
Sugar Beet Seed Institute, Karadj, Iran
Effect of Water-Deficit Stress on Germination and Early Seedling Growth in Sugar
Beet
S. Y. Sadeghian and N. Yavari
AuthorsÕ address: Dr S. Y. Sadeghian (corresponding author; e-mail: sadeghian@sbsi.ir) and N. Yavari, Sugar Beet Seed
Institute, PO Box 31585-4114, Karadj, Iran
With 2 figures and 3 tables
Received May 9, 2003; accepted August 29, 2003
Abstract
Sugar beet progeny lines screened for both high water use
efficiency and high sugar yield under drought stress
conditions in the field were assessed for the rate of seed
germination and early seedling growth in water deficit
stress, induced by mannitol solutions. Seeds of nine
different sugar beet progeny lines were grown in three
experimental conditions using filter paper, perlite and water
agar as substrate. Three levels of 0.0, 0.2 and 0.3 m
mannitol concentrations were applied in each experiment.
A factorial design was used with three replications.
Germination percentage was determined in all experiments.
Seedling growth parameters such as cotyledon fresh weight,
cotyledon dry weight, root fresh weight, root dry weight
(RDW) and root length (RL) were measured experiment-
ally. Abnormality was only recorded in the filter paper
experiment. The results showed that drought stress could
be simulated by mannitol solution and significant differ-
ences were found between stress levels for seedling charac-
teristics. Distinct genetic variances were found among
progeny lines with respect to germination and early seedling
growth characteristics, except for cotyledons and RDW.
Seedling growth and germination rates severely declined at
the highest concentration of mannitol. The rate of abnor-
mality was increased progressively at the germination stage
with an increase in mannitol concentration but it was more
pronounced in the drought-susceptible progeny lines. The
highest values of relative germination % and relative
growth % of RL were obtained for the most tolerant line.
In conclusion, seedling characteristics, in addition to other
physiological components involved in the seed germination
process under specific stress conditions, may be considered
for breeding purposes.
Key words: germination — in vitro — line-seedling-
characteristics — mannitol — stress — sugar beet
Introduction
Spring-sown sugar beet crop in semi-arid condi-
tions needs to be irrigated. This coincides with the
full growing period of other economically import-
ant crops such as wheat. Each year the sugar beet
seedlings suffer due to lack or delay of irrigation
that hampers plant growth, affects final plant
density and ultimately the root yield of the crop.
Seed germination is the most important seed
quality, and germination under laboratory condi-
tions and field establishment are closely related
characteristics that are influenced by genetic (vari-
etal), environmental (location and year) and seed-
processing (treatments) effects (Apostolides and
Goulas 1998). Studies on abiotic stress tolerance in
sugar beet have been undertaken for the identifi-
cation of physiological and environmental factors.
Germination percentage of sugar beet measured
under standard conditions correlated with the
seedling establishment in the field under stress
conditions (Durrant et al. 1985, Durrant and
Gummerson 1990).
Unfavourable
germination
conditions such as low temperature and water
stress have serious impacts on the results of vigour
tests and plant establishment of diploid and trip-
loid sugar beets in the field (Van Swaaij et al. 2001).
Delayed seedling emergence of sugar beet was
found to occur as a result of water stress and
increasing salinity (Ayers 1952). The effects of
abiotic stresses were studied on young sugar beet
seedlings in the laboratory using osmotic agents
such as mannitol, polyethylene glycol (PEG) and
salts in Petri dishes containing Whatman filter
paper (Ghoulam and Fares 2001).
Small differences in the concentration of NaCl
did not change number of germinated seeds but
greatly affected water uptake and seedling growth
(Durrant et al. 1974). Kaffka and Hembree (1999),
investigating the effect of seed priming on emer-
gence rate and seedling growth of sugar beet in
J. Agronomy & Crop Science 190, 138—144 (2004)
2004 Blackwell Verlag, Berlin
ISSN 0931-2250
U.S. Copyright Clearance Centre Code Statement:
0931–2250/2004/9002–0138 $15.00/0
www.blackwell-synergy.com

saline soils, reported a similar rate of germination
for primed seeds in saline plots and non-primed
seeds in plots with low electric conductivity of soil
(ECe). This practice is certain to become a seed-
enhancing requirement for crop production under
stressed conditions.
