lagen chain undergo uniform amino-terminal processing. Ma-
trix Biol. 17, 393–396.
Moradi-Ame´li, M., Rousseau, J. C., Kleman, J. P., Champliaud,
M. F., Boutillon, M. M., Bernillon, J., Wallach, J., and Vander-
rest, M. (1994) Diversity in the processing events at the N-
terminus of type-V collagen. Eur. J. Biochem. 221, 987–995.
Myllyharju, J., and Kivirikko, K. I. (2001) Collagens and collagen-
related diseases. Ann. Med. 33, 7–21.
Orgel, J. P., Wess, T. J., and Miller, A. (2000) The in situ confor-
mation and axial location of the intermolecular cross-linked
non-helical telopeptides of type I collagen. Struct. Fold. Des 8,
137–142.
Orgel, J. P. R. O., Miller, A., Irving, T. C., Fischetti, R. F.,
Hammersley, A. P., and Wess, T. J. (2001) The in situ super-
molecular structure of type I collagen. Struct. Fold. Des. 9,
1061–1069.
Ottani, V., Raspanti, M., and Ruggeri, A. (2001) Collagen struc-
ture and functional implications. Micron 32, 251–260.
Oxford, J. T., Doege, K. J., and Morris, N. P. (1995) Alternative
exon splicing within the amino-terminal nontriple-helical do-
main of the rat pro-alpha 1(XI) collagen chain generates mul-
tiple forms of the mRNA transcript which exhibit tissue-depen-
dent variation. J. Biol. Chem. 270, 9478 –9485.
Parry, D. A. D., and Craig, A. S. (1984) Growth and development
of collagen fibrils in connective tissue. In Ruggeri, A., and
Motta, P. M. (Eds.), Ultrastructure of the Connective Tissue
Matrix. pp. 34 – 64, Nijhoff, Boston.
Prockop, D. J., and Hulmes, D. J. S. (1994) Assembly of collagen
fibrils de novo from soluble precursors. In Yurchenco, P. D.,
Birk, D. E., and Mecham, R. P., (Eds.), Extracellular Matrix
Assembly and Structure, pp. 47–90, Academic Press, San Di-
ego.
Prockop, D. J., Sieron, A. L., and Li, S.-W. (1998) Procollagen
N-proteinase and procollagen C-proteinase. Two unusual met-
alloproteinases that are essential for procollagen processing
probably have important roles in development and cell signal-
ing. Matrix Biol. 16, 399 – 408.
Ricard-Blum, S., Dublet, B., and van der Rest, M. (2000) Uncon-
ventional collagens. Oxford Univ. Press, Oxford.
Rousseau, J. C., Farjanel, J., Boutillon, M. M., Hartmann, D. J.,
van der Rest, M., and Moradi-Ame´li, M. (1996) Processing of
type XI collagen—Processing of the matrix forms of the alpha
1(XI) chain. J. Biol. Chem. 271, 23743–23748.
Svensson, L., Aszu
` di, A., Reinholt, F. P., Fe`ssler, R., Heinega¨rd,
D., and Oldberg, A
˚ . (1999) Fibromodulin-null mice have abnor-
mal collagen fibrils, tissue organization, and altered lumican
deposition in tendon. J. Biol. Chem. 274, 9636 –9647.
Weber, I. T., Harrison, R. W., and Iozzo, R. V. (1996) Model
structure of decorin and implications for collagen fibrillogen-
esis. J. Biol. Chem. 271, 31767–31770.
Zhidkova, N. I., Justice, S. K., and Mayne, R. (1995) Alternative
mRNA processing occurs in the variable region of the pro-alpha
1(XI) and pro-alpha 2(XI) collagen chains. J. Biol. Chem. 270,
9486 –9493.
10
DAVID J. S. HULMES
Document Outline
Wyszukiwarka
Podobne podstrony:
Molecular spectroscopy and structure
Gravitational Entropy and Global Structure
Collagen stability, hydration and native state
Eurocode 2 Part 3 2006 UK NA Design of concrete structures Liquid retaining and containing struc
part3 19 Pragmatics and Argument Structure
0090 Suspension and body structure inspection
[Open Life Sciences] Genetic diversity and population structure of wild pear (Pyrus pyraster (L ) Bu
Syntactic doubling and the structure of wh chains
Eurocode 2 Part 3 2006 Design of concrete structures Liquid retaining and containing structures
tech view inode and metadata structure ext3
Fibrillar Structure and Mechanical Properties of Collagen
SCI03 Model Making Workshop Structure of Tall Buildings and Towers
Collagens structure, function, and biosynthesis
Mechanical Properties of Native and Cross linked Type I Collagen Fibrils Yang
SCI03 Model Making Workshop Structure of Tall Buildings and Towers
więcej podobnych podstron