
Using ultrasonic vacuum spray dryer to produce highly viable dry probiotics
David Semyonov, Ory Ramon, Eyal Shimoni
Faculty of Biotechnology and Food Engineering, Technion - Israel Institute of Technology, Haifa 32000, Israel
a r t i c l e i n f o
Article history:
Received 25 October 2009
Received in revised form
17 March 2011
Accepted 18 March 2011
Keywords:
Spray dryer
Probiotic
Microencapsulation
Storage
a b s t r a c t
Ultrasonic vacuum spray dryer was used to produce a dry powder of highly viable probiotic cells. The
drying was performed through two stages: Vacuum spray drying of the solution followed by
fluidized-
bed drying of the powder. The embedding matrix was a combination of trehalose and maltodextrin. The
effects of external and internal variables on cell survival during the drying process and storage were
investigated. The hypothesis was that by minimizing the oxidative and thermal stresses in the drying
stages, in addition to adequate formulation choice, the cell viability during the drying and storage will
increase. It was concluded that during the drying process the faster the embedding matrix reaches
a glassy state the higher was the probiotic survival. Evaluating water activity and moisture limit of the
glassy matrix concluded that maltodextrin DE5 is a better encapsulating matrix than maltodextrin DE19.
Combining trehalose to maltodextrin in the encapsulating matrix resulted in a signi
ficant increase in the
survival up to 70.6
6.2%.
Ó 2011 Elsevier Ltd. All rights reserved.
1. Introduction
Probiotics are described as
“live micro-organisms which when
administered in adequate numbers confer a health bene
fit on the
host
” (
). They are commonly included in fermented
milks, yoghurts and cheese, but are also available in the form of
dietary supplements where the probiotic is in the form of a dried
product. Probiotic-containing foods can be categorized as functional
foods, and along with prebiotics represent the largest segment of
the functional foods market in Europe, Japan and Australia. The
market for this food category continues to expand, in parallel with
growing consumer awareness of the role of diet in health mainte-
nance (
’Donnell, 1998; Stanton et al., 2001
).
Probiotics such as Lactobacillus and Bi
fidobacteria species are
added to foods mainly to improve intestinal microbial balance.
Lactobacillus, a genus of Gram-positive facultative anaerobic
bacteria, are a major part of the Lactic Acid Bacteria group. Such
probiotics are common and usually benign, even essential, inhabi-
tants of humans and other animals. Lactobacillus and Bi
fidobacte-
rium species are the most commonly used probiotics in foods for
human consumption given the signi
ficant health benefits associated
with ingestion of these micro-organisms. These micro-organisms
share a number of common traits, such as generally regarded as safe
(GRAS) status, acid and bile tolerance, and ability to adhere to
intestinal cells (
). It is recommended that the
probiotic culture must be present in the product at minimum
numbers of 10
7
CFU/ml and even higher numbers have been rec-
ommended (
Ishibashi & Shimamura, 1993; Lee & Salminen, 1995
).
Probiotic cultures for food applications are frequently supplied in
frozen or dried form, either as freeze-dried or spray-dried powders
(
’t Riet,1993; Holzapfel, Haberer, Geisen, Bjorkroth, &
). Relatively successful drying of lactobacilli and
bi
fidobacteria has previously been reported for a number of
different strains (
), including Lactoba-
cillus paracasei (
). An adequate solution to
improve probiotic survival during their processing is their micro-
encapsulation (
). In order to extend the pro-
biotic storage stability, techniques such as spray drying, freeze
drying and
fluidized bed spray coating were employed resulting in
a dry powder. However, most probiotic lactobacilli do not survive
well during the temperature and osmotic extremes to which they
are exposed during the conventional drying and encapsulation
processes (
Goderska & Czarnecki, 2008; Ross, Desmond, Fitzgerald,
).
The energy consumption of spray drying is 6
e10 times lower
compared to freeze drying, since both mass and energy are fast
transferred in a very short time without using an exchange surface
(
). Spray drying technique was applied mostly in dairy
industry and in food products where original properties can be
preserved. However the use of spray drying to produce dry pro-
biotics is questionable because of high bacterial mortality due to
simultaneous dehydration, thermal and oxygen stresses imposed
* Corresponding author. Tel.: þ972 4 8292484; fax: þ972 4 8293399.
E-mail address:
(E. Shimoni).
Contents lists available at
LWT - Food Science and Technology
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / l w t
0023-6438/$
e see front matter Ó 2011 Elsevier Ltd. All rights reserved.
during the spray drying process (
Anal & Singh, 2007; Ross et al.,
). A technique based on spray drying incorporation of
L. paracasei NFBC 338 in a carrier, by a combination of protein and
carbohydrate, contributed to retain a high viability after spray
drying and to extend survival rates during storage (
’Callaghan, Fitzgerald, & Stanton, 2002
).
Our basic hypothesis is that by minimizing the thermal and
oxidative stresses during the drying process the cell viability at the
end of the drying and/or storage stages will increase signi
ficantly.
In the present study we evaluate a new spray drying technique and
process using a newly developed ultrasonic vacuum spray drier. In
order to obtain high survival rates of Lactobacillus casei subsp.
paracasei LMG P-21380 were embedded in a maltodextrin-treha-
lose matrix (
). We expect that it can be ach-
ieved due to the short residence time of the uniform droplets
generated by the ultrasonic nozzle, as well due the low tempera-
ture and vacuum atmosphere in the drier chamber space (
Merkle, & Gander, 2004; Sadykhov & Kish, 1997
). The role of the
maltodextrin-trehalose embedding matrix was to increase the
survival by maintaining the probiotic cells membrane integrity
during the drying process and storage of the dried probiotics as
well as to promote the stabilizing effect of the bacteria
’s proteins
Crowe, Crowe, Rudolph, Womersley, & Appel, 1985; Leslie, Israeli,
Lighthart, Crowe, & Crowe, 1995; Castro, Teixeira, & Kirby, 1997
The main objective of this study was therefore to explore the
application of ultrasonic vacuum spray drying process in the
formation of dried probiotic powder (Lactobacillus casei subsp.
paracasei LMG P-21380) with high survival rate and extended shelf
life. We studied the effect of the drying temperature, matrix
composition, solids and probiotics concentrations, and dextrose
equivalent on their survival. The effect of storage temperature and
oxygen level on the physical state and viability in the dried pro-
biotic powder of various matrix formulations was also measured.
2. Materials and methods
2.1. Materials
2.1.1. Bacterial culture
The bacterial strain used in this study was pure freeze dried
culture of Lactobacillus casei subsp. paracasei LMG P-21380 provided
by Probiotical s.r.l, Novara, Italy.
The following compounds were tested for their protecting
effect: Trehalose (Cargill, Minneapolis, USA) and Maltodextrins DE5
(Mw
w150000) and DE19 (Mw w13000) (Galam, Kibbutz Maanit,
Israel).
2.2. Methods
2.2.1. Preparation of the probiotic solutions
Solutions of maltodextrin (MD) and trehalose formulation of
various ratios were prepared as follows: distilled water was heated
to at least 93
C prior to addition of maltodextrins and trehalose in
order to obtain complete dissolution of the maltodextrin. The
solutions were then cooled to room temperature. The dry probiotic
cultures were dissolved in the formulation solution for at least one
hour before their drying by the ultrasonic vacuum spray drying
process. Oxidative stress was minimized by storing the solutions in
closed vials. Several different solutions of Lactobacillus paracasei
were prepared for the examination.