Nevertheless, breeders assume that there is con-
siderable variability in abiotic stress tolerance in
sugar beet germplasm. Hanson and Wyse (1982)
evaluated sugar beet, fodder beet and Beta mari-
tima
under increasing salinity conditions, and
concluded that salinization increased betaine levels
of roots and shoots two- to three-fold. Beta
maritima
accessions accumulated 40 % less betaine
in shoots than other progeny lines. Studies on
abiotic stress tolerance in sugar beet have been
undertaken for the identification of physiological
and environmental factors. Productivity and ger-
mination are linked to key properties of varieties
used by beet growers and tend to decide the
ultimate crop yield (Tugnoli and Bettini 2001).
Significant variations were found for total dry
matter, root yield, sugar yield, juice purity and
impurities of roots among sugar beet, fodder beet
and red beet when exposed to various periods of
drought stresses (Sadeghian et al. 2000).
The possibility of growing plants in controlled
conditions of greenhouse and laboratory are of
great convenience for the evaluation of genetic
traits. In many crops, seed germination and early
seedling growth are the stages most sensitive
to environmental stresses (Cook 1979). Foolad
and Lin (1999) studied the germination response
of tomato accessions in Petri dishes containing
agar media treated either with 0 mm NaCl (non-
stress and cold-stress treatments) or 150 mm
NaCl + 15 mm CaCl
2
(for salt-stress treatment).
They concluded that some of the same genetic and
physiological parameters contributing to rapid seed
germination under non-stress conditions could also
facilitate rapid seed germination under stress.
Experiments performed at germination and seed-
ling emergence stages of sugar beet under various
temperatures and NaCl concentrations indicated
that the effect of salinity on seedling emergence was
increasingly inhibitory as temperature increased
from 10–15
C to 25 and 35 C (Mahmoud and
Hill 1981). Small differences in the concentration of
NaCl did not change the rate of germinated seeds
but greatly affected water uptake and seedling
growth (Durrant et al. 1974).
The common approach in breeding for drought
tolerance of crops is to select for drought tolerance
components. As several of these components are
difficult to measure, indirect selection should be
applied (Visser 1994). Screening for drought toler-
ance has been reported in vegetable crops using
mannitol as the stress agent (Grezesiak et al. 1996).
Simulated drought condition with mannitol solu-
tion was used to estimate drought resistance in
18 cultivars of field been (Vicia faba), soya bean
(Glycine max), field pea (Pisum sativum), and lupin
(Lupinus albus). The results support the varietal
differences in seed germination and seedling growth
parameters. Osmotic adjustment in tolerant plant
helps maintain leaf metabolism and root growth at
relatively low leaf water potentials by controlling
turgor pressure in the cells. The trait can be assayed
easily by measuring growth rate of seedlings in
PEG solution (Morgan and Condon 1986). More-
over, tolerance ratings under laboratory conditions
were consistent with ratings of the effects of soil
drought on yield in the field experiments, suggest-
ing that nutrient solution containing mannitol
could be used in screening for drought tolerance
in growing seedlings.
Water deficit is one of the main limiting factors
of sugar beet production in arid and semi-arid
lands, therefore breeding programmes should be
explicitly
directed
towards
development
of
drought-tolerant varieties. We report the effects of
an increasing stress agent (mannitol concentra-
tions) on the germination and seedling growth in
sugar beet lines, with an aim to choose an
evaluation procedure for the identification of sugar
beet tolerant to water stress at germination and
early growth phase.
Materials and Methods
Nine sugar beet progeny lines – one very drought tolerant,
four tolerant, two moderate, one rather sensitive and one
sensitive (S
1
and S
2
families) – were previously identified
(Sadeghian et al. 2000) under field condition for their good
yield performances under drought conditions (Table 1).
Seeds of these progeny lines were produced in the same seed
production region, and were graded, standardized, washed,
sterilized and dried at room temperature prior to the
experiments. Germination tests were conducted in three
experiments consisting of filter paper (ISTA standard seed
testing conditions and at 22 ± 2
C), box containing
perlite (greenhouse conditions with night/day temperature
fluctuations from 15 to 23
C), and 0.8 % water-agar
medium in plates (in vitro growth conditions at 25 ± 2
C).
