Contents
lists
available
at
Agriculture,
Ecosystems
and
Environment
j o
u r
n
a l
h o m e p a g e :
w w w . e l s e v i e r . c o m / l o
Chemical
properties
of
anaerobic
digestates
affecting
C
and
N
dynamics
in
amended
soils
José
Antonio
Alburquerque
,
Carlos
de
la
Fuente,
María
Pilar
Bernal
Department
of
Soil
and
Water
Conservation
and
Organic
Waste
Management,
Centro
de
Edafología
y
Biología
Aplicada
del
Segura,
CSIC,
P.O.
Box
164,
30100
Murcia,
Spain
a
r
t
i
c
l
e
i
n
f
o
Article
history:
Received
11
October
2010
Received
in
revised
form
1
March
2011
Accepted
14
March
2011
Available online 8 April 2011
Keywords:
Anaerobic
digestion
Animal
slurries
Organic
matter
mineralisation
Biodegradability
Nitrogen
immobilisation
a
b
s
t
r
a
c
t
The
optimisation
of
digestate
recycling
as
fertilisers,
based
on
both
environmental
and
agricultural
crite-
ria,
requires
an
evaluation
of
the
effects
on
C
and
N
dynamics
in
soil.
In
the
present
paper,
six
digestates
from
several
anaerobic
co-digestion
experiments,
using
pig
or
cattle
slurry
as
the
main
substrate,
were
evaluated
in
short-term
incubations
in
soil.
Digestate
properties
such
as
dissolved
organic-C
(DOC),
biochemical
oxygen
demand
(BOD)
and
digestate
organic-C
mineralised
in
the
soil
during
the
first
7
days
represented
properly
the
digestate
biodegradability.
These,
together
with
their
ratios
with
respect
to
the
total
nitrogen
(TN)
concentration
in
the
digestate,
were
reliable
parameters
with
respect
to
defining
the
C
and
N
dynamics
in
the
soil
and
hence
the
N-fertiliser
potential
of
the
digested
materials.
Therefore,
highly
biodegradable
digested
materials,
represented
in
the
present
study
by
digestates
from
cattle
slurry–glycerine
mixtures
were
not
suitable
for
agricultural
use
as
they
caused
a
high
CO
2
–C
production
and
led
to
N-immobilisation
and/or
denitrification
after
their
application
to
soil.
Contrastingly,
for
less
biodegradable
digested
mate-
rials
(BOD
5
d
<
6.0
g
O
2
L
−1
fresh
weight,
DOC
<
5.5
g
L
−1
fresh
weight
and
DOC/TN
<
1.5),
less
CO
2
–C
was
evolved
and
ammonium
was
rapidly
nitrified
in
soil—being
an
available
N
source
for
crops.
© 2011 Elsevier B.V. All rights reserved.
1.
Introduction
Nowadays,
there
is
increasing
interest
in
Europe
in
the
imple-
mentation
of
anaerobic
digestion
in
productive
sectors
such
as
livestock
and
agroindustry,
where
vast
amounts
of
biodegradable
wastes
(animal
manure
and
slurries,
agricultural
and
food
industry
wastes,
etc.)
must
be
adequately
managed,
due
to
the
demand
for
renewable
energy.
Co-digestion
can
enhance
the
energy
produc-
tion
from
animal
manure
and
slurries,
as
co-digestible
materials
such
as
slaughterhouse
wastes,
glycerine
and
energy
crops
or
silage
can
increase
the
amount
of
biodegradable
organic
matter,
dilute
potential
toxic
compounds,
improve
the
nutrient
balance
and
favour
synergistic
effects
of
microorganisms,
thereby
raising
biogas
production
However,
the
sustainability
of
biogas
production
also
depends
on
an
appropriate
end-use
of
the
digested
material
(digestate)—which
should
be
treated,
disposed
of
or
re-used
in
a
proper
way,
avoiding
any
negative
environmental
impact.
The
use
of
digestates
as
organic
fertilisers
in
agricultural
sys-
tems
seems
the
best
option
for
their
recycling
since
they
contain
considerable
amounts
of
residual
organic-C
and
plant
nutrients.
∗ Corresponding
author.
Tel.:
+34
968
396200;
fax:
+34
968
396213.
addresses:
(J.A.
Alburquerque).
Digestates
also
present
advantages
in
comparison
with
untreated
waste,
such
as
greater
microbial
stability
and
hygiene
and
a
higher
amount
of
N
present
as
ammonium
So,
land
spreading
of
digestate
can
lead
to
benefits
if
integrated
into
good
agricultural
practices,
by
controlling
the
N
application
rate
and
heavy
metal
load,
and
by
securing
digestate
hygiene
Nevertheless,
the
biodegradability
of
these
materials,
which
determines
organic
matter
(OM)
mineralisation
and
thus
nutrient
turnover
in
soil,
is
not
well
characterised.
The
complete
exhaustion
of
the
most
labile
organic
fraction
during
the
anaerobic
process,
in
order
to
obtain
digestates
with
a
high
stability
degree,
is
not
easy
to
achieve
at
the
industrial
level,
the
main
objective
of
the
anaerobic
co-digestion
being
the
pro-
duction
of
a
high
rate
of
biogas,
rich
in
methane.
This
process
is
conditioned
mainly
by
the
composition
of
the
raw
materials
and
the
development
of
the
anaerobic
process,
leading
in
some
cases
to
the
production
of
unstable
digested
materials
which
may
exert
negative
impacts
on
the
plant–soil
system
In
this
context,
aerobic
respiration
indices
based
on
oxygen
uptake
are
considered
the
most
suitable
param-
eters
for
assessing
the
biodegradability
of
organic
amendments
(
In
wastewaters
and
animal
slurries
the
biochemical
oxygen
demand
(BOD)
test
is
a
reliable
and
commonly
used
method
to
determine
readily
biodegradable
OM
(
0167-8809/$
–
see
front
matter ©
2011 Elsevier B.V. All rights reserved.

16
J.A.
Alburquerque
et
al.
/
Agriculture,
Ecosystems
and
Environment
160 (2012) 15–
22
Although
there
is
no
threshold
value
established
as
a
stability
and
quality
criterion
for
the
agricultural
use
of
waste
materials
based
on
BOD
data,
it
is
possible
with
some
caution
to
compare
it
with
the
limits
established
in
the
solid
waste
field
based
on
oxygen
uptake
rate
and/or
cumulative
oxygen
consumption
indices.
Dif-
ferent
methodologies
for
characterising
the
biological
stability
of
organic
materials
have
been
used,
such
as
SOUR
(specific
oxygen
uptake
rate
as
the
maximum
rate
of
oxygen
consumption),
OD
20
h
(cumulative
oxygen
demand
for
the
first
20
h
of
the
test;
DRI
24
h
(dynamic
respiration
index,
which
is
the
average
oxygen
uptake
rate
at
24
h
of
maximum
biological
activity;
Also,
little
information
is
available
about
the
degree
of
stabil-
ity
of
digestates
and
their
C
and
N
dynamics
in
amended
soils
The
information
gained
from
decomposition
studies
in
digestate-treated
soil
(OM
mineral-
isation,
N
mineralisation–immobilisation,
etc.)
may
be
useful
for
assessing
N
availability,
and
for
optimising
the
digestate
applica-
tion
rate
to
agricultural
soils.
Thus,
defining
the
main
digestate
properties
affecting
such
C
and
N
dynamics
in
soil
can
help
achieve
the
sustainable
use
of
digestates
as
fertilisers
in
soil–plant
system,
which
will
have
both
agricultural
and
environmental
benefits.
The
present
paper
evaluates
the
dynamics
of
C-mineralisation
and
inorganic-N
in
soil
amended
with
six
digestates
produced
from
representative
anaerobic
co-digestion
processes
in
Spain.
These
effects
were
investigated
in
aerobic
incubation
experiments,
an
appropriate
tool
to
evaluate
the
feasibility
of
the
use
of
organic
amendments
in
agricultural
soils
(
Based
on
these
considerations,
the
main
objective
of
this
paper
is
to
identify
the
most
relevant
parameters
related
to
digestate
composition
for
assessing
the
maximum
benefits
of
these
materials
as
fertilisers.
2.
Materials
and
methods
2.1.
Incubation
experiments
in
soil
Six
digestates
were
collected
as
representative
samples
from
anaerobic
co-digestion
experiments
based
on
cattle
or
pig
slurry
mixed
with
agro-industrial
wastes
(co-digestion
mixtures):
cat-
tle
slurry
+
4%
glycerine
(CG4),
cattle
slurry
+
6%
glycerine
(CG6),
cattle
slurry
+
4.3%
cattle
manure
+
11.6%
maize–oat
silage
(CMS),
cattle
slurry
+
5%
orange
peel
waste
(CO),
pig
slurry
+
1.0%
sludge
from
a
slaughterhouse
wastewater
treatment
plant
+
6.5%
biodiesel
wastewaters
(PSB),
and
pig
slurry
+
0.6%
pasteurised
slaughter-
house
waste
(PS).
Digestates
were
sampled
directly
after
anaerobic
digestion
(without
post-treatments),
stored
at
a
temperature
<4
◦
C
and
processed
quickly
to
prevent
any
chemical
or
biological
alter-
ation.
