
Ž
.
Fitoterapia 71 2000 501
᎐506
Factors affecting volatile terpene and
non-terpene biotransformation products in
plant cell cultures
W. Zhu, G. Asghari, G.B. Lockwood
U
School of Pharmacy & Pharmaceutical Scienes, Uni
¨
ersity of Manchester, Manchester M13 9PL,
UK
Received 7 January 2000; accepted in revised form 16 February 2000
Abstract
Suspension cultures from Peganum harmala were shown to carry out biotransformations
of a number of terpenes and non-terpenes. The rate of biotransformation was dependant
upon substrate concentration, density of cell suspensions, and the structure and isomeric
form of the substrates.
䊚 2000 Elsevier Science B.V. All rights reserved.
Keywords: Peganum harmala; Biotransformation; Essential oil constituents
1. Introduction
The production and accumulation of volatile essential oil constituents in plant
w x
cell cultures has been reviewed 1,2 . Although plant cells cultured in vitro are
considered to be totipotent, i.e. contain all necessary genetic material to carry out
any or all of the functions shown by the intact plant, in practice many either fail to
produce essential oil constituents or produce a few in only very low levels. It is
often accepted that as undifferentiated cultures contain no structures such as
w x
trichomes or vittae for storage of these constituents 3 none will accumulate. In
U
Corresponding author. Tel.:
q44-161-275-2334; fax: q44-161-275-2396.
Ž
.
E-mail address: lockwood@fsl.pa.man.ac.uk G.B. Lockwood .
0367-326X
r00r$ - see front matter 䊚 2000 Elsevier Science B.V. All rights reserved.
Ž
.
PII: S 0 3 6 7 - 3 2 6 X 0 0 0 0 1 6 0 - X

(
)
W. Zhu et al.
rFitoterapia 71 2000 501᎐506
502
many systems particular enzyme systems needed for a biosynthetic step have been
w x
shown to be present 1 , but inoperative, and this inhibits production of the end
Ž .
product s . A number of workers have attempted to improve production and
w x
accumulation of these compounds by feeding precursors 1,2 , but levels are still
well below those of intact plants. We decided to use a range of techniques to
investigate if levels of biotransformation products could be increased. Biotransform-
ation of geraniol acetate to geraniol by plant cell cultures has not previously been
Table 1
Volatile substrates and their major biotransformation products produced by P. harmala suspension
cultures
Substrate
Product
Substrate
Products
Benzyl acetate
Benzyl alcohol
Bornyl acetate
Borneol
Cinnamyl acetate
Cinnamyl alcohol
Dihydrocarvyl
Dihydrocarveol
acetate
Farnesyl acetate
Farnesol
Fenchyl acetate
Fenchol
Geranyl acetate
Geraniol
Linalyl acetate
Linalool
␣-Terpineol
Geranyl acetate
Menthyl acetate
Menthol
Neryl acetate
Nerol
Geraniol
Phenethyl acetate
Phenethyl alcohol
5-Acetoxymethyl-2-
5-Acetoxymethyl-2-
furaldehyde
furfuryl alcohol
m, p and o-
m, p & o-Anisyl
Benzaldehyde
Benzyl alcohol
Anisaldehyde
alcohol
Cinnamaldehyde
Cinnamyl alcohol
Citral
Geraniol
Hydrocinnamyl
Nerol
alcohol
Citronellal
Citronellol
Cumic aldehyde
Cumic alcohol
3,5-Dimethoxy-
3,5-Dimethoxy-
benzaldehyde
benzyl alcohol
Ethylvanillin
Ethylvanillyl
Furfural
Furfuryl alcohol
alcohol
Hydrocinnamal-
Hydrocinnamyl
Isovanillin
Isovanillyl alcohol
dehyde
alcohol
Myristicin aldehyde
Myristicin alcohol
Myrtenal
Myrtenol
Myrtanol
Perillaldehyde
Perilla alcohol
Phenethylaldehyde
Phenethyl alcohol
Piperonal
Piperonol
Salicylaldehyde
Salicyl alcohol
-Ionone
-Ionol
Menthone
Menthol
7-Hydro-
-ionone
Piperitol
Geraniol
Nerol
Verbenol
Verbenone
d-Limonene
Limonene epoxide
␣-Pinene
Verbenol
p-2,8-Menthadien-1-
Verbenone
ol
Carveol
-Pinene
Myrtenol
␥-Terpinene
p-Cymene
Myrtenal
p-Cymen-8-ol
(
)
W. Zhu et al.
rFitoterapia 71 2000 501᎐506
503
reported, although there is one report of the reverse reaction occurring, in a study
w x
of the biogenesis of monoterpenes using cultures derived from Muscat grapes 4 .
However, a few reports have described the biotransformation of other terpene
w x
acetates into their parent alcohols by plant cell cultures 5,6 , but in both instances
the parent alcohol was only the major product, not the sole product. In an attempt
to show variation in levels of biotransformation of volatile terpenoids and non-
Ž
.
terpenoids, we chose suspension cultures of Peganum harmala L. Zygophyllaceae
for the feeding experiments.
2. Experimental
2.1. Cell culture
Seeds of P. harmala from Esfahan Botanical Research Centre, Iran, were
surface-sterilised with 30% w
rv hydrogen peroxide containing one drop of Triton,
Ž
.
w x
and a primary callus was maintained in Murashige & Skoog media MS
7
containing 3% sucrose, 5 mg
rl ascorbic acid, 1.0 mgrl 2,4-dichlorophenoxyacetic
Ž
.
acid
2,4-D , and 0.1 mg
rl kinetin. The suspension culture from the fourth
Ž
generation of callus culture was maintained in the same media as callus except
.
without the agar in 24-h continuous light at 25
" 2⬚C and subcultured every 3
weeks. Subcultures were done by transferring 10 ml of the old suspension to 90 ml
of the new media.
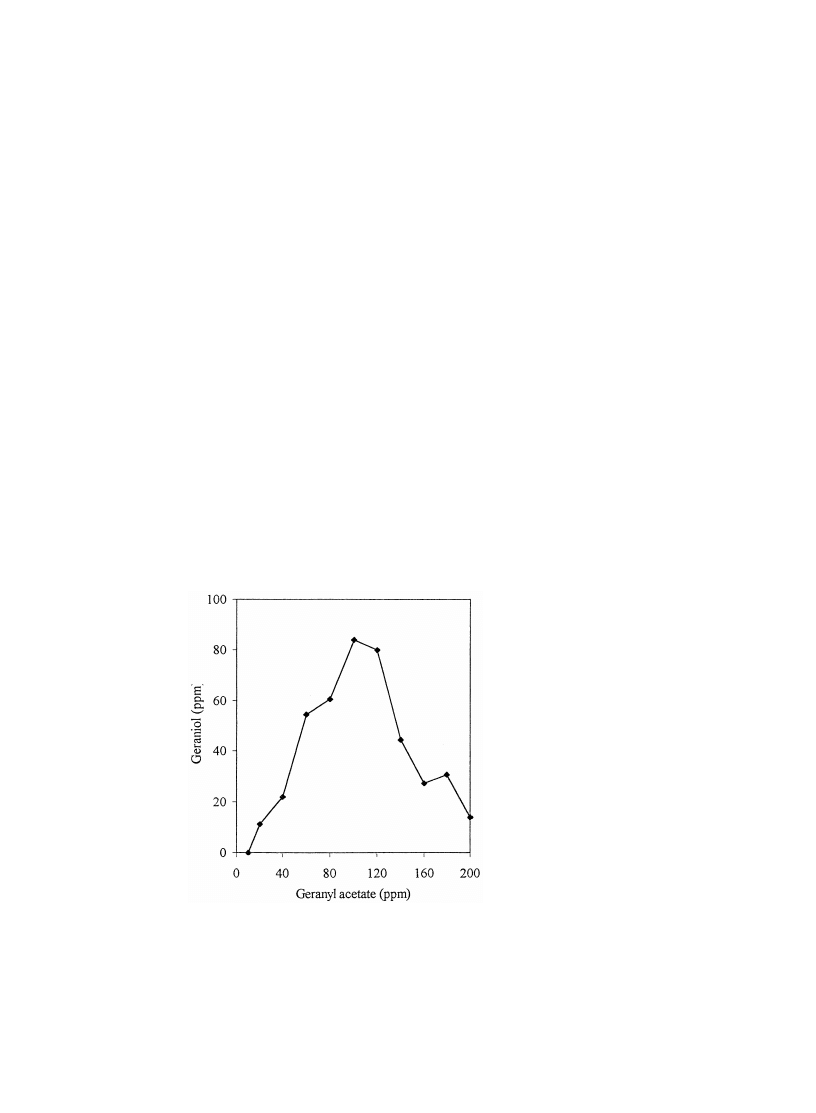
Fig. 1.
Effect of geranyl acetate substrate concentration on product levels in P. harmala suspension
cultures.
(
)
W. Zhu et al.
rFitoterapia 71 2000 501᎐506
504
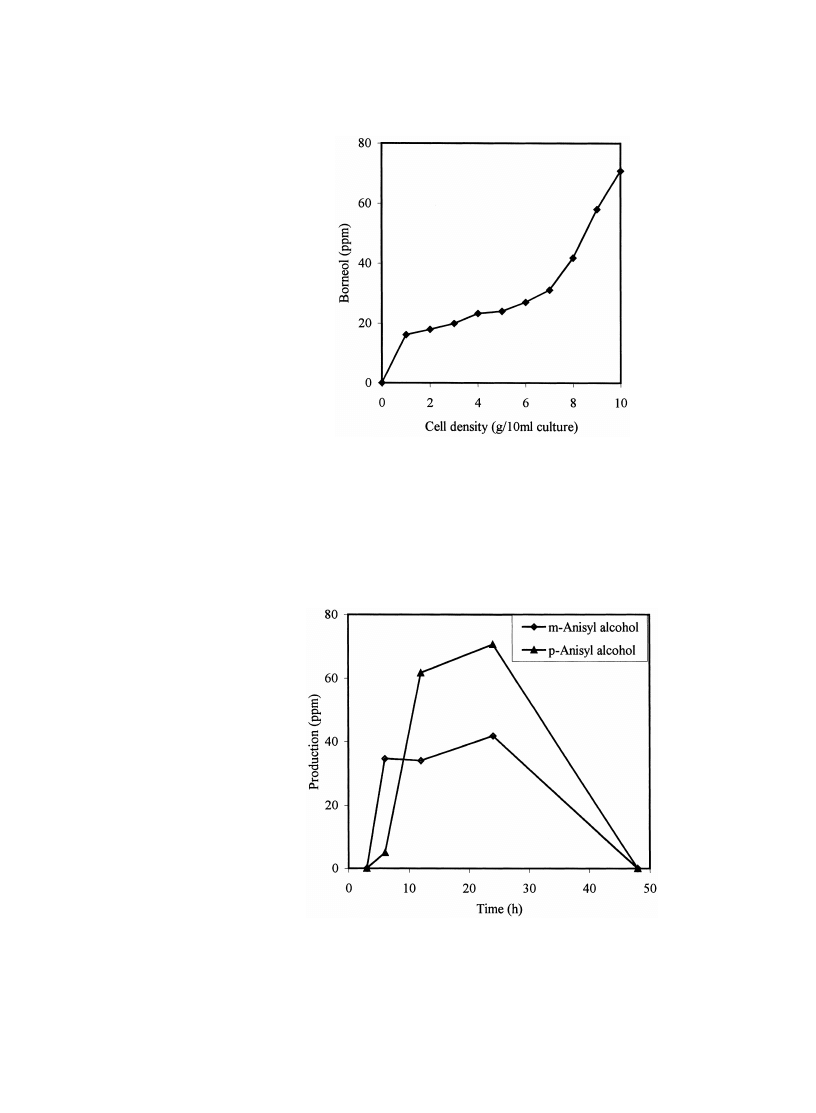
Fig. 2.
Effect of cell density on levels of product after feeding bornyl acetate to P. harmala suspension
cultures.
2.2. Biotransformation
Ž
.
Substrates Fluka and Aldrich were mixed with 70% EtOH and injected through
Ž
.
a preautoclaved Mobile Phase Filter 0.45-mM pore size, Whatman to the suspen-
Fig. 3.
Levels of products after feeding structural isomers of anisaldehyde to P. harmala suspension
cultures.

(
)
W. Zhu et al.
rFitoterapia 71 2000 501᎐506
505
sion, 5 days after subculture. Feeding concentration was 100 ppm unless otherwise
Ž
.
stated. Samples 5 ml were removed from the suspensions after 24 h inoculation,
and analysed.
2.3. Analysis
Ž
.
Aliquots 5 ml were extracted with 5 ml CH Cl and pentadecane as an internal
2
2
standard was added prior to extraction. The CH Cl
layer was passed through
2
2
Whatman No.1 filter paper, and dried with anhydrous sodium sulfate. The organic
layer was evaporated under nitrogen to reduce the volume to 100
l, and subjected
to analysis by gas chromatography. Gas chromatography analysis used a HP 5890A
Ž
with an FID detector. The column was BP1 fused-silica column 25 m
= 0.32 mm,
.
film thickness 0.25 mm . Oven temperature programming was 50
᎐280⬚C, at
10
⬚Crmin, and held isothermal for 5 min. The injector and detector temperatures
were 250 and 280
⬚C, respectively. Identification of components was checked using
standards where available, and GC-MS using the same column.
3. Results and discussion
Table 1 shows substrates and the major biotransformation products produced by
freely suspended cells of P. harmala. Many of these biotransformations have been
w
x
reported for other suspension cultures 1,2,8 , but the biotransformations of
-
ionone to
-ionol and 7-hydro--ionone, and neryl acetate to nerol and geraniol
were previously unreported in plant cell cultures. The effects of substrate concen-
tration upon biotransformation levels can be seen in Fig. 1. Optimum turnover
occurs at a substrate concentration of 100 ppm. Previous reports showed that this
w x
optimum level varied from 80 to 200 ppm in Catharanthus roseus cultures 9 , while
Brown and co-workers showed that feeding from 250 to 420 ppm limonene to
w x
cultures of Pelargonium fragrans reduced viability to zero 10 . Fig. 2 shows the
effect of increasing suspension cell density on levels of biotransformation product
after feeding with bornyl acetate. Levels continue increasing up to cell densities of
10 g
r10 ml. Structural isomers have been reported to be metabolised regioselec-
w x
tively 11 , and this was also noted here with isomers of anisaldehyde. In addition,
Fig. 3 shows distinct differences in the levels of m- and p-anisaldehyde metabolites,
the anisyl alcohol isomers, over the 50 h since substrate feeding.
References
w x
1
Mulder-Krieger TH, Verpoorte R, Svendsen AB, Scheffer JJC. Plant Cell Tissue Organ Cult
1988;13:85.
w x
2
Shin S. Kor J Pharmacogn 1995;26:227.
w x
3
Everitt Z, Lockwood GB. Plant Physiol Life Sci Adv 1989;8:75.
w x
4
Ambid C, Moisseeff M, Fallot J. Plant Cell Rep 1982;1:91.
w x
5
Hirata T, Aoki T, Hiranno Y, Ito T, Suga T. Bull Chem Soc Jpn 1981;54:3527.

(
)
W. Zhu et al.
rFitoterapia 71 2000 501᎐506
506
w x
6
Hook I, Lecky R, Mckenna B, Sheridan H. Phytochemistry 1990;29:2143.
w x
7
Murashige T, Skoog F. Physiol Plant 1962;15:473.
w x
8
Suga T, Hirata T. Phytochemistry 1990;29:2393.
w x
9
Balsevich J. Planta Med 1985;49:128.
w x
10
Brown JT, Hegarty PK, Charlwood BV. Plant Sci 1987;48:195.
w x
11
Hirata T, Koya K, Sarfo KJ et al. J Mol Catal B Enzymatic 1999;6:67.
Wyszukiwarka
Podobne podstrony:
Cadmium and Other Metal Levels in Autopsy Samplesfrom a Cadmium Polluted Area and Non polluted Contr
Ziyaeemehr, Kumar, Abdullah Use and Non use of Humor in Academic ESL Classrooms
Microwaves in organic synthesis Thermal and non thermal microwave
127729 5 4a Factors affecting economic growth16 01 03
blocking and non blocking
Factors affecting
Microwaves in organic synthesis Thermal and non thermal microwave
Linear and non Linear SR 2012
part 2 7 Information Structure and Non canonical Syntax
Do Big Five personality Factors aFFect inDiviDual
ImplicituresCancelability and Non detachability
0739116452 10 17 African American Slave Medicine Herbal and non Herbal Treatments
Co existence of GM and non GM arable crops the non GM and organic context in the EU1
Performance Parameters of Explosives Equilibrium and Non Equilibrium Reactions
2005 Diet and Age Affect Intestinal Morphology and Large Bowel Fermentative End Product Concentratio
The role of child sexual abuse in the etiology of suicide and non suicidal self injury
meaning H P Grice natural and non natural meaning
A Comparison of Linear Vs Non Linear Models of Aversive Self Awareness, Dissociation, and Non Suicid
Kant and Non Euclidean Geometry
więcej podobnych podstron