Stress treatments were performed at 0.0 (control), 0.2 and
0.3 m concentrations of mannitol approximately corres-
ponding to
)1, )5 and )7 bar osmotic pressures. In the
greenhouse experiment, boxes (37
· 55 · 13 cm) were
Effect of Water-Deficit Stress on Germination and Early Seedling Growth
139

watered with 1/2 diluted Hoagland solution (Hoagland and
Arnon 1950) for each different mannitol level. The amount
of this solution was adjusted daily to keep a 2-cm level at
the bottom. A factorial design with three replications was
used for each experiment and each treatment replicate
contained 100 seeds/box except in the agar medium, where
25 seeds were grown individually in culture tubes.
Germination was recorded in all the three experiments,
and percentage of abnormal seedlings (germinated seeds
failed in growing standard roots and shoots) was also
calculated in the filter paper test. Germination percentage
was recorded after 14 days in paper, 24 days in perlite and
28 days in agar medium. Seedling characteristics such as
cotyledon fresh weight (CFW), cotyledon dry weight
(CDW), root fresh weight (RFW), root dry weight
(RDW) and root length (RL) were measured in the water
agar medium. Assessments of seedling characteristics were
made 28 days after sowing. The relative germination was
determined by the following calculation: number of germi-
nated seeds in stress medium/number of germinated seeds
in control medium
· 100 (Smith and Dobrenz 1987).
Similarly, relative growth % for RL, RFW and CFW
was calculated for various progeny lines in the in vitro
experiment.
All measured variables were subjected to analysis of
variance (anova) and SAS software was used for the
correlation analysis (SAS Institute, Inc. 1996). Sugar beet
lines were compared for germination %, abnormal seedling
% and relative growth % of seedling characteristics.
Results and Discussion
Records were made on the number of germinated
seed in paper, perlite, and agar medium and of
seedling growth characteristics under in vitro con-
dition. Analysis of variance showed significant
effects of mannitol treatments and progeny lines
(P < 0.001) on the rate of germination in the three
experiments (Table 2). Germination was progres-
sively inhibited by the increase in mannitol
concentration. The strongest inhibition occurred
at the second mannitol concentration (0.3 m) par-
ticularly in perlite and water agar medium. The
response of seeds to an increase in mannitol con-
centration varied among progeny lines (Fig. 1a–c).
Drought stress simulated by mannitol and its
effects on all seedling characteristics were signifi-
cant (Table 2). The seedling growth parameters
were significantly affected by an increase in the
osmotic
pressure
of
mannitol
concentrations
(Fig. 2b–d). Except for very small values obtained
for CDW and RDW, significant differences among
the progeny lines (P < 0.01) were obtained for the
seedling growth traits (Table 2). The effect of
decreasing water potential in 0.2 and 0.3 m mann-
itol concentrations resulted in seedlings with less
fresh weight during the processes of germination
and early growth when compared with the control
treatment (Figs 1 and 2). Analysis of variance for
CFW and RFW indicated that stress condition and
progeny lines had significant differences for these
parameters. Stresses decreased water content and
Table 1: Brief descriptions of sugar beet lines evaluated
in mannitol-stressed conditions
Line no.
Identity no.
Seed type
Drought
tolerance
1
7112
mm
S
2
191
MM
RS
3
181
mm
MT
4
436m
MM
MT
5
7233-17
MM
T
6
7233-12
MM
T
7
BP-Karadj
MM
T
8
463-I-BMM
T
9
7219-69
MM
VT
MM, multigerm; mm, monogerm; S, sensitive; RS, rather
sensitive; MT, medium tolerant; VT, very tolerant.
Table 2: Mean squares from analysis of variance of sugar beet lines tested for the germination rate in filter paper,
perlite and agar medium together with seedling growth characteristics of in vitro seedlings at three levels of mannitol
concentrations
Source of
variation
d.f.
Ger.
in paper
Ger.
in perlite
Ger.
in vitro
CFW
CDW
RFW
RDW
RL
Replication
2
6.5679
14.2716
255.864
0.00526
0.00026
0.00023 0.00005
54. 583
Genotype
8
808.9753
***
676.20
***
1873.688
***
0.0429
**
0.0003
0.0011
**
0.00005
117.544
**
Mannitol
2 1901.3827
***
31071.457
***
16775.308
***
0.8719
***
0.00304
***
0.092
***
0.00022
**
4600.513
***
Variety
·
mannitol
16
134.8827
***
230.734
***
399.964
***
0.01356
0.0002
0.0005
0.00005
43.217
Error
52
37.0807
24.989
6438.272
0.7755
0.0088
0.0188
0.00168
1828.935
Ger., germination %; CFW, cotyledon fresh weight; CDW, cotyledon dry weight; RFW, root fresh weight; RDW,
root dry weight; RL, root length.