Their
main
characteristics
and
the
specifications
of
anaerobic
co-digestion
performance
are
shown
in
and
PSB
sam-
ples
came
from
industrial-scale
co-digestion
processes,
while
the
rest
of
the
samples
came
from
laboratory-scale
experiments,
run
mainly
to
optimise
the
production
of
biogas
through
anaerobic
co-digestion.
From
an
agricultural
soil
at
La
Alberca
(Murcia,
Spain),
soil
was
taken
from
the
top
20
cm,
air-dried
and
sieved
to
2
mm
before
use.
Its
main
characteristics
were:
24%
CaCO
3
,
pH
7.5
and
electrical
conductivity
(EC)
1.72
dS
m
−1
(both
saturated
paste,
with
water),
24.3
g
kg
−1
OM,
14.1
g
kg
−1
total
organic-C
(TOC)
and
1.85
g
kg
−1
total
nitrogen
(TN),
with
14.8%
clay,
22.3%
silt
and
62.9%
sand.
The
digested
materials
were
mixed
thoroughly
with
the
soil
in
a
proportion
of
4
g
of
fresh
digestate
per
100
g
of
dry
soil
(equiva-
lent
to
a
field
application
of
96
m
3
ha
−1
).
This
application
rate
was
selected
in
order
to
avoid
excessively
low
inputs
of
organic-C
to
the
soil
with
some
digestate
samples,
which
could
limit
the
accu-
racy
of
the
C-mineralisation
study,
while
keeping
the
N
addition
(140–380
kg
N
ha
−1
)
realistic
for
the
requirements
of
agricultural
crops
(
The
digestate-soil
mixtures
were
incubated
in
darkness
under
aerobic
conditions,
at
26
±
1
◦
C
for
56
days.
Each
treatment
was
run
in
triplicate,
and
soil
without
digestate
was
used
as
the
control.
Soil
moisture
was
maintained
at
60%
of
the
water-holding
capac-
ity
during
incubation,
with
deionised
water.
To
follow
N
dynamics,
a
set
of
destructive
samples
of
the
digestate-soil
mixtures
were
placed
in
50-mL
tubes
without
drainage
holes;
then,
they
were
closed
with
parafilm
–
which
allows
gas
exchange
–
to
retain
soil
moisture
and
avoid
anaerobic
conditions
Periodically,
three
replicates
per
treatment
were
removed
from
the
incubator
(at
0,
2,
7,
14,
28,
42
and
56
days)
for
analysis
of
inorganic-
N
(NH
4
–N
and
NO
3
–N).
Since
the
digestate
samples
were
mixed
homogeneously
with
the
soil
at
the
time
of
application
and
there
was
no
airflow
at
the
soil
surface
during
incubation,
N-loss
through
volatilisation
was
negligible—as
demonstrated
by
organic-N
mineralisation
was
evaluated
by
the
accu-
Table
1
Main
characteristics
of
the
digestate
samples
(mean
value
±standard
deviation,
data
expressed
on
a
fresh
weight
basis).
Parameter
CG4
CG6
CMS
CO
PSB
PS
BOD
24
h
(g
L
−1
)
7.5
±
1.5
35.0
±
3.0
1.6
±
0.2
0.5
±
0.1
0.7
±
0.1
1.1
±
0.1
BOD
5
d
(g
L
−1
)
37.5
±
3.5
52.5
±
3.5
5.9
±
0.7
1.3
±
0.1
2.2
±
0.2
2.3
±
0.2
pH
5.64
±
0.01
7.35
±
0.03
7.50
±
0.01
7.86
±
0.01
8.20
±
0.02
7.95
±
0.03
EC
(dS
m
−1
)
14.5
±
0.3
11.7
±
0.2
25.7
±
0.8
8.7
±
0.2
30.3
±
0.9
21.1
±
0.1
DM
(g
L
−l
)
38.3
±
0.5
72.9
±
5.8
90.1
±
0.2
24.4
±
0.3
19.5
±
0.1
21.0
±
2.4
OM
(g
L
−l
)
26.4
±
0.1
56.4
±
0.9
66.4
±
0.2
18.0
±
0.1
8.5
±
0.1
11.4
±
0.2
TOC
(g
L
−l
)
17.8
±
0.1
42.8
±
0.1
33.7
±
0.5
9.4
±
0.1
5.9
±
0.1
5.8
±
0.1
DOC
(g
L
−l
)
10.6
±
0.3
27.6
±
0.9
5.4
±
0.1
1.2
±
0.1
2.4
±
0.1
1.2
±
0.1
TN
(g
L
−l
)
1.88
±
0.02
2.32
±
0.07
3.97
±
0.02
1.44
±
0.01
3.96
±
0.03
2.89
±
0.02
NH
4
–N
(g
L
−l
)
0.97
±
0.03
0.89
±
0.01
2.43
±
0.01
0.76
±
0.01
3.46
±
0.04
2.21
±
0.01
TOC/TN
ratio
9.5
±
0.1
18.5
±
0.5
8.5
±
0.1
6.6
±
0.1
1.5
±
0.1
2.0
±
0.1
Anaerobic
co-digestion
performance
Operation
Discont
Discont
Cont
Discont
Cont
Cont
Scale
L
L
I
L
I
L
Temperature
(
◦
C)
35
35
38.5
38
37
35
HRT
(days)
40
40
25
28
21
20
BOD
24
h
and
BOD
5
d
:
24-h
and
5-d
biochemical
oxygen
demand,
respectively.
EC:
electrical
conductivity,
DM:
dry
matter,
OM:
total
organic
matter,
TOC:
total
organic-C,
DOC:
dissolved
organic-C
and
TN:
total
nitrogen.
Discont:
discontinuous
operation,
Cont:
continuous
operation,
L:
laboratory-scale
(2–6
L
digester),
I:
industrial-scale
(3000
m
3
digester)
and
HRT:
hydraulic
residence
time
CG4:
cattle
slurry
+
4%
glycerine,
CG6:
cattle
slurry
+
6%
glycerine,
CMS:
cattle
slurry
+
4.3%
cattle
manure
+
11.6%
maize–oat
silage,
CO:
cattle
slurry
+
5%
orange
peel
waste,
PSB:
pig
slurry
+
1.0%
sludge
from
a
slaughterhouse
wastewater
treatment
plant
+
6.5%
biodiesel
wastewaters,
and
PS:
pig
slurry
+
0.6%
pasteurised
slaughterhouse
waste.

J.A.
Alburquerque
et
al.
/
Agriculture,
Ecosystems
and
Environment
160 (2012) 15–
22
17
mulation
of
inorganic-N
in
the
soil,
since
small
changes
in
organic
pools
are
usually
difficult
to
detect
and
gaseous
losses
were
consid-
ered
negligible
under
these
experimental
conditions
(
Net
N-mineralisation
(net
N-min)
in
each
treatment
(in
digestate
amended
and
non-amended
soil)
was
calculated
by
subtracting
the
soil
inorganic-N
content
at
day
0
from
the
amount
present
in
soil
after
56
days
of
incubation,
and
expressed
as
g
inorganic-N
g
−1
dry
soil.
The
N-mineralisation
from
the
digestates
(N
m
)
was
calculated
as:
N
m
(%)
=
100
×
[(inorg
−
N
56
d
−
inorg-N
0
d
)
soil
+
digestate
−
(inorg
-
N
56
d
−
inorg-N
0
d
)
soil
]/added
TN.while
nitrification
(nitrate
conver-
sion)
was
determined
as
the
percentage
of
the
added-N
from
the
digestate
that
had
been
converted
into
nitrate
after
56
days.
NC
(%)
=
100
×
[(NO
3
-N
56
d
−
NO
3
-N
0
d
)
soil
+digestate
−
(NO
3
-N
56
d
−
NO
3
-N
0
d
)
soil
]
/added
TN.
The
dynamics
of
C-mineralisation
was
determined
in
a
sepa-
rate
set
of
incubations
lasting
56
days,
using
500-mL,
hermetically
closed
glass
vessels.
A
small
vial
with
10
mL
of
0.1
M
NaOH
was
placed
inside
each
vessel
to
trap
the
CO
2
evolved
during
the
incuba-
tion,
and
empty
vessels
were
used
as
blanks.
These
were
opened
for
several
minutes
when
the
NaOH
vials
were
replaced,
to
maintain
adequate
aerobic
conditions.
The
CO
2
was
measured
periodically
(after
2,
4,
7,
14,
28,
42
and
56
days),
by
titration
of
the
NaOH
solution
with
0.1
M
HCl
in
an
excess
of
BaCl
2
to
precipitate
car-
bonates.
The
mineralisation
of
the
organic-C
from
the
digestates
(C
m
)
was
calculated
as
the
difference
between
the
CO
2
–C
evolved
in
the
amended
soils
and
that
produced
in
the
control
(unamended)
soil,
and
was
expressed
as
a
percentage
of
the
TOC
added
with
the
digestates.
The
data
for
the
C-mineralisation
from
the
diges-
tates
were
fitted
to
kinetic
functions
by
the
non-linear
least-square
technique
(Marquardt–Levenberg
algorithm),
using
the
Sigma-Plot
computer
programme
(SPSS
Inc.).
