from the association
Position of the American Dietetic Association:
Functional Foods
ABSTRACT
All foods are functional at some phys-
iological level, but it is the position of
the American Dietetic Association
(ADA) that functional foods that in-
clude whole foods and fortified, en-
riched, or enhanced foods have a po-
tentially beneficial effect on health
when consumed as part of a varied
diet on a regular basis, at effective
levels. ADA supports research to fur-
ther define the health benefits and
risks of individual functional foods
and their physiologically active com-
ponents. Health claims on food prod-
ucts,
including
functional
foods,
should be based on the significant sci-
entific agreement standard of evi-
dence and ADA supports label claims
based on such strong scientific sub-
stantiation. Food and nutrition pro-
fessionals will continue to work with
the food industry, allied health pro-
fessionals, the government, the scien-
tific community, and the media to en-
sure that the public has accurate
information
regarding
functional
foods and thus should continue to ed-
ucate themselves on this emerging
area of food and nutrition science.
Knowledge of the role of physiologi-
cally active food components, from
plant, animal, and microbial food
sources, has changed the role of diet
in health. Functional foods have
evolved as food and nutrition science
has advanced beyond the treatment
of deficiency syndromes to reduction
of disease risk and health promotion.
This position paper reviews the defi-
nition of functional foods, their regu-
lation, and the scientific evidence
supporting this evolving area of food
and nutrition. Foods can no longer be
evaluated only in terms of macronu-
trient
and
micronutrient
content
alone. Analyzing the content of other
physiologically
active
components
and evaluating their role in health
promotion will be necessary. The
availability of health-promoting func-
tional foods in the US diet has the
potential to help ensure a healthier
population. However, each functional
food should be evaluated on the basis
of scientific evidence to ensure appro-
priate integration into a varied diet.
J Am Diet Assoc. 2009;109:735-746.
POSITION STATEMENT
All foods are functional at some phys-
iological level, but it is the position of
the American Dietetic Association
(ADA) that functional foods that in-
clude whole foods and fortified, en-
riched, or enhanced foods have a po-
tentially beneficial effect on health
when consumed as part of a varied
diet on a regular basis, at effective
levels. ADA supports research to fur-
ther define the health benefits and
risks of individual functional foods
and their physiologically active com-
ponents. Health claims on food prod-
ucts,
including
functional
foods,
should be based on the significant sci-
entific agreement standard of evidence
and ADA supports label claims based
on such strong scientific substantia-
tion. Food and nutrition professionals
will continue to work with the food
industry, allied health professionals,
the government, the scientific commu-
nity, and the media to ensure that the
public has accurate information re-
garding functional foods and thus
should continue to educate themselves
on this emerging area of food and nu-
trition science.
F
unctional foods are an exciting
current trend in the food and nu-
trition field (
). However, the
term functional foods has no legal
meaning in the United States. It is
currently a marketing, rather than a
regulatory, idiom. The rapidly evolv-
ing trend of functional foods creates
significant new issues and opportuni-
ties for public health, especially in
providing reliable information. In
1753, Lind suggested in “A Treatise of
the Scurvy,” that limes might prevent
scurvy in British sailors. His recom-
mendations to the Royal Navy physi-
cian were not incorporated until ap-
proximately 40 years later, even
though some people continued to die
due to scurvy (
). Nutrition research
progressed slowly until the 1940s,
when many deficiency diseases and
the nutrients to “cure” them were the
central focus. During this golden age
of biochemistry, Nobel Prize winners
and others discovered nutrients that
were responsible for many life-threat-
ening medical conditions. Throughout
World War I and II, many potential
soldiers failed military physicals due
to protein and vitamin/mineral defi-
ciencies. The US government re-
sponded by establishing the first Rec-
ommended Dietary Allowances and
initiating a health era emphasizing
the
importance
of
nutrition
for
growth (especially protein) and devel-
opment. In the 1960s, the pendulum
swung to the other side, when exces-
sive (fat, cholesterol, sodium), and or
imbalanced (lack of calcium, dietary
fiber) nutrient intakes started to be
recognized as related to chronic dis-
eases such as heart disease, cancer,
diabetes, osteoporosis, diverticulosis,
and others. The US government be-
came involved again, but this time
with an emphasis on reducing fat and
increasing
complex
carbohydrates.
Twenty years later the focus shifted
toward essential and a few nonessen-
tial nutrients and their possible ben-
efit in treating disease, such as fiber;
vitamins A, C, and E; and the mineral
selenium in cancer chemoprevention.
An American cereal company collabo-
ration with the National Cancer In-
stitute to market a bran cereal as a
functional food broke new regulatory
ground and led to a radical change in
food labeling laws, including Food
0002-8223/09/10904-0018$36.00/0
doi: 10.1016/j.jada.2009.02.023
© 2009 by the American Dietetic Association
Journal of the AMERICAN DIETETIC ASSOCIATION
735
and
Drug
Administration
(FDA)
health claims regulations (
Dietary supplement companies also
experienced rapid economic growth as
they promoted links between n-3
fatty acids and heart disease, lyco-
pene and prostate cancer, probiotics
and certain gastrointestinal condi-
tions, and other nutrient-health con-
nections. Functional food and dietary
supplement companies, focused on in-
creasing their profit margins, began
to collide with researchers calling for
scientific proof. Natural food stores
grew in such numbers and strength
that they began to spawn larger chain
stores that started competing with
mainstream grocery stores (
). In
1998, official recognition of the alter-
native proactive health path sup-
ported by functional foods was fur-
ther solidified by the formation of the
National Center for Complementary
and Alternative Medicine within the
National Institutes of Health, which
funded university-associated research
centers. The path of nutrition science
had come full circle back to the tenet
Hippocrates espoused more than 2,500
years ago: “Let food be thy medicine
and medicine be thy food.”
Registered dietitians (RDs) follow
in Hippocrates footsteps by recom-
mending food as medical nutrition
therapy (MNT) to treat myriad health
conditions and/or to maintain, re-
store, and optimize health. As a re-
sult, defining and utilizing functional
foods is an important component of
their practice. They are consumers’
bridge between evidence-based re-
search and optimal health. RDs will
continue working to update MNT in
terms of incorporating functional food
information into academic curricula
and
evidence-based
practice.
The
number of functional food products
will continue to expand. It is critical
that RDs understand how these foods
reach the marketplace and are pro-
moted. RDs must work to ensure that
all the necessary steps and regulatory
procedures are followed.
DEFINING FUNCTIONAL FOODS
What exactly is a functional food?
There is no FDA or universally ac-
cepted definition of this evolving
food category (
). Several working
definitions
used
by
professional
groups and marketers have been
proposed by various organizations
in several countries as outlined be-
low along with the American Die-
tetic Association’s (ADA’s) definition
of functional foods.
ADA’s Definition
As the largest organization of food
and nutrition professionals in the
United States, ADA classifies all
foods as functional at some physiolog-
ical level (
) because they provide nu-
trients or other substances that fur-
nish
energy,
sustain
growth,
or
maintain/repair vital processes. How-
ever, functional foods move beyond
necessity to provide additional health
benefits that may reduce disease risk
and/or promote optimal health. Func-
tional
foods
include
conventional
foods, modified foods (ie, fortified, en-
riched, or enhanced), medical foods,
and foods for special dietary use (
United States’ Definition
Functional foods are not officially rec-
ognized as a regulatory category by
the FDA in the United States. How-
ever, several organizations have pro-
posed definitions for this rapidly
growing food category, most notably
the International Food Information
Council (IFIC) and the Institute of
Food Technologists. The IFIC consid-
ers functional foods to include any
food or food component that may have
health benefits beyond basic nutrition
(
). Similarly, a recent Institute of
Food Technologists expert report (
defined functional foods as “foods and
food
components
that
provide
a
health benefit beyond basic nutrition
(for the intended population). These
substances provide essential nutri-
ents often beyond quantities neces-
sary for normal maintenance, growth,
and development, and/or other biolog-
ically active components that impart
health benefits or desirable physio-
logical effects.”