**P
¼ 0.01, ***P ¼ 0.001.
140
Sadeghian and Yavari
assimilate accumulation in the seedling as a conse-
quence of osmotic pressure induced by mannitol.
Interaction effects between genotype and mannitol
concentration were significant for the germination
rates in all cases, pointing to the fact that differ-
ences in genetic components affecting the germina-
tion response of sugar beet are expressed at early
stages under specific stress conditions. As shown in
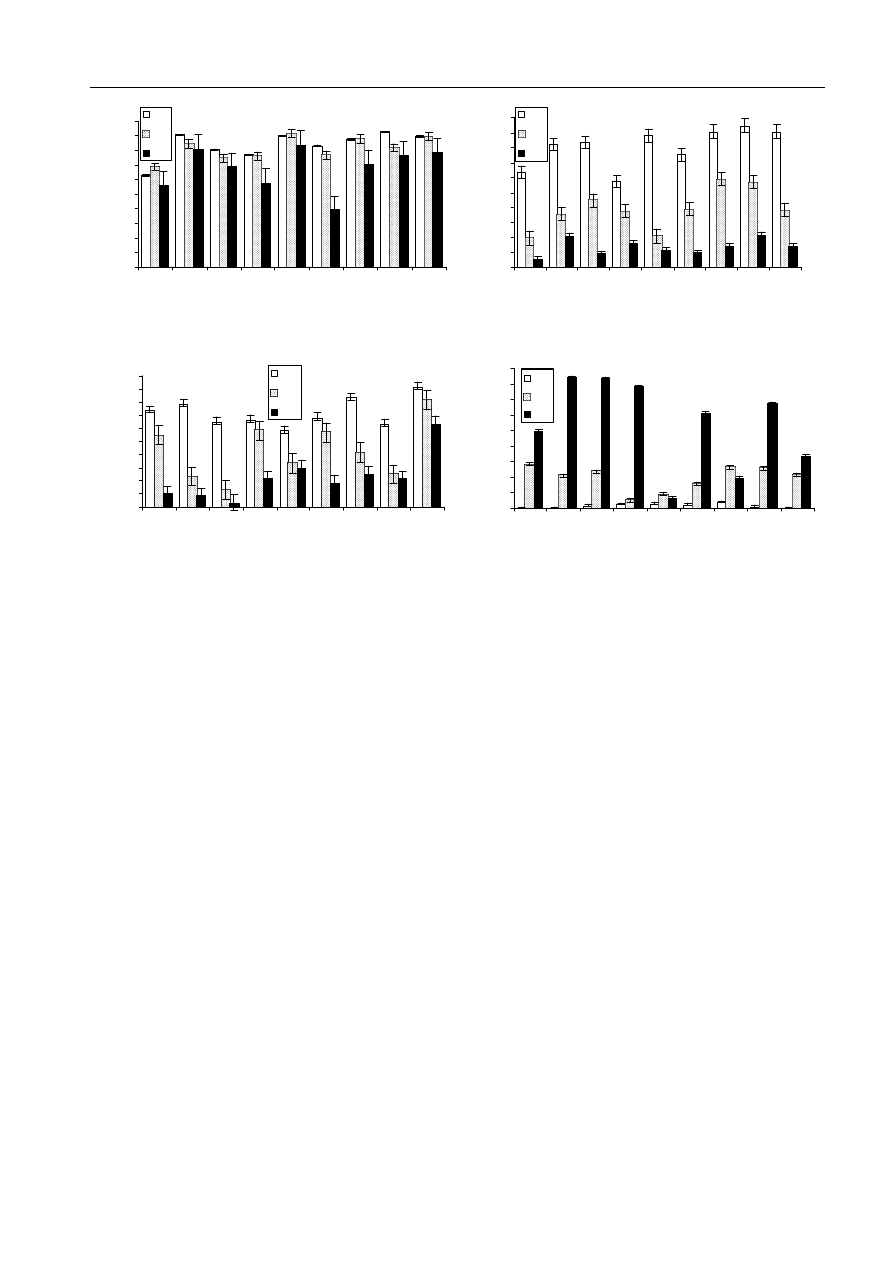
Fig. 1a, the germination percentage recorded in
paper was only slightly affected but number of
abnormal seedlings augmented, as the stress was
intensified (Fig. 1d). The differences in germination
percentage of seeds subjected to stress levels were
more detectable in the perlite and water agar
experiments (Fig. 1b,c). Germination of seed in line
nos 2 (191), 1 (7112) and 3 (181) proved to be most
sensitive to water restriction at the higher level of
osmotic potential in the in vitro experiment, but
seeds of line no. 9 (7219) revealed a relatively stable
tolerance in both stress levels (Fig. 1c).