The
statistical
significance
of
the
curve-fitting,
residual
mean
square
(RMS)
and
F-values
were
also
calculated.
2.2.
Analytical
methods
The
following
parameters
were
determined
in
the
digestate
samples:
EC
and
pH
(directly,
after
sample
homogenisation);
mois-
ture
content,
after
drying
to
constant
weight
at
105
◦
C;
the
volatile
solids,
which
reflect
the
OM
content,
by
loss
on
ignition
at
500
◦
C
for
24
h.
The
TOC
and
TN
were
measured
by
automatic
microanalysis
(EuroVector
elemental
analyser,
Milan,
Italy)
of
freeze-dried
sam-
ples
and
the
dissolved
organic-C
(DOC)
using
an
automatic
analyser
for
liquid
samples
(TOC-V
CSN
Analyzer,
Shimadzu)
after
sample
filtration
(0.45
m
pore-diameter).
Ammonium
was
extracted
by
steam-distillation
of
fresh
samples
alkalised
with
MgO,
trapped
in
boric
acid
and
titrated
with
HCl.
The
5-day
biochemical
oxy-
gen
demand
(BOD
5
d
)
was
measured
with
a
respirometric
Oxitop
®
IS
6
(WTW,
Germany)
based
on
pressure
measurement,
which
is
automatically
transformed
into
mg
O
2
L
−1
.
In
the
Oxitop
®
system,
cumulative
oxygen
consumption
measurements
were
made
each
day
during
a
5-day
period.
The
soil
TOC
and
TN
were
determined
with
an
automatic
microanalyser.
The
CaCO
3
content
was
measured
with
a
Bernard
calcimeter.
As
incubation
progressed,
a
two-step
sequential
extrac-
tion
procedure
was
carried
out
for
inorganic-N
determination:
ultrapure
water
(1:5
w/v)
for
NO
3
-N
and
2
M
KCl
(1:5
w/v)
for
NH
4
–N.
The
NO
3
–N
was
measured
using
a
nitrate-ion
selective
electrode
(
while
NH
4
–N
was
determined
by
a
colori-
metric
method
based
on
Berthelot’s
reaction
All
values
refer
to
soil
dried
at
105
◦
C
for
24
h.
2.3.
Statistical
analyses
Basic
statistical
analyses
of
data,
correlation
coefficients
and
regression
equations
were
calculated
using
the
SPSS
18.0
pro-
gramme
for
Windows.
The
normal
distribution
of
the
data
was
checked
by
the
Shapiro–Wilk’s
test;
when
data
failed
this
test,
they
were
adjusted
to
a
normal
distribution
through
log-transformation.
3.
Results
and
discussion
3.1.
Digestate
microbial
stability
In
the
present
study,
all
digestate
samples
showed
an
initial
phase
of
maximum
respirometric
activity
within
the
first
24
h
of
testing.
As
a
result,
the
BOD
24
h
accounted
for
20,
67,
27,
39,
30
and
47%
of
the
BOD
5
d
for
CG4,
CG6,
CMS,
CO,
PSB
and
PS,
respectively
(
This
indicated
the
presence
of
an
easily
biodegradable
organic
fraction
probably
including
decaying
microbial
biomass
from
the
digestate.
When
the
BOD
24
h
data
were
expressed
as
the
average
oxygen
uptake
rate
(mg
O
2
g
−1
OM
h
−1
),
the
following
results
were
obtained:
1.0,
1.2,
3.2,
3.9,
11.8
and
25.9
for
CMS,
CO,
PSB,
PS,
CG4
and
CG6,
respectively.
Our
results
exceed
the
limit
value
established
by
0.5
mg
O
2
g
−1
OM
h
−1
as
the
average
oxygen
uptake
over
a
24-h
period
of
the
most
intense
biological
activity
(DRI
24
h
)
for
highly
stable
materials
(mature
compost);
only
the
CMS
digestate
behaved
like
a
material
of
medium
stability
(limit
of
1.0
mg
O
2
g
−1
OM
h
−1
).
By
using
the
less
restrictive
classification
proposed
by
based
on
DRI
24
h
data,
the
CMS,
CO
and
PSB
showed
a
low
biodegradabil-
ity
(<2
mg
O
2
g
−1
DM
h
−1
):
0.8,
0.9
and
1.4
mg
O
2
g
−1
DM
h
−1
,
respectively.
The
PS
had
a
moderate
biodegradability
(2–5
mg
O
2
g
−1
DM
h
−1
)
of
2.1
mg
O
2
g
−1
DM
h
−1
and
the
CG
samples
showed
a
high
biodegradability
(>5
mg
O
2
g
−1
DM
h
−1
):
8.2
and
20.0
mg
O
2
g
−1
DM
h
−1
for
CG4
and
CG6,
respectively.
These
results
are
in
agreement
with
those
of
and
who
compared
stability
data
from
digested
and
non-digested
materials
based
on
cumulative
oxygen
demand
after
20
h
using
the
SOUR-test
(OD
20
h
).
They
noted
clear
decreases
in
the
OD
20
h
from
235
to
264
mg
O
2
g
−1
DM
in
the
non-digested
mixtures
to
30–95
mg
O
2
g
−1
DM
after
anaerobic
digestion,
the
latter
values
being
comparable
to
those
shown
by
stabilised
materials.
When
these
values
were
compared
to
the
BOD
24
h
data
obtained
in
this
work
(
the
CG
samples
showed
values
of
oxygen
demand
similar
to
those
reported
for
non-digested
mixtures,
while
the
rest
of
the
digestates
had
lower
values,
within
the
range
proposed
for
stabilised
materials.
These
results
can
be
attributed
to
the
high
content
in
CG
digestates
of
OM
easily
degradable
by
microorganisms
(DOC,
which
greatly
affected
the
degree
of
stability
of
the
digestate.
The
DOC
accounted
for
60,
64,
16,
13,
41
and
21%
of
the
TOC
for
CG4,
CG6,
CMS,
CO,
PSB
Table
2
Biochemical
oxygen
demand
(BOD)
values,
expressed
as
mg
O
2
g
−1
DM,
obtained
after
24
h
and
2,
3,
4
and
5
days
of
testing.
Digestate
BOD
24
h
BOD
2
d
BOD
3
d
BOD
4
d
BOD
5
d
CG4
195.8
456.9
587.4
718.0
979.1
CG6
479.9
514.2
651.3
685.6
719.8
CMS
18.0
33.3
45.8
55.5
66.0
CO
20.5
32.8
41.0
45.1
53.2
PSB
33.4
54.0
82.3
100.3
110.5
PS
50.0
73.8
85.7
97.6
107.1
CG4:
cattle
slurry
+
4%
glycerine,
CG6:
cattle
slurry
+
6%
glycerine,
CMS:
cattle
slurry
+
4.3%
cattle
manure
+
11.6%
maize–oat
silage,
CO:
cattle
slurry
+
5%
orange
peel
waste,
PSB:
pig
slurry
+
1.0%
sludge
from
a
slaughterhouse
wastewater
treat-
ment
plant
+
6.5%
biodiesel
wastewaters,
and
PS:
pig
slurry
+
0.6%
pasteurised
slaughterhouse
waste.
18
J.A.
Alburquerque
et
al.