Canada’s Definition
Health Canada defines functional
foods as “similar in appearance to, or
may be, a conventional food, is con-
sumed as part of a usual diet, and is
demonstrated to have physiological
benefits and/or reduce the risk of
chronic disease beyond basic nutri-
tional functions” (
Europe’s Definition
The European Commission Concerted
Action on Functional Food Science in
Europe regards a food as functional if
it is satisfactorily demonstrated to af-
fect beneficially one or more target
functions in the body, beyond ade-
quate nutritional effects, in a way
that is relevant to either an improved
Functional food category
Selected functional food examples
Conventional foods (whole foods)
Garlic
Nuts
Tomatoes
Modified foods
Fortified
Calcium-fortified orange juice
Iodized salt
Enriched
Folate-enriched breads
Enhanced
Energy bars, snacks, yogurts, teas, bottled water,
and other functional foods formulated with
bioactive components such as lutein, fish oils,
ginkgo biloba, St John’s wort, saw palmetto,
and/or assorted amino acids
Medical foods
Phenylketonuria (PKU) formulas free of
phenylalanine
Foods for special dietary use
Infant foods
Hypoallergenic foods such as gluten-free foods,
lactose-free foods
Weight-loss foods
Figure 1. Functional food categories along with selected food examples.
736
April 2009 Volume 109 Number 4

state of health and well-being and/or
reduction of risk of disease. In this
context, functional foods are not pills
or capsules, but must remain foods
and they must demonstrate their ef-
fects in amounts that can normally be
expected to be consumed in the diet
(
Japan’s Definition
The functional food concept first de-
veloped in Japan in the late 1980s
when
the
Japanese
Ministry
of
Health and Welfare devised a regula-
tory framework for a category of foods
that provided specific health benefits,
clearly separating them from drugs.
Japan is the only country that recog-
nizes functional foods as a distinct
category, and the Japanese functional
food market is now one of the most
advanced in the world (
). Known as
Foods for Specified Health Use, these
are foods composed of functional in-
gredients that affect the structure
and/or function of the body and are
used to maintain or regulate specific
health conditions, such as gastroin-
testinal health, blood pressure, and
blood cholesterol levels (
Functional Foods vs Dietary Supplements
The Dietary Supplement Health and
Education Act of 1994 specifically de-
fines a dietary supplement as a spe-
cial category of food, but excludes any
product that is “represented for use as
a conventional food or as a sole item of
a meal or the diet” (
). In other
words, dietary supplements are le-
gally classified as foods, but not con-
sidered conventional foods consumed
in a diet such as a fruit or an entire
meal. ADA supports the FDA’s regu-
latory definition of dietary supple-
ments as foods, as long as they meet
the following criteria outlined in the
Dietary Supplement Health and Ed-
ucation Act: a product (other than to-
bacco) that is intended to supplement
the diet, which contains one or more
of
the
following
dietary
ingredi-
ents—a vitamin, a mineral, an herb
or other botanical, an amino acid, a
dietary substance to supplement the
diet by increasing the total daily in-
take, or a concentrate, metabolite,
constituent, extract, or combinations
of these ingredients; is ingested in
pill, capsule, tablet, or liquid form; is
not represented for use as a conven-
tional food or as the sole item of a
meal or diet; and is labeled as a “di-
etary supplement.”
One unofficial moniker for dietary
supplements in the United States is
“nutraceuticals.” Health Canada has
officially defined nutraceuticals as “a
product isolated or purified from
foods, and generally sold in medicinal
forms not usually associated with
food and demonstrated to have a
physiological benefit or provide pro-
tection against chronic disease” (
Functional Food Categories
In response to the increasing num-
bers of consumers interested in max-
imizing their health, the food indus-
try now offers an unprecedented
variety of new functional food prod-
ucts (
). The term functional food
should not be used to imply that there
are good foods and bad foods, but
rather that all foods can be incorpo-
rated into a healthful, varied diet.
The functional food categories defined
by ADA are now further explained:
Conventional Foods.
Unmodified whole
foods or conventional foods such as
fruits and vegetables represent the
simplest form of a functional food. For
example, tomatoes, raspberries, kale,
or broccoli are considered functional
foods because they are, respectively,
rich in such bioactive components as
lycopene, ellagic acid, lutein, and sul-
foraphane. Interestingly, the IFIC
2007 Consumer Attitudes Toward
Functional Foods/Foods for Health
survey found that fruits and vegeta-
bles were the top functional foods
identified by consumers (
). A few of
the many examples of emerging evi-
dence that links conventional foods to
health benefits include cancer risk re-
duction. Cruciferous vegetables re-
duced risk of several types of cancer
in experimental and epidemiologic
studies (
). Tomato products rich in
lycopene may reduce the risk of pros-
tate, ovarian, gastric, and pancreatic
cancers (
). Citrus fruit may reduce
the risk of stomach cancer (
). For
heart health, dark chocolate im-
proved endothelial function (
), and
tree nuts and peanuts reduce the risk
of sudden cardiac death (
). For in-
testinal
health
maintenance,
fer-
mented dairy products (probiotics)
may improve irritable bowel syn-
drome (
); and for urinary tract
function, cranberry juice reduced bac-
teriuria (
Modified Foods.
Functional foods can
also include those that have been
modified through fortification, enrich-
ment, or enhancement. These include
calcium-fortified
orange
juice
(for
bone health), folate-enriched breads
(for proper fetal development), or
foods enhanced with bioactive compo-
nents, such as margarines containing
plant stanol or sterol esters (for cho-
lesterol lowering), and beverages en-
hanced with energy-promoting ingre-
dients marketed to consumers such
as ginseng, guarana, or taurine. How-
ever, some of these beverages, as well
as various snacks containing such bo-
tanicals as St John’s wort, kava kava,
and echinacea, have been criticized
for making fraudulent medical claims
and safety concerns (
). As a result,
some commercially sold functional bo-
tanical products have been removed
from the market (
). Modifying foods
through biotechnology to improve
their nutritional value and health at-
tributes may also bring new func-
tional foods to the marketplace, such
as n-3 fatty acid enhanced or no-
trans-fat oils (
), although the topic
remains controversial.
Medical Foods.
The term medical food,
as defined by the Orphan Drug Act is
“a food which is formulated to be con-
sumed or administered enterally un-
der the supervision of a physician and
which is intended for the specific di-
etary management of a disease or
condition for which distinctive nutri-
tional requirements, based on recog-
nized scientific principles, are estab-
lished by medical evaluation” (
Examples of medical foods include
oral supplements in the form of phe-
nylketonuria formulas free of phenyl-
alanine, and diabetic, renal, and liver
formulations. Claims determine the
regulatory status of a product. For
example, a canned or bottled oral sup-
plement used under medical supervi-
sion, is a medical food; however, it
becomes a food for special dietary use
when sold to consumers at the retail
level.
Foods for Special Dietary Use.
The Fed-
eral Food, Drug, and Cosmetic Act
[Section 411(c)(3)] defines “special di-
etary use” as “a particular use for
which a food purports or is repre-
April 2009
● Journal of the AMERICAN DIETETIC ASSOCIATION
737
sented to be used, including but not
limited to the following: 1. Supplying
a special dietary need that exists by
reason of a physical, physiological,
pathological, or other condition . . . ;
2. Supplying a vitamin, mineral, or
other ingredient for use by humans to
supplement the diet by increasing the
total dietary intake. 3. Supplying a
special dietary need by reason of be-
ing a food for use as the sole item of
the diet . . .” (
). Examples of such
foods include infant foods, hypoaller-
genic foods such as gluten-free foods
and lactose-free foods, and foods of-
fered for reducing weight.