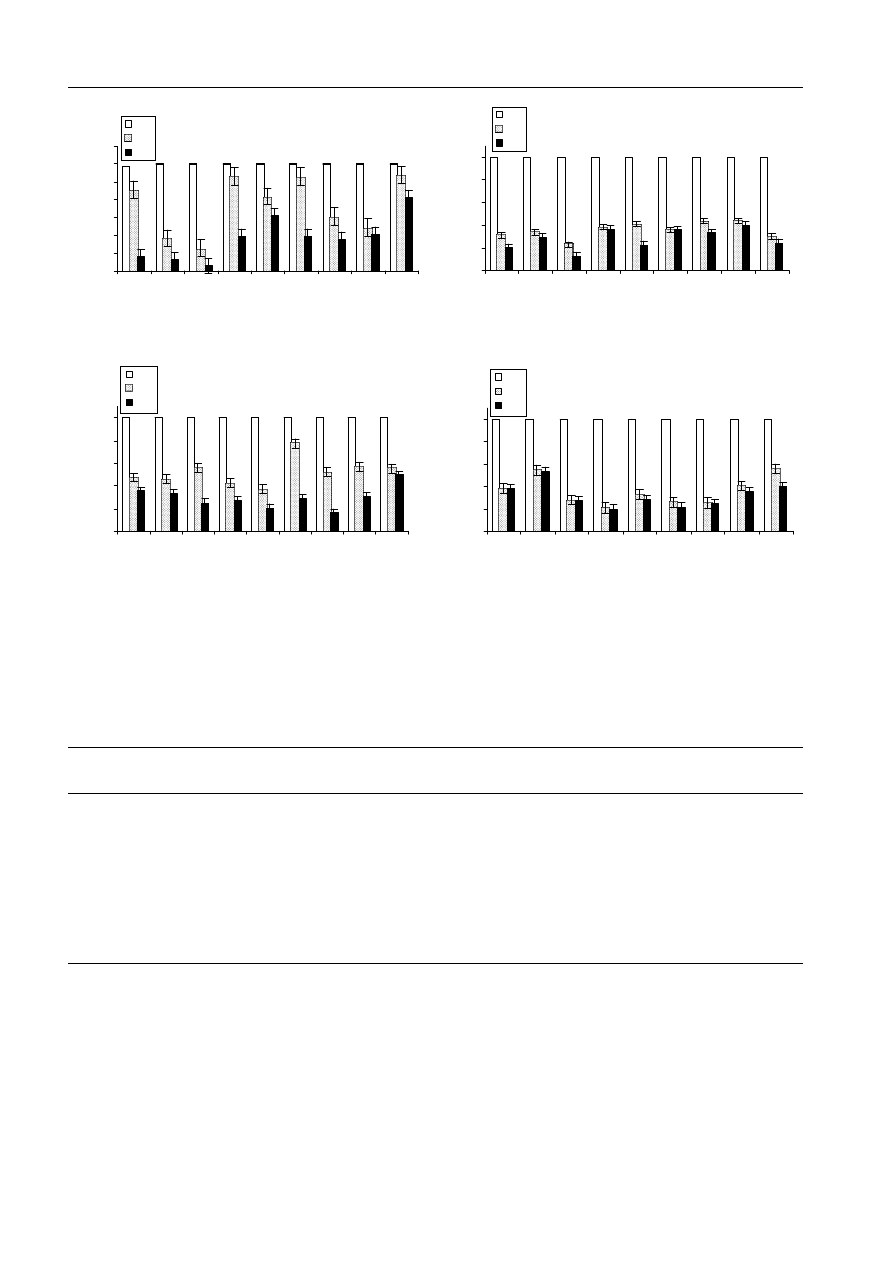
Evaluation of seedling characteristics presented
as a percentage of the control (Fig. 2a–d) showed
a decrease for all growth parameters as stress
levels intensified. The most tolerant seedlings
showed a better biological efficiency (increase in
size and weight) under increased water deficiency,
while a different distribution of biomass in leaves
and roots were noted (Fig. 2b,d). Here again
line no. 9 (7219–69) responded best for relative
germination and relative growth of RL. Various
progeny lines responded differently for the relat-
ive growth of CFW and RFW (Fig. 2b–d). A
positive correlation was found between germina-
tion rates and seedling characteristics in the three
experiments, except for the percentage of abnor-
mal seedling (Table 3). Coefficients of correlation
between seed germination in paper with that of
perlite and agar medium were strongly significant
(P < 0.001). Abnormality always had a negative
correlation with germination rates and other
seedling characteristics like CDW, CFW, RDW,
RL and RFW, indicating that seedling water
potential and growth efficiency have an essential
role in the development of normal plants. Seed
0
10
20
30
40
50
60
70
80
90
7112
191
181
436mo
7233-107 7233-12 BPKaraj
436-I
7219-69
Lines
A
bnor
. %
0
0.2
0.3
0
10
20
30
40
50
60
70
80
90
100
7112
191
181
436mo
7233-107 7233-12 BPKaraj
436-I
7219-69
Lines
GER. % in Paper
0
0.2
0.3
0
10
20
30
40
50
60
70
80
90
100
7112
191
181
436mo
7233-1077233-12 BPKaraj
436-I
7219-69
Lines
GER. % in perlite
0
0.2
0.3
0
10
20
30
40
50
60
70
80
90
100
7112
191
181
436mo
7233-107 7233-12 BPKaraj
436-I
7219-69
Lines
GER. % in Vitro
0
0.2
0.3
(a)
(b)
(d)
(c)
Fig. 1: Nine sugar beet lines examined for germination percentage (GER. %) in paper (a), perlite (b) and in vitro