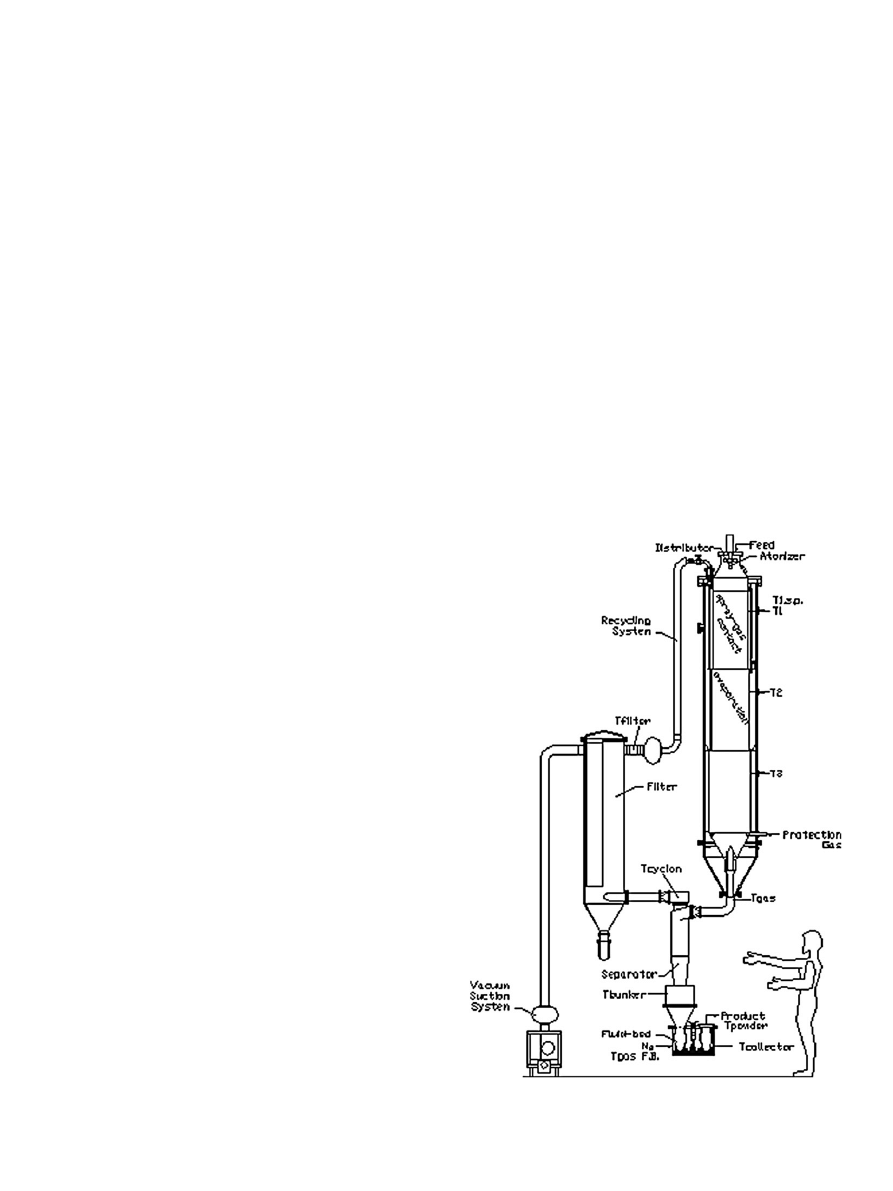
2.2.2. The drying setup
Ultrasonic Vacuum Spray Dryer (UVSD) (Nanosol, Israel) is
a spray dryer that operates under vacuum conditions. The system
includes a special designed ultrasonic atomizer which can operate
in a vacuum environment, dispersing the product solution evenly,
to the vacuum drying zone. The particle size distribution of the
drops is signi
ficantly narrower than in conventional spraying
systems, and can be controlled within certain limits. UVSD includes
a vacuum chamber with three adjustable heating zones (
). The
atomized drops are directed into a vacuum chamber. The internal
temperature in the drying chamber is adjusted by several heating
coils according to the speci
fic task. The heating of the falling drops
inside the drying chamber occurs though the convection of heat
from the chamber wall towards the atomized drops.
The drying was performed through two stages. In the
first stage,
the narrow size distribution of the drops (from 10
m
m to 50
m
m
depending on solution viscosity) fall free with low velocities within
3
e4 s and evaporate the majority of the free water. The solution
feeding and the vacuum in the drying chamber were maintained at
9 ml/min and 2.27 or 3.33 kPa. In that pressure conditions the drops
temperature does not exceed 20
e30
C. Using this technique the
heating is gentle and the vacuum in the drier space signi
ficantly
reduces the temperature of the product, the oxidative stress, as well
as the residence time of the particles in the drying camber.
The remaining water was evaporated during the second drying
stage that was performed in a cooled (10
e15
C) vacuum and
Nitrogen environment
fluidized-bed, for 20e60 min. After this
stage the product with the required water activity was removed
from the collector without stopping the process.
Fig. 1. Schematic representation of Ultrasonic Vacuum Spray Dryer (UVSD). Presented
with permission of Dr. Sadykhov (Nanosol, Israel).
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1845

The internal parameters of the feed solutions were: cell
concentration (0.75, 2.5, 5 and 10 g/100 g), solids concentration
(10
e30 g/100 g), MD dextrose equivalent (DE5 and DE19), and
maltodextrin - trehalose ratios (1:0, 2:1, 1:1 respectively).
External variables that were investigated were: vacuum pres-
sure in the drying chamber that effect directly the temperature of
the product and the heat pro
file in the dryer, and the water activity
of the dry product after a known residence time in the UVSD second
stage, i.e. the
fluidized-bed vacuum chamber.
The
first variable investigated was the vacuum magnitude in the
drying chamber (2.27 and/or 3.33 kPa). Two solutions containing
(20 g/100g maltodextrin DE19) 0.75 g/100g Lactobacillus paracasei
were dried under vacuum pressures of 2.27 or 3.33 kPa, which
represents pure water boiling temperatures of 19.3 and 25.7
C
respectively. The dry probiotic powder was retrieved from the
powder collection site and then dissolved to determine cells
survival. In order to determine the effect of solids concentration in
the feed solution on probiotics survival during the drying stage of
the UVSD process (at 2.27 kPa vacuum), Lactobacillus paracasei
0.25 g/100g cells were embedded in a matrix of four different
maltodextrin DE19 concentrations: 10, 15, 20 and 25 g/100g. In
order to determine effect of trehalose on the survival Lactobacillus
paracasei 0.75 g/100g was embedded and microencapsulated by
the UVSD process in various matrixes: maltodextrin DE5/DE19-
trehalose [1:1] (20 and 30 g/100g) and maltodextrin DE5/DE19-
trehalose [2:1] (30 g/100g). To examine the in
fluence of probiotic
concentration in the formulation on their survival, four solutions
were prepared: maltodextrin DE5-trehalose (1:1) with 0.75, 2, 5
and 10 g/100g of Lactobacillus paracasei (all at 20 g/100g total
solids). All four were microencapsulated using the UVSD process
and the vacuum in the drying chamber was 3.33 kPa.
2.2.3. Shelf life evaluation
The in
fluence of three storage temperatures and two types of
storage atmospheres (air and N
2
) on the dried encapsulated pro-
biotics stability was studied. For standard atmosphere storage, 5 g of
dried granules were placed in glass containers in dark rooms at 4
C
(typical refrigerating temperature), 25
C (proper room temperature
storage), and 37
C (simulation temperature abuse conditions). For N
2
atmosphere storage, 2 g of dry probiotic microcapsules were placed
in glass containers and compressed N
2
gas was allowed to purge the
container for 3 min. The top was quickly closed after the tube was
withdrawn. The containers were placed in a dark room at 25
C.
Samples were taken on a weekly basis to determine the concentra-
tion of L. paracasei survival.
