Ground green coffee beans as a functional food
supplement
e Preliminary study
Dariusz Dziki
, Urszula Gawlik-Dziki
*
,
Łukasz Pecio
, Renata R
o_zyło
, Micha
ł Swieca
,
Andrzej Krzykowski
, Stanis
ław Rudy
a
Thermal Engineering Department, University of Life Sciences, Do
swiadczalna Str. 44, 20-280 Lublin, Poland
b
Department of Biochemistry and Food Chemistry, University of Life Sciences, Skromna Str. 8, Lublin, Poland
c
Department of Biochemistry and Crop Quality, Institute of Soil Science and Plant Cultivation, State Research Institute, Czartoryskich Str. 8, 24-100 Pu
ławy,
Poland
d
Department of Equipment Operation and Maintenance in the Food Industry, University of Life Sciences, Do
swiadczalna Str. 44, 20-280 Lublin, Poland
a r t i c l e i n f o
Article history:
Received 7 April 2014
Received in revised form
17 March 2015
Accepted 18 March 2015
Available online 27 March 2015
Keywords:
Green coffee
Grinding
Functional food
Antioxidants
Bioaccessibility
a b s t r a c t
The paper presents the study on possibilities of using green coffee beans (GCB) from Ethiopia, Kenya,
Brazil and Colombia as a functional additive. The dominant compound identi
fied was 5-caffeoylquinic
acid. Caffeine content was comparable in all samples and averaged from 4.36 mg/g dw to 4.99 mg/
g dw. The grinding characteristics of GCB was studied and the sensory properties of bread enriched with
GCB
flour were evaluated. GCB was characterized by high grinding energy requirements. Phenolics
released during simulated digestion were highly bioavailable in vitro. Simulated digestion released
phytochemicals acting as chelating and reductive agents, free radical scavengers and lipid-preventers.
Results of a preliminary study concerning the proposed functional product indicate that phenolic
compounds from bread enriched with powdered GCB were highly mastication-extractable, which may
predict their high bioaccessibility and bioavailability. The content of phenolics was strongly correlated
with powdered GCB addition. The sensory characteristics linking results indicated that a partial
replacement of wheat
flour in bread with up to 3% ground GCB powder gives satisfactory overall con-
sumer acceptability. Bread enriched with GCB possessed higher antiradical activity than control samples.
The results of our study clearly show that powdered GCB may be used directly, without extract prepa-
ration, for food supplementation.
© 2015 Elsevier Ltd. All rights reserved.
1. Introduction
Current food production trends include not only the protection
of food components, but also the production of products with pro-
health properties through the introduction of antioxidants (
). Due to growing evidence that diets rich in phenols
and polyphenols may have potential health bene
fits for consumers,
the nutritional supplement and food industries have developed
numerous products forti
ficated with phenolics (
).
Recently, due to its unique composition and properties,
growing consumer interest has been directed towards green
coffee. Scienti
fic studies have revealed that both bioactive com-
ponents of coffee (phenolic acids and caffeine) play a preventive
role against various degenerative diseases of modern society
(
). Green coffee
has a mild, green, bean-like aroma; the characteristic aroma of
coffee develops during the roasting process. Green coffee beans
(GCB) are rich in phenolic acids, especially in chlorogenic acid
(CGA), and its related compounds that show hypotensive effects
(
). Chlorogenic and caffeic acids, the
main phenolics of green coffee, exhibit antimutagenic, anticarci-
nogenic and antioxidant activities in vitro, which are linked with
the ability to scavenge reactive oxygen species. Additionally, these
compounds have been suggested as inhibitors of in
flammation
and tumor promotion via deactivation of a range of pro-oxidative
enzymes such as lipoxygenase (LOX)
e a key enzyme of the
arachidonic acid metabolism (
). Besides
this, green coffee bean phytochemicals show a tendency to reduce
visceral fat and body weight (
).
* Corresponding author. Tel.: þ48 81 4610061.
E-mail address:
(U. Gawlik-Dziki).
Contents lists available at
LWT - Food Science and Technology
j o u r n a l h o me p a g e :
w w w . e l s e v i e r . c o m / l o ca t e / l w t
http://dx.doi.org/10.1016/j.lwt.2015.03.076
0023-6438/
© 2015 Elsevier Ltd. All rights reserved.

During roasting, there is a progressive destruction and trans-
formation of CGAs with some 8
e10% being lost for every 1% loss of
dry matter (
). Thus, GCB seem to be a better source of
these compounds.
The particle size distribution and size reduction of ground ma-
terial determine the properties of the
final product and the influ-
ence of many processes such as mixing, extraction, kneading or
baking. The extractability of bioactive compounds strongly depends
on solvents and the degree of
fineness. This is the reason why
grinding is a very important process in the food industry. There is
no information in the literature concerning the grinding charac-
teristics of GCB. The production of plant extracts is usually costly
and requires energy inputs; some concerns have also been raised
about the safety of their use. A de
finitely cheaper and, according to
some, safer method is to enrich food products with less processed
supplements. Thus, we examined the potential bioaccessibility and
bioavailability of antioxidative compounds derived directly from
ground GCB based on an in vitro gastrointestinal model. In vitro
models based on human physiology were elaborated as simple,
cheap and repeatable tools for the study of food component bio-
accessibility. These are widely used for the study of structural
changes, digestibility and food component release in simulated
alimentary tract conditions (
Thus, the aim of this study was an estimation of the potential
possibilities of using ground coffee beans as a functional additive.
The grinding characteristics of GCB were also studied and the
sensory properties of bread enriched with GCB were evaluated.
2. Material and methods
2.1. Chemicals
Ferrozine (3-(2-pyridyl)-5,6-bis-(4-phenyl-sulfonic acid)-1,2,4-
triazine),
ABTS
(2,2
ediphenyl-1-picrylhydrazyl)
a
-amylase,
pancreatin, pepsin (from porcine stomach mucosa, pepsin A, EC
3.4.23.1), bile extract, Folin
eCiocalteu reagent, linoleic acid,
ammonium thiocyanate, hemoglobin, pepsin, gallic acid, chloro-
genic acid, sinapinic acid, ferulic acid, benzoic acid, quercetin,
kaempferol, and PBS (phosphate buffered saline, 0.01 mol/L phos-
phate buffer, 0.0027 mol/L potassium chloride and 0.137 mol/L
sodium chloride, pH 7.4, at 25
C.) were purchased from Sigma
e
Aldrich Company (Poznan, Poland). All others chemicals were of
analytical grade.
2.2. Material
Green coffee (Coffea arabica) beans (GCB) derived from various
plantation (from Ethiopia, Kenya, Brazil and Colombia) were ob-
tained from company Cofeina
eRomuald Zalewski, Marki, Poland.
The initial moisture content of GCB ranged from 8.7 g/100 g to 9.0 g/
100 g wet basis (w.b.).
2.3. The grinding process
The samples of GCB were prepared by adding water to adjust
moisture content to 10 g/100 g (w.b.) and storing for 48 h. The
beans of individual coffee samples were ground using the labora-
tory hammer mill (POLYMIX-Micro-Hammermill MFC, Kinematica.
AG, Littau/Lucerne, Switzerland) equipped with round holes
3.0 mm. The detailed procedure of grinding method and grinding
equipment was described by
. The speci
fic grinding energy (E
r
) was
determined as the ratio of the grinding energy to the mass of the
material taken for grinding. The sieving test was used to determine
the particle size distribution of the pulverized material. Sieving was
carried out for 5 min, by using a laboratory screen (Thyr 2, SASKIA,
Essen, Germany), and separated into fractions using sieves of sizes,
0.800, 0.630, 0.500, 0.400, 0.315, and 0.200 mm. On the basis of the
particle size distribution, the average particle size (d) was calcu-
lated. The grinding ability index (E
f
) was calculated as a ratio of the
grinding energy to the surface area of the ground material. The
Soko
łowski's grinding index (K) was calculated on the basis of the
size reduction theory described by
. Details of the
procedure used in determining these indices can be found in
. The distribution of the particle size was
evaluated thrice and the values of grinding indices were calculated
from the average particle size.
2.4. Extraction procedures
For qualitative analysis the powdered samples of GCB (100 mg,
particle size
< 0.2 mm) were extracted using an automated accel-
erated solvent extractor, ASE 200 (Dionex, Sunnyvale, CA, USA).
Extraction was performed with 70 mL/100 mL MeOH at 1500 psi
(10.3 mPa) solvent pressure, 100
C cell temperature,
flush 150%,
and three static cycles for 2 min each. Extracts (25 mL) were
collected in vials. The solvent was evaporated under reduced
pressure at 40
C. The dried extracts were dissolved in 2 mL of Milli-
Q water (Millipore Corp., Billerica, MA, USA). All extractions were
performed in triplicate for three independent samples and stored in
a freezer at
20
C before analysis.
2.4.1. In vitro digestion and absorption
In vitro digestion and absorption were performed according to
. The samples (1 g) were homogenized in a
stomacher laboratory blender for 1 min to simulate mastication
with the presence of 15 mL of simulated salivary
fluid (prepared by
dissolving 2.38 g Na
2
HPO
4
, 0.19 g KH
2
PO
4
, and 8 g NaCl, 100 mg of
mucin in 1 L of distilled water). The solution was adjusted to
pH
¼ 6.75 and
a
-amylase (E.C. 3.2.1.1.) was added to obtain 200 U/
mL of enzyme activity). The samples were adjusted to pH
¼ 1.2
using 5 mol/L HCl, and subsequently, 15 mL of simulated gastric
fluid (300 U/mL of pepsin in 0.03 mol/L NaCl, pH ¼ 1.2) was added.
The samples were shaken for 120 min at 37
C. After that the
samples were adjusted to pH
¼ 6 with 0.1 mol/L of NaHCO
3
and
then 15 mL of simulated intestinal juice (prepared by dissolving
0.05 g of pancreatin (activity equivalent 4
USP) and 0.3 g of bile
extract in 35 mL 0.1 mol/L NaHCO
3
) was added. The extracts were
adjusted to pH
¼ 7 with 1 mol/L NaOH and finally 5 mL of
120 mmol/L NaCl and 5 mL of mmol/L KCl were added. The pre-
pared samples were submitted for in vitro digestion for 60 min, at
37
C in the darkness. After that samples were centrifuged and
supernatants (extracts after simulated digestion) were used for
further analysis.
In vitro absorption. Considering that antioxidants absorption
takes place mainly at the intestinal digestion stage,
fluids obtained
after in vitro digestion was transferred to the dialysis sacks (D9777-
100FT, Sigma
eAldrich), placed in an Erlenmeyer flask containing
50 mL of PBS buffer and incubated in a rotary shaker (2 times per
2 h, 37
C). The PBS buffer together with the compounds that
passed through the membrane was treated as an equivalent of the
raw material absorbed in the intestine after digestion.
2.5. Analytical procedures
2.5.1. Ultra-performance liquid chromatography
Compounds of interest were analyzed using a Waters ACQUITY
UPLCTM system (Waters Corp., Milford, MA, USA), consisting of a
binary pump system, sample manager, column manager and PDA
detector (also from Waters Corp.). Waters MassLynx software v.4.1
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
692

was used for acquisition and data processing. The samples were
separated on a BEH C18 column (100 mm
2.1 mm i.d., 1.7
m
m,
Waters Corp., Milford, MA, USA), which was maintained at 40
C.
The
flow rate was adjusted to 0.40 mL/min. The following solvent
system: mobile phase A (0.1 mL/100 mL formic acid in Milli-Q
water, v/v) and mobile phase B (0.1 mL/100 mL formic acid in
MeCN, v/v) was applied. The gradient program was as follows:
0
e1.0 min, 5% B; 1.0e24.0 min, 5e50% B; 24.0e25.0 min, 50e95% B;
25.0
e27.0 min, 95% B; 27.0e27.1 min, 95-5% B; 27.1e30.0 min, 5% B.
Samples were kept at 8
C in the sample manager. The injection
volume of the sample was 2.0
m
L (full loop mode) and samples were
analyzed in triplicate. Strong needle wash solution (95:5, meth-
anol
ewater, v/v) and weak needle wash solution (5:95, acetoni-
trile
ewater, v/v) were used. The detection wavelength was set at
250 nm for caffeine and 325 for phenolic acids at a 5 point/s rate, at
3.6 nm resolution. The separation was completed in 30 min. Peaks
were assigned on the basis of their UV spectra, mass to charge ratio
(m/z) and ESI-MS/MS fragmentation patterns. Chlorogenic acid (5-
caffeoyl-quinic acid) was used as a group standard for determina-
tion of phenolic compounds. The MS analyses were carried out on a
TQD mass spectrometer (Waters Corp.) equipped with a Z-spray
electrospray interface. The following instrumental parameters
were used for ESI-MS analysis of phenolic compounds (negative
ionization mode): capillary voltage, 2.8 kV; cone voltage, 40 V;
desolvation gas, N2 800 L/h; cone gas, N2 100 L/h; source temp.
140
C, desolvation temp. 350
C. Compounds were analyzed in full
scan mode (mass range of 100
e1600 amu was scanned).
Total phenolics (TPC) content was estimated according to
and calculated as a gallic acid (GAE)
equivalent (mg/g dw).
2.5.2. Analyses of antioxidant activities of extracts
Inhibition of lipid peroxidation (LPO) was performed according
to
. For antiradical activity (AA) analyses,
the improved ABTS decolorisation assay was performed (
). Chelating (CHEL) and ferric reducing antioxidant power
(FRAP) was determined according to the methods described by
Guo, Lee, Chiang, Lin, and Chang (2001)
and
respectively. Antioxidant activities were expressed as EC
50
(Ef
fi-
cient Concentration): the amount of sample (mg dry weight, dw)
needed to obtain 50% activity per 1.0 mL of the initial solution.
2.6. Bread preparation and sensory evaluation
The
flour used in the formula of control bread (C) was wheat
bread
flour (600 g), type 750 (average 0.75 g/100 g ash content,
humidity 14 g/100 g). The
flour was replaced with GCB flour (par-
ticles of ground CGB from Brazil
< 0.2 mm; based of the highest BAV
values) at 1 g/100 g, 2 g/100 g, 3 g/100 g, 4 g/100 g, 5 g/100 g levels
(GC1, GC2, GC3, GC4 and GC5, respectively). Besides this 6 g of
instant yeast and 12 g of salt were used for dough preparation. The
general quantity of water necessary for the preparation of the
dough was established through the marking of water absorption
properties in
flour of a consistency of 350 Brabender units. The
batches of dough were mixed in a spiral mixer for 6 min. After
fermentation, the pieces of dough (300 g) were put into an oven
heated up to a temperature of 230
C. The baking time was 30 min.
After baking, the bread was left to stand for 24 h at room temper-
ature. Sensory evaluation was carried out on bread samples with
the different percentages of GCB. Subsequently, the samples were
sliced (slices about 1.5 cm thick), coded with a number and served
to untrained consumers. The panel consisted of 33 consumers
(24
e45 years old; 21 women and 12 man), who evaluated the
bread's crumb color, aroma, texture, taste overall acceptability. This
hedonic test was used to determine the degree of overall liking for
the different types of bread based on degree of liking or disliking
according to a nine-point hedonic scale (1: dislike extremely, 5:
neither like nor dislike, 9: like extremely). Plain water was used for
mouth rinsing before and after each sample testing (
).
2.6.1. Bread extracts preparations
The breads were sliced (slices about 1.5 cm thick). The crust was
removed aseptically and kept frozen (at
20
C) until analysis. After
thawing, the slices were dried and then manually crumbed,
grounded in a mill and screened through 0.5 mm sieve to obtain
bread powder.