(c) at 0.0, 0.2 and 0.3 m concentrations of mannitol. The percentage of seedling abnormality (Abnor. %) in the
paper test is also presented (d). Error bars represent standard error
Effect of Water-Deficit Stress on Germination and Early Seedling Growth
141
germination was closely related to the RL and in
severe stress condition the highest value of RL
was allocated to the drought-resistant line no. 9
(7219–69). Absolute increases in root elongation
rate are strongly related to a high water status in
plant organs.
Here we should distinguish between seed germi-
nation – which is completed when the radical
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
7112
191
181
436mo
7233-107 7233-12 BPKaraj
436-I
7219-69
Lines
Relative germination %
0
0.2
0.3
0
20
40
60
80
100
7112
191
181
436mo
7233-107 7233-12 BPKaraj
436-I
7219-69
Lines
Relative growth % CFW
0
20
40
60
80
100
7112
191
181
436mo
7233-1077233-12 BPKaraj
436-I
7219-69
Lines
Relative growth % RL
0
0.2
0.3
0
20
40
60
80
100
7112
191
181
436mo
7233-107 7233-12 BPKaraj
436-I
7219-69
Lines
Relative growth % RFW
0
0.2
0.3
0
0.2
0.3
(a)
(b)
(c)
(d)
Fig. 2: Seedlings of nine sugar beet lines compared at 0.0, 0.2, and 0.3 m concentrations of mannitol: (a) for the
relative germination %, (b) relative growth % of cotyledon fresh weight (CFW), (c) relative growth % of root length
(RL), and (d) relative growth % of root fresh weight (RFW). Error bars represent standard error
Table 3: Coefficients of correlation estimated between germination rate and in vitro seedling characteristics of sugar
beet lines
CDW
CFW
Ger.
in vitro
Ger.
in paper
Ger.
in perlit
Abnor.
RDW
RL
RFW
CDW
1
–
–
–
–
–
–
–
–
CFW
0.387
***
1
–
–
–
–
–
–
–
Ger. in vitro
0.512
***
0.648
***
1
–
–
–
–
–
–
Ger. in paper
0.347
***
0.214
0.42
***
1
–
–
–
–
–
Ger. in perlite
0.569
***
0.645
***
0.676
***
0.532
***
1
–
–
–
–
Abnor.