FACTORS DRIVING THE GROWTH OF THE
FUNCTIONAL FOODS CATEGORY
The health and nutrition paradigm
has changed significantly during the
past 2 decades. Today, food is not
merely viewed as a vehicle for essen-
tial nutrients to ensure proper growth
and development, but as a route to
optimal wellness. According to IFIC,
85% of consumers agree that certain
foods have health benefits that may
reduce the risk of chronic disease or
other health concerns (
). This food
as medicine paradigm will continue to
be driven by several key factors, in-
cluding:
●
increased consumer interest in con-
trolling their own health;
●
demographics including increases
in elders and ethnic subpopula-
tions;
●
escalating health care costs;
●
highly competitive food market with
small profit margins;
●
advances in technology, such as bio-
technology and nutrigenomics;
●
changes in food regulations; and
●
evidence-based science linking diet
to chronic disease risk reduction.
It is well established that poor di-
etary practices are linked with the
Figure 2. Schematic detailing how the Food and Drug Administration defines significant scientific agreement. Source:
738
April 2009 Volume 109 Number 4
major causes of morbidity and mortal-
ity, including cardiovascular disease,
hypertension, type 2 diabetes, over-
weight and obesity, and osteoporosis
(
). Certain types of cancer are also
strongly linked to diet as emphasized
in the second Expert Report from the
World Cancer Research Fund and the
American Institute for Cancer Re-
search: Food, Nutrition, Physical Ac-
tivity, and the Prevention of Cancer: A
Global Perspective (
). As the re-
search supporting the role of diet in
disease prevention and health promo-
tion continues to grow, the breadth
Dietary component
Disease
Petitioner
Clinical trial
support
Allowed health claim
Effective level
Sugar alcohols
Dental caries
NA
a
(in vivo
studies)
Frequent eating of foods high in sugars and
starches as between-meal snacks can
promote tooth decay. The sugar alcohol
[name of product] used to sweeten this
food may reduce the risk of dental
caries.
NA
Whole oat soluble
fiber
CHD
b
The Quaker Oats
Company
c
37 submitted
33 reviewed
d
17 priority
e
Diets low in saturated fat and cholesterol
that include soluble fiber from whole
oats may reduce the risk of heart
disease.
3 g/d; 0.75 g/serving
four times/d
Psyllium seed husk
soluble fiber
CHD
Kellogg Company
f
21 submitted
21 reviewed
17 priority
Soluble fiber from foods such as [name of
food], as part of a diet low in saturated
fat and cholesterol, may reduce the risk
of heart disease. A serving of [name of
food] supplies [x] grams of the soluble
fiber necessary per day to have this
effect.”
7g/d; 1.7 g/serving
four times/d
Oatrim
g
CHD
The Quaker Oats
Company
c
and
Rhodia, Inc
h
5 submitted
1 reviewed
Diets low in saturated fat and cholesterol
that include soluble fiber from Oatrim
may reduce the risk of heart disease.
0.75 g/serving
Barley soluble fiber
CHD
National Barley
Foods Council
11 submitted
5 reviewed
Soluble fiber from foods such as [name of
food], as part of a diet low in saturated
fat and cholesterol, may reduce the risk
of heart disease. A serving of [name of
food] supplies [x] grams of the soluble
fiber necessary per day to have this
effect.
3 g/d
Soy protein
CHD
Protein Technologies
International, Inc
i
43 submitted
41 reviewed
14 priority
Diets low in saturated fat and cholesterol
that include 25 g soy protein a day may
reduce the risk of heart disease. One
serving of [name of food] provides 6.25
g soy protein.
25 g/d; 6.25g/serving
Plant sterol esters
CHD
Lipton Tea Company
j
15 submitted
9 reviewed
8 priority
Plant sterols: Foods containing at least 0.65
g per serving of plant sterols, eaten
twice a day with meals for a daily total
intake of at least 1.3 g as part of a diet
low in saturated fat and cholesterol, may
reduce the risk of heart disease. A
serving of [name of food] supplies [x] g
vegetable oil sterol esters.
1.3 g/d; 0.65
g/serving
Plant stanol esters
CHD
McNeil Consumer
Healthcare
k
24 submitted
15 reviewed
14 priority
Plant stanol esters: Foods containing at
least 1.7 g per serving of plant stanol
esters, eaten twice a day with meals for
a total daily intake of at least 3.4 g, as
part of a diet low in saturated fat and
cholesterol, may reduce the risk of heart
disease. A serving of [name of food]
supplies [x] g plant stanol esters.
3.4 g/d; 1.7g/serving
a
NA
⫽not applicable.
b
CHD
⫽coronary heart disease.
c
Chicago, IL.
d
The FDA conducts its own independent review of the literature.
e
The FDA excludes studies that do not meet critical study design criteria.
f
Battle Creek, MI.
g
The soluble fraction of
␣-amylase hydrolyzed oat bran or whole oat flour.
h
Cranbury, NJ.
i
St Louis, MO.
j
Unilever USA, Englewood Cliffs, NJ.
k
Johsohn&Johnson Services, Inc, New Brunswick, NJ.
Figure 3. Health claims authorized under the Nutrition Labeling and Education Act (NLEA) between 1997 and 2006 resulting from Food and Drug
Administration (FDA) review of industry or trade association petitions.
April 2009
● Journal of the AMERICAN DIETETIC ASSOCIATION
739

and variety of functional foods on the
market will also increase. Consumers
interested in self-care and self-medi-
cation will increasingly seek diet-
based solutions to health and well-
ness. According to a recent IFIC
survey, 60% or more of Americans ei-
ther somewhat or strongly believe
that certain foods and beverages can
provide multiple health benefits and
more than 80% say they are currently
consuming or would be interested in
consuming foods and/or beverages for
such benefits (
Aging Baby Boomers will continue
to seek functional foods tailored for
specific health needs, including heart
disease, bone and joint health, cogni-
tive function, and eye health. This
personalized approach to nutrition
will become more feasible as the tech-
nology associated with nutrigenomics
continues to develop (
). In a recent
IFIC survey, more than two thirds
(71%) of Americans were favorable to-
ward the idea of using genetic infor-
mation to provide nutrition and/or di-
et-related
recommendations
Functional foods are viewed as one
option available to Americans seek-
ing cost-effective, preventative ap-
proaches to health care and im-
proved
health
status,
and
will
continue to transform the food sup-
ply of the 21st century.
REGULATION OF FUNCTIONAL FOODS IN
THE UNITED STATES
The boundaries between what is a
food and what is a medicine have
been challenged by both consumers
and manufacturers since the mid-
1980s. This has led to dramatic
changes in food regulations that have
fueled a so-called functional foods
revolution (
). The FDA has no spe-
cific definition for functional foods
and, unlike Japan, has no regulatory
framework for foods being marketed
as such. A functional food can be reg-
ulated as a conventional food, a food
additive, dietary supplement, drug,
medical food, or food for special di-
etary use depending on how the man-
ufacturer chooses to market the prod-
uct and, in particular, the type of
claims used on the package label or in
labeling. Thus, the regulation of func-
tional foods remains confusing and
has been criticized by a public advo-
cacy group (
). In response, in Octo-
ber 2006 the FDA announced a public
hearing to solicit information and
comments from interested persons on
how the FDA should regulate these
foods (
Three Claim Categories
There are currently three categories
of claims that food and dietary sup-
plement manufacturers can use on la-
bels to communicate health informa-
tion to consumers:
●
nutrient content claims;
●
structure/function claims; and
●
health claims.