Powdered samples of breads (1 g) were extracted for 30 min
with 20 mL of methanol: water mixture (1:1, v/v), pH
¼ 2 (chemical
extracts, CE) or 20 mL of PBS (phosphate buffered saline, pH 7.4),
(buffer extracts, BE). The extracts were separated by decantation
and the residues were extracted again with 20 mL of proper solvent.
Extracts were combined and stored in darkness at
20
C. Obtained
extracts were used for determining total lipophilic (CE) and
potentially mastication-extractable hydrophilic phenolics (BE) and
antiradical activity.
2.7. Calculations
Better
estimation
of
extractability,
bioaccessibility
and
bioavailability of phenolic compounds and antioxidants the
following factors were determined:
- the relative phenolics bioaccessibility index (RBC) which is an
indication of potential bioaccessibility of phenolic compounds:
RBC
¼ C
GE
=C
CE
- the relative phenolics bioavailability index (RBV) which is an
indication of potential bioavailability of phenolic compounds:
RBV
¼ C
AE
=C
CE
- the phenolics bioavailability index (PAV) which is an indication
of potential bioavailability of phenolics released during diges-
tion in vitro:
PAV
¼ C
GE
=C
AE
where, C
GE
e total phenolic contents in gastrointestinal digested
extracts, C
CE
e total phenolic contents in chemical extracts, C
AE
e
total phenolic contents in extracts after simulated absorption.
- the antioxidant bioavailability (BAV) index which is an indica-
tion of the potential bioavailability of antioxidative compounds:
BAV
¼ A
GE
=A
AE
where, A
GE
is EC
50
of extract after simulated gastrointestinal
digestion (GE), A
AE
is EC
50
of extract after simulated intestinal ab-
sorption (ABE).
2.8. Statistical analysis
All experimental results are displayed as
± S.D. of three parallel
experiments (n
¼ 9) and data were evaluated by one-way analysis
of variance (one-way Anova). The statistical differences between
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
693
the groups were estimated using the Tukey test. Statistical tests
were evaluated using Statistica 6.0 software (StatSoft, Inc., Tulsa,
USA). All the statistical tests were carried out at a signi
ficance level
of
a
¼ 0.05.
3. Results and discussion
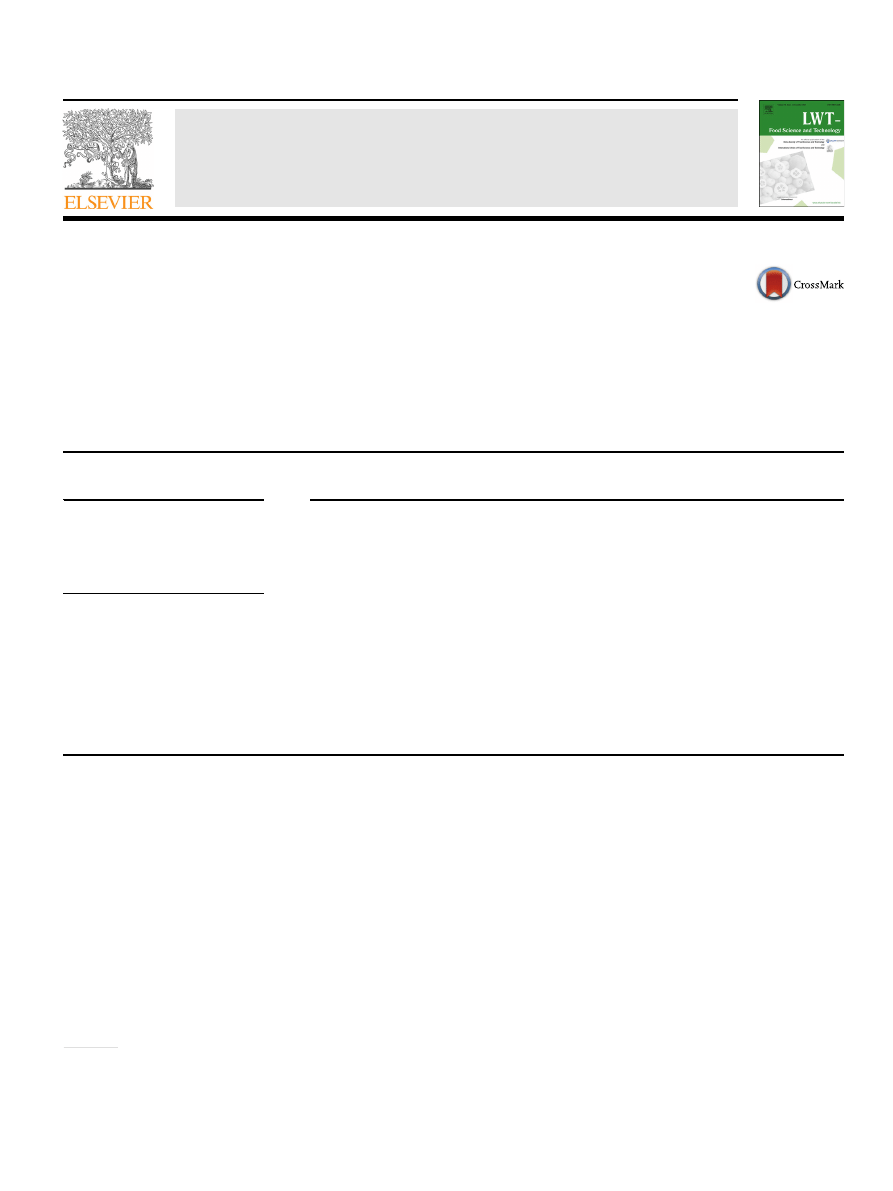
3.1. Grinding results
The particle size distributions of ground GCB are given in
For all samples, the highest mass fraction was obtained for coarse
particles: 1.0
e1.6 mm. The share of this fraction ranged from 50.7%
(GCB from Kenya) to 58.3% (sample from Ethiopia). The highest
mass fraction of the smallest particles (
<0.2 mm) was obtained for
Kenyan coffee samples (average 13.5%) and the lowest in the case of
ground Colombian coffee (average 4.6%). In the case of cereal grains,
the amount of the
fine fraction correlated with grain hardness. In
particular, soft wheat kernels are characterized by a lower degree of
adhesion between starch granules and the protein matrix and thus
a higher mass fraction of
fine particles is produced (
). Analyzing the grinding pattern of size reduced GCB,
this is quite different from ground cereal grain obtained in the same
grinding conditions (
), especially in terms of the
mass fraction of coarse particles. The average particle size (d) of
ground GCB ranged from 0.95 mm (Kenyan coffee) to 1.05 mm
(Ethiopian coffee). However, signi
ficant differences were found
only between d for Kenyan samples and other samples.
There is no information in the literature concerning the grinding
characteristics of green coffee. Most studies in to the size reduction
process of coffee are devoted to a study of the grinding process of
roasted beans and especially the in
fluence of particle size and
particle uniformity after grinding on coffee extraction.
reported that the key to good roasted coffee brewing is
good coffee grinding, which is obtained by optimizing the extrac-
tion of the soluble solids into the hot brewing water.
Thomann, Perren, and Escher (2008)
found that the water con-
tent of roasted coffee in
fluenced grinding behavior, extraction, and
aroma retention dynamics.
showed that grinding roasted CB is a critical step in the prepara-
tion of espresso coffee.
Baggenstoss, Thomann, Perren, and Escher
studied the aroma recovery from roasted coffee by wet
grinding. They found that a two-step process involving cold wet
grinding and subsequent hot extraction in a closed system
increased the yield of aroma compounds in the resulting coffee
compared to conventionally ground coffee. The results of grinding
energy requirements showed that E
r
ranged from 73.3 kJ kg
1
(Brazilian coffee) to 106.9 kJ kg
1
(Colombian coffee). Also, for these
samples the lowest and the highest values of E
f
and K were ob-
tained, i.e. from 16.2 kJ m
2
to 23.4 kJ m
2
and from
120.3 kJ kg
1
mm
0.5
to 173.4 kJ kg
1
mm
0.5
, respectively (
These values are between two and three times higher than the
values of these indices obtained for wheat grain under the same
grinding conditions (
). Both particle size distribution and
grinding indices suggested that GCB is a relatively dif
ficult raw
plant material for size reduction by impact grinding.
3.2. Qualitative
equantitative analysis of phenolics and caffeine
Green coffee beans are high in chlorogenic acids (CGAs); their
contents are 3.5
e7.5% (w/w dry matter) for C. arabica and 7.0e14.0%
(w/w dry matter) for Coffea canephora (
). The
nomenclature of CGAs is based on the IUPAC numbering system
(1976), and 5-caffeoylquinic acid (5-CQA) is generally called
chlorogenic acid. Thirty-four kinds of CGAs have been reported in
green coffee beans. The CGAs in green coffee beans consist of three
main classes: caffeoylquinic acids (CQAs) with three isomers (3-, 4-,
and 5-CQA), dicaffeoylquinic acids (diCQAs) with three isomers
(3,4-, 3,5-, and 4,5-diCQA), and feruloylquinic acids (FQAs) with
three isomers (3-, 4-, and 5-FQA). These nine kinds of CGAs account
for 80% of the content of total CGAs in green coffee beans (
The dominant compound identi
fied in all analyzed samples was
5-CQA. The richest source of this compound was GCB from Kenya,
Table 1
Particle size distribution (%) and the average particle size of the GCB
samples.
Range of class, mm
Sample
Brazil
Colombia
Ethiopia
Kenya
>1.6
3.4
± 0.11
A
4.8
± 0.18
B
4.4
± 0.21
C
3.4
± 0.15
A
1.0
e1.6
53.1
± 1.72
A
51.4
± 2.78
A
58.3
± 1.23
B
50.7
± 2.21
A
0.8
e1.0
16.9
± 0.42
B
16.4
± 0.32
B
11.7
± 0.48
C
14.1
± 0.36
A
0.63
e0.8
9.7
± 0.32
B
6.6
± 0.25
A
9.8
± 0.21
B
6.5
± 0.18
A
0.5
e0.63
3.0
± 0.16
B
2.9
± 0.08
B
2.5
± 0.18
A
2.6
± 0.12
A
0.4
e0.5
1.4
± 0.10
A
2.9
± 0.16
B
1.1
± 0.06
C
1.5
± 0.09
A
0.315
e0.4
2.0
± 0.17
C
1.8
± 0.11
BC
1.6
± 0.14
B
2.5
± 0.13
A
0.2
e0.315
4.0
± 0.27
D
8.7
± 0.33
B
2.8
± 0.26
C
5.2
± 0.28
A
<0.2
6.4
± 0.33
D
4.6
± 0.27
B
7.8
± 0.29
C
13.5
± 0.65
A
d
[mm]
1.02
± 0.011
B
1.01
± 0.018
B
1.05
± 0.012
B
0.95
± 0.014
A
The values are expressed as mean
± SD (n ¼ 3).
a
GCB
e green coffee beans.
b
The values designated by the different letters in the rows of the table are signi
ficantly different (
a
¼ 0.05).
c
d
e Average particle size.
Fig. 1. The grinding energy indices of green coffee beans.
e specific grinding energy
(E
r
),
e grinding efficiency index (E
f
),
e Sokołowski's grinding index (K); B e Brazil,
C
e Colombia, E e Ethiopia, K e Kenya; the values are expressed as mean ± SD (n ¼ 9);
means with different letter superscript are signi
ficantly different (
a
< 0.05).
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
694

whereas the lowest amount was determined in GCB from Colombia.
Moreover, signi
ficant amounts of other phenolic acids (3-CQA; 4-
CQA; 3-FQA; 5-FQA and 3,5-diCQA) were determined. The
analyzed extracts did not differ in terms of their composition;
however, the levels of individual compounds differed signi
ficantly.
The caffeine content was comparable in all samples and averaged
from 4.36 mg/g dw (Ethiopian coffee) to 4.99 mg/g dw (Kenyan
coffee) (
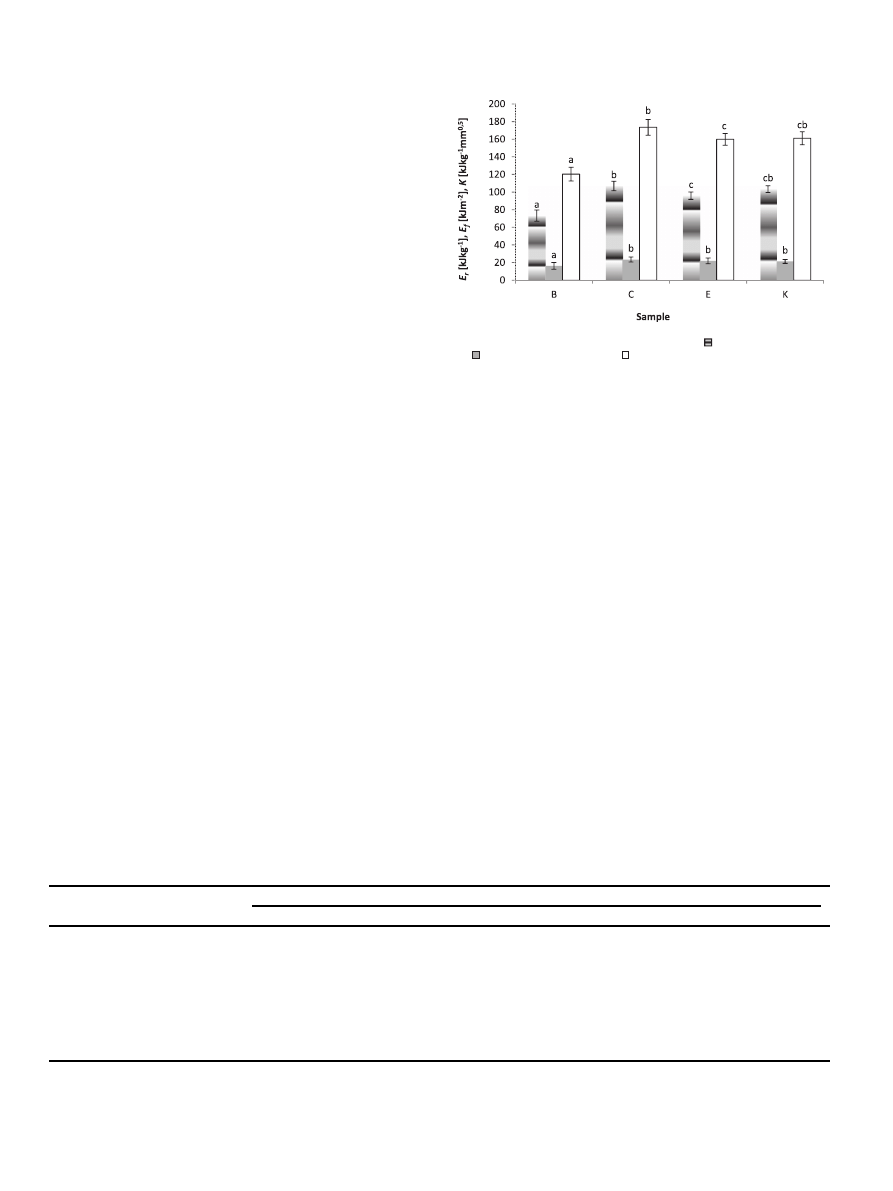
3.3. Extractability of phenolics
For the estimation of the extractor-like activity of a simulated
gastrointestinal tract, the phenolics content in chemical extracts,
and
fluids after simulated digestion and absorption were compared.
As presented in
, the levels of lipophilic phenolics (chemical
extracts) in all coffee samples were comparable and averaged from
183.20 to 197.13 mg GAE/g dw. Most importantly, phenolic com-
pounds were better extracted by simulated gastrointestinal diges-
tion, which may indicate their hydrophilic character and high
bioaccessibility. Additionally, phenolics released during simulated
digestion were highly bioavailable in vitro. The weakest source of
chemical-extractable and potentially bioaccessible phenolic com-
pounds was Colombian coffee, whereas the highest values were
determined for GCB from Ethiopia.
To better understand the potential bioaccessibility and
bioavailability of coffee phenolics, the mathematical indices were
calculated (
).
Relative phenolics bioaccessibility and relative phenolics
bioavailability indices (RBC and RBV, respectively) were calculated
in foothold of results obtained for chemical extracts and thus may
indicate the extraction ef
ficiency of the simulated gastrointestinal
tract. Their values con
firm previous observations e in all cases RBC
values exceed 1, which indicates a high potential bioaccessibility.
Especially high RBV values were found in the case of Ethiopian and
Colombian coffee. The PAV factor indicates the potential bioavail-
ability of compounds released during digestion in vitro. The highest
value was found for GCB from Ethiopia, whereas the lowest for GCB
from Kenya. Interestingly, in the case of GCB from Colombia, despite
a relatively low content of phenolics, high values for parameters
describing bioaccessibility and bioavailability were obtained.
3.4. Antioxidant activity of potentially bioaccessible and
bioavailable fraction
Brewed coffee has been consumed for many centuries, due to its
stimulating properties and other health bene
ficial activities. As is
presented here, powdered GCBs are excellent sources of anti-
oxidative compounds with multidirectional activity. Irrespective of
GCB source, simulated digestion of GCB released phytochemicals
acting as chelating and reductive agents, free radical scavengers
and lipid-preventers (
). The highest capacity for metal ions
chelation was determined for GCB from Brazil (EC
50
¼ 2.07 mg dw/
mL), whereas the lowest for GCB from Ethiopia (EC
50
¼ 7.01 mg dw/
mL).
Most importantly, active compounds were bioavailable in the
model system. The activity of extracts obtained after simulated
absorption was signi
ficantly higher than that determined for
samples obtained after simulated digestion. Especially high
bioavailability was determined for GCB derived from Ethiopia
(BAV
¼ 7.14). Particularly noteworthy is the fact that GCBs were an
excellent source of potentially bioaccessible reductive compounds.
EC
50
values ranged from 0.97 mg dw/mL to 2.32 mg dw/mL for
GCBs from Kenya and Brazil, respectively. Activities of extracts
obtained after simulated absorption were comparable with those
determined for extracts after digestion. This fact may indicate a
potential high bioavailability of active compounds, which was
additionally con
firmed by BAV values. Relatively low bioavailability
was found only in the case of reductive compounds released for
Kenyan GCBs (BAV
¼ 0.42). The present results confirmed those
obtained by
Farah, Monteiro, Donangelo, and Lafay (2008)
which
proved that CQA and diCQA (major CGA compounds in coffee) are
highly bioavailable in humans and are differentially absorbed and/
or metabolized throughout the whole gastrointestinal tract. How-
ever,
Stalmach, Williamson, and Crozier (2014)
showed trends to-
wards a reduced bioavailability of chlorogenic acids associated with
the highest dose ingested, when expressed as percentages of
intake.
The antiradical activity of samples obtained after digestion
in vitro of powdered GCB was comparable and averaged about 4 mg
dw/mL. The potential bioavailability of antiradical compounds
differed signi
ficantly. The highest activity was found for Brazilian
GCB whereas the lowest was found for GCB from Colombia. Low
bioavailability of antiradical compounds from Colombian GCB was
con
firmed by BAV values (0.52). In other cases their values averaged
about 1.
The ability to prevent lipids against oxidation determined for
extracts obtained after simulated digestion was relatively low (in
comparison to other activities). Probably, this is because lipophilic
compounds are less extractable in the gastrointestinal model sys-
tem used. Activity averaged from 30.01 to 16.06 mg dw/mL. Most
importantly, the potential bioavailability of these phytochemicals
was surprisingly high. The activity of extracts obtained after
Table 2
Qualitative-quantitative analysis of phytochemicals of coffee derived from various plantations (n
¼ 9).
No.
Compound
Content [mg/g d.m.]
Brazil
Colombia
Ethiopia
Kenya
1
3.19
± 0.22
2.61
± 0.24
B
1.62
± 0.19
C
2.95
± 0.29
A
2
5-CQA
36.9
± 3.11
AC
27.64
± 3.91
B
30.86
± 3.82
AB
39.92
± 4.69
C
3
Caffeine
4.83
± 0.12
AB
4.40
± 0.38
A
4.36
± 0.11
A
4.99
± 0.32
B
4
4-CQA and 3-FQA
5.01
± 0.21
A
4.03
± 0.33
B
2.98
± 0.30
C
4.92
± 0.49
A
5
5-pCoQA
0.95
± 0.02
A
0.79
± 0.12
B
0.75
± 0.03
B
1.00
± 0.10
A
6
5-FQA
4.22
± 0.11
A
4.14
± 0.42
A
4.32
± 0.33
A
5.48
± 0.52
B
7
3,4-diCQA
0.97
± 0.21
A
0.63
± 0.41
AB
0.45
± 0.11
C
0.82
± 0.22
AB
8
3,5-diCQA
2.47
± 0.28
A
1.79
± 0.13
B
2.45
± 0.62
A
2.98
± 0.41
A
9
4,5-diCQA
1.07
± 0.18
A
0.68
± 0.07
B
0.43
± 0.12
C
0.90
± 0.19
A
The values are expressed as mean
± SD (n ¼ 9).
a
3-CQA
e 3-caffeoylquinic acid, 5-CQA e 5-caffeoylquinic acid, 4-CQA e 4-caffeoylquinic acid, 3-FQA e 3-feruloylquinic acid, 5-FQA e 5-feruloylquinic acid, 4-FQA e 4-
feruloylquinic acid, 5-pCoQA
e 5-p-coumaroylquinic acid, 3-CQL e 3-caffeoylquinic-1,5-lactone, 4-CQL e 4-caffeoylquinic-1,5-lactone, 3,4-diCQA e 3,4-dicaffeoylquinic
acid, 3,5-diCQA
e 3,5-dicaffeoylquinic acid, 4,5-diCQA e 4,5-dicaffeoylquinic acid.
b
The values designated by the different letters in the rows of the table are signi
ficantly different(
a
¼ 0.05).
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
695
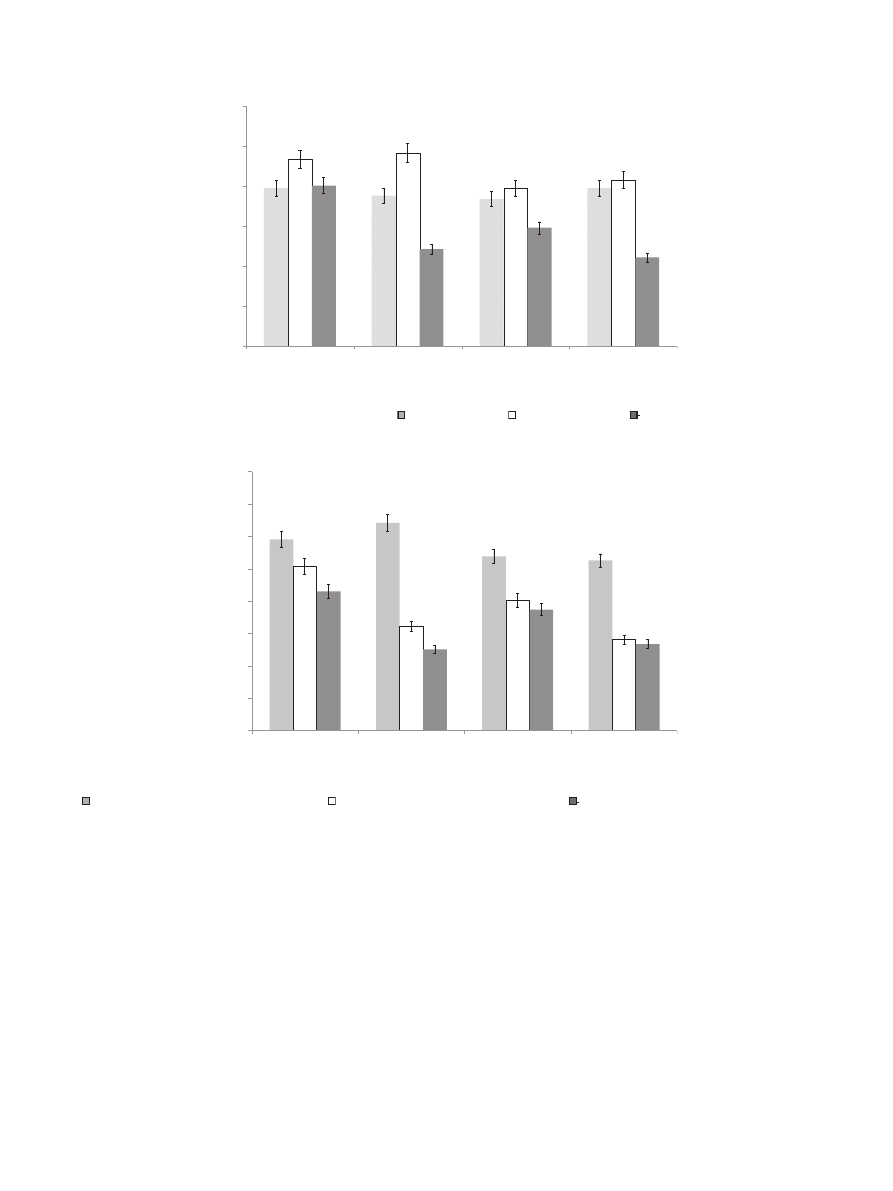
simulated absorption averaged from 8.33 to 5.74 mg dw/mL. The
high bioavailability of lipid-preventive compounds was con
firmed
by BAV values (
The multidirectional antioxidant activity of coffee has been
proved by other investigators (
Budryn et al., 2013; Sato et al., 2011
).
Administration of antioxidants may therefore help to remove
reactive oxygen species and thus improve the clinical outcome. It is
thought that dietary antioxidants can enhance cellular defense and
help to prevent oxidation damage to cellular components (
).
The obtained result may con
firm previous reports concerning
the protective effect of coffee phytochemicals on the central ner-
vous system (CNS), especially for the brain, containing a very high
content of phospholipids. CNS is most susceptible to the effects of
hydroxyl radicals (
). Formation of hydroxyl radicals
(formed from the reaction of hydrogen peroxide with iron and
copper ions) can be delayed by the chelation and deactivation of
transition metal ions. Thus, both activities
e chelating power and
the ability to prevent lipids from oxidation are complementary.
Additionally, these compounds were bioavailable in vitro.
3.5. Sensory properties of bread
Bread is one of the main products consumed in our cultural area
and resignation from it for many people is impossible. Commer-
cially produced bread is an important component in the everyday
diet of Central and Eastern Europe. In many countries, bread is the
staple food product and, depending on regional traditions, it may
be eaten with some or even all meals of which it is the basis or a
valuable supplement. The attempts at enriching bread in materials
abundant in bioactive ingredients seem, therefore, to be well
targeted.
The results of hedonic tests on different types of bread are given
in
. The color of both crust and crumb of the enriched bread
cd
c
c
cd
e
e
cd
d
cd
a
b
a
0
50
100
150
200
250
300
E
K
C
B
To
tal ph
en
olic
s
co
nt
en
t
[m
g
GA
E
/g
dw]
Coffee source
Fig. 2. Total phenolic contents in extracts from powdered green coffee beans.
chemical extract (CE),
e digested in vitro (DE),
e absorbed in vitro (ABE); B e Brazil, C e
Colombia, E
e Ethiopia, K e Kenya; the values are expressed as mean ± SD (n ¼ 9); means with different letter superscript are significantly different (
a
< 0.05).
ab
b
a
a
c
a
b
a
c
a
a
a
0,00
0,20
0,40
0,60
0,80
1,00
1,20
1,40
1,60
E
K
C
B
RBC, RBV
, P
A
V
Coffee source
Fig. 3. Comparison of potential bioaccessibility and bioavailability of phenolic compounds from powdered green coffee beans (n
¼ 9). B e Brazil, C e Colombia, E e Ethiopia, K e
Kenya;
e the relative phenolics bioaccessibility index (RBC),
e the relative phenolics bioavailability index (RBV),
e the phenolics bioavailability index (PAV); means with
different letter superscript (within the index) are signi
ficantly different (
a
< 0.05).
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
696
was a little greener than that of the control bread. However, it had a
slight negative in
fluence on bread acceptability. The taste, aroma
and overall acceptability of bread at substitution levels of 1
e3% had
the highest linking score. Generally higher levels of GCB addition
caused a less acceptable aroma and taste. For texture characteris-
tics, no statistically signi
ficant differences were observed in any
samples. The sensory characteristics linking results indicated that a
partial replacement of wheat
flour in bread with up to 3 g/100 g
ground GCB powder gives satisfactory overall consumer accept-
ability (on average more than 7 points on 9 maximum possible).
However, bread which contained 4 g/100 g and 5 g/100 g GCB was
rated comparatively lower (below 7 points), which is due to
excessive amounts of GCB compounds which negatively affected
the aroma and taste of products.
Although traditional bread uses only four ingredients, most
recipes also add some sweetener, some oil, multiple types of
flour,
seeds, and other bread additives which can improve the nutritional
and nutraceutical value of bread. The biological advantages of
chlorogenic acid and ferulic acids appear useful for the develop-
ment of functional foods, which could contribute to a healthy diet.
It must be taken into account, however, that any functional sub-
stance that is effective on its own (in vitro or in vivo) may have
different or no effects when it becomes an ingredient in food.
Therefore, it is necessary to investigate not only the single agent but
also the whole food (
Glei, Kirmse, Habermann, Persin,
). Another aspect of our research is the potential us-
age of GCB for obesity treatment. A variety of natural products,
including crude extracts and isolated compounds from plants, can
induce body weight reduction and prevent diet-induced obesity.
Therefore, they have been widely used in treating obesity. A wealth
of information indicates that numerous bioactive components from
nature are potentially useful in obesity treatments. A good example
of one such component are the polyphenols which show strong
anti-obesity activity (
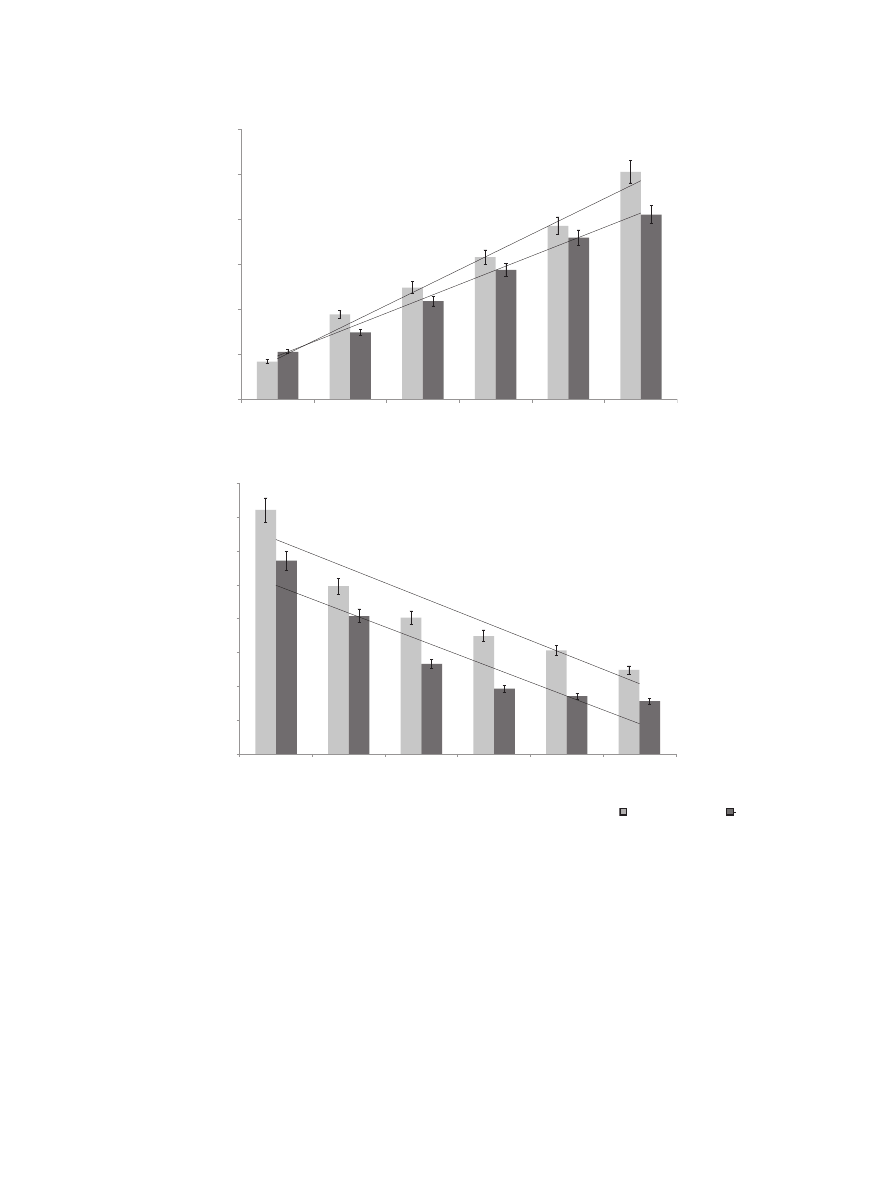
Results of a preliminary study concerning the proposed func-
tional product indicate that phenolic compounds from bread
enriched with powdered GCB were highly mastication-extractable
(buffer extract), which may predict their high bioaccessibility and,
thus, bioavailability. The content of phenolic compounds was
strongly correlated with powdered GCB addition (
A). Data
presented in
con
firmed previous results concerning the
extractability of phenolic compounds from powdered GCB.
Oxidative stress in accumulated fat is an early instigator of
metabolic syndrome and the redox state in adipose tissue is a
potentially useful therapeutic target for obesity-associated meta-
bolic syndrome (
). Our preliminary study
clearly showed that bread enriched with powdered GCB possessed
higher antiradical activity than control samples (bread without GCB
addition). Most importantly, higher activity was found for buffer
extracts containing potentially mastication-extractable compounds
(
B).
proved that the antioxidant activity
increased by the addition of extracts with green coffee mostly in
sponge
cake,
caramel
candies,
marshmallows,
and
also
Table 3
Bioaccessibility and bioavailability in vitro of antioxidants from powdered green coffee beans.
Activity
Coffee samples
Bioaccessible in vitro phytochemicals
EC
50
[mg dw/mL]
Bioavailable in vitro phytochemicals
EC
50
[mg dw/mL]
Bioavailability
(BAV) factor
Chelating power
Brazil
2.07
± 0.09a
0.56
± 0.13aB
3.71
Colombia
3.34
± 0.79bA
1.27
± 0.55bB
2.63
Ethiopia
7.01
± 1.90cA
0.98
± 0.50cB
7.14
Kenya
3.65
± 1.01bA
1.48
± 0.59bB
2.47
Reducing power
Brazil
2.32
± 0.13aA
0.93
± 0.54aB
2.48
Colombia
0.99
± 0.10bA
1.03
± 0.01bA
0.96
Ethiopia
1.85
± 0.34cA
1.50
± 0.85bA
1.23
Kenya
0.97
± 0.10bA
2.30
± 1.10cB
0.42
Antiradical activity
Brazil
3.76
± 0.18aA
2.99
± 1.20aB
1.26
Colombia
3.76
± 0.13aA
7.25
± 3.20bB
0.52
Ethiopia
4.11
± 0.37bA
4.75
± 0.63cA
0.86
Kenya
3.67
± 0.10aA
3.95
± 1.58dA
0.93
Ability to lipids
prevention
Brazil
17.71
± 1.81aA
8.33
± 0.72aB
2.13
Colombia
16.06
± 0.74bA
6.34
± 0.27bB
2.53
Ethiopia
16.64
± 1.45bA
7.29
± 2.73cB
2.28
Kenya
30.01
± 2.34cA
5.74
± 0.27bB
5.23
The values are expressed as mean
± SD (n ¼ 9).
a
The values designated by the different small letters in the columns are signi
ficantly different(
a
¼ 0.05).
b
The values designated by the different capital letters in the lines are signi
ficantly different(
a
¼ 0.05).
Table 4
Sensory evaluation of bread prepared by the substitution of wheat
flour with GCB.
GCB addition, [g/100 g]
Sensory evaluation
Crumb color
Aroma
Texture
Taste
Overall
C
8.5
± 0.38
8.8
± 0.38
A
7.8
± 0.24
A
8.4
± 0.42
A
8.4
± 0.42
A
GC1
8.4
± 0.26
AB
8.4
± 0.54
AB
7.6
± 0.48
A
8.2
± 0.40
A
8.2
± 0.53
A
GC2
8.2
± 0.34
AB
7.8
± 0.29
B
7.9
± 0.42
A
7.5
± 0.66
AB
7.9
± 0.61
AB
GC3
8.3
± 0.42A
B
6.8
± 0.61
C
7.8
± 0.69
A
6.2
± 0.44
BC
7.3
± 0.35
BC
GC4
7.9
± 0.42
BC
6.0
± 0.60
D
7.6
± 0.58
A
5.7
± 0.58
CD
6.8
± 0.28
C
GC5
6.8
± 0.31
C
5.8
± 0.56
D
7.3
± 0.37
A
5.2
± 0.39
D
6.3
± 0.45
C
a
GCB
e green coffee beans, nine-point hedonic scale of sensory evaluation with 1, 5 and 9 representing extremely dislike, neither like nor dislike, and extremely like,
respectively.
b
Means with different letter superscript within a same column are signi
ficantly different (
a
< 0.05).
c
C
e control bread, GC1eGC5, wheat bread with 1e5 g/100 g of powdered GCB addition, respectively.
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
697
mayonnaise.
demonstrated that the supplemen-
tation of bread with 1% commercial green coffee extract resulted in
enhanced chemopreventive in vitro properties in comparison with
normal bread. Enriched bread contains more chlorogenic acid and
has a higher antioxidant activity than normal bread. These prop-
erties were associated with an increased resistance of colon and
liver cells against H
2
O
2
-mediated genotoxicity, which is an impor-
tant mechanism of chemoprotection. However, in the recent liter-
ature there is a lack of
findings regarding the usefulness of whole
powdered GCB in food forti
fication.
Potential functional properties of proposed products may be
supported by caffeine. Caffeine's mechanism of lipolytic action
might be due to its binding to the phospholipid phosphate groups
and the subsequent interactions between the lipase and triglycer-
ide portions of lipid droplets, eliciting lipolysis (
). Besides this, caffeine inhibits pancreatic
lipase, and has been found to be a suppressor of fat absorption
(
4. Conclusion
Results of our study clearly show that powdered GCB may be
used directly, without extract preparation, for food supplementa-
tion. An important aspect of the proposed research is to try to
determine the suitability of GCB to obtain a functional product
dedicated to people suffering from/at risk of metabolic syndrome.
An innovative solution is to offer a product containing both
phenolic acids to hinder the absorption of fat and caffeine which
contributes to energy expenditure and improved mood. Bread is
one of the main products consumed in the cultural area of many
a
b
c
d
e
f
a
b
c
d
e
f
R² = 0.987
R² = 0.995
0
2
4
6
8
10
12
C
GC1
GC2
GC3
GC4
GC5
To
tal ph
en
olics
con
ten
t
[m
g
GA
E
/g
dw]
Sample
a
b
c
d
e
f
a
b
c
d
d
d
R² = 0.883
R² = 0.865
0
10
20
30
40
50
60
70
80
C
GC1
GC2
GC3
GC4
GC5
E
C
50 [
m
g
dw/m
L
]
Sample
A
B
Fig. 4. In
fluence of powdered green coffee beans addition on total phenolics content (A) and antiradical activity (B) of wheat bread.
chemical extract (CE),
e buffer extract (BE),
the values are expressed as mean
± SD (n ¼ 9); means with different letter superscript (within the extract) are significantly different (
a
< 0.05); C e control bread, GC1eGC5, wheat
bread with 1
e5 g/100 g of powdered green coffee beans addition, respectively.
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
698

countries and resignation from its consumption for many people is
impossible. The proposed product is mainly targeted at this group
of consumers
e it is a compromise between “traditional” and pro-
health food.
Acknowledgments
This scienti
fic study was financed by the Polish National Science
Centre (grant 2013/09/B/NZ9/01801). We are grateful to Romuald
Zalewski that provided the raw material for research.
References
~na, M. P., & Cid, C. (2003). Chemical and sensorial characteristics
e7039
Baggenstoss, J., Perren, R., & Escher, F. (2008). Water content of roasted coffee:
.
Baggenstoss, J., Thomann, D., Perren, R., & Escher, F. (2010). Baggenstoss aroma
recovery from roasted coffee by wet grinding. Journal of Food Science, 75,
C697
Budryn, G., Nebesny, E., Rachwa
ł-Rosiak, D., & Oracz, J. (2013). Fatty acids, essential
amino acids, and chlorogenic acids pro
files, in vitro protein digestibility and
.
Clifford, M. N. (1999). Chlorogenic acids and other cinnamates: nature, occurrence
and dietary burden. Journal of the Science of Food and Agriculture, 79, 362
e372
.
Dziki, D. (2008). The crushing of wheat kernels and its consequence on the grinding
process. Powder Technology, 185, 181
Dziki, D. (2011). Effect of preliminary grinding of the wheat grain on the pulverizing
process. Journal of Food Engineering, 104, 585
Dziki, D., Cacak-Pietrzak, G., Mi
s, A., Jonczyk, K., & Gawlik-Dziki, U. (2014). Influence
e2655
.
Dziki, D., & Laskowski, J. (2010). Study to analyze the in
wheat grain on the grinding process. Journal of Food Engineering, 96, 562
e567
Ephraim, D. (2007). What you should know about grinding coffee. Indian Coffee, 71,
e33
Farah, A., Monteiro, M., Donangelo, C. M., & Lafay, S. (2008). Chlorogenic acids from
green coffee extract are highly bioavailable in humans. Journal of Nutrition, 138,
2309
Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., et al.
Gawlik-Dziki, U. (2012). Changes in the antioxidant activities of vegetables as a
consequence of interactions between active compounds. Journal of Functional
Foods, 4, 872
.
łkowski, M., Dziki, D., Baraniak, B., & Czy_z, J. (2013).
Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts
in vitro study. Food and Chemical Toxicology, 5, 154
e160
Glei, M., Kirmse, A., Habermann, N., Persin, C., & Pool-Zobel, B. L. (2006). Bread
e192
Greffeuille, V., Abecassis, J., Rousset, M., Oury, F.-X., Faye, A., Bar L'Helgouac'h, C.,
.
Guo, J.-T., Lee, H.-L., Chiang, S.-H., Lin, H.-I., & Chang, C.-Y. (2001). Antioxidant
Halliwell, B. (2001). Role of free radicals in the neurodegenerative diseases: ther-
apeutic implications for antioxidant treatment. Drugs
.
Han, L. K., Takaku, T., Li, J., Kimura, Y., & Okuda, H. (1999). Anti-obesity action of
oolong tea. International Journal of Obesity and Related Metabolic Disorders, 23,
98
Harland, B. F. (2000). Caffeine and nutrition. Nutrition, 16, 522
e526
Igho, O., Rohini, T., & Edzard, E. (2011). The use of green coffee extract as a weight
.
Kuo, J.-M., Yeh, D.-B., & Pan, B. (1999). Rapid photometric assay evaluating anti-
oxidative activity in edible plant material. Journal of Agricultural and Food
Chemistry, 47, 3206
e3209
Lim, H. S., Park, S. H., Ghafoor, K., Hwang, S. Y., & Park, J. (2011). Quality and anti-
e1582
Maat, J., Rossi, D., Babuchowsk,i, A., Beekmans, F., Castenmiller, J., Fenwick, R., et al.
(2005). European technology platform on food for life. The vision for 2020 and
beyond. Downloaded from
http:platformazywnosci.pl/pliki/BAT_Brochure_ETP.
. on 23.01.13.
Narita, Y., & Inouye, K. (2011). Inhibitory effects of chlorogenic acids from green
coffee beans and cinnamate derivatives on the activity of porcine pancreas
amylase isozyme I. Food Chemistry, 127, 1532
Oomen, A. G., Hack, A., Minekus, M., Zeijdner, E., Cornelis, C., Schoeters, G., et al.
five in vitro digestion models to study the bio-
accessibility of soil contaminants. Environmental Science and Technology, 36,
3326
Oyaizu, M. (1986). Studies on products of browning reaction
.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999).
e1237
Sato, Y., Itagaki, S., Kurokawa, T., Ogura, J., Kobayashi, M., Hirano, T., et al. (2011).
e138
Shimoda, H., Seki, E., & Aitani, M. (2006). Inhibitory effect of green coffee bean
.
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phospho-
ephosphotungstic acid reagents. American Journal of Enology and
e158
łowski, M. (1996). Energy consumed in grinding e a new idea of a general law
e new tests stands and testing results. Recents Progress en
Stalmach, A., Williamson, G., & Crozier, A. (2014). Impact of dose on the bioavail-
ability of coffee chlorogenic acid in humans. Food and Function, 5, 1727
e1737
Yun, J. W. (2010). Possible anti-obesity therapeutics from nature
e1641
D. Dziki et al. / LWT - Food Science and Technology 63 (2015) 691
e699
699
Document Outline
- Ground green coffee beans as a functional food supplement – Preliminary study
- 1. Introduction
- 2. Material and methods
- 3. Results and discussion
- 4. Conclusion
- Acknowledgments
- References
Wyszukiwarka
Podobne podstrony:
Attitudes toward Affirmative Action as a Function of Racial ,,,
Attitudes toward Affirmative Action as a Function of Racial ,,,
The challenge of developing green tea polyphenols as therapeutic agents
Green tea catechins as a BACE1 inhibitor
Wheat bread enriched with green coffee – In vitro bioaccessibility and
Functional Food
Green tea catechins as brain permeable, natural iron chelators antioxidants for the treatment of neu
BoyerTiCS Religious Thought as a By Product of Brain Function
ECN, GREEN GAS AS SNG (SYNTHETIC NATURAL GAS) rx04085
Cookery And Food Preparation Manual Beans
BBC Good Food Recipes The ultimate makeover Thai green chicken curry
As the Green Star Rises Lin Carter
Fulling Green s functions, heat kernels, Kasimir effect(10s)
PREZENTacja dla as
L 3 Complex functions and Polynomials
3 1 Krzywa podazy AS ppt
3 ABAP 4 6 Basic Functions
więcej podobnych podstron