)0.466
***
)0.477
***
)0.685
***
)0.498
***
)0.665
***
1
–
–
–
RDW
0.273
*
0.325
**
0.449
***
0.177
0.332
*
)0.392
***
1
–
–
RL
0.553
***
0.715
***
0.698
***
0.275
*
0.750
***
)0.620
***
0.326
*
1
–
RFW
0.502
***
0.766
***
0.800
***
0.379
**
0.801
***
)0.621
***
0.403
**
0.799
***
1
Ger., germination %; Abnor., abnormality %; CFW, cotyledon fresh weight; CDW, cotyledon dry weight; RFW,
root fresh weight; RDW, root dry weight; RL, root length.
*P
¼ 0.05, **P ¼ 0.01, ***P ¼ 0.001.
142
Sadeghian and Yavari

expands and penetrates the medium, consisting of
only cell elongation – and the cell division and
radical growth (RL), which starts later on. Experi-
ments performed on Brassica oleracea var. italica
seeds in water stress medium demonstrated that the
sensitivity of radical expansion and radical growth
to water stress is markedly different (Bewley and
Black 1985). While germination % of these seeds
decline as water stress increase from 0 to
)8 bar
and finally stopped at
)14 bar, radical growth
started at
)8 bar water potential, declined at )16
bar and stopped at
)22 bar.
Our preliminary test of sugar beet germination
using high mannitol concentrations up to
)12 bar
(data not shown) indicated that the stress levels
exceeding
)8 bar did not permit seed imbibition
and distinction among the seed lines. Therefore,
experimental mannitol concentrations (0.2 and
0.3 m) were chosen according to a better distribu-
tion of stress effects observed among the seed lines.
The most tolerant lines were to be discriminated for
their better performance at both levels of water
deficiency.
These results are in accordance with the results of
field evaluation trials (Sadeghian et al. 2000). These
findings agreed fairly with those reported for
legumes in which drought-tolerant lines could be
screened during seedling stages using nutrient
solutions containing mannitol (Grezesiak et al.
1996).
Distinction of significant differences in sugar beet
seedling growth and physiological performance in
water restriction stress lead to the conclusion that
these parameters, specifically germination rate and
seedling RL, could be used as criteria in screening
the most tolerant progeny lines against abiotic
stresses. In vitro controlled conditions seem to be
more amenable for evaluation of genetic materials
at early growth phase. Progeny lines having stable
germination and seedling growth properties against
a range of induced osmotic pressures may then be
included in breeding programmes for yield poten-
tial and stability under water-restricted conditions
in experimental plots.
Acknowledgements
Financial assistance by Agricultural Research and Educa-
tion Organisation (AREO) and the assistance extended by
Seed Laboratory Technology and Tissue Culture Laborat-
ory of the Sugar Beet Seed Institute (SBSI) are gratefully
acknowledged. The authors also thank H. Ghaffari for his
critical comments on the manuscript.
References
Apostolides, G., and C. Goulas, 1998: Seed crop
environment and processing effects on sugar beet
(Beta vulgaris L.) certified hybrid variety seed quality.
Seed Sci. Technol. 26, 223—235.
Ayers, A. D., 1952. Seed germination as affected by soil
moisture and salinity. Agron. J. 44, 82—84.
Bewley, J. D., and M. Black, 1985: Seeds: Physiology
of Development and Germination, pp. 124—125.
Plenum Publishing Corporation, NY.
Cook, R. E., 1979: Patterns of juvenile morbidity and
recruitment in plants. In: O. T. Solbrig, S. Jain, G. B.
Johnson, and P. H. Raven (eds), Topics in Plant
Population Biology, pp. 207–-301, Columbia Univer-
sity Press, Los Angles.
Durrant, M. J., and R. J. Gummerson, 1990: Factors
associated with germination of sugar beet seed in the
standard test and establishment in the field. Seed Sci.
Technol. 18, 561—575.
Durrant, M. J., A. P. Draycott, and P. A. Payne, 1974:
Some effects of sodium chloride on germination and
seedling
growth
of
sugarbeet.
Ann.
Bot.
38,
1045—1051.
Durrant, M. J., S. J. Brown, and A. Bould, 1985: The
assessment of the quality of sugar beet seed. J. Agric.
Sci. 104, 71—84.