Without question, most of the activ-
ity and controversy surrounding US
food regulations during the past 25
years has centered on health claims
(
), defined as statements that de-
scribe a relationship between a food
substance and a disease or other
health-related condition authorized
under the Nutrition Labeling and Ed-
ucation Act (NLEA) of 1990 (
The NLEA enables a product to
bear a health claim following the
FDA’s extensive review of the scien-
tific evidence summarized in petitions
submitted to the agency. Such claims
are authorized only if significant sci-
entific agreement exists among ex-
perts qualified to evaluate the totality
of the publicly available evidence re-
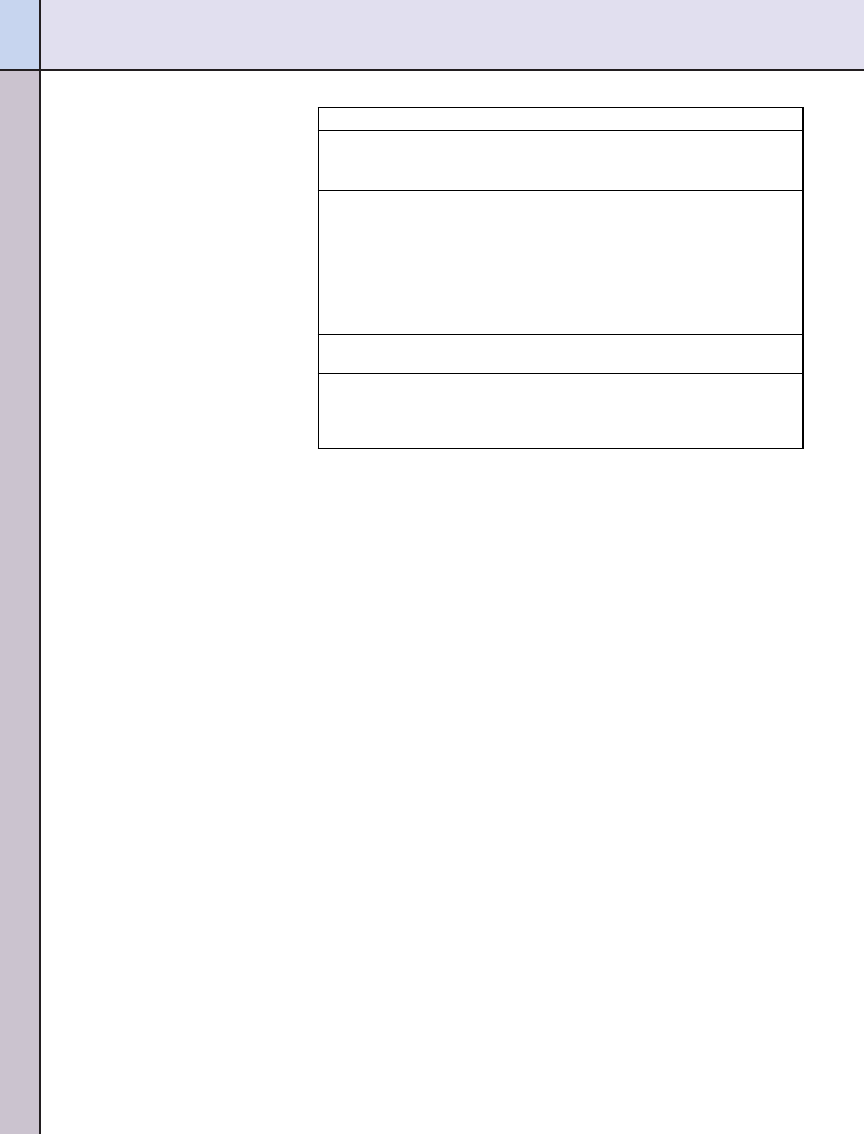
lated to the proposed claim. A scheme
outlining the significant scientific
agreement standard is shown in
Health claims must be authorized by
the FDA before they can be used on
food labels. Currently, health claims
that meet the significant scientific
agreement standard are allowed for 12
diet– disease relationship categories
(
). Substantial clinical efficacy is ab-
solutely essential for the approval of a
health claim under the NLEA.
More specifically, several well-de-
signed,
randomized,
placebo-con-
trolled intervention trials published
in
highly
regarded
peer-reviewed
journals are a critical component of a
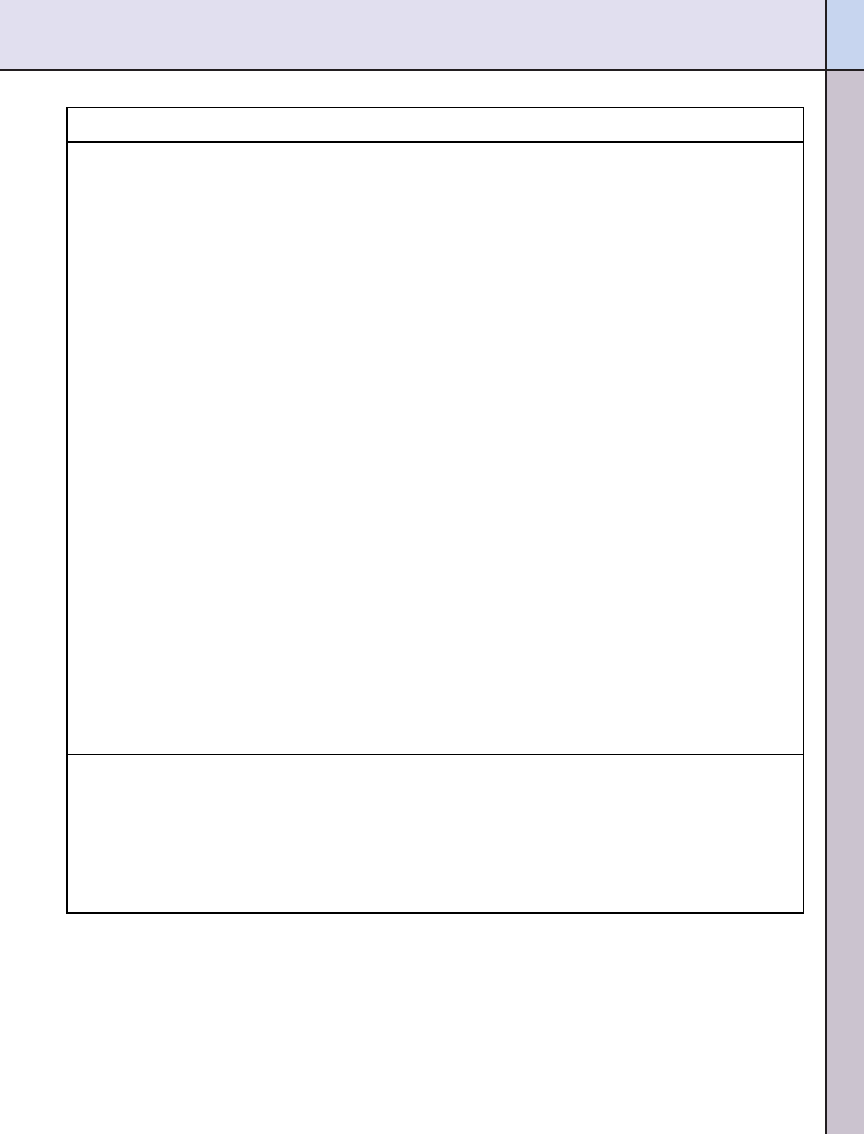
health claim petition to the FDA. For
example, 11 clinical trials were in-
cluded in the petition submitted by
the National Barley Foods Council to
the FDA requesting a health claim
linking barley soluble fiber to reduced
risk of coronary heart disease (
). However, the FDA also conducts
its own rigorous review of the litera-
ture supporting a proposed health
claim when a petition is submitted. In
the case of the barley health claim,
the FDA excluded six of the 11 clinical
trials due to various design flaws (
Due to the strong scientific substanti-
ation required for health claims au-
thorized under the NLEA, ADA sup-
ports the use of such preauthorized
claims on food products, including
functional foods.
Scientific ranking
Level of scientific evidence
FDA
a
category
Proposed qualifying language
First level
High
A
Category A health claims are unqualified claims that meet
the current standard of significant scientific agreement
b
Second level
Moderate/good
B
“Scientific evidence suggests but does not prove . . .”
Third level
Low
C
“Some scientific evidence suggests . . . however; FDA has
determined that this evidence is limited and not
conclusive.”
Fourth level
Lowest
D
“Very limited and preliminary scientific research suggests . . .
FDA concludes that there is little scientific evidence
supporting this claim.”
a
FDA
⫽Food and Drug Administration.
b
Note that “significant scientific agreement” is equivalent to First Level, High, or FDA Category A.
Figure 4. Scientific ranking, level of scientific evidence, and proposed language for qualified health claims.
740
April 2009 Volume 109 Number 4

FDA can also authorize health
claims under the Food and Drug
Administration
Modernization
Act
(FDAMA) of 1997. Such claims are
based on current, published authori-
tative statements from “a scientific
body of the United States with official
responsibility for public health pro-
tection or research directly related to
human nutrition” such as the Na-
tional Institutes of Health, the Na-
tional Academy of Sciences, and the
Dietary component(s)
Qualifying source
Qualified health claim language
Claim level
Folic acid, vitamins
B-6 and B-12
Supplements containing vitamin
B-6, B-12, and/or folic acid
As part of a well-balanced diet that is low in saturated fat
and cholesterol, folic acid and vitamins B-6 and B-12
may reduce the risk of vascular disease. The FDA
a
evaluated the claim and found that, although it is
known that diets low in saturated fat and cholesterol
reduce the risk of heart disease and other vascular
diseases, the evidence in support of the above claim is
inconclusive.
B
Almonds, hazelnuts,
some pine nuts,
peanuts, pecans,
and pistachios
Whole or chopped nuts
Scientific evidence suggests but does not prove that
eating 1.5 oz/d of most nuts such as [name of specific
nut] as part of a diet low in saturated fat and
cholesterol may reduce the risk of heart disease. [See
nutrition information for fat content.]
B
Walnuts
Whole or chopped walnuts
Supportive but not conclusive research shows that eating
1.5 oz/d walnuts, as part of a low-saturated-fat and
low-cholesterol diet and not resulting in increased
energy intake, may reduce the risk of CHD
b
. See
nutrition information for fat [and energy] content.
B
n-3 fatty acids
Fish, other conventional foods,
and supplements
Supportive but not conclusive research shows that
consumption of EPA
c
and DHA
d
n-3 fatty acids may
reduce the risk of CHD. One serving of [name of the
food] provides [x] g EPA and DHA n-3 fatty acids. [See
nutrition information for total fat, saturated fat, and
cholesterol content.]
B
Monounsaturated fat
from olive oil
Salad dressings, vegetable oil,
olive oil-containing food, and
shortenings
Limited and not conclusive scientific evidence suggests
that eating about 2 T (23 g) olive oil daily may reduce
the risk of CHD due to the monounsaturated fat in olive
oil. To achieve this possible benefit, olive oil is to
replace a similar amount of saturated fat and not
increase the total amount of daily energy intake. One
serving of this product contains [x] g olive oil.
C
Unsaturated fatty acids
from canola oil
Canola oil, vegetable oil
spreads, dressings for
salads, shortenings, and
canola oil-containing foods
Limited and not conclusive scientific evidence suggests
that eating about 1
1
⁄
2
T (19 g) canola oil daily may
reduce the risk of CHD due to the unsaturated fat
content in canola oil. To achieve this possible benefit,
canola oil is to replace a similar amount of saturated
fat and not increase the total amount of daily energy
intake. One serving of this product contains [x] g
canola oil.
C
Corn oil and corn oil-
containing products
Very limited and preliminary scientific evidence suggests
that eating about 1 T (16 g) corn oil daily may reduce
the risk of heart disease due to the unsaturated fat
content in corn oil. The FDA concludes that there is
little scientific evidence supporting this claim. To
achieve this possible benefit, corn oil is to replace a
similar amount of saturated fat and not increase the
total amount of daily energy intake. One serving of this
product contains [x] g corn oil.
D
a
FDA
⫽Food and Drug Adminstration.
b
CHD
⫽coronary heart disease.
c
EPA
⫽eicosapentaenoic acid.
d
DHA
⫽docosahexaenoic acid.
Figure 5. Qualified health claims for cardiovascular disease in terms of dietary component, qualifying source, language, and claim level. B, C, and
D level claims correspond, respectively, to moderate, low, and lowest level of scientific evidence as outlined in
April 2009
● Journal of the AMERICAN DIETETIC ASSOCIATION
741

Centers for Disease Control and Pre-
vention (
). FDAMA provisions are in-
tended to expedite the process by which
the scientific basis for such claims is
established. Currently, one nutrient
content claim and five health claims
have been approved under the FDAMA
(
). ADA was actively involved in dis-
cussions surrounding the passage of
the FDAMA and continues to support
the need for all claims, regardless of
whether they are authorized via the
NLEA process or the FDAMA notifica-
tion process, to be based on significant
scientific agreement.
The significant scientific agreement
standard of evidence required under
the NLEA was radically altered with
the Consumer Health Information for
Better Nutrition Initiative issued by
the FDA in July 2003 (
). This ini-
tiative was FDA’s response to the
1999 Pearson v Shalala court deci-
sion, which successfully challenged
the rigid standards of evidence ap-
plied to NLEA health claims (
This decision clarified the need to al-
low health claims based on less scien-
tific evidence as long as the claims do
not mislead the consumer. As part of
this initiative, the agency established
interim procedures whereby qualified
health claims can be made for dietary
supplements and conventional foods
(
). The body of data required for a
qualified health claim does not meet
the significant scientific agreement
standard; rather it must demon-
strate, based on the totality of infor-
mation available, that the weight of
the scientific evidence supports the
proposed
claim.
Qualified
health
claims are intended to provide infor-
mation about diet-disease relation-
ships to consumers even though the
scientific support has not reached the
highest level of scientific evidence
(
A primary objective of qualified
health claims is to benefit consumers
by providing more information on food
labels concerning diet and health. Un-
fortunately, recent evidence suggests
that consumers are unable to distin-
guish the different categories of claims.
A survey conducted by IFIC in 2004
showed that consumers have difficulty
distinguishing among four levels of sci-
entific evidence, especially with lan-
guage-only claims (
). In addition, the
survey found that consumers rate the
scientific evidence and other attributes
of a product containing an unqualified
claim similar to those products contain-
ing a structure-function claim or a di-
etary guidance statement.
Currently qualified health claims
are allowed for six disease categories:
cancer, cardiovascular disease, cogni-
tive function, diabetes, hypertension,
and neural tube defects. Qualified
health claims for cardiovascular dis-
ease are presented in
and
demonstrate the difference in qualify-
ing language among B-, C-, and D-
level claims. Qualified health claims
for other diseases are shown in
. More than two dozen qualified
health claim petitions have been de-
nied by FDA.
ADA strongly supports the signifi-
cant scientific agreement standard of
evidence and believes all claims made
on conventional foods and dietary
supplements should meet this stan-
dard. However, given FDA’s mandate
to allow qualified health claims, ADA
recommends an evidence-based rank-
ing system to facilitate a thorough re-
view of scientific data that supports
both qualified and unqualified health
claims, as well as mechanisms to com-
municate strength of evidence more
clearly to the public.
SCIENTIFIC SUBSTANTIATION
Functional foods differ with regard to
the quantity and quality of evidence-
based science supporting their pur-
ported health benefits. As a result,
consumers can be confused about
which products are truly beneficial.
The strength of evidence is based on
the current cumulative summary of
all studies and is weighted by their
number, quality, and type of which
only the latter will be discussed. The
FDA’s system of substantiation that
serves as a guide for the industry has
been previously described (
Types of Research
The type of research used to deter-
mine the level of evidence can range
from “soft” to “hard” science and var-
ies from: in vitro (in the test tube)
studies, in vivo (in the living) animal
studies, and in vivo human studies.