2.2.4. Survival determination of Lactobacillus paracasei
2.2.4.1. Viable cell counts in the probiotic solutions. The viable pro-
biotic cells counts in the feed solutions were determined as follows:
probiotic samples were spread plated on MRS agar plates (DifcoTM
Lactobacilli MRS agar, BD, Sparks, MD, USA), after appropriate 10-
fold serial dilutions in saline solution. Viable cells counts were
determined after 48 h incubation under anaerobic conditions at
37
C. Anaerobic jars and gas generating kits (Oxoid Ltd.) were used
for creating anaerobic conditions. Plates containing 20350 colonies
were measured and recorded as colony forming units (CFU) per g of
the product or ml of solution (N
B
).
2.2.4.2. Viable cell counts in dry samples. Dry samples in four
replicates (100
e300 mg) were rehydrated at ambient temperature
and dissolved in 4.5 ml saline (NaCl 0.85 g/100g water). Dissolved
samples were spread plated on MRS agar as described above. The %
survival of the samples tested was calculated as follows:
Survival percentage
¼ ð100 N=N
0
Þ;
(1)
Where N
0
is the number of bacteria per g of dry matter before
drying and N is the number of bacteria per g of dry matter in the
capsules.
2.2.5. Water activity
Water activity of the dry probiotic microcapsules at the end of
the second stage of the UVSD process was measured by using an
“Hygropalm Aw1” water activity indicator (Rotronic Instrument
corp., Basserdorf, Germany).
2.2.6. Scanning electron microscopy
Encapsulated and coated probiotic capsules were stored in
desiccators (silica gel), and goldcoated by Polaron SC515 (Fisons
Instruments, UK) prior the observation by JSM-5400 SEM (Jeol,
Japan). Digital images were obtained using an EDS-unit with
Voyager II software (Moran, Netherlands).
2.2.7. Data collection and statistical analysis
All the experiments were performed with at least three repli-
cates. The results hereto are expressed as their means
the stan-
dard deviation (SD). Where necessary, the number of repetition is
noted in the text. The signi
ficance of the differences between
groups was tested using t-test analysis. A probability level (p value)
of
<0.05 was considered to be statistically significant unless stated
otherwise. Statistical analysis was performed by the data analysis
tool pack of Microsoft Excel 2003 software.
3. Results and discussion
The aim of this investigation was to evaluate a new method for
producing dry microcapsules (MC), containing high concentration
of viable Lactobacillus paracasei cells by an ultrasonic vacuum spray
drier (UVSD). Our hypothesis was that since UVSD process operates
under vacuum and low temperature, along with a gentle heating
mode by radiation, thus product temperature, oxidative stress, and
the residence time of the drying drop/microcapsule in the drying
chamber would be reduced signi
ficantly.
In the UVSD setup, an ultrasonic atomizer creates droplets with
low velocities that drift under the in
fluence of gravity down the
drying chamber. The height and diameter of the chamber and
the heating system are designed to ensure that the droplets reach the
powder collection site in a dry state. The droplet size (10
e50
m
m) is
a function of several factors, primarily the nozzle vibration frequency
but also on the liquid solution viscosity. The liquid outlet is large
compared with that of a pressure nozzle without moving parts pre-
venting clogging, and it enables creating drops from high viscosity
solutions. The UVSD drying chamber is built of three heat controlled
zones that can be adjusted to produce an appropriate heat pro
file,
and a vacuum system that can operate under several vacuum pres-
sures keeping the temperature as low as possible.
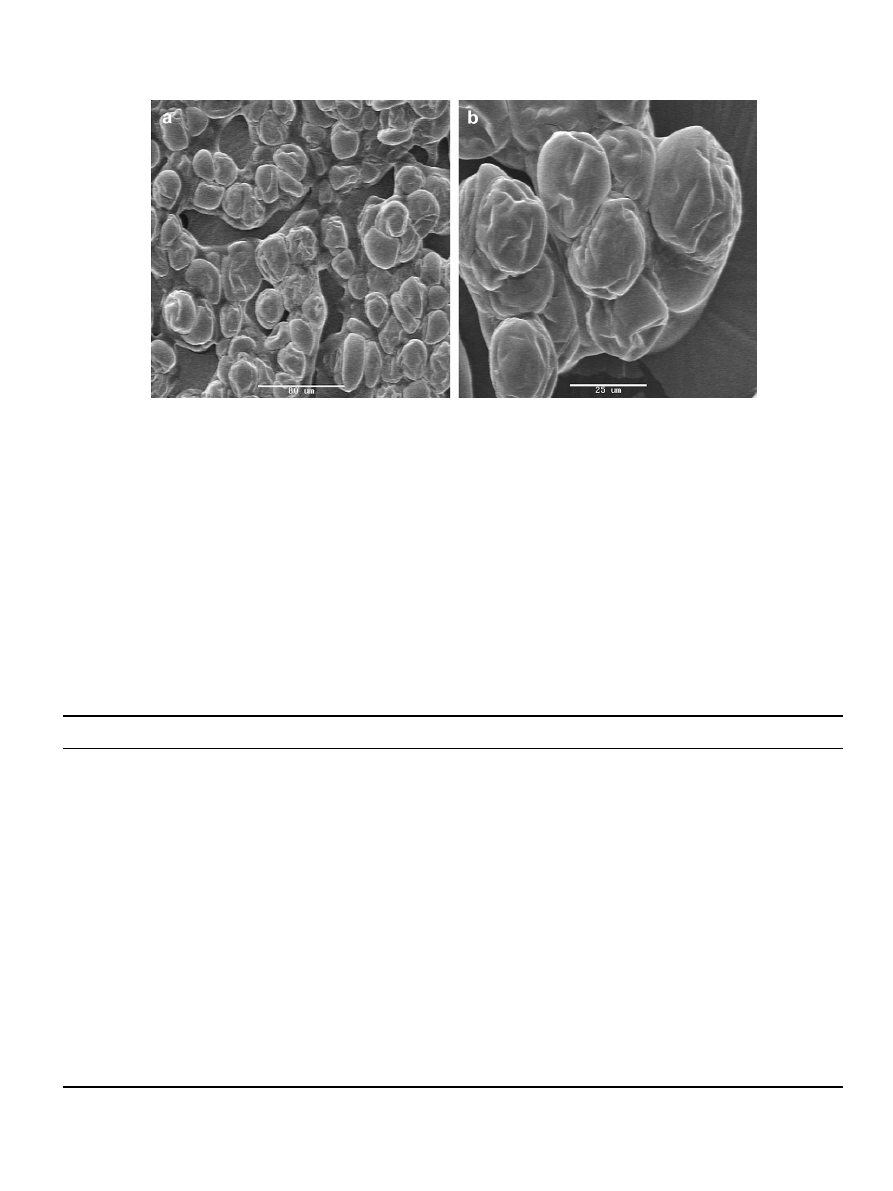
The dried Lactobacillus paracasei powder had features like high
flow ability, mono-dispersive, and immediate solubility. The
microcapsules were rod shaped particles with particle size ranging
from 20 to 50
m
m depend on solids concentration in the sprayed
solution (
).
3.1. Effects of matrix composition
To protect the probiotic cells from dehydration induced damage,
such as disruption of cells membranes due to irreversible phase
transitions, and in order to enhance the probiotic stability during
storage of the dried powder, we encapsulated the Lactobacillus par-
acasei cells in a matrix of maltodextrin and/or in a mixture of the later
with trehalose. Polysaccharides such as maltodextrin are used as
matrix wall materials due to their ability to form glasses of a very high
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1846
viscosity (10
7
e10
11
kPa s
1
). It is known that in the glassy state
chemical reactions that require diffusion are practically stopped
(
Oldenhof, Wolkers, Fonseca, Passot, & Marin, 2005
), thus increasing
the stability of the dried cells during drying and storage. Large poly-
mers cannot act as osmotic and volumetric spacers preventing
fluid
-gel phase transition temperature (Tm) of cells membranes (
). Thus, high M
w
maltodextrins such as
MD DE19 and MD DE5 are excluded from the intra-membrane space
(intra lamellar region) and their main role is by causing spacing
between the cells and increasing the strength of the glassy matrix
that embed them (
Koster et al., 2003; Oldenhof et al., 2005
The effect of the drying temperature (vacuum) on L. paracasei
survival is shown in
A. From this table one can note that
there was no signi
ficant difference in probiotic survival after drying
in vacuum of 2.27 or 3.33 kPa, as in both matrix formulations of
Maltodextrin DE19 the survival was 29.2
4.9 and 30.8 3.1%
respectively. Thus it can be concluded that the difference in drying
survival between vacuum pressures of 2.27 or 3.33 kPa which affect
the temperature of the product during the drying process, in the
UVSD system, is not signi
ficant.
3.2. Effect of solids concentration
The effect of the maltodextrin concentration on L. paracasei
survival is presented in
A, showing that the survival of the
probiotic was signi
ficantly high solids concentration of maltodextrin
Fig. 2. SEM images of microcapsules produced by UVSD process. L. paracasei (0.75 g/100g) encapsulated in maltodextrin DE5-trehalose (1:1) matrix.
Table 1
Survival of Lactobacillus casei subsp. paracasei LMG P-21380 after the encapsulation process using the UVSD process. (A) Encapsulation in maltodextrin; (B) Effect of trehalose;
(C) Effect of L. paracasei concentration; and (D) Effect of high
final water activity of the powder.
Matrix composition
Solids
concent. [g/100g]
Drying
vacuum [KPa]
Initial cell
concent. [CFU/g d m]
Final water
activity
Survival [%]
Signi
ficance
of differences
b
A
MD DE19
10
2.27
1.4
10
8
0.11
20.4
0.8
a
A
MD DE19
15
2.27
2.6
10
8
0.04
18.2
3.0
A
MD DE19
20
2.27
2.7
10
8
0.12
29.2
4.9
B
MD DE19
25
2.27
2.6
10
8
0.25
33.8
3.9
B
MD DE19
20
3.33
2.6
10
8
0.16
30.8
3.1
B
MD DE5
20
3.33
2.4
10
8
0.13
51.6
8.9
C
B
MD DE19 : Trehalose (2:1)
30
2.27
3.2
10
8
0.19
49.8
7.3
C
MD DE19 : Trehalose (2:1)
30
3.33
5.5
10
8
0.19
48.3
5.3
C
MD DE19 : Trehalose (1:1)
20
2.27
5.0
10
8
0.11
29.6
4.0
B
MD DE19 : Trehalose (1:1)
20
3.33
3.3
10
8
0.20
39.8
8.1
B,C
MD DE19 : Trehalose (1:1)
30
2.27
2.4
10
8
0.21
47.5
6.9
C
MD DE19 : Trehalose (1:1)
30
3.33
3.1
10
8
0.31
49.9
7.0
C
MD DE5 : Trehalose (2:1)
30
3.33
5.3
10
8
0.25
62.8
7.9
C,D
MD DE5 : Trehalose (1:1)
20
3.33
3.4
10
8
0.21
70.6
6.2
D
MD DE5 : Trehalose (1:1)
30
3.33
5.2
10
8
0.28
64.3
4.1
D
C
MD DE19 : Trehalose (1:1)
20
3.33
1.6
10
9
0.17
56.0
8.3
C,D
MD DE19 : Trehalose (1:1)
20
3.33
3.3
10
9
0.13
50.8
8.0
C
MD DE19 : Trehalose (1:1)
20
3.33
7.0
10
9
0.20
47.2
10.0
B,C
D
MD DE19
25
2.27
2.6
10
8
0.42
28.4
4.6
B
MD DE19 : Trehalose (2:1)
30
2.27
3.2
10
8
0.44
17.1
2.8
A
MD DE19 : Trehalose (2:1)
30
3.33
6.4
10
8
0.33
45.9
5.6
C
MD DE19 : Trehalose (1:1)
30
2.27
2.4
10
8
0.49
27.5
4.4
A,B
MD DE19 : Trehalose (1:1)
30
3.33
3.0
10
9
0.40
27.4
4.5
A,B
a
The error represent standard deviation of means (n
3).
b
p
< 0.05.
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1847

DE19 (20
e30 g/100g), than at lower solids concentration (10e15 g/
100g). Since the size of the droplets created by the ultrasonic nozzle
are in the range 10
e50
m
m, and the inlet
flow rate and the nozzle
vibration frequency were kept constant during the drying experi-
ments, the main factor that affect the droplets size was the viscosity
of the inlet solution. The solution viscosity at 25
C (room temper-
ature) increases with solid concentration, therefore increasing the
droplets size. We may therefore suggest that the larger the droplets
are the more protection can be provided.
3.3. The role of trehalose
In order to form a glassy matrix and provide optimal protection
to probiotic cells during dehydration, we combined the maltodex-
trins with trehalose. The effect of the maltodextrin DE and treha-
lose concentrations on L. paracasei survival is shown in
B.
Apparently, trehalose presence in the matrix with maltodextrin
DE5 increased signi
ficantly (p < 0.05) the probiotic survival during
drying (
A and B). However, increase of trehalose concen-
tration from 33% to 50% g/100g did not increase the L. paracasei
survival.
It was suggested that damage to probiotic cells following drying
can be related to: 1) Changes in physical state of membrane lipids;
2) Changes in structure of sensitive proteins (
).
Preservation of the structure and functionality of lipid membranes
and proteins during drying may be achieved by several mecha-
nisms (
Carpenter, Martin, Crowe, & Crowe, 1987; Crowe, Crowe,
). One mechanism is the disaccharide
ability to form a glass of a very high viscosity and low mobility.
Trehalose posses a high Tg in dry state (much higher than other
disaccharides) and has the capacity to form a dehydrate that enable
trehalose to remain in glassy state at a higher moisture content
(
Crowe, Reid, & Crowe, 1996; Patist & Zoerb, 2005
). During the
preparation of the matrix solution the low molecular weight
trehalose (Mw
¼ 342.3) can penetrate into the cells inter-
membrane space prior to drying, turning into a glassy state during
the dehydration process. This glassy state provides mechanical
resistance to the membrane bilayer (
).
During the dehydration process, trehalose vitri
fies, at a tempera-
ture lower than Tm
e the fluid membrane, and the vitrified sugar in
the inter-membrane space acts as a volumetric spacer. Reduction of
T
m
by trehalose glassy state prevents packing defects and phase
transitions accompanied by cytoplasm leakage upon rehydration.
In addition, the glassy trehalose restricts the mobility of cytoplasm
proteins, as well as the protein unfolding (
Trehalose interaction with the polar groups of the lipid
membrane, may replace the hydration water at the membrane
interface, thus lowering the phase transition temperature T
m
during drying processes (
Crowe et al., 1988; Crowe, Carpenter, &
). This stabilizing effect of trehalose is related to its
structure, providing the most favorable
fit with the polar head
groups of the phospholipids membrane (
), thus maintaining the polar groups at
their hydrated position. Besides lowering T
m
of membranes,
trehalose has the ability to preserve the structure and functionality
of cell proteins during drying. This ability is associated to formation
of the hydrogen bonds between the hydroxyl groups and polar
residues of the protein (
The results (
B) indicate that the survival at 1:1 and 2:1
maltodextrin-trehalose ratio is similar. At higher amounts of
trehalose, the plasticizing effect of the added sugar is more
accentuated, the glass transition temperature of the mixture is
lower as well the a
wg
limit (water activity at glass transition) for
stabilization while the moisture stabilization limit m
g
(moisture
content at glass transition) is lower too (
). On the other hand the water replacement
capability of trehalose due hydrogen bonding will be more signif-
icant at higher trehalose fractions. Therefore we suggest that the
latter effect compensates for the lower glass transition temperature
and different moisture stability limit.
3.4. Effect of water activity on microcapsule stability
The effect of water activity and moisture content on the stability
of the microcapsule was tested in formulations composed of mal-
todextrins DE5 and DE19, both as mixed maltodextrin-trehalose
matrixes. Increase in
final water activity (a
w
>0.4) in maltodextrin-
trehalose matrix lead to a decrease in probiotic survival (p
< 0.05)
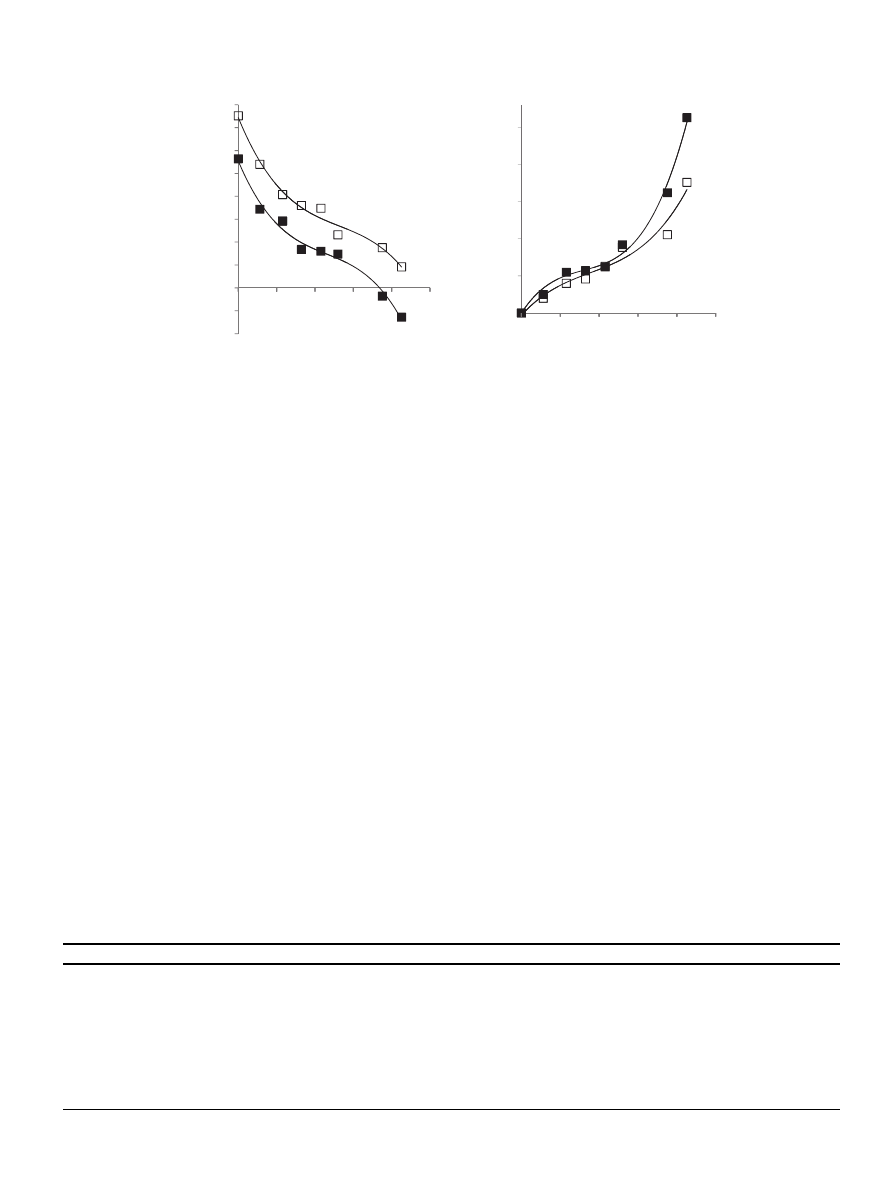
D). The effect of water activity on the glass transition of MD
DE19 was previously evaluated by determining the moisture
sorption isotherm (at 25
C) and DSC determination of the glass
transition, T
g
for maltodextrin DE20 (
A) (
). This
use of existing data on maltodextrin DE20 for calculations related to
DE19 was carried out assuming that for the purpose of this
discussion, the difference is insigni
ficant. Bouquerand (
) also determined the limit of maltodextrin DE20
moisture stability as an encapsulation carrier. They demonstrated
that as long as the Tg is higher than the storage temperature, it
remains in a glassy state, and the molecular mobility is restricted at
that water activity limit a
wg
. Using this concept we evaluated the
moisture content corresponding to 25
C as the glass transition
temperature, Tg vs. the moisture content (m) curve of MD DE20
(
). Once determining the moisture content at
glass transition (m
g
), the stability limit value of the water activity
(a
wg
) is evaluated from the sorption isotherms curve of maltodex-
trin DE20 at 25
C presented in the form of moisture content m
(g water/g solids) as function of the water activity a
w
(
B).
Bellow this m
g
maltodextrin DE20 remains in a glassy state. This
way we calculated the estimated stability limits of maltodextrin
DE20 as m
g
(DE20)
¼ 0.11 (g water/g solids), and a
wg
(DE20)
¼ 0.59.
Based on polymer physics principles, we used an additional
method to determine a
wg
and m
g
. The water sorption isotherms
data can be transformed using the equations below into a new
form, representing the osmotic pressure
P
osm
(MPa) of the polymer
(MD DE 20) as a function of solids concentration C
* (g/ml solution)
The relationship between polysaccharide concentration and
moisture content m can be expressed as follow (
SilberbergBouhnik, Eichler, & Cohen, 1997
).
C
* ¼
1
m
y
w
þ
y
s
(2)
m
¼
1
C*
y
s
C
*
y
w
(3)
Where: C
* is the solids concentration in g solids/ml solution;
m is the moisture content in g
water
/g
solids
n
s,
n
w
, are the speci
fic volume of the solids and of the water in
ml/g
solids
.
The osmotic pressure is calculated from the water activity data
according to the following expression:
p
osm
¼
RT
V
w
lna
w
(4)
Where
T is the temperature in K,
R is the gas constant,
V
w
is molar volume of liquid water 18 ml/mol.
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1848
The values of C
* can be evaluated from the sorption isotherm
data and from Eq.
.
By plotting the sorption isotherm data in the form of log (
P
osm
)
versus log(C
*) one can see two distinct straight lines. One, repre-
senting the rubbery region of the water sorption isotherm and the
second representing its glassy region. The obtained straight lines
intersect each other (
), and from that intersection one can
evaluate the concentration C
g
* and osmotic pressure
P
osm
magni-
tudes at the onset glassi
fication. From C*
g
and Eq.
the moisture
limit value, m
g
the moisture content at the glassy onset is evalu-
ated, assuming that in the limit of the rubbery region
y
w
y1.
From the magnitude of log(
P
osm
) at the intersection of the
straight lines (see the horizontal line in
) it is possible to
estimate a
wg
. The stability limits of maltodextrin DE 20 were
calculated from (
A) are: m
g
(DE20)
¼ 0.108 (g water/g solids),
and a
wg
(DE20)
¼ 0.63. Thus, by employing this method we
obtained very similar stability limits, m
g
and a
wg
for MD DE20, as
those found by
.
In maltodextrin-trehalose mixtures, trehalose crystallization
may be inhibited due the presence of the polysaccharide malto-
dextrin, as suggested for amorphous sucrose (
and lactose (
Labrousse, Roos, & Karel, 1992
). By adding high Mw
maltodextrin DE5 (Mw 150000) or DE20 (Mw 9000), we expected
the anhydrous Tg of the mixture maltodextrin-trehalose to
increase. We assumed that trehalose acts as a plasticizer for the
large maltodextrin molecule, thus lowering the Tg as function of
moisture content. This would lead to a decrease in the stability limit
of the water activity, a
wg
and the moisture content needed for the
matrix to be in glassy state (m
g
).
Plotting the water sorption isotherm of maltodextrin-trehalose
mixture 1:1 one can estimate the moisture and water activity limit
as previously described. We therefore transformed the sorption
isotherm of maltodextrin-trehalose 1:1 data, determined at 25
C
by Iglesias (
Iglesias, Chirife, & Buera, 1997
), to osmotic pressure vs
water activity (
) and plot it in the form log(
P
osm
) vs. log(C
*)
(
B). The calculated moisture and water activity limits, m
g
and
a
wg
for the 1:1 maltodextrin
etrehalose mixture were a
wg
¼ 0.59
and m
g
¼ 0.11(g water/g solids) respectively. It can be therefore
seen that the a
wg
limit (0.59) is lower than for MD alone
(DE5
¼ 0.77 and DE20 ¼ 0.63). However the trehalose presence and
its additional protection lead to an overall higher survival in the
trehalose-maltodextrin mixture than for MD alone.
3.5. The effect of the dextrose equivalent
The survival of the probiotic cells was signi
ficantly higher when
the encapsulation matrix was based on maltodextrin with low DE
rather than high DE (p
< 0.05 maltodextrin and maltodextrin-
trehalose formulations) (
A and B). The viability difference
between DE5 and DE19 was up to 20%. In addition, we have found
that while at similar solids concentration (30 g/100g) the survival at
maltodextrin DE5-tehalose 2:1 ratio was similar to 1:1 ratio
(
B), both were higher than in the mixtures of maltodextrin
DE19-trehalose 2:1 and 1:1. The reason to the 20% increase in cell
survival is probably the higher glass transition temperature of DE5
(anhydrous Tg is 188
C versus 141
C for DE20). This effect was
even more prominent at 20 g/100g solids concentration. Here the
survival was almost doubled for the high MW maltodextrin (DE5).
Table 2
The osmotic pressure
P
osm
(MPa) of the formulation as function of solids concentration C
* (g/ml solution) (based on
Maltodextrin DE5
Maltodextrin DE20
Maltodextrin-Trehalose
Concentration C
*
(g solids/ml solution)
Osmotic pressure
(MPa)
Concentration C
*
(g solids/ml solution)
Osmotic pressure
(MPa)
Concentration C
*
(g solids/ml solution)
Osmotic pressure (MPa)
0
0
0
0
0
0
1.18
22.1
1.07
22.1
1.25
37.8
1.25
30.7
1.15
30.7
1.27
55.2
1.29
39.4
1.20
39.4
1.28
72.7
1.32
90.2
1.31
90.2
1.30
92.7
1.36
115.9
1.37
115.9
1.32
106.9
1.40
152.9
1.38
152.9
1.33
133.3
1.41
201.8
1.38
201.8
1.35
165.8
1.45
301.0
1.44
301.0
1.37
208.5
Tg (°C)
A
B
200
175
150
125
100
75
50
25
0
0
0.2
0.4
0.6
0.8
1
0
0.2
0.4
0.6
0.8
1
0.25
0.20
0.15
0.10
0.05
0.00
25-
50-
Water activity
m (gr water / gr d.m.)
Water activity
Fig. 3. (A) The dependency of glass transition (Tg) on the water activity (a
w
) (based on
). The curves were
fitted to a third degree polynom (R
2
0.987). (B) Sorption
isotherms at 25
C of maltodextrins DE5 and DE20. Both curves were
fitted to a third degree polynom (R
2
(DE5)
¼ 0.963, R
2
(DE20)
¼ 0.991). , Maltodextrin DE5,
-
maltodextrin
DE20. From these graphs we estimated the water activity and moisture content limit (aw
g
and m
g
) for both polymers: m
g
(DE20)
¼ 0.108 (g water/g solids), a
wg
(DE20)
¼ 0.63;
m
g
(DE5)
¼ 0.17 (g water/g solids), a
wg
(DE5)
¼ 0.80.
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1849
In order to determine the water activity and moisture content
stability limit for maltodextrin DE5 we employed again the two
methods used for determining the a
wg
and m
g
for DE 20. The
evaluated stability limits of maltodextrin DE5 m
g
and a
wg
:
m
g
(DE5)
¼ 0.17 (g water/g solids), a
wg
(DE5)
¼ 0.80. The sorption
isotherm of maltodextrin DE5 was plotted in the same manner in
the form of
P
osm
vs C
* (
) and (
). Here too, we observed
two distinct straight lines, and at their intersection we evaluated
the concentration C
*
g
and osmotic pressure
P
osm
at the glassy state
onset, and then calculated a
wg
. The stability limits of the malto-
dextrins were therefore m
g
(DE5)
¼ 0.11 (g water/g solids),
a
wg
(DE5)
¼ 0.77 for DE5 and m
g
(DE20)
¼ 0.11 (g water/g solids),
a
wg
(DE20)
¼ 0.63 for DE20.
Two different methods thus, showed very close stability limits,
m
g
and a
wg
were obtained for MD DE20 and DE5. These results
indicate that MD DE5 is in a glassy state at a higher water activity
than MD DE20. This implies that during the moisture removal the
MD DE5 falling drops will reach faster the glassy state due the
vitri
fication mechanism. This in turn may lead to better protection
of the probiotic cells than with MD DE19. This observation may
therefore explain the increase in the survival percentage when the
probiotics were embedded in a maltodextrin DE5 matrix.
3.6. The effect of the probiotic concentration
The survival of L. paracasei in relation to its concentration in the
matrix is shown in
C. There was a decrease in the survival
with the increase in cell concentration. Hence, part of the cells can
be located at the drop surface; therefore, the matrix formulation
cannot protect them during the drying stages. At low probiotic
concentrations, fewer cells are at the droops surface and the
survival in both methods is similar. Despite the decreased viability,
UVSD process provided an opportunity to encapsulate more than
3
10
9
CFU/g L. paracasei with near 50% survival.
3.7. Stability of the encapsulated probiotics
Probiotic bacteria integrated in food products should retain
their viability during storage, thus ensuring adequate number of
cells in the consumed product. We therefore examined changes in
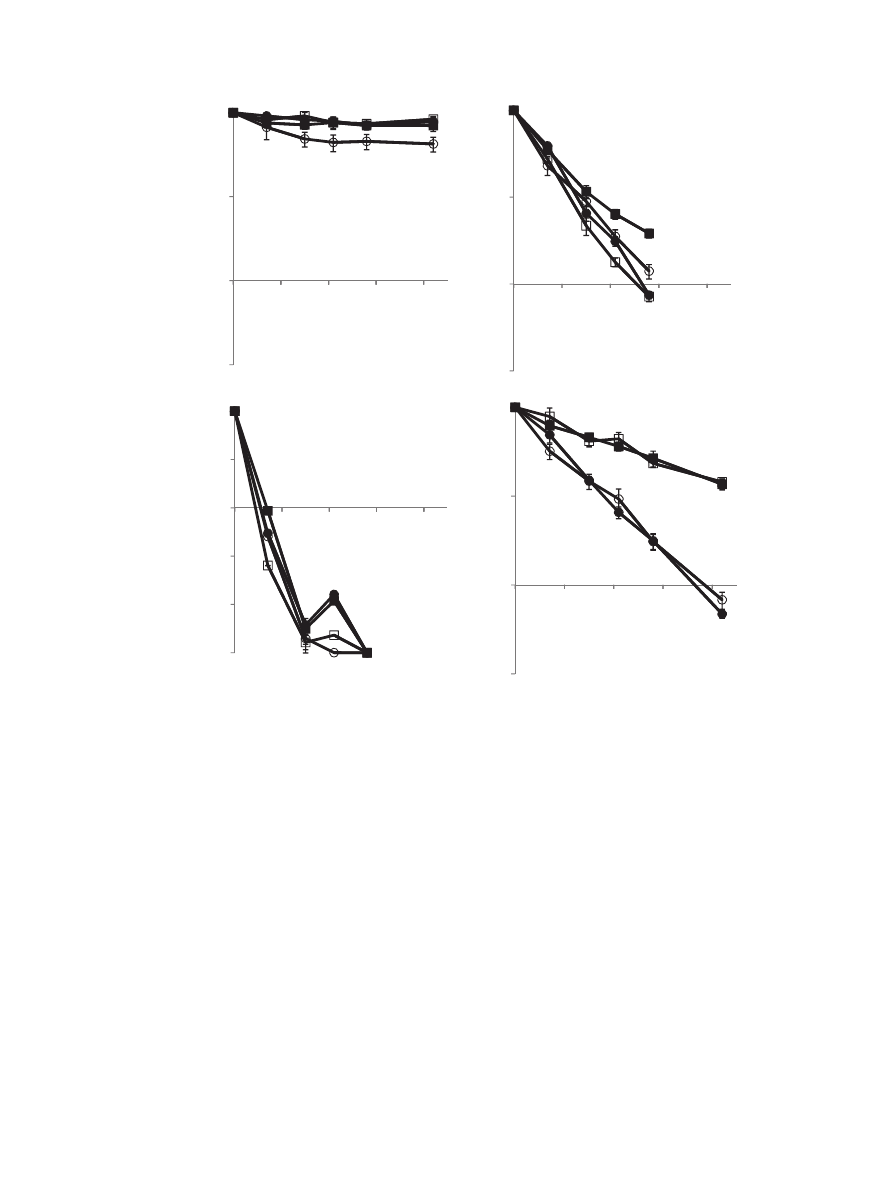
the probiotic survival during storage. The effect of storage
temperature on L. paracasei stability in matrixes composed of
maltodextrins and various dextrose equivalent (DE) and malto-
dextrin-trehalose ratio is shown in
. Indeed, storage temper-
ature affects probiotic survival in a very signi
ficant manner. Similar
results were reported by other researchers (
Tailliez, 1993; Gardiner et al., 2000; Teixeira, Castro, Malcata, &
Kirby, 1995
). In addition, the survival was considerably lost after 7
days and 28 days of storage at 37 and 25
C respectively. The results
also indicate that in most compositions the probiotic survival was
above 70% for at least 42 days when stored at 4
C.
The presence of trehalose in the matrix increased viability,
especially in combination with low oxygen level (N
2
). At low
oxygen level, using trehalose as part of the encapsulation matrix
provided better protection than encapsulation in a maltodextrin
matrix alone (p
< 0.01) (
). Interestingly, dextrose equivalent,
DE, did not have signi
ficant effect on probiotic survival during
storage at 25 and 37
C. However in combination with trehalose the
best results were obtained with lower DE, probably because the
higher T
g
of the mixture. This would increase the difference
between the T
g
and the storage temperature T, (T - T
g
) that is known
to control the rate of physical, chemical and biological changes
(
As noted brie
fly before, we also explored the effect of oxygen
level as an external factor affecting probiotic survival. Samples that
were stored in vessels
flushed with nitrogen and stored under
nitrogen at 25
C maintained higher viability for longer time than
samples stored at 25
C in air (p
< 0.01) as described in
B and D.
Several studies claimed that reduction of cell viability during
storage can be related to oxidation of membrane lipids that initiate
the production of hydroperoxides which have been shown to
induce also DNA damage (
Akasaka, 1986; Inouye, 1984; Marnett
). Thus chemical stability is also an important factor to
be considered to increase preservation of the dried probiotic cells
during storage. From this point of view the glassy state of the
encapsulating matrix reduce the rate of chemical reactions and
decrease the molecular mobility in the cytoplasm (
). In light of this study, the fast decay of viability at
37
C is likely due to the fact that the carrier matrix is not in a glassy
state.
1.
1.
1.
1.
2.
2.
2.
2.
log(Osm. Pres.)
A
B
1
.2
.4
.6
.8
2
.2
.4
.6
.8
3
0
0..05
0.1
log(
0.15
C*)
0.2
1.
1.
1.
2.
2.
log(Osm. Pres.)
.4
.6
.8
2
.2
.4
0.08
0.1
0.1
log(C*)
12
0.14
)
Fig. 4. Evaluation of moisture content and water activity limit (aw
g
and m
g
) from curves of osmotic pressure as function of the concentration. (A) Maltodextrin DE5:
, rubbery,
-
glassy; and maltodextrin DE20:
B rubbery, C glassy. From these graphs we estimated the water activity and moisture content limit (aw
g
and m
g
) for both polymers:
m
g
(DE5)
¼ 0.11 (g water/g solids), a
wg
(DE5)
¼ 0.77; and m
g
(DE20)
¼ 0.11 (g water/g solids), a
wg
(DE20)
¼ 0.63 (based on
). (B) Evaluation of moisture content and
water activity limit (aw
g
and m
g
) from curves of osmotic pressure as function of the concentration of maltodextrin-trehalose mixture (based on
):
6
rubbery,
: glassy. From these graphs we estimated the water activity and moisture content limit: a
wg
¼ 0.59 and m
g
¼ 0.11 (g water/g solids).
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1850
4. Conclusions
The aim of this investigation was to study a process for the
formation of dry-encapsulated probiotics, using ultrasonic vacuum
spray drying (UVSD), and microcapsule matrix composed of mal-
todextrin and trehalose. The results of this study demonstrate that
using UVSD brought the matrix rapidly to a glassy state and
provided high survival of the probiotic cells - 3.3
10
9
CFU/g d m,
that was achieved with maltodextrin DE19-trehalose (1:1) 20%
g/100g matrix and 7.0
10
9
CFU/g d.m. initial L. paracasei
concentration. It was found that MD DE5 was a better encapsula-
tion matrix than MD DE19, probably due to the fact that DE5 matrix
maintained its glassy state at a higher a
w
. The addition of trehalose
increased the viability signi
ficantly during the drying and during
storage of the dried powder. MD DE5-trehalose combination (1:1)
resulted with the highest survival (70.6
6.2%). Evidently, further
protection should be provided to the cells against oxidation, as
storage in nitrogen was essential in order to gain storage stability.
Further studies should be conducted to provide further protection
to the probiotics by better control of (T - T
g
), and enhancement of
the chemical stability during storage by employing additional
compatible solutes and coatings.
Acknowledgment
The research was supported by the Israeli Ministry of Industry
Commerce and Trade.
References
Akasaka, S. (1986). Inactivation of transforming activity of plasmid DNA by lipid-
peroxidation. Biochimica and Biophysica Acta, 867(4), 201
e208.
Anal, A. K., & Singh, H. (2007). Recent advances in microencapsulation of probiotics
for industrial applications and targeted delivery. Trends in Food Science &
Technology, 18(5), 240
e251.
Berner, L. A., & O
’Donnell, J. A. (1998). Functional foods and health claims legisla-
tion: applications to dairy foods. International Dairy Journal, 8(5
e6), 355e362.
Bouquerand, P.-E., Maio, S., Meyer, F., & Normand, V. (2008). Moisture stability of
maltodextrin-based delivery systems. Food Biophysics, 3(2), 182
e185.
Buitink, J., & Leprince, O. (2004). Glass formation in plant anhydrobiotes: survival in
the dry state. Cryobiology, 48(3), 215
e228.
Carpenter, J. F., & Crowe, J. H. (1989). An Infrared spectroscopic study of the inter-
actions of carbohydrates with dried proteins. Biochemistry, 28(9), 3916
e3922.
Carpenter, J. F., Martin, B., Crowe, L. M., & Crowe, J. H. (1987). Stabilization of
phosphofructokinase during air-drying with sugars and sugar transition-metal
mixtures. Cryobiology, 24(5), 455
e464.
Castro, H. P., Teixeira, P. M., & Kirby, R. (1997). Evidence of membrane damage in
Lactobacillus bulgaricus following freeze drying. Journal of Applied Microbiology,
82(1), 87
e94.
0
1
Viabil
ity [
%
]
A
B
D
C
0.0
0
0
Viability [%]
0.1
1
10
00
0
1
01
.01
.1
1
10
0
1
10
20
Time (days)
0
20
Time (days)
30
4
30
4
40
0
0.
1
10
Viability [
%
]
0
1
100
Viab
ility [%]
.1
1
10
0
0
10
.1
1
0
0
10
0
20
Time (days)
0
20
Time (days)
30
40
30
4
0
40
Fig. 5. Effect of the storage temperature and oxygen level on L. paracasei survival. (A) microcapsules stored in air at 4
C; (B) microcapsules stored in air at 25
C; (C) microcapsules
stored in air at 37
C; and (D) microcapsules stored in vessels
flashed with nitrogen at 25
C
B maltodextrin DE5, , maltodextrin DE5 [1:1] trehalose, C maltodextrin DE19, and
-
maltodextrin DE19 [1:1] trehalose. The error bars represent standard deviation of means (n
3).
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1851

Crowe, J. H., Carpenter, J. F., & Crowe, L. M. (1998). The role of vitri
fication in
anhydrobiosis. Annual Review of Physiology, 60, 73
e103.
Crowe, J. H., Crowe, L. M., Carpenter, J. F., Rudolph, A. S., Wistrom, C. A., Spargo, B. J.,
et al. (1988). Interactions of sugars with membranes. Biochimica and Biophysica
Acta (BBA) - Reviews on Biomembranes, 947(2), 367
e384.
Crowe, J. H., Crowe, L. M., Carpenter, J. F., & Wistrom, C. A. (1987). Stabilization of dry
phospholipid-bilayers and proteins by sugars. Biochemical Journal, 242(1), 1
e10.
Crowe, L. M., Crowe, J. H., Rudolph, A., Womersley, C., & Appel, L. (1985). Preser-
vation of freeze-dried liposomes by trehalose. Archives of Biochemistry and
Biophysics, 242(1), 240
e247.
Crowe, L. M., Reid, D. S., & Crowe, J. H. (1996). Is trehalose special for preserving dry
biomaterials? Biophysical Journal, 71(4), 2087
e2093.
Desmond, C., Ross, R. P., O
’Callaghan, E., Fitzgerald, G., & Stanton, C. (2002).
Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders
containing gum acacia. Journal of Applied Microbiology, 93(6), 1003
e1011.
Dunne, C., O
’Mahony, L., Murphy, L., Thornton, G., Morrissey, D., O’Halloran, S., et al.
(2001). In vitro selection criteria for probiotic bacteria of human origin:
correlation with in vivo
findings. American Journal of Clinical Nutrition, 73(2),
386S
e392S.
FAO/WHO. (2001). Evaluation of health and nutritional properties of powder milk with
live lactic acid bacteria. Report from FAO/WHO Expert Consultation.
Freitas, S., Merkle, H. P., & Gander, B. (2004). Ultrasonic atomisation into reduced
pressure atmosphere
eenvisaging aseptic spray-drying for microencapsulation.
Journal of Controlled Release, 95(2), 185
e195.
Gardiner, G. E., O
’Sullivan, E., Kelly, J., Auty, M. A. E., Fitzgerald, G. F., Collins, J. K.,
et al. (2000). Comparative survival rates of human-derived probiotic Lactoba-
cillus paracasei and L-salivarius strains during heat treatment and spray drying.
Applied and Environmental Microbiology, 66(6), 2605
e2612.
Goderska, K., & Czarnecki, Z. (2008). In
fluence of microencapsulation and spray
drying on the viability of Lactobacillus and Bi
fidobacterium strains. Polish
Journal of Microbiology, 57(2), 135
e140.
Hector, A.,I., & Jorge, C. (1978). Delayed crystallization of amorphous sucrose in
humidi
fied freeze dried model systems. International Journal of Food Science and
Technology, 13(2), 137
e144.
Holzapfel, W. H., Haberer, P., Geisen, R., Bjorkroth, J., & Schillinger, U. (2001).
Taxonomy and important features of probiotic microorganisms in food and
nutrition. American Journal of Clinical Nutrition, 73(2), 365S
e373S.
Iglesias, H. A., Chirife, J., & Buera, M. P. (1997). Adsorption isotherm of amorphous
trehalose. Journal of the Science of Food and Agriculture, 75(2), 183
e186.
Inouye, S. (1984). Site-speci
fic cleavage of double-strand DNA by hydroperoxide of
linoleic acid. FEBS Letters, 172(2), 231
e234.
Ishibashi, N., & Shimamura, S. (1993). Bi
fidobacteria - research-and-development in
Japan. Food Technology, 47(6), 126
e134.
Knorr, D. (1998). Technology aspects related to microorganisms in functional foods.
Trends in Food Science & Technology, 9(8
e9), 295e306.
Koster, K. L., Maddocks, K. J., & Bryant, G. (2003). Exclusion of maltodextrins from
phosphatidylcholine multilayers during dehydration: effects on membrane
phase behaviour. European Biophysics Journal with Biophysics Letters, 32(2),
96
e105.
Labrousse, S., Roos, Y. H., & Karel, M. (1992). Collapse and crystallization in amor-
phous matrices with encapsulated compounds. Sciences Des Aliments, 12(4),
757
e769.
Lee, Y. K., & Salminen, S. (1995). The coming of age of probiotics. Trends in Food
Science & Technology, 6(7), 241
e245.
Leslie, S. B., Israeli, E., Lighthart, B., Crowe, J. H., & Crowe, L. M. (1995). Trehalose and
sucrose protect both membranes and proteins in intact bacteria during drying.
Applied and Environmental Microbiology, 61(10), 3592
e3597.
Lievense, L. C., & Van
’t Riet, K. (1993). Convective drying of bacteria. I: the drying
processes. Advances in Biochemical Engineering/ Biotechnology, vol. 50(82 ref.), 45
e63.
Marnett, L. J., Hurd, H. K., Hollstein, M. C., Levin, D. E., Esterbauer, H., & Ames, B. N.
(1985). Naturally-occurring carbonyl-compounds are mutagens in Salmonella
tester strain Ta104. Mutation Research, 148(1
e2), 25e34.
Mary, P., Moschetto, N., & Tailliez, R. (1993). Production and survival during storage
of spray-dried Bradyrhizobium japonicum cell concentrates. Journal of Applied
Microbiology, 74(3), 340
e344.
Menashe, E. (1995). Physical models to invistigate water sorption isotherms and their
relation to the glass transition temperature, mobility and stabality of food systems.
Biotechnology and Food Engineering. Haifa, Technion - Israel Institute of
Technology. B.Sc.
Mizrahi, S., Ramon, O., SilberbergBouhnik, M., Eichler, S., & Cohen, Y. (1997). Scaling
approach to water sorption isotherms of hydrogels and foods. International
Journal of Food Science and Technology, 32(2), 95
e105.
Oldenhof, H., Wolkers, W. F., Fonseca, F., Passot, S. P., & Marin, M. (2005). Effect of
sucrose and maltodextrin on the physical properties and survival of air-dried
Lactobacillus bulgaricus: an in situ Fourier transform infrared spectroscopy
study. Biotechnology Progress, 21(3), 885
e892.
Patist, A., & Zoerb, H. (2005). Preservation mechanisms of trehalose in food and
biosystems. Colloids Surf B Biointerfaces, 40(2), 107
e113.
Roos, Y., & Karel, M. (1991). Phase transitions of mixtures of amorphous poly-
saccharides and sugars. Biotechnology Progress, 7, 49
e53.
Ross, R. P., Desmond, C., