Wheat bread enriched with green coffee – In vitro bioaccessibility and
bioavailability of phenolics and antioxidant activity
Michał S´wieca
, Urszula Gawlik-Dziki
, Dariusz Dziki
, Barbara Baraniak
Department of Biochemistry and Food Chemistry, University of Life Sciences, Skromna Str. 8, 20-704 Lublin, Poland
Department of Thermal Technology, University of Life Sciences, Dos´wiadczalna Str. 44, 20-280 Lublin, Poland
a r t i c l e i n f o
Article history:
Received 21 June 2016
Received in revised form 7 October 2016
Accepted 1 November 2016
Available online 2 November 2016
Keywords:
Bioaccessibility
Bioavailability
Bread
Fortification
Green coffee
In vitro
Phenolics
a b s t r a c t
The potential bioaccessibility and bioavailability of phenolics, caffeine and antioxidant activity of wheat
bread enriched with green coffee were studied. Supplementation enhanced nutraceutical potential by
improving phenolic content and lipid protecting capacity. The simulated-digestion-released phenolics
(mainly caffeic acid, syringic acid and vanillic acid) from bread, also caused significant qualitative
changes (chlorogenic acids were cleaved and significant amounts of caffeic acid and ferulic acid were
determined). Compared to the control, for the bread with 1% and 5% of the functional component the con-
tents of phenolics were 1.6 and 3.33 times higher. Also, an approximately 2.3-fold increase in antioxidant
activity was found in bread containing 5% of the supplement. The compounds responsible for antioxidant
potential have high bioaccessibility but poor bioavailability. The qualitative composition of the phenolic
fraction has a key role in developing the antioxidant potential of bread; however, caffeine and synergism
between antioxidants are also important considerations.
Ó 2016 Elsevier Ltd. All rights reserved.
1. Introduction
Fortification (enrichment) of food is one of the most popular
strategies aimed at achieving products that are characterized by
an increased content of health-promoting components. It includes
the addition of one or more functional components to particular
foods, thereby preventing their deficiency and/or providing addi-
tional benefits (
Allen, de Benoist, Dary, & Hurrell, 2006
). Fortifica-
tion of foods, especially of widely consumed products (e.g. bakery
products, pasta, juices), makes this strategy more effective and
allows it to reach a larger number of consumers (
Gawlik-Dziki, & S´wieca, 2014; Takahama, Tanaka, & Hirota,
2011
). Additionally, targeted as well as market-driven fortification
enables new food products to be designed, that usually meet the
criteria of the so-called ‘‘functional food” (
Lambert, 2004; Siró, Kápolna, Kápolna, & Lugasi, 2008
Phenolics, a group of plant secondary metabolites with well-
documented antioxidant, anti-inflammatory and anticancer activi-
ties, are widely used for food fortification (
Wang, Melnyk, Tsao, & Marcone, 2011
). So far, there is no substan-
tial evidence as to which method is the most appropriate for mea-
suring the bioaccessibility/bioavailability of these bioactive
ingredients; however these two factors are strongly determined
by phenolics’ chemical structure, hydrophobicity as well as the
current status of an organism, including microbiota action
(
).
It is a well-known fact that the effectiveness of fortification and
the final effect observed after consumption of phenolic-rich foods
is a result of many factors (
). A high activity
in vitro is not always translated into comparable activity in vivo
(
González-Sarrías et al., 2015; Siviero et al., 2015
), thus, it is very
important to determine the bioaccessibility and bioavailability of
functional supplements whilst evaluating the quality of new prod-
ucts. As in vivo studies are very expensive and usually difficult to
perform, some in vitro strategies based on simulated digestion
and absorption have been developed and successfully introduced
(
Hur, Lim, Decker, & McClements, 2011; Minekus et al., 2014
). In
general, in vitro methods are somewhat limited due to the omitted
role of the colon in digestion and absorption (
). It has been proven that Caco-2 systems are useful to
study the bioaccessibility of phenolics (
).
Unfortunately, they may only be applied to samples characterized
by a relatively low content of phenolics, as there is some evidence
that a high concentration of phenolics - up to 50
l
M (e.g. like in
coffee and coffee-based products) is toxic and by destroying the
monolayer of cells causes an unspecific transfer of phenolics,
http://dx.doi.org/10.1016/j.foodchem.2016.11.006
0308-8146/
Ó 2016 Elsevier Ltd. All rights reserved.
⇑
Corresponding author.
E-mail addresses:
(M. S´wieca),
(U. Gawlik-Dziki),
(D. Dziki),
(B. Baraniak).
Food Chemistry 221 (2017) 1451–1457
Contents lists available at
Food Chemistry
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / f o o d c h e m

which overestimates their bioaccessibility (
).
The absorption system used in this study (based on the passive
transport) allows estimation of the minimal bioavailability and
minimizes the risk of overestimation. So far, the intestinal absorp-
tion of 5-caffeoylquinic acid (5-CQA) has been studied in a cell cul-
ture, using the human colon carcinoma cell line Caco-2. The
absorption rate for 5-CQA was found to be about 0.10% at physio-
logical concentrations equivalent to gut lumen concentrations
(0.1
1 mM). Transepithelial transport experiments with chloro-
genic acid (CGA) using Caco-2 intestine epithelia cultured mono-
layer demonstrated bidirectional permeation with no transport
into the basolateral side, regardless of pH gradient. The permeation
rate was concentration-dependent and not saturable, thus indicat-
ing passive diffusion. In addition, the transport was inversely cor-
related with transepithelial electrical resistance, indicating limited
passive diffusion when intestinal junctions are tight (
). On the other hand, according to absorption studies in the
Caco-2 system and in vivo experiments in a rat model, in which
CQA was studied together with a realistic food matrix, it has been
reported that CQA is poorly absorbed in its native form (
Baglieri, Ordonaud, & Tomø, 2006
). An important role has also been
proven for the interactions of phenolics with other active compo-
nents and/or food matrices. Such relationships may significantly
diminish the bioactivity of phenolics in the upper parts of the
digestive tract and may also lower the digestibility of nutrients
(
Budryn et al., 2016; Dupas et al., 2006; Swieca, Gawlik-Dziki,
).
Green coffee is a rich source of chlorogenic acids and caffeine
(
Budryn, Zaczyn´ska, & Rachwał-Rosiak, 2015; Dziki et al., 2015;
). Chlorogenic acids, due to a high antiradical
and reducing potential as well as the ability to modify the activity
of pro-oxidative enzymes, exhibit in vitro many biological activities
including antimutagenic, anticarcinogenic, anti-inflammatory and
antioxidant properties (
2014; Dziki et al., 2014; Liang & Kitts, 2015
). In the last years, green
coffee has also been considered as a functional ingredient useful in
regulating the metabolism aiming at reducing body weight.
According to literature data, this effect is ambiguous (
). It has been proven that in some cases green
coffee bean phytochemicals show a tendency to reduce visceral fat
and body weight (
); however, another study provides
opposite findings (
The aim of this study was to evaluate the in vitro bioaccessibil-
ity and absorption of phenolics and caffeine. The antioxidant
potential of green coffee bean as well as wheat bread enriched with
this functional component, was also explored.
2. Materials and methods
2.1. Chemicals
-amylase, pancreatin, pepsin, bile extract, linoleic acid, Tween-
20 and haemoglobin were purchased from Sigma–Aldrich Com-
pany (Poznan, Poland). All others chemicals were of analytical
grade.
2.2. Green coffee flour preparation
The samples of green coffee (Coffea arabica) beans from Kenya
(GCB) were purchased from Caffeine Co. Marki, Poland. They were
prepared by adding water to adjust moisture content to 10 g/100 g
(w.b.) and storing for 48 h. The beans were ground using the labo-
ratory hammer mill (POLYMIX-Micro-Hammermill MFC, Kinemat-
ica. AG, Littau/Lucerne, Switzerland) equipped with round holes of
3.0 mm (
2.3. Bread making
Wholemeal wheat flour (type 2000; average 1.95% ash content,
humidity 14%) used in the formula of control bread (CB) (600 g)
was purchased in a local market from Lublin, Poland. The flour
was replaced with GCB at 1%, 2%, 3%, 4% and 5% levels (B1-B5,
respectively). The percentage of green coffee flour addition was
chosen on the basis of previous test concerning consumer accep-
tance (data previously published (
)). Besides this,
6 g of instant yeast and 12 g of salt were used for dough prepara-
tion. The general quantity of water necessary for the preparation
of the dough was established through the marking of water
absorption properties of flour to a consistency of 350 Brabender
units. The batches of dough were mixed in a spiral mixer for
6 min. After fermentation (optimal time was about 2 h), the pieces
of dough (300 g) were put into an oven at a temperature of 230
°C.
The baking time was 30 min. After baking, the bread was left to
stand for 24 h at room temperature, lyophilized and ground
(
).
2.4. Extract preparation
2.4.1. Buffer extracts (BE)
The samples of wheat and green coffee flours as well as
enriched bread (1 g of dry weight (DW)) were extracted for 1 h
with 20 ml of PBS buffer (phosphate buffered saline, pH 7.4). The
extracts were separated by decantation and the residues were
extracted again with 20 ml of PBS buffer. Extracts were combined
and stored in the dark at
20 °C.
2.4.2. Digestion in vitro – potentially bioaccessibility (GE)
In vitro digestion was performed as described previously by
with some modifications. Artificial saliva
solution was prepared by dissolving 2.38 g Na
2
HPO
4
, 0.19 g KH
2
-
PO
4
, 8 g NaCl and 100 mg of mucin in 1 L of distilled water. The
solution was adjusted to pH 6.75 and
a
-amylase (E.C. 3.2.1.1.)
was added to obtain 200 U/ml of enzyme activity. For gastric diges-
tion, 300 U/ml of pepsin (from porcine stomach mucosa, pepsin, EC
3.4.23.1) was prepared in 0.03 mol/l NaCl, pH 1.2. Simulated
intestinal juice was prepared by dissolving 0.05 g of pancreatin
(activity equivalent 4
USP) and 0.3 g of bile extract in 35 ml
0.1 mol/l NaHCO
3
. The samples were subjected to simulated gas-
trointestinal digestion as follows: 1 g of powdered sample (wheat
and green coffee flours as well as enriched bread) was homoge-
nized in a Stomacher laboratory blender for 1 min to simulate mas-
tication in the presence of 15 ml of simulated salivary fluid. The
samples were then shaken for 10 min. at 37
°C. The samples were
adjusted to pH 3 using 5 mol/l HCl, and then 15 ml of simulated
gastric fluid was added. The samples were shaken for 60 min at
37
°C. After digestion with the gastric fluid, the samples were
adjusted to pH 6 with 0.1 mol/l of NaHCO
3
and then 15 ml of a mix-
ture of bile extract and pancreatin was added. The extracts were
adjusted to pH 7 with 1 mol/l NaOH and finally 5 ml of
120 mmol/l NaCl and 5 ml of 5 mmol/l KCl were added to each
sample. Once prepared, the samples were submitted for in vitro
digestion for 120 min., at 37
°C and in the dark. Thereafter, the
samples were centrifuged and the supernatants were used for fur-
ther analysis.
2.4.3. Absorption in vitro – potentially bioavailability (AE)
Fluids obtained after in vitro digestion were transferred to dial-
ysis sacks (D9777–100FT, Sigma-Aldrich), placed in an Erlenmeyer
flask containing 50 ml of PBS buffer and incubated in a rotary sha-
ker (2 times per 2 h, 37
°C). The PBS buffer, together with the com-
pounds that passed through the membrane (dialysate, GDA), was
treated as an equivalent of the raw material absorbed in the intes-
1452
M. S´wieca et al. / Food Chemistry 221 (2017) 1451–1457

tine after digestion (at least 75% efficiency) (
).
2.5. Phenolics and caffeine content
Samples were analyzed with a Varian ProStar high-performance
liquid chromatography (HPLC) system separation module (Varian,
Palo Alto, CA, USA) equipped with a Varian ChromSpher C18
reverse phase column (250 mm
4.6 mm) and ProStar DAD detec-
tor (
). The column thermostat was set at
40
°C. The mobile phase consisted of 4.5% acetic acid (solvent A)
and 50% acetonitrile (solvent B), and a flow rate of 0.8 ml min
1
was used. At the end of the gradient, the column was washed with
50% acetonitrile and equilibrated to the initial conditions for
10 min. The gradient elution was used as follows: 0 min, 92% A;
30 min, 70% A; 45 min, 60% A; 80 min, 60% A; 82 min, 0% A;
85 min, 0% A; 86 min, 92% A; and 90 min, 92% A. Phenolics detec-
tion was carried out at 270 and 370 nm (250 nm for caffeine).
Spectrum analysis and a comparison of their retention times with
those of the standard compounds enabled identification of the phe-
nolics in a sample. Quantitative determinations were carried out
by means of the external standard calculation, using calibration
curves of the standards. Phenolics and caffeine were expressed in
micrograms per gram of dry mass (DW).
2.6. Inhibition of linoleic acid peroxidation (LPO)
The inhibition of the haemoglobin-catalyzed peroxidation of
linoleic acid was determined according to
. Ten microliters of the extract obtained
after digestion in vitro was mixed with 0.37 ml of 5 mM phosphate
buffer (pH 7) containing 0.05% Tween 20 and 4 mM linoleic acid
and then equilibrated at 37
°C for 3 min. The peroxidation of lino-
leic acid in the above-mentioned reaction mixture was initiated by
adding 20
l
l of 0.035% bovine haemoglobin (in water) followed by
incubation in a shaking water bath at 37
°C for 10 min. The reac-
tion was stopped by adding 5 ml 0.6% HCl (in ethanol). Hydrox-
yperoxide
formation
was
assayed
according
to
a
ferric
thiocyanate method that consists of mixing first with 0.02 mol/l
FeCl
2
(0.1 ml) and then with 30% ammonium thiocyanate
(0.1 ml). Absorbance (A
s
) at 480 nm was measured (Lambda 40
UV–vis spectrophotometer, Perkin-Elmer Inc. Waltham, USA). The
absorbance of blank (A
o
) was obtained without the addition of hae-
moglobin to the above reaction mixture; the absorbance of control
(A
100
) was obtained with no sample addition to the above mixture.
Thus, the antioxidative activity of the sample was calculated
according to the following equation:
LPO
½% ¼ ½1 ðA
s
A
0
Þ=ðA
100
A
0
Þ 100
ð1Þ
The activity was expressed as Trolox equivalent in mg per g of
dry mass (DW).
2.7. Theoretical calculations
The following factors were determined to permit better under-
standing of the relationships between biologically active com-
pounds in the light of their bioaccessibility, bioavailability, and
bioefficiency (
):
The phenolics bioaccessibility index (PAC), which is an indica-
tion of the bioaccessibility of phenolic compounds:
PAC
¼ C
GE
=C
BE
ð2Þ
The phenolics bioavailability index (PAV), which is an indication
of the bioavailability of phenolic compounds:
PAC
¼ C
AE
=C
GE
ð3Þ
where C
BE
is the phenolic content in raw extract (BE), C
GE
is the phe-
nolic content in extracts after simulated gastrointestinal digestion
(GE), and C
AE
is the phenolic content in extracts after simulated
intestinal absorption (AE),
The antioxidant bioaccessibility index (BAC), which is an indica-
tion of the bioaccessibility of antioxidative compounds:
BAC
¼ A
GE
=A
BE
ð4Þ
The antioxidant bioavailability index (BAV), which is an indica-
tion of the bioavailability of antioxidative compounds:
BAV
¼ A
AE
=A
GE
ð5Þ
where, A
BE
is the activity of raw extract (BE), A
GE
is the activity of
extract after simulated gastrointestinal digestion (GE) and A
AE
is
the activity of extract after simulated intestinal absorption (AE).
2.8. Statistical analysis
All experimental results were expressed as mean ± S.D. of three
independent experiments (n = 18). One-way analysis of variance
(ANOVA) and Turkey’s post hoc test were used to compare groups
(STATISTICA 6, StatSoft, Inc., Tulsa, USA). Differences were consid-
ered significant at p < 0.05.
3. Results and discussion
The phenolic composition of wheat flour and green coffee bean
is well characterized; however, there are only a few studies con-
cerning the bioavailability and bioaccessibility of phenolics from
these sources. As shown in
, wheat flour was characterized
by a high content of bound phenolics that were effectively released
during in vitro digestion. Such an observation confirms the results
of previous studies conducted by
and
, who demonstrated that free phenolics
(regardless of wheat variety) account for only 10–40% of total
wheat phenolics. Green coffee contained high amounts of chloro-
genic acids, mainly 3-caffeoylquinic acid, which are bioavailable
and rapidly metabolizable in the human body (
). In the cultured gastric epithelial model,
multiple chlorogenic acid isomers showed intact transfer across
the gastric barrier at an acidic apical pH, with dicaffeoylquinic
acids showing a relatively higher permeability coefficient com-
pared to CQA. Experiments conducted in a rat model showed that
CGAs are not hydrolyzed in the stomach but absorbed in an intact
form. This could explain the early detection of CGA in plasma
within 30 min of coffee consumption (
). Also,
according to previous studies (
) concerning green
coffee from different locations (Brazil, Columbia, Ethiopia and
Kenya), these compounds are easily bioaccessible in vitro. The
results obtained after digestion in this study were comparable with
those obtained for the chemical extraction by the cited researchers.
The relatively low bioaccessibility and bioavailability determined
in these studies may be due to the fact that the results obtained
after in vitro digestion were compared to those achieved for buffer
extraction - chlorogenic acid is excellently isolated using an extrac-
tion system based on water.
showed that about 10% and 12% of CGA were
bound to gastric or intestinal enzymes during in vitro digestion,
respectively. Additionally, according to a study by
Williamson, and Crozier (2014)
, the lowered bioavailability of phe-
nolics from green coffee may also be associated with a higher
ingested dose. Considering the above and by comparing these
two factors, it may be pointed out that the approximately 30 and
20 times higher contents of potentially bioaccessible and bioavail-
able phenolics predispose green coffee beans to be a functional
M. S´wieca et al. / Food Chemistry 221 (2017) 1451–1457
1453

ingredient for food enrichment. However, the influence of the type
of food matrix, which affected CGA digestion and bioavailability,
remains unclear and represents an interesting area for more
research on factors that influence the bioaccessibility of CGAs
and other important dietary polyphenols. There is some evidence
that CGAs exhibit high affinity to proteins and Maillard reaction
products, which may lower their bioaccessibility (
). Strong evidence has shown that the majority of CGA is not
absorbed in the proximal part of the gastrointestinal tract, unless
it is transformed into caffeic and ferulic acids before being
absorbed (
). Another aspect is that even when
absorption occurs in the small intestine, substantial quantities pass
to the large intestine where the parent compounds and their
catabolites can impact both colonic health and colonic microbiota.
Some of these compounds may play a key role in the protective
effects of phenolic rich foods (
Crozier, Del Rio, & Clifford, 2010
).
It this study, green coffee beans were used for the enrichment of
wheat bread. For better estimation of the fortification, systems
based on in vitro digestion and absorption were used to mirror
the amount of potentially bioaccessible and bioavailable bioactive
compounds. The contents of buffer extractable (BE), potentially
bioaccessible (extracts after in vitro digestion, DE) and potentially
bioavailable (extracts after in vitro absorption, AE) phenolics and
caffeine in the studied bread are presented in
, respec-
tively. The control bread (BC) contained all of the phenolics previ-
ously found in wheat flour; however, their contents were about 2
times higher than in flour (
). These phenomena may be par-
tially explained by the fact that during dough fermentation some
phenolics are released from flour, which has previously been
described by
Chandrasekara and Shahidi (2012)
. On analysis of
the buffer extract, it was found that the addition of green coffee
flour into the bread formula significantly increased the phenolic
content - up to 4.17–times for the bread with 5% of supplement.
Also, the caffeine content significantly increased (about 3.3-fold
for the 5% bread). Syringic acids and (+)-catechin dominated in
the phenolic profile of the control bread. The enriched bread also
Table 1
Potentially bioaccessible and bioavailable phenolics and caffeine in green coffee beans and wheat flour.
Compounds
[
l
g/g DM]
Green coffee
Wheat flour
Buffer extract
Extract after digestion
in vitro
Extracts absorbed
in vitro
Buffer
extract
Extract after digestion
in vitro
Extracts absorbed
in vitro
(+)-catechin
481 ± 12.5c
Nd
75.2 ± 2.9b
49.9 ± 14.7a
84.1 ± 6.9b
75.2 ± 2.9b
Gallic acid
Nd
134 ± 29.9
a
121.4 ± 7.2
a
Nd
Nd
Nd
Caffeic acid
Nd
9803 ± 758
c
3687 ± 240
b
5.8 ± 4.4
a
19.4 ± 13.3
a
Nd
Protocatechuic acid
580 ± 172
Nd
Nd
Nd
Nd
Nd
Syringic acid
Nd
183 ± 17
b
86.5 ± 2.4
a
87.9 ± 6.4
a
377.7 ± 30
c
77.5 ± 11
a
Ferulic acid
Nd
2628 ± 554
c
225.4 ± 96
b
Nd
15.9 ± 13.3
a
6.21 ± 0.98
a
p-coumaric acid
Nd
123 ± 6.8
b
71.6 ± 5.8
a
Nd
Nd
Nd
Vanillic acid
Nd
Nd
Nd
Nd
128.7 ± 45
b
46.2 ± 4.2
a
3-caffeoylquinic acid
35,574 ± 743
c
1380 ± 63
b
446 ± 75.5
a
Nd
Nd
Nd
5-caffeoylquinic acid
4239 ± 796
c
287 ± 30
b
188 ± 15
a
Nd
Nd
Nd
4-caffeoylquinic acid
2435 ± 616
c
282 ± 62
b
193 ± 5.3
a
Nd
Nd
Nd
3-feruloylquinic acid
1082 ± 294
b
227 ± 42.3
a
195 ± 46
a
Nd
Nd
Nd
5-p-coumaroylquinic
acid
1894 ± 948
b
188 ± 61.4
a
167 ± 120
a
Nd
Nd
Nd
5-feruloylquinic acid
968 ± 327
Nd
Nd
Nd
Nd
Nd
3.5-dicaffeoylquinic
acid
6855 ± 721c
241 ± 77
b
84.1 ± 19.8
a
Nd
Nd
Nd
4.5-dicaffeoylquinic
acid
5682 ± 345
Nd
Nd
Nd
Nd
Nd
Sum of phenolics
66,794
16,803
7919
160.9
562.8
381.8
Caffeine
31,460 ± 5589
b
9683 ± 692
a
9231 ± 576
a
–
–
–
Results were expressed as mean ± SD (n = 9). The values designated by the different letters (a,b,c) are significantly different (p < 0.05). Nd – not detected.
Table 2
Phenolics and caffeine content in the control and bread enriched with green coffee - buffer extracts.
Compounds
[
l
g/g DM]
Bread
CB
B1
B2
B3
B4
B5
Gallic acid
8.7 ± 5.0
b
8.2 ± 1.0
b
5.0 ± 0.2
a
5.0 ± 0.4
a
5.1 ± 2.0
a
5.1 ± 2.0
a
(+)-catechin
92.4 ± 31
a
72.8 ± 24
a
94.0 ± 11
a
103.3 ± 15
a
89.1 ± 29
a
96.2 ± 13
a
Caffeic acid
3.4 ± 0.8
a
81.0 ± 3.2
b
157.8 ± 12
c
192.3 ± 21
d
278.4 ± 28
e
397.7 ± 94
f
Ferulic acid
13.8 ± 2.1
a
15.9 ± 0.8
a
13.8 ± 1.6
a
24.8 ± 2.4
a
12.2 ± 3.9
a
15.1 ± 2.0
a
Syringic acid
107 ± 7.2
c
86.2 ± 1.4
b
83.3 ± 8.9
b
87.6 ± 6.6
b
43.8 ± 8.6
a
47.3 ± 10.0
a
3-caffeoylquinic acid
Nd
40.6 ± 4.5
a
99.8 ± 13
b
135.5 ± 16
c
187.1 ± 50
cd
229.1 ± 59
d
5-caffeoylquinic acid
Nd
26.3 ± 3.1
a
31.7 ± 10.9
ab
37.3 ± 3.3
b
42.3 ± 6.0
b
45.7 ± 11.9
b
4-caffeoylquinic acid
Nd
2.0 ± 0.6
a
57.7 ± 6.6
c
45.1 ± 3.6
b
37.9 ± 9.5
b
64.0 ± 13.6
d
3-feruloylquinic acid
Nd
4.1 ± 2.3
a
12.6 ± 2.1
bc
9.0 ± 2.1
b
17.1 ± 3.5
c
9.0 ± 4.7
bc
5-p-coumaroylquinic acid.
Nd
1.4 ± 1.7
a
1.7 ± 1.0
a
1.4 ± 1.6
a
1.3 ± 0.5
a
1.6 ± 0.3
a
5-feruloylquinic acid
Nd
0.5 ± 0.15
a
0.4 ± 0.15
a
2.6 ± 0.9
b
3.1 ± 0.9
b
2.0 ± 0.4
b
3.5-dicaffeoylquinic acid
Nd
0.3 ± 0.03
a
0.9 ± 0.08
c
0.5 ± 0.05
b
1.4 ± 0.4
a
2.7 ± 0.5
e
4.5-dicaffeoylquinic acid
Nd
6.3 ± 1.8
a
21.6 ± 2.9
bc
18.9 ± 1.8
b
15.3 ± 3.6
b
26.2 ± 3.6
c
Sum of phenolics
225.8
445.4
619.5
569.4
734.0
941.6
Caffeine
Nd
71 ± 5.7
a
133 ± 32.5
bc
163 ± 0.5
c
175 ± 56.8
cd
225 ± 56.8
d
CB – control bread; B1–B5 – breads supplemented with green coffee beans (1–5%).
Results were expressed as mean ± SD (n = 9). The values designated by the different letters (a,b,c,d,e,f) in the rows of the table are significantly different (p < 0.05). Nd – not
detected.
1454
M. S´wieca et al. / Food Chemistry 221 (2017) 1451–1457

contained significant amounts of chlorogenic acids derived from
the functional ingredient (
). The in vitro digestion effectively
released both phenolics and caffeine from the studied breads
(
). The phenolics bioaccessibility index (PAC) being signifi-
cantly higher than 1, indicates that phenolics from the studied
bread were highly bioaccessible in vitro. Most importantly, the
PAC values determined for the enriched bread were lower than
those recorded for the control (2.11) (
), which may indicate
the occurrence of phenolic – bread matrix interactions previously
described for wheat bread enriched with a phenolic-rich supple-
ment (
Swieca et al., 2013; Swieca et al., 2014
). Effectiveness of for-
tification was obvious – compared to the control, for the bread
with 1% and 5% of the functional component phenolic contents
were 1.6 and 3.33 times higher, respectively. Conditions similar
to those occurring in the human digestive tract caused significant
qualitative changes in the phenolics. Caffeic acid, syringic acid
and vanillic acid were released from the wheat matrix. Addition-
ally, ester linkages of chlorogenic acids in the enriched bread were
cleaved – those samples were characterized by significant contents
of caffeic and ferulic acids. Compared to the buffer extract, caffeine
contents were significantly higher – about 4.3-fold for the bread
with 5% of supplement (
). The values of the phenolics
bioavailability index (PAV) confirmed that potentially bioactive
compounds from the studied breads were poorly bioavailable
in vitro. The highest bioavailability in vitro was found for
the bread with 1%–3% (PAV = 0.44), whereas the lowest value
was determined for those with 5% of supplement (PAV = 0.37)
(
). The diminished potential bioavailability of phenolics
may be partially explained by the use of model systems (exhibiting
by definition only 82.5% of passive transport). Despite this, supple-
mentation of bread increased contents of both phenolics as well as
caffeine (
). Comparing these samples (AE), it was found that
3-caffeoylquinic acid was very effectively absorbed; however, the
sum of phenolics after absorption was about 2.7 times lower com-
pared to the potentially bioaccessible fraction. As mentioned
above, some quantitative changes were found, however, there
were no qualitative changes in the phenolics (generally phenolics
present in the extract obtained after in vitro digestion were also
found after the simulated absorption).
As the antioxidant potential of food is mainly created by low-
molecular weight antioxidants, thus the effect of incorporation of
green coffee phenolics as well as caffeine into wheat bread on
the ability to protect lipids against induced oxidation was also
studied (
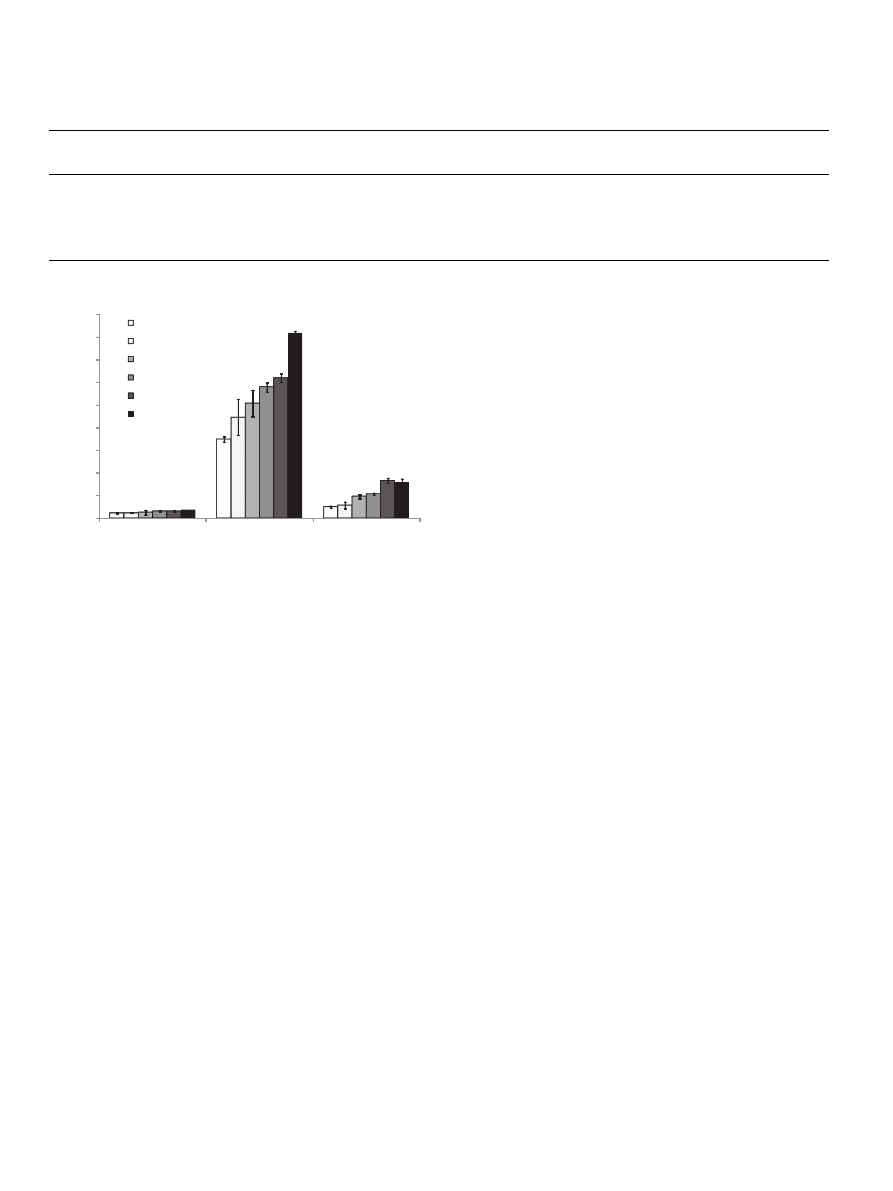
). The enriched bread exhibited a significantly higher
antioxidant activity (compared to the control) probably due to a
significantly increased content of caffeine and phenolics acid –
antioxidants with well–documented antioxidant capacity (
et al., 2015; Farah et al., 2008; Li Kwok Cheong et al., 2014
).
Between the studied fractions, the highest activity was found for
the extracts obtained after in vitro digestion (DE). The activity of
bread with 5% of supplement was about 2.3-fold higher than that
of the control (
). Most importantly, the bioactive compounds
Table 3
Phenolics and caffeine content in the control and bread enriched with green coffee – extracts obtained after digestion in vitro.
Compounds
[
l
g/g DM]
Bread
CB
B1
B2
B3
B4
B5
Gallic acid
Nd
5.3 ± 1.8
a
5.6 ± 2.0
a
6.3 ± 0.8
a
6.5 ± 0.7
a
8.7 ± 3.1
a
(+)-catechin
105.1 ± 8.4
c
59.7 ± 10.8
a
67.3 ± 6.9
ab
77.8 ± 8.0
ab
63.5 ± 10.8
ab
77.3 ± 3.6
b
Caffeic acid
25.8 ± 1.3
a
220.5 ± 26.2
b
351.4 ± 20.9
c
532.2 ± 95.8
d
733.4 ± 111.7
de
844.6 ± 122.6
e
Ferulic acid
12.3 ± 2.6a
11.3 ± 1.1
a
14.3 ± 1.2
ab
14.9 ± 1.7
b
21.5 ± 1.7
c
25.9 ± 2.5
c
Syringic acid
287 ± 15.9
a
296 ± 45.9
ab
288 ± 44.4
ab
3091 ± 12.0
ab
324 ± 15.4
b
327 ± 11.5
b
p-coumaric acid
Nd
1.5 ± 0.6
a
4.1 ± 1.2
b
4.4 ± 1.0
b
4.4 ± 1.0
b
7.4 ± 1.3
c
Vanillic acid
46.9 ± 3.3
b
48.6 ± 3.5
b
46.4 ± 3.2
b
39.6 ± 5.2
a
41.2 ± 1.58
a
38.6 ± 2.6
a
3-caffeoylquinic acid
Nd
38.6 ± 22.3
a
90.1 ± 17.4
b
123.9 ± 30.4
bc
128.7 ± 49.0
c
146.4 ± 9.7
c
5-caffeoylquinic acid
Nd
46.2 ± 7.6
a
65.5 ± 9.5
b
68.7 ± 3.9
b
69.3 ± 2.3
b
120.0 ± 12.1
c
4-caffeoylquinic acid
Nd
36.2 ± 2.5
a
69.2 ± 4.1
b
64.9 ± 2.1
b
72.4 ± 7.8
b
93.7 ± 10.4
c
3-feruloylquinic acid
Nd
11.4 ± 2.7
a
13.3 ± 3.6
a
11.8 ± 1.9
a
18.1 ± 1.2
b
19.3 ± 2.3
b
Sum of phenolics
477.3
763.6
977.5
1212.2
1464.9
1694.3
Caffeine
Nd
304 ± 9.4a
714 ± 10.9
b
1072 ± 24.3
c
1186 ± 49.9
c
1296 ± 111
d
CB – control bread; B1–B5 – breads supplemented with green coffee beans (1–5%).
Results were expressed as mean ± SD (n = 9). The values designated by the different letters (a,b,c,d,e) in the rows of the table are significantly different (p < 0.05). Nd -not
detected.
Table 4
Phenolics and caffeine content in the control and bread enriched with green coffee – extracts obtained after absorption in vitro.
Compounds
[
l
g/g DM]
Bread
CB
B1
B2
B3
B4
B5
Gallic acid
7.3 ± 1.1ab
5.9 ± 1.1a
5.4 ± 1.3a
7.0 ± 2.6ab
8.4 ± 1.5b
9.0 ± 2.0b
(+)-catechin
43.2 ± 12.16a
58.5 ± 13.6ab
69.9 ± 12.7bc
73.7 ± 2.9bc
76.8 ± 7.5 cd
88.1 ± 3.0d
Caffeic acid
Nd
82.3 ± 34.6a
157.3 ± 26.0b
191.4 ± 26.0bc
202.4 ± 23.6bc
238.3 ± 56.3c
Ferulic acid
5.4 ± 0.7a
5.1 ± 1.8ab
8.6 ± 3.4abc
6.9 ± 3.3abc
8.0 ± 1.5bc
9.4 ± 1.3c
Syringic acid
77.5 ± 11.7a
71.2 ± 9.2a
75.2 ± 13.0a
77.5 ± 20.0a
66.9 ± 6.6a
58.7 ± 10.1a
Vanillic acid
36.9 ± 3.3bc
38.6 ± 3.5c
35.4 ± 3.2bc
29.6 ± 2.7ab
38.0 ± 3.4c
27.5 ± 2.5a
3-caffeoylquinic acid
Nd
26.6 ± 2.8a
52.4 ± 6.8b
82.2 ± 11.2c
110.1 ± 13.0d
131.3 ± 10.0e
5-caffeoylquinic acid
Nd
3.9 ± 0.0a
12.6 ± 3.8b
18.8 ± 0.9c
21.4 ± 2.2 cd
29.1 ± 6.9d
4-caffeoylquinic acid
Nd
10.6 ± 2.6a
12.1 ± 4.3ab
15.9 ± 2.6b
25.7 ± 5.3c
27.8 ± 3.0c
3-feruloylquinic acid
Nd
3.4 ± 0.1a
4.6 ± 0.1b
6.3 ± 0.2c
7.4 ± 0.2d
9.2 ± 0.3e
Sum of phenolics
197.1
333.3
444.6
535.8
583.6
633.6
Caffeine
Nd
66.6 ± 3.5a
296.5 ± 14.4b
366.0 ± 7.5c
561.9 ± 24.0d
836.3 ± 13.9e
CB – control bread; B1–B5 – breads supplemented with green coffee beans (1%–5%).
Results were expressed as mean ± SD (n = 9). The values designated by the different letters (a,b,c,d,e) in the rows of the table are significantly different (p < 0.05). Nd – not
detected.
M. S´wieca et al. / Food Chemistry 221 (2017) 1451–1457
1455
responsible for the lipid protecting activity were highly bioaccessi-
ble in vitro – the values of the antioxidant bioaccessibility index
(BAC) ranged from 15.2 to 21.7 (the effect of supplementation
was not clearly visible) (
). Unfortunately, according to the
antioxidant bioavailability index (BAV) it was shown that antioxi-
dant compounds were very poorly bioavailable in vitro. It may be
suggested that antioxidants realized during digestion in vitro are
not able to permeate the membrane due to polarity and/or com-
pounds size (
). It is also well documented that
phenolics are able to interact with the food matrix and form indi-
gestible complexes that cannot be absorbed (
The highest bioactivity is not always directly translated into
higher values of factors describing potential bioavailability and
bioaccessibility. Comparing the level of activity with the content
of bioactive compounds it may be stated that the most important
role in creating the antioxidant potential is ascribed to the qualita-
tive composition of phenolics; however, the role of caffeine as well
as interactions of bioactive components seem to be important as
well. The studied activity was significantly higher in the absorbed
extracts compared to the buffer extracts. The buffer and absorbed
extracts were characterized by a similar content of phenolics; how-
ever, their qualitative composition varied. In turn, the potentially
bioaccessible and bioavailable fractions had the same phenolics
but the extracts after in vitro digestion exhibited much higher
activity – they had much higher amounts of phenolics. This con-
firms earlier findings reported by
concerning
the protective effect of green coffee hydroxycinnamic acids against
oxidative stress in a human HepG2 cells model. Moreover, the
chemical composition (phenolics as well as caffeine) only partially
explains differences between the extracts. There are no linear cor-
relations, which may indicate that also interactions (e.g. synergism,
antagonism) between them are important. Such an observation
was previously described by
in different plant
formulations.
4. Conclusion
The addition of green coffee flour into wheat bread significantly
improved the phenolic content and their ability to protect lipids
against oxidation. The in vitro digestion induced the release of phe-
nolics from bread, causing significant qualitative changes. Most
importantly, the introduced phenolics were potentially bioaccessi-
ble and bioavailable in vitro, however the influence of the food
matrix and its interaction with CGAs also played an important role
in the bioactivity of the functional product. According to the
results, it may be concluded that the qualitative composition of
the phenolic fraction plays a key role in creating antioxidant poten-
tial; however caffeine and potential synergism between low-
molecular weight antioxidants are important as well. To sum up,
the fortification of wheat bread with green coffee is an effective
tool that allows obtaining functional food with a significantly
enhanced nutraceutical potential to be obtained.
Conflict of interests
The authors declare that there is no conflict of interest regard-
ing the publication of this paper.
Acknowledgement
This scientific study was financed by the National Science
Centre, Poland (grant 2013/09/B/NZ9/01801). We are grateful to
Romuald Zalewski who provided the raw material for research.
Allen, L., de Benoist, B., Dary, O., & Hurrell, R. (2006). Guidelines on food fortification
with micronutrients. World Health Organization, Food and Agricultural
Organization of the United Nations, 1–341.
http://dx.doi.org/10.1242/jeb.02490
.
Arts, I. C. W., & Hollman, P. C. H. (2005). Polyphenols and disease risk in
epidemiologic studies 1–4. The American Journal of Clinical Nutrition, 81,
317–325
Baeza, G., Amigo-Benavent, M., Sarriá, B., Goya, L., Mateos, R., & Bravo, L. (2014).
Green coffee hydroxycinnamic acids but not caffeine protect human HepG2
cells against oxidative stress. Food Research International, 62, 1038–1046.
dx.doi.org/10.1016/j.foodres.2014.05.035
.
Barrington, R., Williamson, G., Bennett, R. N., Davis, B. D., Brodbelt, J. S., & Kroon, P.
A. (2009). Absorption, conjugation and efflux of the flavonoids, kaempferol and
galangin, using the intestinal CACO-2/TC7 cell model. Journal of Functional
Foods, 1(1), 74–87.
http://dx.doi.org/10.1016/j.jff.2008.09.011
Budryn, G., Nebesny, E., _Zy
_zelewicz, D., & Oracz, J. (2014). Properties of model
systems of sunflower oil and green coffee extract after heat treatment and
storage. LWT - Food Science and Technology, 59(1), 467–478.
.
Budryn, G., Zaczyn´ska, D., Pałecz, B., Rachwał-Rosiak, D., Belica, S., Den-Haan, H., &
Ellipsis Pérez-Sánchez, H. (2016). Interactions of free and encapsulated
hydroxycinnamic acids from green coffee with egg ovalbumin, whey and soy
Table 5
Relationships between biologically active compounds in the light of their bioaccessibility, bioavailability and bioefficiency.
Bread sample
Phenolics
bioavailability
index (PAV)
Phenolics bioaccessibility
index (PAC)
Antioxidant bioavailability
index (BAV)
Antioxidant
bioaccessibility
index (BAC)
CB
0.41
2.11
0.13
17.69
B1
0.44
1.71
0.18
15.19
B2
0.45
1.58
0.21
21.42
B3
0.44
1.83
0.18
17.84
B4
0.40
2.00
0.28
19.20
B5
0.37
1.80
0.19
21.71
CB – control bread; B1–B5 – breads supplemented with green coffee beans (1–5%).
a
i
d
a
ij
de
ab
jk
f
b
kl
fg
b
l
h
c
m
h
0
10
20
30
40
50
60
70
80
90
Buffer extractable
compounds
Potentially bioaccessible
compounds
Potentially bioavailable
compounds
Inhi
b
it
ion of
li
pi
ds
pe
roxi
da
ti
o
n
[m
g T
E
/ g]
CB
B1
B2
B3
B4
B5
Fig. 1. Ability of buffer extractable, potentially bioaccessible and bioavailable
fractions of control and enriched bread to inhibit lipid peroxidation CB- control
bread; B1-B5 – breads supplemented with green coffee beans (1%–5%) Results were
expressed as mean ± SD (n = 9). The values designated by different letters are
significantly different (p < 0.05).
1456
M. S´wieca et al. / Food Chemistry 221 (2017) 1451–1457

protein hydrolysates. LWT - Food Science and Technology, 65, 823–831.
doi.org/10.1016/j.lwt.2015.09.001
.
Budryn, G., Zaczyn´ska, D., & Rachwał-Rosiak, D. (2015). Changes of free and
nanoencapsulated hydroxycinnamic acids from green coffee added to different
food products during processing and in vitro enzymatic digestion. Food Research
International.
http://dx.doi.org/10.1016/j.foodres.2015.12.011
.
Calvello, R., Aresta, A., Trapani, A., Zambonin, C., Cianciulli, A., Salvatore, R., & Ellipsis
Panaro, M. A. (2016). Bovine and soybean milk bioactive compounds: Effects on
inflammatory response of human intestinal Caco-2 cells. Food Chemistry, 210,
276–285.
http://dx.doi.org/10.1016/j.foodchem.2016.04.067
.
Chandrasekara, A., & Shahidi, F. (2012). Bioaccessibility and antioxidant potential of
millet grain phenolics as affected by simulated in vitro digestion and microbial
fermentation. Journal of Functional Foods, 4(1), 226–237.
Crozier, A., Del Rio, D., & Clifford, M. N. (2010). Bioavailability of dietary flavonoids
and phenolic compounds. Molecular Aspects of Medicine, 31(6), 446–467.
dx.doi.org/10.1016/j.mam.2010.09.007
Dupas, C., Baglieri, M., Ordonaud, C., & Tomø, D. (2006). Chlorogenic acid is poorly
absorbed, independently of the food matrix : A Caco-2 cells and rat chronic
absorption study. Molecular Nutrition & Food Research, 50, 1053–1060.
doi.org/10.1002/mnfr.200600034
.
Dziki, D., Gawlik-Dziki, U., Pecio, Ł., Ró
_zyło, R., S´wieca, M., Krzykowski, A., & Rudy, S.
(2015). Ground green coffee beans as a functional food supplement –
Preliminary study. LWT - Food Science and Technology, 63(1), 691–699.
dx.doi.org/10.1016/j.lwt.2015.03.076
Dziki, D., Ró
_zyło, R., Gawlik-Dziki, U., & S´wieca, M. (2014). Current trends in the
enhancement of antioxidant activity of wheat bread by the addition of plant
materials rich in phenolic compounds. Trends in Food Science & Technology, 40
(1), 48–61.
http://dx.doi.org/10.1016/j.tifs.2014.07.010
.
Etcheverry, P., Grusak, M. A., & Fleige, L. E. (2012). Application of in vitro
bioaccessibility and bioavailability methods for calcium, carotenoids, folate,
iron, magnesium, polyphenols, zinc, and vitamins B(6), B(12), D, and E. Frontiers
in Physiology, 3, 317.
http://dx.doi.org/10.3389/fphys.2012.00317
.
Farah, A., Monteiro, M., Donangelo, C. M., & Lafay, S. (2008). Chlorogenic acids from
green coffee extract are highly bioavailable in humans. The Journal of Nutrition,
138(12), 2309–2315.
http://dx.doi.org/10.3945/jn.108.095554
.
Fletcher, R. J., Bell, I. P., & Lambert, J. P. (2004). Public health aspects of food
Gawlik-Dziki, U., Dziki, D., S´wieca, M., Se˛czyk, Ł., Ró
_zyło, R., & Szymanowska, U.
(2015). Bread enriched with Chenopodium quinoa leaves powder – The
procedures for assessing the fortification efficiency. LWT - Food Science and
Technology, 62(2), 1226–1234.
http://dx.doi.org/10.1016/j.lwt.2015.02.007
Gawlik-Dziki, U., S´wieca, M., Dziki, D., Kowalska, I., Pecio, Ł., Durak, A., & Se˛czyk, Ł.
(2014). Lipoxygenase inhibitors and antioxidants from green coffee—
mechanism of action in the light of potential bioaccessibility. Food Research
International, 61, 48–55.
http://dx.doi.org/10.1016/j.foodres.2014.05.002
.
González-Sarrías, A., García-Villalba, R., Núñez-Sánchez, M. Á., Tomé-Carneiro, J.,
Zafrilla, P., Mulero, J., & Ellipsis Espín, J. C. (2015). Identifying the limits for
ellagic acid bioavailability: A crossover pharmacokinetic study in healthy
volunteers after consumption of pomegranate extracts. Journal of Functional
Foods, 19, 225–235.
http://dx.doi.org/10.1016/j.jff.2015.09.019
Goupy, P., Vulcain, E., Caris-Veyrat, C., & Dangles, O. (2007). Dietary antioxidants as
inhibitors of the heme-induced peroxidation of linoleic acid: mechanism of
action and synergism. Free Radical Biology & Medicine, 43(6), 933–946.
doi.org/10.1016/j.freeradbiomed.2007.06.013
Hung, P. Van., Hatcher, D. W., & Barker, W. (2011). Phenolic acid composition of
sprouted wheats by ultra-performance liquid chromatography (UPLC) and their
antioxidant activities. Food Chemistry, 126(4), 1896–1901.
10.1016/j.foodchem.2010.12.015
.
Hur, S. J., Lim, B. O., Decker, E. A., & McClements, D. J. (2011). In vitro human
digestion models for food applications. Food Chemistry, 125(1), 1–12.
doi.org/10.1016/j.foodchem.2010.08.036
.
Jakobek, L. (2015). Interactions of polyphenols with carbohydrates, lipids and
proteins.
Food
Chemistry,
175,
556–567.
Laparra, J. M., & Sanz, Y. (2010). Interactions of gut microbiota with functional food
components and nutraceuticals. Pharmacological Research, 61(3), 219–225.
http://dx.doi.org/10.1016/j.phrs.2009.11.001
Li, H., Tsao, R., & Deng, Z. (2012). Factors affecting the antioxidant potential and
health benefits of plant foods. Canadian Journal of Plant Science, 92(6),
1101–1111.
http://dx.doi.org/10.4141/cjps2011-239
.
Li Kwok Cheong, J. D., Croft, K. D., Henry, P. D., Matthews, V., Hodgson, J. M., & Ward,
N. C. (2014). Green coffee polyphenols do not attenuate features of the
metabolic syndrome and improve endothelial function in mice fed a high fat
diet. Archives of Biochemistry and Biophysics, 559, 46–52.
.
Liang, N., & Kitts, D. D. (2015). Role of chlorogenic acids in controlling oxidative and
inflammatory stress conditions. Nutrients, 8(1), 1–20.
Mehari, B., Redi-Abshiro, M., Chandravanshi, B. S., Combrinck, S., Atlabachew, M., &
McCrindle, R. (2015). Profiling of phenolic compounds using UPLC-MS for
determining the geographical origin of green coffee beans from Ethiopia. Journal
of Food Composition and Analysis, 45, 16–25.
.
Minekus, M., Alminger, M., Alvito, P., Ballance, S., Bohn, T., Bourlieu, C., & Ellipsis
Brodkorb, A. (2014). A standardised static in vitro digestion method suitable for
food – An international consensus. Food & Function, 5(5), 1113–1124.
Onakpoya, I., Terry, R., & Ernst, E. (2011). The use of green coffee extract as a weight
loss supplement: A systematic review and meta-analysis of randomised clinical
trials. Gastroenterology Research and Practice, 2011, 1–6.
Siró, I., Kápolna, E., Kápolna, B., & Lugasi, A. (2008). Functional food. Product
development, marketing and consumer acceptance–a review. Appetite, 51(3),
456–467.
http://dx.doi.org/10.1016/j.appet.2008.05.060
Siviero, A., Gallo, E., Maggini, V., Gori, L., Mugelli, A., Firenzuoli, F., & Vannacci, A.
(2015). Curcumin, a golden spice with a low bioavailability. Journal of Herbal
Medicine, 5(2), 57–70.
http://dx.doi.org/10.1016/j.hermed.2015.03.001
.
Stalmach, A., Williamson, G., & Crozier, A. (2014). Impact of dose on the
bioavailability of coffee chlorogenic acids in humans. Food & Function, 5(8),
1727–1737.
http://dx.doi.org/10.1039/c4fo00316k
.
S´wieca, M., & Baraniak, B. (2014). Nutritional and antioxidant potential of lentil
sprouts affected by elicitation with temperature stress. Journal of Agricultural
and Food Chemistry, 62(14), 3306–3313.
http://dx.doi.org/10.1021/jf403923x
.
Swieca, M., Gawlik-Dziki, U., Dziki, D., Baraniak, B., & Czy
_z, J. (2013). The influence of
protein-flavonoid interactions on protein digestibility in vitro and the
antioxidant quality of breads enriched with onion skin. Food Chemistry, 141
(1), 451–458.
http://dx.doi.org/10.1016/j.foodchem.2013.03.048
Swieca, M., Se˛czyk, L., Gawlik-Dziki, U., Dziki, D., S´wieca, M., S
ȩczyk, Ł., & Ellipsis
Dziki, D. (2014). Bread enriched with quinoa leaves – the influence of protein-
phenolics interactions on the nutritional and antioxidant quality. Food
Chemistry, 162, 54–62.
http://dx.doi.org/10.1016/j.foodchem.2014.04.044
Takahama, U., Tanaka, M., & Hirota, S. (2011). Flour and breads and their fortification
in health and disease prevention. Elsevier. 10.1016/B978-0-12-380886-8.10013-
3
Thom, E. (2007). The effect of chlorogenic acid enriched coffee on glucose
.
Wang, S., Melnyk, J. P., Tsao, R., & Marcone, M. F. (2011). How natural dietary
antioxidants in fruits, vegetables and legumes promote vascular health. Food
Research
International,
44(1),
14–22.
.
Williamson, E. (2001). Synergy and other interactions in phytomedicines.
Phytomedicine, 8(5), 401–409.
http://dx.doi.org/10.1078/0944-7113-00060
.
M. S´wieca et al. / Food Chemistry 221 (2017) 1451–1457
1457
Document Outline
- Wheat bread enriched with green coffee – In vitro bioaccessibility and bioavailability of phenolics and antioxidant activity
- 1 Introduction
- 2 Materials and methods
- 3 Results and discussion
- 4 Conclusion
- Conflict of interests
- Acknowledgement
- References
Wyszukiwarka
Podobne podstrony:
in vitro, studia rolnictwo, rok IV
Kultury in vitro roslin rozmnazanie klonalne
In vitro antitumor actions of extracts
In vitro truskawka id 212540 Nieznany
1 1 Podstawowe definicje; główne kierunki przemian rozwojowych roślinnych tkanek in vitro(1)
Życie ludzkie świętość czy zabawka nt in vitro
Greenshit go home Greenpeace, Greenland and green colonialism in the Arctic
In vitro, Sem 1, TMR3
6 Hodowle komórek skóry w warunkach in vitro
Kościół katolicki wobec zapłodnienia In vitro, Etyka, Bioetyka
IN VITRO GRZECH CZY SZANSA
In vitro tulipan
in vitro 2
In vitro liliowce
In vitro a współczesna cywilizacja, RODZINA
In vitro groźne, godność życia-in vitro
wyklad V in vitro
więcej podobnych podstron