Human studies can be further subdi-
vided into subjective sensory evalua-
tions; epidemiologic studies; migra-
tion studies; observational; cohort
studies; and finally randomized, pla-
cebo-controlled clinical trials, which
are the gold standard. All studies are
strengthened by including a control,
using a single- or double-blind or
cross-over design, and validating the
bioactive being tested or evaluation
tool being utilized. A meta-analysis is
conducted when smaller studies are
pooled to increase their statistical
power. However, sometimes only cer-
tain studies in a meta-analysis meet
the qualifying criteria and not all
studies are homogeneous in treat-
ment or measurable outcomes. Ulti-
mately, the weight of the evidence ob-
tained through literature reviews
should be based on a sufficient num-
ber of clinical trials. This information
is then used to make claims commu-
nicating the functional food’s benefit
to consumers.
Disease
Food component(s)
Qualifying source
Prostate, ovarian, gastric, and
pancreatic cancers
Tomatoes
Cooked, raw, dry, or canned
tomatoes
Colorectal cancer and polyps
Calcium
Supplements
Breast and prostate cancers
Green tea
Green tea and supplements
containing green tea
Certain cancers
Selenium
Supplements
Certain cancers
Antioxidant vitamins
Supplements
Cognitive function and
dementia
Phosphatidylserine
Supplements
Insulin resistance
Chromium picolinate
Supplements
Essential and gestational
hypertension and
preeclampsia
Calcium
Supplements
Neural tube defects
Folic acid
Supplements
Figure 6. Qualified health claims for various diseases based on food components and their
qualifying sources.
742
April 2009 Volume 109 Number 4
Consistent Reporting Standards
Although randomized, double-blind,
placebo-controlled clinical studies
are the gold standard for determin-
ing efficacy, a person can potentially
compare results of multiple studies
involving functional foods when re-
searchers and/or the journal editors
ensure that a standard check list of
information is reported. Such infor-
mation would include, but is not
limited to subject (ie, type, number,
and age), use of a control, dosage
form (ie, pill, powder, plant part,
plant genus and species, liquid, or
solvent), standardized source (ie,
compositional verification via chro-
matographic analysis or some other
standardization test, including ge-
nus, species and variety/accession
for plants/herbs, or supplier identi-
fication),
frequency,
and/or
even
measurable outcomes dependent on
quantitative end points.
presents an example of a study with
thorough reporting of critical pa-
rameters (
). Measurable outcomes
must also relate to the hypotheses
proposed in the study design when
evaluating the efficacy of functional
foods. This is necessary to safeguard
the scientific advancement of com-
plementary and alternative medi-
cine. Comparison or duplication of
research results is not possible with-
out this critical information that
should be included in all publica-
tions, including abstracts.
Ideally, measurable outcomes or
end points for any research topic
should be standardized. Whenever
possible, researchers should choose
well-defined
bioactives
), biomarkers (often laboratory
Study
author,
year
Subjects
Design
Dosage
(substance,
form,
amount,
frequency)
Standardized
source
Duration
End
points
Jenkins
and
colleagues,
2008
(46)
27
healthy
men
and
postmenopausal
women
with
hyperlipidemia
Hyperlipidemia
defined
as
elevated
low-density
lipoprotein
cholesterol
(⬎
158
mg/dL
[⬎
4.1
mmol/L])
Randomized
cross-over
design
(subjects
served
as
own
controls)
Each
1-mo
diet
phase
separated
by
a
minimum
2-wk
washout
period
Substance/form:
whole
raw
unblanched
almonds
daily
for
4
wk
in
the
form
of
muffins
Three
1-mo
diet
phases:
1)
Treatment
-
full
dose
almonds
in
muffins
(73
g/dL)
2)
Treatment
-
half-dose
almonds
in
muffins
(37
g/dL)
3)
Control.
No
almonds
in
muffins
Almond
source
(brand)
not
specified
1
mo
for
each
of
the
three
diet
phases
Total
serum
cholesterol
concentration
was
significantly
lower
in
half-
and
full-dose
almond
treatments
compared
to
controls
(P
⬍
0.05).
Serum
low-density
lipoprotein
cholesterol
was
lower
and
serum
high-density
lipoprotein
cholesterol
was
higher
in
full-dose
almond
group
compared
to
controls
(P
⬍
0.05).
Full
dose
almonds
reduced
serum
malondialdehyde
concentrations
(P
⫽
0.040)
and
creatinine-adjusted
urinary
isoprostane
output
(P
⫽
0.026).
No
significant
difference
occurred
in
serum
triglyceride
concentrations.
Figure
7.
Suggested
comprehensive
data
to
report
when
publishing
results
of
clinical
trials
on
functional
foods.
Bioactive ingredient
Lignans
Polyphenols
Anthocyanins
Ellagitannins
Flavonoids
Proanthocyanidins
Gallotannins
Phenolic acids
Stilbenoids
Triterpenoids
Figure 8. Selected classes of bioactives found
in berries, namely, blackberries, black raspber-
ries, blueberries, cranberries, red raspberries,
and strawberries. Data from reference
April 2009
● Journal of the AMERICAN DIETETIC ASSOCIATION
743
tests such as serum cholesterol, blood
pressure, and prostate specific anti-
gen) or clinical endpoints (myocardial
infarction, bone fractures, tumors,
metastasis, overall survival, or polyp
size and number) to evaluate their
hypotheses (
). However, it is not
always feasible to evaluate all mea-
surable outcomes due to financial con-
straints that limit the type and
amount of data collection.
Another problem facing evidenced-
based research in the substantiation of
a functional food claim is that clear
cause and effect relationships are not
always easily deciphered in the com-
plex web of individual variability and
human physiology. Differences among
population subgroups further compli-
cate the identification of clear cause
and effect relationships. Increasingly,
evidence for functional foods is based
on well-designed clinical trials.
TAKE HOME MESSAGE FOR FOOD AND
NUTRITION PROFESSIONALS
The landscape of the food and nutri-
tion field changes constantly. Foods
are no longer solely viewed in terms of
macro- or micronutrients or even nu-
trient deficiencies or excesses. The
possibility of other potential health-
promoting components found in foods
has created a new wave of functional
food information that will continue to
expand.
Staying Informed
There are many ways for food and nu-
tritional professionals to keep well in-
formed about this growing field within
the food and nutrition arena. Recent
research can be accessed either directly
through PubMed or the Journal of the
American Dietetic Association’s re-
search abstracts in the back of each
issue called “New in Review,” reading
science-based books on the subject,
and/or the Daily News from ADA’s
Knowledge Center, which is “a daily
newsletter informing ADA members of
news affecting food, nutrition and
health.” (To get the Daily News via e-
mail each day, go to
). It may also be helpful to
stay current with functional food ad-
vancements in other countries. Other
avenues of staying connected include
joining the ADA’s Nutrition in Comple-
mentary Care dietetic practice group
where functional foods are part of their
scope. Additional resources for re-
search and/or regulations are the Na-
tional Center for Complementary and
Alternative Medicine, Natural Medi-
cines Comprehensive Database, Co-
chrane Reviews, and FDA.
MNT Client Education
Enhancing the health of the individ-
ual can occur by continuing to incor-
porate functional food information
into the current mainstream MNT
contained in ADA’s Nutrition Care
Manual. ADA’s Evidence Analysis Li-
brary (
is also a unique source of information
that will continue to be expanded. It
is the role of RDs and researchers to
search the literature and incorporate
solid evidence-based scientific sup-
port into the manual. RDs familiar
with the three current categories of
health claims can now start incorpo-
rating such foods into the diets of pa-
tients with certain medical conditions
and or optimizing the health of clients
interested in disease prevention and
health promotion.