/
Agriculture,
Ecosystems
and
Environment
160 (2012) 15–
22
Incubation
time
(da
ys)
0
7
14
21
28
35
42
49
56
Cumulative CO
2
-C ev
olv
e
d (µg C
g
-1
)
0
250
500
750
1000
1250
1500
1750
2000
2250
Soil
Soil
+ CG6
Soil
+ P
S
Soil+ CO
Soil
+ CG4
Soil+ CMS
Soil
+ P
SB
Incuba
tio
n time (da
ys)
0
7
14
21
28
35
42
49
56
Cumulativ
e mi
neralised-C (% of
T
O
C)
0
10
20
30
40
50
60
70
80
90
100
110
CG6
PS
CO
CG4
CMS
PSB
(a)
(b)
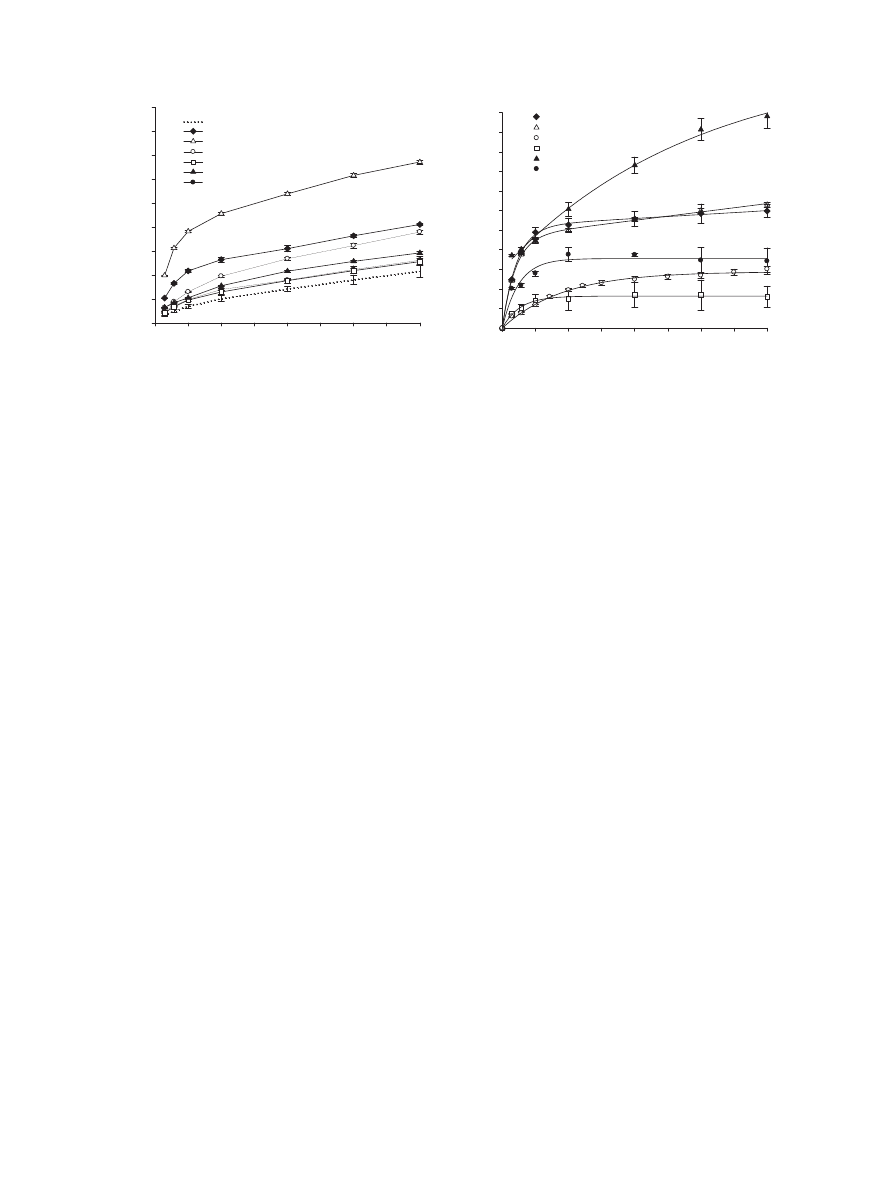
Fig.
1.
Cumulative
CO
2
–C
evolved
from
soil
during
incubation
(mean
value
±standard
deviation;
where
absent,
bars
fall
within
symbols)
(a)
and
cumulative
mineralised-C
from
digestate
samples
added
to
soil:
symbols
are
experimental
data
(mean
value
±standard
deviation,
n
=
3)
and
lines
represent
the
curve-fitting
(b).
CG4:
cattle
slurry
+
4%
glycerine,
CG6:
cattle
slurry
+
6%
glycerine,
CMS:
cattle
slurry
+
4.3%
cattle
manure
+
11.6%
maize–oat
silage,
CO:
cattle
slurry
+
5%
orange
peel
waste,
PSB:
pig
slurry
+
1.0%
sludge
from
a
slaughterhouse
wastewater
treatment
plant
+
6.5%
biodiesel
wastewaters,
and
PS:
pig
slurry
+
0.6%
pasteurised
slaughterhouse
waste.
and
PS,
respectively.
Therefore,
the
higher
such
percentage
was,
the
lower
microbial
stability
exhibited.
Although
a
significant
correlation
between
the
BOD
24
h
and
BOD
5
d
data
was
obtained
(P
<
0.01),
indicating
that
both
param-
eters
can
be
used
to
characterise
the
microbial
stability
of
the
digested
materials,
the
5-day
measurement
can
give
more
reliable
information
since
the
tested
materials
usually
maintained
a
high
respirometric
activity
beyond
the
first
24
h
of
testing.
The
cumula-
tive
data
followed
a
linear
tendency
over
time
in
all
samples
after
the
first
24
h
of
BOD
testing,
reaching
steady
rates
without
showing
clear
decreases
indicating
exhaustion
of
the
easily
biodegradable
OM
fraction
in
the
digestate
The
BOD
5
d
values
varied
considerably
among
the
digestate
sam-
ples
Considering
that
BOD
5
d
values
for
slurry
and
silage
effluents
can
be
in
the
range
of
10–80
g
L
−1
(
and
BOD
reductions
of
around
70%
can
be
reached
after
anaerobic
diges-
tion
the
values
found
in
the
present
study
can
be
considered
normal
for
digestates
with
the
exception
of
CG
samples
which
were
clearly
higher.
3.2.
Carbon
mineralisation
of
digestates
in
soil
The
addition
of
digestate
to
the
soil
caused
a
rapid
development
of
microbial
activity,
reflected
by
the
high
release
of
CO
2
–C
dur-
ing
the
first
days
of
incubation
This
was
related
to
the
presence
of
an
easily
degradable
organic
fraction
in
the
digestate
samples,
already
detected
in
the
BOD
test,
with
clear
differences
among
samples
(the
organic
load
and
its
microbial
stability).
Sub-
sequently,
the
CO
2
–C
production
rates
decreased
rapidly
during
the
first
two
weeks,
reaching
nearly
constant
values
at
the
end
of
the
incubation
(<10
g
C
g
−1
soil
and
day),
similar
to
those
obtained
in
the
unamended
soil
as
the
easily
mineralisable
OM
sources
were
exhausted.
The
amount
of
CO
2
–C
evolved
from
digestate-treated
soil
after
56
days
of
incubation
(mineralised-C)
increased
significantly
in
the
order
(mean
value):
639
<
653
<
730
<
948
<
1027
<
1679
g
C
g
−1
soil
for
soil
treated
with
CO,
PS,
PSB,
CMS,
CG4
and
CG6,
respec-
tively
(
The
TOC
mineralised
from
the
digestate
(C
m
)
also
reflected
the
different
biodegradability
of
the
OM
present
in
the
digestates
At
the
end
of
the
incubation,
CO
showed
the
lowest
percentage
of
C
m
(16%
of
TOC),
followed
by
CMS
and
PS
(30
and
34%,
respectively),
which
were
much
lower
than
for
the
CG4
and
CG6
digestates
(60
and
63%
of
TOC,
respectively).
Thus,
CMS,
PS
and,
especially,
CO
showed
a
more
recalcitrant
nature
than
the
digestates
from
the
glycerine
mixtures,
in
agreement
with
the
BOD
and
DOC
results.
Also,
more
than
100%
of
the
TOC
added
with
PSB
had
been
mineralised
after
56
days
of
incubation,
indicating
degra-
dation
of
native
soil
TOC
during
incubation
(priming
effect).
that
the
addition
to
soil
of
anaerobi-
cally
treated
pig
manure
led
to
105%
mineralised-C
after
70
days,
related
to
the
presence
of
a
high
amount
of
easily
degradable
OM.
The
addition
to
soil
of
organic
materials
with
a
low
C/N
ratio,
such
as
PSB
favours
high
C-mineralisation
rates
as
noted
by
Our
results
agree
well
with
those
obtained
by
soil
treated
with
fresh
and
aerobically
or
anaerobically
treated
ani-
mal
slurry
and
manures,
from
similar
incubation
experiments.
The
percentages
of
TOC
evolved
from
these
materials
after
70
days
of
incubation
ranged
from
23
to
105%,
depending
on
the
biodegradability
of
the
OM
and
the
presence
of
highly
available
organic
compounds
to
microorganisms
under
aerobic
condi-
tions.
Such
compounds
can
accumulate
in
digested
materials
when
degradation
of
complex
substrates
into
simple
and
water-
soluble
compounds
(hydrolysis)
and
their
subsequent
degradation
to
produce
methane
(methanogenesis)
are
not
completely
bal-
anced
during
anaerobic
digestion,
leading
to
the
production
of
unstable
materials
The
presence
of
such
intermediate
products
could
enhance
soil
microbial
activity
and
oxygen
demand
when
digestates
are
added
to
soil,
resulting
in
oxygen
depletion
and
N-immobilisation
(
The
dynamic
of
C-mineralisation
from
the
CG4
and
CG6
diges-
tates
in
the
soil
fitted
to
a
combined
first-
and
zero-order
kinetic
model
(
which
suggests
the
presence
of
two
different
pools
of
OM
in
these
digestate
samples
of
different
degradability:
a
labile
pool,
which
was
quickly
decomposed
in
soil
during
an
initial,
intense
phase
of
microbial
respiration
(about
50%
of
the
added
TOC,
with
high
k
values),
and
another,
more
resistant
to
microbial
degra-
dation
and
hence
mineralised
at
a
low,
constant
rate
with
time.
In
the
CG
samples,
the
addition
of
glycerine
as
co-digestion
substrate
may
have
increased
the
labile
pool
of
TOC,
resulting
in
high
insta-
bility
with
regard
to
microbial
breakdown.
The
high
percentage
of
J.A.
Alburquerque
et
al.
/
Agriculture,
Ecosystems
and
Environment
160 (2012) 15–
22
19
Table
3
Parameters
of
the
kinetic
models
used
to
describe
C-mineralisation
of
the
digestates
(
±
standard
error)
and
the
statistical
significance
of
the
non-linear
curve-fitting
(RMS:
residual
mean
square,
F-value
of
the
ANOVA).
Combined
first-
and
zero-order
function:
C
m
=
C
R
(1
−
e
−k
R
t
)
+
At
Digestate
C
m
(%
of
TOC)
C
R
k
R
A
RMS
F
CG4
59.8
±
2.4
52.0
±
0.6
0.330
±
0.011
0.143
±
0.017
0.498
3015
CG6
63.0
±
0.8
46.5
±
0.5
0.362
±
0.013
0.305
±
0.015
0.436
3577
First
order
function:
C
m
=
C
0
(1
− e
−kt
)+B
Digestate
C
m
(%
of
TOC)
C
0
k
B
RMS
F
CMS
30.1
±
1.2
28.9
±
0.6
0.078
±
0.004
–
0.757
1447
CO
16.1
±
3.8
16.4
±
0.3
0.272
±
0.023
–
0.425
574
PSB
108
±
4.4
106
±
10
0.025
±
0.005
30.2
±
1.9
4.625
568
PS
34.2
±
4.8
35.5
±
1.5
0.289
±
0.049
–
8.119
133
CG4:
cattle
slurry
+
4%
glycerine,
CG6:
cattle
slurry
+
6%
glycerine,
CMS:
cattle
slurry
+
4.3%
cattle
manure
+
11.6%
maize–oat
silage,
CO:
cattle
slurry
+
5%
orange
peel
waste,
PSB:
pig
slurry
+
1.0%
sludge
from
a
slaughterhouse
wastewater
treatment
plant
+
6.5%
biodiesel
wastewaters,
and
PS:
pig
slurry
+
0.6%
pasteurised
slaughterhouse
waste.
‘–’:
parameter
not
included
in
the
curve-fitting.
C
m
:
mineralised-C
(%
of
TOC)
after
56
days
of
incubation,
and
t:
incubation
time
(days).
In
the
combined
first-
and
zero-order
function:
C
R
,
rapid
potentially
mineralisable-C
(%
of
TOC);
k
R
,
rapid
rate
constant
(day
−1
);
A
(%
of
TOC
day
−1
),
slowly
mineralisable-C
rate
(equivalent
to
“C
S
×
k
S
”;
C
S
:
slowly
mineralisable-C
and
k
S
:
the
slow
rate
constant).
In
the
first
order:
C
0
,
potentially
mineralisable-C
(%
of
TOC)
and
k,
rate
constant
(day
−1
).
The
constant
term
(B)
indicates
the
initial
mineralisation
flux
detected
in
the
PSB
sample
(%
of
TOC).
*
Significant
at
probability
level
P
<
0.001.
TOC
from
CG
digestates
evolved
as
CO
2
–C
during
the
first
stage
of
incubation
is
typical
of
non-treated
animal
manure
and
slurries
(
identify-
ing
them
as
unstable
materials.
The
C-mineralisation
of
the
CMS,
CO
and
PS
digestates
fitted
better
to
a
first-order
kinetic
model,
with
a
potentially
mineralisable-C
of
16.4–35.5%
(
indicat-
ing
the
existence
in
these
digestates
of
a
predominant
proportion
of
OM
that
is
hardly
degradable
under
both
anaerobic
and
aerobic
conditions.
The
PSB
dynamic
of
C-mineralisation
needed
an
inde-
pendent
parameter
(B)
to
show
the
initial
flux
of
CO
2
–C
evolved
(30%
of
the
TOC
added),
suggesting
the
elevated
presence
of
an
OM
fraction
susceptible
to
rapid
mineralisation
Although
this
digestate
had
the
lowest
OM
concentration,
it
was
highly
unstable
according
to
the
BOD
5
d
test
(
3.3.
Nitrogen
dynamics
in
digestate-treated
soil
As
shown
in
all
the
digestates
supplied
NH
4
–N
to
the
soil.
During
the
first
week
of
incubation,
the
inorganic-N
concen-
tration
decreased
in
most
of
the
amended
soils,
due
mainly
to
a
reduction
in
NH
4
–N.
However,
during
the
initial
stage
of
incuba-
tion,
the
NO
3
–N
concentration
also
decreased
in
soil
treated
with
CG
samples
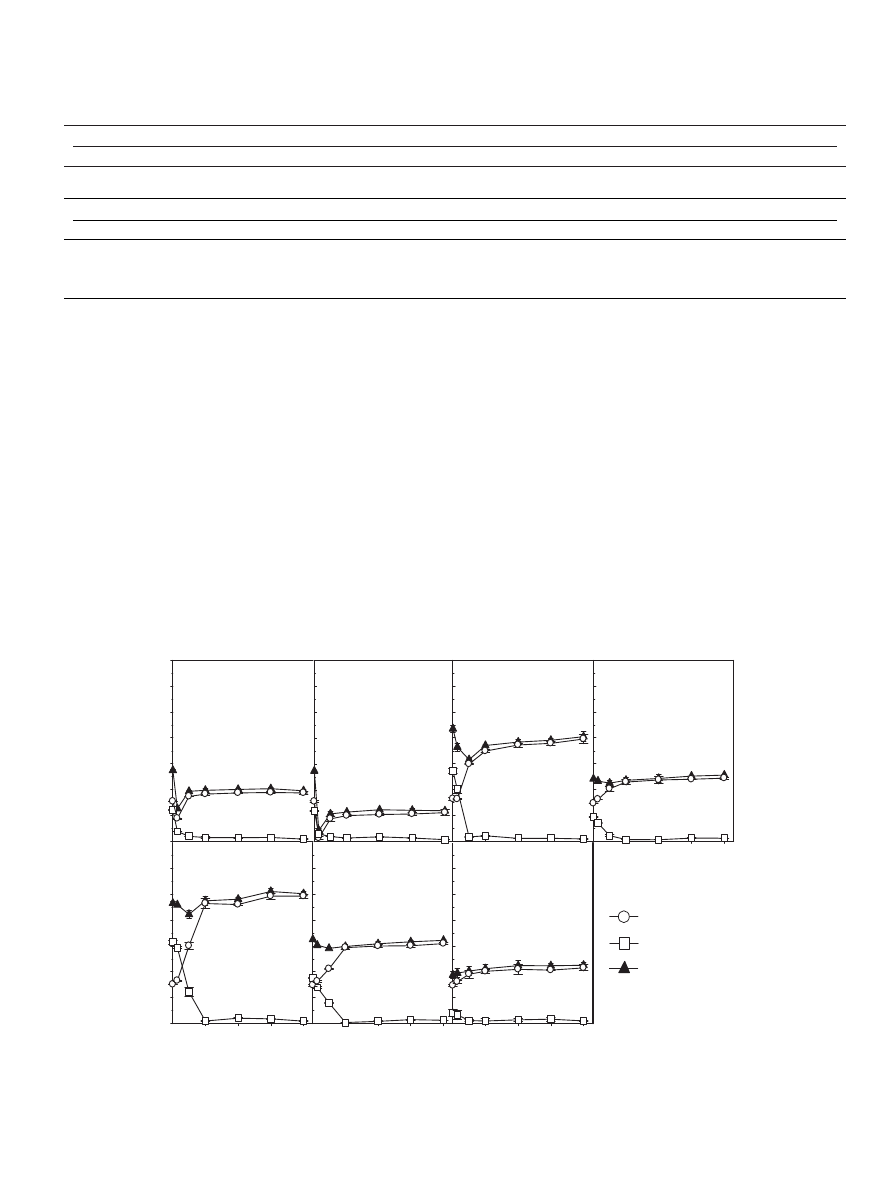
(
The
initial
inorganic-N
had
decreased
after
7
days
and
accounted
for
8,
17,
22
and
60
g
N
g
−1
soil
for
soil
treated
with
CO,
PS,
PSB
and
CMS,
respectively,
while
the
decrease
was
even
faster
after
CG4
or
CG6
addition:
74
and
115
g
N
g
−1
soil,
respectively
(after
2
days,
Such
initial
decreases
in
inorganic-
N
could
be
due
to
microbial
immobilisation,
since
a
concomitant
strong
C-mineralisation
was
found
in
all
treated
soils
which
0
14
28
42
56
µg N g
-1
0
50
100
150
200
250
300
µg N g
-1
0
50
100
150
200
250
300
350
Soil+CG4
Soil+PS
B
0
14
28
42
56
Soil+PS
Soi
l
0
14
28
42
56
Soil+CG6
Soil+CMS
14
28
42
56
Soil+CO
Soil
Incubation time (days)
NO
3
-N
NH
4
-N
Inorganic-
N (NH
4
-N + NO
3
-N)
Fig.
2.
Evolution
of
inorganic-N
in
a
soil
treated
with
digestates
during
incubation
(mean
value
±standard
deviation;
where
absent,
bars
fall
within
symbols).
CG4:
cattle
slurry
+
4%
glycerine,
CG6:
cattle
slurry
+
6%
glycerine,
CMS:
cattle
slurry
+
4.3%
cattle
manure
+
11.6%
maize–oat
silage,
CO:
cattle
slurry
+
5%
orange
peel
waste,
PSB:
pig
slurry
+
1.0%
sludge
from
a
slaughterhouse
wastewater
treatment
plant
+
6.5%
biodiesel
wastewaters,
and
PS:
pig
slurry
+
0.6%
pasteurised
slaughterhouse
waste.

20
J.A.
Alburquerque
et
al.
/
Agriculture,
Ecosystems
and
Environment
160 (2012) 15–
22
also
indicates
high
activity
of
soil
microorganisms.
detected
an
initial
period
of
inorganic-
N
immobilisation,
of
up
to
200
g
N
g
−1
soil,
after
amending
soil
with
animal
slurries.
A
significant,
inverse
correlation
(r
=
−0.949
at
P
<
0.01)
between
the
net
N-mineralisation
and
mineralised-C
(microbial
respiration)
was
obtained
in
the
present
study
(data
at
56
days).
The
addition
to
soil
of
high
amounts
of
easily
degradable
OM
in
the
digestates
should
induce
a
quick
development
of
the
microbial
population,
immobilising
inorganic-N
for
tissue
synthesis
(
Although
preferential
microbial
immobilisation
of
NH
4
over
NO
3
is
generally
accepted,
microbial
assimilation
of
NO
3
as
a
N
source
can
occur
But,
N-losses
by
denitrification
cannot
be
discounted
in
the
soils
treated
with
CG4
or
CG6,
which
sup-
plied
high
amounts
of
unstable
OM
to
the
soil
and
produced
intense
microbial
respiration
(CO
2
–C
production,
This
high
respi-
ration
during
the
first
days
of
incubation
could
have
reduced
the
oxygen
concentration
in
the
soil
system,
leading
to
N-losses
by
den-
itrification
After
the
immobilisation
period,
inorganic-N
increased,
indicating
re-mineralisation
and
nitrification.
The
net
N-mineralisation
after
56
days
of
incubation
was:
+7,
−2,
+18,
−15,
−39
and
−77
g
N
g
−1
soil,
for
soil
amended
with
CO,
PS,
PSB,
CMS,
CG4
and
CG6,
respectively
These
values
were
equivalent
to
net
N-mineralisation
from
the
digestate
(N
m
)
of
−10,
−15,
−8,
−19,
−68
and
−96%
for
CO,
PS,
PSB,
CMS,
CG4
and
CG6,
respectively,
indicating
the
proportion
of
the
TN
from
the
digestate
samples
which
had
been
immobilised
in
the
soil.
The
PSB,
CMS,
CO
and
PS
samples
led
to
fast
NO
3
-N
produc-
tion
in
the
soil
after
an
initial
lag
phase
(related
to
adaptation
and/or
immobilisation
periods)
and,
concomitantly,
to
a
decrease
in
NH
4
–N,
due
to
nitrification
(
The
percentage
of
TN
added
in
digestates
that
had
converted
into
nitrate
after
56
days
(nitrate
conversion:
NC),
was
44%,
50%,
59%
and
84%
for
CO,
CMS,
PS-MW
and
PSB,
respectively.
These
results
indicate
the
N-fertiliser
value
of
the
digestates,
as
nitrate
is
the
main
form
of
N
taken
up
by
plants
from
the
soil.
3.4.
Characteristics
of
the
digestate
defining
the
C
and
N
dynamics
in
soil
The
digestate
composition
and
stability
parameters
(BOD
5
d
and
C-mineralisation
in
soil)
were
highly
interrelated
(
with
highly
significant
correlations
between
DOC
and
BOD
24
h
and
BOD
5
d
and
the
7-day
C-mineralisation
from
digestate
(C
7
d
).
The
latter
is
in
agreement
with
the
results
presented
by
who
related
the
amount
of
TOC
mineralised
from
crop
residues
after
7
days
of
incubation
in
soil
mainly
to
the
water-
soluble
organic
fraction
added
with
the
amendments.
In
addition,
DOC,
BOD
24
h
,
BOD
5
d
and
C
7
d
correlated
significantly
with
key
parameters
which
define
C
and
N
turnover
in
soil
after
digestate
addition,
such
as
mineralised-C
and
both
N
m
and
NC;
however,
for
the
two
latter
parameters,
the
DOC/TN
and
C
7
d
/TN
ratios
showed
the
most
significant
correlations
(
These
results
suggest
that
DOC,
BOD
5
d
and
C
7
d
,
which
represent
the
most
labile
organic
fraction
of
the
digested
materials,
together
with
their
ratios
with
respect
to
the
TN,
can
be
considered
reliable
criteria
to
assess
C
and
N
dynamics
in
soil
after
digestate
addition.
Although
the
com-
position
of
the
digestates
differed
quite
a
lot
in
the
present
study,
significant
regression
equations
were
obtained,
which
allows
cau-
tious
prediction
of
the
C-mineralisation
and
N-fertilising
potential
of
these
materials
in
soil,
especially
from
BOD
data
(in
mg
L
−1
),
avoiding
time
consuming
procedures
of
mineralisation
tests
in
soil:
Mineralised-C
(
g
C
g
−1
soil)
=
523.4
×
log
BOD
24
h
−
812.9;
r
2
=
0.903
(P
<
0.01).
N
m
(%)
=
−51.0
×
logBOD
24
h
+
135.4;
r
2
=
0.970
(P
<
0.001).
N
m
(%)
=
−51.6
×
logBOD
5
d
+
160.8;
r
2
=
0.915
(P
<
0.01).
NC
(%)
=
−69.8
×
logBOD
24
h
+
262.0;
r
2
=
0.892
(P
<
0.01).
NC
(%)
=
−87.7
×
log(DOC/TN)
+
46.5;
r
2
=
0.915
(P
<
0.01).
According
to
the
BOD,
DOC
and
DOC/TN
data,
digestates
from
cattle
slurry–glycerine
mixtures
(CG
samples)
constitute
a
group
clearly
different
from
the
rest
of
the
digestates
and
represent
highly
biodegradable
materials,
indicating
that
anaerobic
co-digestion
in
this
case
did
not
produce
stable
materials.
The
CG
digestates
were
characterised
by
the
highest
DOC
concentrations
(>10
g
L
−1
fresh
weight,
accounting
for
>59%
of
TOC),
DOC/TN
>
5,
BOD
5
d
>
37
g
L
−1
fresh
weight
and
the
lowest
percentages
of
TN
as
NH
4
–N
(52
and
38%
for
CG4
and
CG6,
respectively).
These
digestates
were
charac-
terised
by
an
intense
initial
period,
with
a
high
respiration
activity,
after
their
addition
to
soil—during
which
N
was
mainly
immo-
bilised.
This
limits
their
N-fertilising
potential
and
hence
their
possible
use
in
agriculture,
since
further
stabilisation
is
necessary
before
their
use.
In
contrast,
the
CO
and
PS
samples
showed
the
lowest
DOC
con-
centrations
(1.2
g
L
−1
each,
accounting
for
only
12
and
21%
of
TOC
for
CO
and
PS,
respectively)
and
a
high
stability
degree
according
to
the
BOD
5
d
test
(
which
led
to
the
lowest
CO
2
–C
produc-
tion
in
the
soil
(
These
digestates
showed
DOC/TN
ratios
<1,
clearly
lower
than
those
of
the
CG
samples,
and
high
percent-
Table
4
Significant
correlations
found
among
parameters
related
to
digestate
composition,
and
C-
and
N-mineralisation
dynamics
(n
=
6).
Parameters
C
7
d
C
7
d
/TN
Mineralised-C
DOC
DOC/TN
BOD
24
h
BOD
5
d
Net
N-min
N
m
NC
C
7
d
1
C
7
d
/TN
1
Mineralised-C
0.889
1
DOC
0.914
0.943
1
DOC/TN
0.991
0.912
1
BOD
24
h
0.945
0.950
0.944
1
BOD
5
d
0.944
0.880
0.953
1
Net
N-min
−0.943
−0.949
−0.940
1
N
m
−0.977
−0.918
−0.961
1
NC
−0.981
−0.878
−0.956
0.975
1
C
7
d
:
7-day
C-mineralisation
from
digestate
(mg
L
−1
fresh
digestate
weight),
mineralised-C:
the
amount
of
CO
2
–C
evolved
from
digestate-treated
soil
after
56
days
of
incubation
(
g
C
g
−1
dry
soil),
DOC:
dissolved
organic
carbon
(mg
L
−1
fresh
digestate
weight),
BOD
24
h
:
24-h
biochemical
oxygen
demand
(mg
O
2
L
−1
fresh
digestate
weight),
BOD
5
d
:
5-day
biochemical
oxygen
demand
(mg
O
2
L
−1
fresh
digestate
weight),
TN:
total
nitrogen
(mg
L
−1
fresh
digestate
weight),
net
N-min:
the
net
N-mineralisation
in
the
amended
soils
after
56
days
of
incubation
(
g
N
g
−1
dry
soil),
N
m
:
nitrogen
mineralisation
after
56
days
of
incubation
(%
of
TN
from
digestate)
and
NC:
nitrate
conversion
as
a
percentage
of
added
TN
that
had
been
converted
into
nitrate
from
digestate
after
56
days
of
incubation
in
soil
(%
of
TN
from
digestate).
*
Significant
at
probability
level
P
<
0.05.
**
Significant
at
probability
level
P
<
0.01.
***
Significant
at
probability
level
P
<
0.001.

J.A.
Alburquerque
et
al.
/
Agriculture,
Ecosystems
and
Environment
160 (2012) 15–
22
21
ages
of
TN
as
NH
4
–N
(>50%),
which
improve
the
soil
N
balance
(44
and
59%
of
added
TN
for
CO
and
PS,
respectively,
was
converted
into
nitrate
with
low
N-immobilisation),
representing
an
available
N
source
for
plants.
Based
on
the
characteristics
of
such
diges-
tates,
DOC
<
1.5
g
L
−1
(<25%
of
TOC),
BOD
5
d
<
2.5
g
L
−1
,
C
m
<
35%
and
DOC/TN
<
1
can
be
used
to
define
a
high
quality
digestate
appropri-
ate
for
use
as
a
fertiliser.
The
characteristics
of
the
PSB
and
CMS
digestates
showed
a
higher
similarity
to
CO
and
PS
(stable
digested
materials)
than
to
the
CG
samples
(highly
unstable).
The
PSB
sample
showed
a
relatively
low
BOD
5
d
value,
in
accordance
with
its
low
TOC
content
(5.9
g
L
−1
fresh
weight);
however,
it
was
highly
labile
(41%
of
TOC
as
DOC)
and
had
the
highest
percentage
of
TN
as
NH
4
–N
(87%).
This,
combined
with
its
low
DOC/TN
ratio
(0.6),
could
explain
the
observed
priming
effect
on
C-mineralisation
when
PSB
was
added
to
soil;
however,
negative
effects
on
the
soil
N
dynamic
were
not
seen.
Finally,
the
CMS
sample
showed
an
acceptable
degree
of
stability
considering
its
much
higher
organic
load
relative
to
CO
and
PS
(33.7
g
TOC
L
−1
fresh
weight,
only
16%
as
DOC);
however,
it
showed
a
relatively
high
CO
2
–C
production
at
the
end
of
the
incubation,
similar
to
that
resulting
from
CG4
addition
to
soil.
The
CMS
sample
had
a
DOC/TN
ratio
of
1.4
with
61%
of
its
TN
as
NH
4
–N,
leading
to
a
percentage
conversion
of
TN
into
nitrate
of
about
50%.
Therefore,
digestate
properties
such
as
DOC
<
5.5
g
L
−1
(<45%
of
TOC),
BOD
5
d
<
6.0
g
L
−1
and
DOC/TN
<
1.5
can
be
established
as
less
restrictive
quality
cri-
teria
compared
with
those
defined
previously
for
stable
digested
materials.
The
suitable
land
application
of
these
digestates
may
require
some
precautions
depending
on
the
land
use
and
manage-
ment.
A
curing
or
maturation
period
may
be
necessary
in
order
to
increase
digestate
stability
or
to
allow
digestates
to
stabilise
in
soil
before
sowing.
4.
Conclusions
Our
results
demonstrate
that
digestate
composition
and
stabil-
ity
degree
can
vary
greatly
depending
on
the
raw
materials
used
for
co-digestion
and
process
characteristics.
Therefore,
the
anaerobic
co-digestion,
including
the
selection
of
co-substrates,
must
guaran-
tee
that
a
certain
degree
of
OM
stability
of
the
digested
material
is
reached
in
order
to
avoid
detrimental
effects
on
the
plant–soil
sys-
tem.
These
are
mainly
conditioned
by
the
biodegradability
of
the
digested
material
obtained,
which
determines
whether
the
diges-
tate
can
be
used
directly
(highly
stable),
after
a
curing/maturation
period
(moderately
stable)
or
after
aerobic
post-treatments
to
achieve
stabilisation
(highly
unstable).
In
this
context,
the
biochemical
oxygen
demand
(BOD
24
h
or
BOD
5
d
),
dissolved
organic-C
(DOC)
together
with
the
DOC/TN
can
be
considered
the
most
reliable
parameters
to
describe
digestate
biodegradability
and
hence
provide
a
quick
estimation
of
the
main
effects
on
C
and
N
turnover
produced
by
digestate
addition
to
soil.
Highly
unstable
and
easily
biodegradable
materials,
represented
in
our
study
by
digestate
from
cattle
slurry–glycerine
mixtures
(BOD
5
d
>
37
g
O
2
L
−1
fresh
weight,
DOC
>
10
g
L
−1
fresh
weight
and
DOC/TN
>
5),
gave
rise
to
a
strong
CO
2
–C
production
and
a
loss
of
N-fertiliser
value
through
N-immobilisation
in
soil.
These
materials
need
more
extensive
biological
treatment,
such
as
aerobic
stabilisa-
tion,
in
order
to
reduce
potential
detrimental
impacts.
In
contrast,
ammonium
in
the
more
stable
digested
materials
was
oxidised
by
nitrifiers
in
the
very
short
term.
These
less
biodegradable
digested
materials
(BOD
5
d
<
6.0
g
O
2
L
−1
fresh
weight,
DOC
<
5.5
g
L
−1
fresh
weight
and
DOC/TN
<
1.5)
showed
a
scaled
degree
of
stability,
from
highly
stabilised
materials,
which
can
be
used
directly
in
soils
as
fertilisers,
to
moderately
stabilised
materials,
which
may
need
a
curing/maturation
period
or
stabilisation
in
the
soil
before
sowing.
Acknowledgements
This
research
was
funded
by
the
“Ministerio
de
Ciencia
e
Inno-
vación,
Plan
Nacional
I+D+I
2008-2011”
and
FEDER
Funds
“Fondo
Europeo
de
Desarrollo
Regional,
una
manera
de
hacer
Europa”,
in
the
framework
of
the
project
“singular
estratégico
PROBIOGAS”:
sub-project
3
Agronomical
evaluation
of
digestates;
and
subproject
8,
Co-digestion
of
citric
and
farm
residues
(Refs.:
PSS-120000-2008-58;
PSS-120000-2008-62).
The
authors
thank
all
the
research
groups
involved
in
the
project
PROBIOGAS
(
),
especially
the
GIRO
and
AINIA
Technological
Centres,
the
Univer-
sity
of
Oviedo,
San
Ramón
Group
and
Treatments
of
Juneda
Society
(Tracjusa)
for
providing
the
digested
materials
used
in
this
work.
The
authors
wish
to
thank
Dr.
D.J.
Walker
for
the
English
revision.
References
Abdullahi,
Y.A.,
Akunna,
J.C.,
White,
N.A.,
Hallett,
P.D.,
Wheatley,
R.,
2008.
Investigat-
ing
the
effects
of
anaerobic
and
aerobic
post-treatment
on
quality
and
stability
of
organic
fraction
of
municipal
solid
waste
as
soil
amendment.
Bioresour.
Technol.
99,
8631–8636.
Adani,
F.,
Confalonieri,
R.,
Tambone,
F.,
2004.
Dynamic
respiration
index
as
a
descrip-
tor
of
the
biological
stability
of
organic
wastes.
J.
Environ.
Qual.
33,
1866–1876.
Álvarez,
J.A.,
Otero,
L.,
Lema,
J.M.,
2010.
A
methodology
for
optimising
feed
compo-
sition
for
anaerobic
co-digestion
of
agro-industrial
wastes.
Bioresour.
Technol.
101,
1153–1158.
Al
Seadi,
T.,
2002.
Good
practice
in
quality
management
of
AD
residues
from
biogas
production.
IEA
Bioenergy,
Task
24-Energy
from
Biological
Con-
version
of
Organic
Waste,
January
2002
(Available
at:
bio/pdf/manage.pdf
).
APHA,
2005.
Standard
Methods
for
the
Examination
of
Water
&
Wastewater,
21st
ed.
American
Public
Health
Association,
American
Water
Works
Association
and
Water
Environment
Federation,
Washington,
DC,
USA.
Barrena,
R.,
Vázquez,
F.,
Sánchez,
A.,
2006.
The
use
of
respiration
indices
in
the
composting
process:
a
review.
Waste
Manage.
Res.
24,
37–47.
Bernal,
M.P.,
Kirchmann,
H.,
1992.
Carbon
and
nitrogen
mineralization
and
ammo-
nia
volatilization
from
fresh,
aerobically
and
anaerobically
treated
pig
manure
during
incubation
with
soil.
Biol.
Fert.
Soils
13,
135–141.
Brookman,
S.K.E.,
1997.
Estimation
of
biochemical
oxygen
demand
in
slurry
and
effluents
using
ultra-violet
spectrophotometry.
Water
Res.
31,
372–374.
Clemens,
J.,
Huschka,
A.,
2001.
The
effect
of
biological
oxygen
demand
of
cattle
slurry
and
soil
moisture
on
nitrous
oxide
emissions.
Nutr.
Cycl.
Agroecosys.
59,
193–198.
Copperband,
L.R.,
Stoner,
A.G.,
Fryda,
M.R.,
Ravet,
J.L.,
2003.
Relating
compost
mea-
sures
of
stability
and
maturity
to
plant
growth.
Compost
Sci.
Util.
11,
113–124.
de
la
Fuente,
C.,
Clemente,
R.,
Martínez,
J.,
Bernal,
M.P.,
2010.
Optimisation
of
pig
slurry
application
to
heavy
metal
polluted
soils
monitoring
nitrification
pro-
cesses.
Chemosphere
81,
603–610.
Dendooven,
L.,
Bonhomme,
E.,
Merckx,
R.,
Vlassak,
K.,
1998.
N
dynamics
and
sources
of
N
2
O
production
following
pig
slurry
application
to
a
loamy
soil.
Biol.
Fert.
Soils
26,
224–228.
Drennan,
M.F.,
DiStefano,
T.D.,
2010.
Characterization
of
the
curing
process
from
high-solids
anaerobic
digestion.
Bioresour.
Technol.
101,
537–544.
Drury,
C.F.,
Voroney,
R.P.,
Beauchamp,
E.G.,
1991.
Availability
of
NH
4
+
-N
to
microor-
ganisms
and
the
soil
internal
N
cycle.
Soil
Biol.
Biochem.
23,
165–169.
Fuchs,
J.C.,
Berner,
A.,
Mayer,
J.,
Smidt,
E.,
Schleiss,
K.,
2008.
Influence
of
compost
and
digestates
on
plant
growth
and
health:
potentials
and
limits.
In:
Fuch,
J.S.,
Kupper,
T.,
Tamm,
L.,
Schenk,
K.
(Eds.),
Proceedings
of
the
International
Congress
CODIS
2008.
27–29
February
2008,
Solothurn,
Switzerland
(Available
at:
http://orgprints.org/13135/1/fuchs-etal-proceedings-codis-2008.pdf
Holm-Nielsen,
J.B.,
Al
Seadi,
T.,
Oleskowicz-Popiel,
P.,
2009.
The
future
of
anaerobic
digestion
and
biogas
utilization.
Bioresour.
Technol.
100,
5478–5484.
Kirchmann,
H.,
Lundvall,
A.,
1993.
Relationship
between
N
immobilization
and
volatile
fatty
acids
in
soil
after
application
of
pig
and
cattle
slurry.
Biol.
Fert.
Soils
15,
161–164.
Lasaridi,
K.E.,
Stentiford,
E.I.,
1998.
A
simple
respirometric
technique
for
assessing
compost
stability.
Water
Res.
32,
3717–3723.
MARM,
2010
Guía
practica
de
la
fertilización
racional
de
los
cultivos
en
Espa ˜
na.
Ministerio
de
Medio
Ambiente
y
Medio
Rural
y
Marino.
Myrold,
D.D.,
Posavatz,
N.R.,
2007.
Potential
importance
of
bacteria
and
fungi
in
nitrate
assimilation
in
soil.
Soil
Biol.
Biochem.
39,
1737–1743.
Orzi,
V.,
Cadena,
E.,
D’Imporzano,
G.,
Artola,
A.,
Davoli,
E.,
Crivelli,
M.,
Adani,
F.,
2010.
Potential
odour
emission
measurement
in
organic
fraction
of
municipal
solid
waste
during
anaerobic
digestion:
Relationship
with
process
and
biological
stability
parameters.
Bioresour.
Technol.
101,
7330–7337.
Pesta,
G.,
2007.
Anaerobic
digestion
of
organic
residues
and
wastes.
In:
Oreopoulou,
V.,
Russ,
W.
(Eds.),
Utilization
of
By-products
and
Treatment
of
Waste
in
the
Food
Industry.
Springer,
New
York,
pp.
53–72.
Ponsá,
S.,
Gea,
T.,
Sánchez,
A.,
2010.
Different
indices
to
express
biodegradability
in
organic
solid
wastes.
J.
Environ.
Qual.
39,
706–712.

22
J.A.
Alburquerque
et
al.
/
Agriculture,
Ecosystems
and
Environment
160 (2012) 15–
22
Qiu,
S.,
McComb,
A.J.,
Bell,
R.W.,
2008.
Ratios
of
C,N
and
P
in
soil
water
direct
microbial
immobilisation-mineralisation
and
N
availability
in
nutrient
amended
sandy
soils
in
southwestern
Australia.
Agr.
Ecosyst.
Environ.
127,
93–99.
Riffaldi,
R.,
Saviozzi,
A.,
Levi-Minzi,
R.,
1996.
Carbon
mineralization
kinetics
as
influ-
enced
by
soil
properties.
Biol.
Fert.
Soils
22,
293–298.
Salminen,
E.,
Rintala,
J.,
Härkönen,
J.,
Kuitunen,
M.,
Högmander,
H.,
Oikari,
A.,
2001.
Anaerobically
digested
poultry
slaughterhouse
wastes
as
fertiliser
in
agriculture.
Bioresour.
Technol.
78,
81–88.
Sánchez,
M.,
Gomez,
X.,
Barriocanal,
G.,
Cuetos,
M.J.,
Morán,
A.,
2008.
Assessment
of
the
stability
of
livestock
farm
wastes
treated
by
anaerobic
digestion.
Int.
Biodeter.
Biodegr.
62,
421–426.
Schievano,
A.,
D’Imporzano,
G.,
Malagutti,
L.,
Fragali,
M.,
Ruboni,
G.,
Adani,
F.,
2010.
Evaluating
inhibition
conditions
in
high-solids
anaerobic
digestion
of
organic
fraction
of
municipal
solid
waste.
Bioresour.
Technol.
101,
5728–5732.
Smith,
K.A.,
Metcalfe,
P.,
Grylls,
J.,
Jeffrey,
W.,
Sinclair,
A.,
2007.
Nutrient
value
of
digestate
from
farm-based
biogas
plants
in
Scotland.
Report
for
Scottish
Exec-
utive
Environment
and
Rural
Affairs
Department-ADA/009/06
(Available
at:
http://www.scotland.gov.uk/Resource/Doc/1057/0053041.pdf
Sommer,
S.G.,
Kjellerup,
V.,
Kristjansen,
O.,
1992.
Determination
of
total
ammonium
nitrogen
in
pig
and
cattle
slurry:
sample
preparation
and
anal-
ysis.
Acta
Agriculturae
Scandinavica
Section
B.
Soil
and
Plant
Science
42,
146–151.
Tambone,
F.,
Genevini,
P.,
D’Imporzano,
G.,
Adani,
F.,
2009.
Assessing
amendment
properties
of
digestate
by
studying
the
organic
matter
composition
and
the
degree
of
biological
stability
during
the
anaerobic
digestion
of
the
organic
frac-
tion
of
MSW.
Bioresour.
Technol.
100,
3140–3142.
Trinsoutrot,
I.,
Recous,
S.,
Bentz,
B.,
Linères,
M.,
Chèneby,
D.,
Nicolardot,
B.,
2000.
Biochemical
quality
of
crop
residues
and
carbon
and
nitrogen
mineraliza-
tion
kinetics
under
nonlimiting
nitrogen
conditions.
Soil
Sci.
Soc.
Am.
J.
64,
918–926.
USEPA,
2007.
Method
9210A.
Potentiometric
determination
of
nitrate
in
aque-
ous
samples
with
an
ion-selective
electrode
(Available
at:
epa.gov/epawaste/hazard/testmethods/sw846/pdfs/9210a.pdf
).
Williams,
A.G.,
1983.
Organic
acids,
biochemical
oxygen
demand
and
chemical
oxy-
gen
demand
in
the
soluble
fraction
of
piggery
slurry.
J.
Sci.
Food
Agric.
34,
212–220.
Document Outline
- Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils
Wyszukiwarka
Podobne podstrony:
Właściwości Aroni i jej wpływ na, ZDROWIE-Medycyna naturalna, 01-Do uporządkowania, ZIOŁA
Potencjał nawozowy pofermentu z pozostałości z farmy i przemysłu agro Hiszpania 2012
Wielkie posiadłości ziemskie w Rzeczypospolitej Obojga Narodów i ich wpływ na dzisiejsze środowisko
Stolarczyk, Tomasz Kryzys i reforma życia zakonnego XIV–XVI wieku i jego wpływ na dominikański klas
Koloidy glebowe i ich wpływ na właściwości gleby
3.Obróbka cieplna stali narzędziowej i jej wpływ na właściwości
Właściwości reologiczne półprod. ciastkarskich i ich wpływ na jakośc wyrobów
Własciowosci aeracyjne gleby i ich wpływ na roślinki, Studia - Ochrona Środowiska
Zmiany właściwości fiz półprod piekarskich i ich wpływ na teksturę gotowych produktów
Struktura-wpływ na właściwości, Studia, Materiały Konstrukcyjne - PNOM
Podstawy ogrodnictwa próchnica glebowa i jej wpływ na właściwości gleby
Kurasiński Właściwości współczesnych operacji militarnych i ich wpływ na organizację zabezpieczenia
Wymień i scharakteryzuj nawozy organiczne stosowane w produkcji roślinnej i ich wpływ na właściwości
, laboratorium inżynierii chemicznej, sprawozdanie Wpływ energii mieszania na współczynnik wnikania
Rdzany, Zbigniew Wpływ energii geotermalnej na dynamikę strumieni lodowych lądolody warty w Polsce
Techniki wywierania wplywu oparte na dynamice interakcji
wlasciwosci chemiczne alkenow 1 ppt
więcej podobnych podstron