Foolad, M. R., and G. Y. Lin, 1999: Relationship
between cold- and salt-tolerance during seed germi-
nation in tomato: germplasm evaluation. Plant Breed-
ing 118, 45—48.
Ghoulam, C., and K. Fares, 2001: Effect of salinity on
seed germination and early seedling growth of sugar
beet (Beta vulgaris L.). Seed Sci. Technol. 29,
357—364.
Grezesiak, S., W. Filek, G. Skrudilk, and B. Niziol,
1996: Screening for drought tolerance: evaluation of
seed germination and seedling growth for drought
resistance in legume plants. J. Agron. Crop Sci. 177,
245—252.
Hanson, A. D., and R. Wyse, 1982: Biosynthesis,
translocation, and accumulation of betaine in sugar
beet and its progenitors in relation to salinity. Plant
Physiol. 70, 1191—1198.
Hoagland, D. R., and D. I. Arnon, 1950: The Water–
Culture Method for Growing Plants without Soil.
California Experiment Station Circular No. 347. The
College of Agriculture, University of California,
Berkeley, CA.
Kaffka, S. R., and K. Hembree, 1999: The emergence of
autumn-planted sugar beet seedlings under saline
conditions. Proceedings of the 62th IIRBCongress,
7–11 June, Sevilla, Spain, 195—199.
Mahmoud, E. A., and M. J. Hill, 1981: Salt tolerance of
sugar beet at various temperatures. NZ J. Agric. Res.
24, 67—71.
Morgan, M. J., and A. G. Condon, 1986: Water-use,
grain yield and osmoregulation in wheat. Aust.
J. Plant Physiol. 13, 523—532.
Effect of Water-Deficit Stress on Germination and Early Seedling Growth
143

Sadeghian, S. Y., H. Fasli, D. F. Taleghani, and
M. Mesbah, 2000: Genetic variation for drought
stress in sugar beet. J. Sugar Beet Res. 37, 55—77.
SAS Institute, Inc., 1996: Getting Started with PROC
ANOVA. SAS Institute, Inc., Cary, NC.
Smith, S. E., and A. K. Dobrenz, 1987: Seed age and salt
tolerance at germination in Alfalfa. Crop Sci. 27,
1053—1056.
Tugnoli, D. V., and D. G. Bettini, 2001: Verifying the
germinability of commercial sugar beet seeds under
laboratory conditions and from emergence in the
field. Proceedings of the 64th IIRBCongress, 26–27
June, Bruges, pp. 333—340.
Van Swaaij, A. C. P. M., W. Heijbroek, and J. L.
Basting, 2001: Testing and improving seed vigour in
sugar beet. Proceedings of the 64th IIRBCongress,
26–27 June, Bruges, pp. 237—246.
Visser, B., 1994: Technical aspects of drought tolerance.
Biotechnol. Dev. Monit. 18, 5.
144
Sadeghian and Yavari
Wyszukiwarka
Podobne podstrony:
the effect of water deficit stress on the growth yield and composition of essential oils of parsley
Effect of various drying methods on texture and color of tomato halves (Gholam Reza Askari, Zahra Em
71 1021 1029 Effect of Electron Beam Treatment on the Structure and the Properties of Hard
Effect of vacuum microwave drying on selected mechanical and rheological properties of carrot
Jóźwiak, Małgorzata; Warczakowska, Agnieszka Effect of base–acid properties of the mixtures of wate
Microwave drying characteristics of potato and the effect of different microwave powers on the dried
Understanding the effect of violent video games on violent crime S Cunningham , B Engelstätter, M R
The Effect of Childhood Sexual Abuse on Psychosexual Functioning During Adullthood
Zieliński, Marek i inni Effects of Constant Magnetic Field on Electrodeposition of Co W Cu Alloy (2
Effects of a Group Exercise Program on Strength,
The effects of social network structure on enterprise system success
THE VACCINATION POLICY AND THE CODE OF PRACTICE OF THE JOINT COMMITTEE ON VACCINATION AND IMMUNISATI
Dos Santos Ferreira D , Staubach W Global and local regularity of Fourier integral operators on weig
The Effect of Back Squat Depth on the EMG Activity of 4 Superficial Hip
United Nations Conference on Trade and Development Economic Development in Africa (2007)
Clive Staples Lewis The Grand Miracle; And Other Selected Essays On Theology And Ethics From God In
więcej podobnych podstron