Consumer Education
Consumers need to be advised on the
appropriate intake of functional foods
in the context of a healthful diet to
optimize their health. Their major
source of information on this topic
should be through RDs and dietetic
technicians, registered, knowledge-
able about functional foods.
Corporate Consulting
Food and nutritional professionals fa-
miliar with functional foods can con-
sult corporations on the latest or pos-
sible future trends in functional
foods. Working in research and devel-
opment departments will enable RDs
to be involved in the formation of new
functional foods that maximize the
potential health benefits to consum-
ers. Learning how a functional food
product is brought to market allows
RDs to do the same or assist both
small and large corporations starting
to enter the market.
Research
It is also the role of food and nutri-
tion professionals conducting re-
search to continue expanding the
knowledge base of functional foods
and/or complementary medicine to
provide high-quality evidence-based
research. Promoting a standardized
reporting method that includes sub-
ject type, study design (preferably
double-blind,
placebo-controlled),
dose (ie, source and amount), dura-
tion, and measurable outcomes or
endpoints is crucial. Research find-
ings then need to be translated into
practical information for both con-
sumers and clinicians.
Government Policy
Functional food regulation is para-
mount to public policy formation in-
volving foods that may optimize human
health. The role of RDs is to safeguard
the public by protecting the definition,
use, and regulation of functional foods.
Getting involved politically through
ADA and Congress is key to developing
and enhancing regulatory standards
for functional foods. Ensuring their
safety and making sure label claims
and marketing are based on scientifi-
cally sound data are critical.
The functional foods category is
just one aspect of complementary
medicine that is increasingly recog-
nized in the history of food and nutri-
tion as an advance toward optimizing
health. Food and nutritional profes-
sionals play important roles in edu-
cating new RDs, clients, consumers,
corporations, and public policy mak-
ers about functional foods and their
roles in human health.
CONCLUSIONS
In 1907 an editorial appeared in the
Journal of the American Medical Asso-
ciation calling for practicing physicians
“to give [e]special attention to the study
of dietetics so that he may appropriate
and put to practical use the latest de-
velopments of physiological research”
(
). One hundred years later, the
study of how diet impacts disease pre-
vention and health promotion is more
important than ever. Consumer inter-
est in the health benefits of foods and
food components is at an all time high
and will continue to grow. Food and
nutrition professionals are uniquely
qualified to interpret scientific findings
on functional foods and translate such
findings into practical dietary applica-
tions for consumers, other health pro-
fessionals, policy makers and the me-
dia. Food and nutrition professionals
744
April 2009 Volume 109 Number 4

must continue to be leaders in this ex-
citing and ever-evolving area of food
and nutrition.
The authors thank the reviewers for
their many constructive comments
and suggestions. The reviewers were
not asked to endorse this position or
the supporting paper.
References
1. Sloan AE. The top 10 functional food trends.
Food Technol. 2008;62:24-44.
2. Cook GC. Scurvy in the British Mercantile
Marine in the 19th century, and the contri-
bution of the Seamen’s Hospital Society.
Postgrad Med J. 2004;80:224-229.
3. Fulgoni V. Health claims: A US perspective.
In: Hasler CM, ed. Regulation of Functional
Foods and Nutraceuticals. Ames, IA: Black-
well Publishing; 2005:79-88.
4. Wrick KL. The impact of regulations on the
business of nutraceuticals in the United
States: Yesterday, today, and tomorrow. In:
Hasler CM, ed. Regulation of Functional
Foods and Nutraceuticals. A Global Perspec-
tive. Ames, IA: Blackwell Publishing; 2005:
3-36.
5. Ross S. Functional foods: The Food and Drug
Administration perspective. Am J Clin Nutr.
2000;71(suppl):1735S-1738S.
6. Position of The American Dietetic Associa-
tion: Functional foods. J Am Diet Assoc.
2004;104:814-826.
7. International
Food
Information
Council.
Functional foods: Attitudinal research. Inter-
national Food Information Council Web site.
http://www.ific.org/research/funcfoodsres02.
cfm.
Accessed January 9, 2009.
8. Institute of Food Technologists. Functional
foods: Opportunities and challenges. Insti-
tute of Food Technologists Web site.
members.ift.org/IFT/Research/IFTExpert
Reports/functionalfoods_report.htm.
Ac-
cessed January 9, 2009.
9. Policy paper—Nutraceuticals/functional foods
and health claims on foods. Health Canada
Web site.
http://www.hc-sc.gc.ca/fn-an/label-
etiquet/claims-reclam/nutra-funct_foods-nutra-
fonct_aliment-eng.php.
Accessed January 9,
2009.
10. European Commission Concerted Action on
Functional Food Science in Europe. Scien-
tific concepts of functional foods in Europe.
Consensus document. Br J Nutr. 1999;
81(suppl 1):S1-S27.
11. Yamaguchi P. Japan’s nutraceuticals today—
Functional foods Japan 2006. NPIcenter Web
site.
http://www.npicenter.com/anm/templates/
Accessed January 9, 2009.
12. Arai S, Morinaga Y, Yoshikawa T, Ichishi E,
Kiso Y, Yamazaki M, Morotomi M, Shimizu
M, Kuwata T, Kaminogawa S. Recent trends
in functional food science and the industry
in Japan. Biosci Biotechnol Biochem. 2002;
66:2017-2029.
13. Dietary Supplement Health and Education
Act of 1994, 21 USC §321.
14. Hsieh YH, Ofori JA. Innovations in food
technology for health. Asia Pac J Clin Nutr.
2007;16(suppl 1):65-73.
15. Consumer attitudes toward functional foods/
foods for health—Executive summary. Inter-
national Food Information Council Web site.
http://www.ific.org/research/funcfoodsres07.
cfm.
Accessed January 9, 2009.
16. Moriarty, Naithani R, Surve B. Orgaosulfur
compounds in cancer chemoprevention. Mini
Rev Med Chem. 2007;7:827-838.
17. Kavanaugh CJ, Trumbo PR, Ellwood KC.
The U.S. Food and Drug Administration’s
evidence-based review for qualified health
claims: Tomatoes, lycopene, and cancer.
J Natl Cancer Inst. 2007;99:1074-1085.
18. Bae J-M, Lee AJ, Guyatt G. Citrus fruit
intake and stomach cancer risk: A quantita-
tive systematic review. Gastric Cancer.
2008;11:23-32.
19. Faridi Z, Njike VY, Dutta S, Ali S, Katz DL.
Acute dark chocolate and cocoa ingestion
and endothelial function: A randomized con-
trolled crossover trial. Am J Clin Nutr. 2008;
88:58-63.
20. Kris-Etherton PM, Hu FB, Ros E, Sabate J.
The role of tree nuts and peanuts in the
prevention of coronary heart disease: Multi-
ple potential mechanisms. J Nutr. 2008;
138(suppl):1746S-1751S.
21. Quigley EM. Bacteria: A new player in
gastrointestinal motility disorders-infec-
tions, bacterial overgrowth, and probiotics.
Gastroenterol Clin North Am. 2007;6:735-
748.
22. Nowack R. Cranberry juice—A well-charac-
terized folk-remedy against bacterial uri-
nary tract infection. Wien Med Wochenschr.
2007;157:325-330.
23. Center for Science in the Public Interest.
Functional food named in the Center for Sci-
ence in the Public Interest’s complaints to the
Food and Drug Administration. Center for Sci-
ence in the Public Interest Web site.
www.cspinet.org/reports/funcfoodcomplaint.
htm.
Accessed January 9, 2009.
24. Jacobson MF, Silverglade B, Heller IR.
Functional foods: Health boon or quackery?
West J Med. 2000;172:8-9.
25. Pew Initiative on Food and Biotechnology. Ap-
plication of biotechnology for functional foods.
Pew Charitable Trusts Web site.
pewagbiotech.org/research/functionalfoods/.
Accessed January 9, 2009.
26. Food and Drug Admnistration. Orphan
Drug Act (as amended).
Accessed January 9, 2009.
27. Tailoring your diet to fit your genes: A global
quest. International Food Information Council
Web site.
http://www.ific.org/foodinsight/2006/
Accessed January 9, 2009.
28. Dietary Guidelines for Americans, 2005.
US Food and Drug Administration Web
site.
Accessed January 9, 2009.
29. World Cancer Research Fund, American
Institute for Cancer Research. Food, nutri-
tion, physical activity, and the prevention
of cancer: A global perspective. Diet and
Cancer
Report
Web
site.
Accessed
January 9, 2007.
30. 2008 Food and health survey: Consumer
attitudes
toward
food,
nutrition,
and
health. International Food Information
Council
Web
site.
research/foodandhealthsurvey.cfm.
Ac-
cessed January 15, 2009.
31. Fogg-Johnson N, Kaput J. Moving forward
with nutrigenomics. Food Technol. 2007;61:
50-57.
32. Heasman M, Mellentin J. The Functional
Foods Revolution: Healthy People, Healthy
Profits? London, UK: Earthscan Publi-
cations Ltd; 2001.
33. Citizen ptition 2002P-0122, petition for rule-
making on functional foods and request to
establish an advisory committee. Center for
Science in the Public Interest Web site.
www.cspinet.org/reports/funcgaopt_032102.pdf.
Accessed January 22, 2009.
34. Conventional foods being marketed as “func-
tional foods”; public hearing; request for
comments. Food and Drug Administration
Center for Food Safety and Applied Nutri-
tion
Web
site.
Accessed January
9, 2009.
35. Taylor CL, Wilkening VL. How the nutrition
food label was developed, Part 2: The pur-
pose and promise of nutrition claims. J Am
Diet Assoc. 2008;108:618-623.
36. Nutrition Labeling and Education Act. USC
343(4)(3)(B)(i), implemented at 21 CFR
§101.14.
37. Label claims: Health claims that meet sig-
nificant scientific agreement. US Food and
Drug Administraiton Center for Food Safety
and Applied Nutrition Web site.
Ac-
cessed January 9, 2009.
38. Food labeling: Health claims; soluble dietary
fiber from certain foods and coronary heart
disease. US Food and Drug Administration
Center for Food Safety and Applied Nutri-
tion
Web
site.
Accessed January 15,
2009.
39. Guidance for industry: Notification of a
health claim or nutrient content claim based
on an authoritative statement of a scientific
body. US Food and Drug Administration
Center for Food Safety and Applied Nutri-
tion
Web
site.
Accessed January 9,
2009.
40. Label claims: FDA Modernization Act of
1997 (FDAMA) claims. US Food and Drug
Administration Center for Food Safety and
Applied Nutrition Web site.
Ac-
cessed January 9, 2009.
41. Consumer Health Information for Better Nu-
trition Initiative. Task force final report. US
Food and Drug Administration Center for
Food Safety and Applied Nutrition Web site.
Accessed January 9, 2009.
42. Pearson v Shalala. Bureau of National Af-
fairs
Web
site.
Accessed January
9, 2009.
43. Consumer Health Information for Better
Nutrition Initiative: Task force final report:
Attachment E—Interim procedures for qual-
ified health claims guidance: Interim proce-
dures for qualified health claims in the la-
beling of conventional human food and
human dietary supplements. US Food and
Drug Administration Center for Food Safety
and Applied Nutrition Web site.
Ac-
cessed November 5, 2008.
44. Qualified health claims consumer research
project executive summary. International
Food Information Council Foundation Web
site.
http://www.ific.org/research/qualhealth
Accessed January 9, 2009.
45. Guidance for industry and FDA: Interim ev-
idence-based ranking system for scientific
data. US Food and Drug Administration
April 2009
● Journal of the AMERICAN DIETETIC ASSOCIATION
745
Center for Food Safety and Applied Nutri-
tion
Web
site.
Accessed January 9,
2009.
46. Jenkins DJ, Kendall CW, Marchie A, Josse
AR, Nguyen TH, Faulkner DA, Lapsley KG,
Blumberg J. Almonds reduce biomarkers of
lipid peroxidation in older hyperlipidemic
subjects. J Nutr. 2008;138:908-913.
47. Seeram NP. Berry fruits for cancer preven-
tion: Current status and future prospects. J
Agric Food Chem. 2008;56:630-635.
48. Weber P, Fluhmann B, Eggersdorfer M. De-
velopment of bioactive substances for func-
tional foods—Scientific and other aspects.
In: Heinrich M, Muller WE, Galli C, eds.
Local
Mediterranean
Food
Plants
and
Nutraceuticals (Forum of Nutrition). Vol 59.
Basel, Switzerland: Karger; 2006:171-181.
49. Anonymous. Diet and health. J Am Med As-
soc. 1907;198:2687.
ADA Position adopted by the House of Delegates Leadership Team October 16, 1994 and reaffirmed on September
7, 1997; June 15, 2001; and June 11, 2006. This position is in effect until December 31, 2012. ADA authorizes
republication of the position, in its entirety, provided full and proper credit is given. Readers may copy and distribute
this paper, providing such distribution is not used to indicate an endorsement of product or service. Commercial
distribution is not permitted without the permission of ADA. Requests to use portions of the position must be
directed to ADA headquarters at 800/877-1600, ext 4835, or
Authors: Clare M. Hasler, PhD, MBA (Robert Mondavi Institute for Wine and Food Science, University of
California, Davis); Amy C. Brown, PhD, RD (Department of Complementary and Alternative Medicine, John A
Burns School of Medicine, University of Hawaii at Manoa, Honolulu).
Reviewers: Sharon Denny, MS, RD (ADA Knowledge Center, Chicago, IL); Mary H. Hager, PhD, RD, FADA (ADA
Government Relations, Washington, DC); Oncology dietetic practice group (Kathryn Keifer Hamilton, MA, RD,
Morristown Memorial Hospital, Morristown, NJ); Andrea Hutchins, PhD, RD (University of Colorado at Colorado
Springs, CO); Rosalyn Franta Kulik, MS, RD, FADA (Rosalyn Franta Kulik Consulting, Tampa, FL); Esther Myers,
PhD, RD, FADA (ADA Scientific Affairs, Chicago, IL); Leila Saldanha, PhD, RD, (President, NutrIQ LLC, Alexan-
dria, VA); Research dietetic practice group (Kim S. Stote, PhD, MPH, RD, State University of New York, College at
Oneonta, Oneonta, NY); Jennifer A. Weber, MPH, RD (ADA Government Relations, Washington, DC); Robert
Wildman, PhD, RD (Demeter Consultants LLC, Pittsburgh PA, and Kansas State University, Manhattan). Associ-
ation Positions Committee Workgroup: Helen W. Lane, PhD, RD (chair); Debe Nagy-Nero, MS, RD; Suzanne
Hendrich, PhD (content advisor).
746
April 2009 Volume 109 Number 4
Wyszukiwarka
Podobne podstrony:
Ground green coffee beans as a functional food
L 3 Complex functions and Polynomials
3 ABAP 4 6 Basic Functions
Aging skin and food suplements Nieznany (2)
Functional Origins of Religious Concepts Ontological and Strategic Selection in Evolved Minds
Applications and opportunities for ultrasound assisted extraction in the food industry — A review
MEDC17 Special Function Manual
FOOD AND DRINKS, SŁOWNICTWO
Verb form and function
Food ćwiczenia angielski Starland 1 module 8
food
dpf doctor diagnostic tool for diesel cars function list
Euler’s function and Euler’s Theorem
Attitudes toward Affirmative Action as a Function of Racial ,,,
nutritional modulation of immune function
moto suzuki motorbike scanner with bluetooth function list
NMR in Food
Changes in passive ankle stiffness and its effects on gait function in
Functional improvements desired by patients before and in the first year after total hip arthroplast
więcej podobnych podstron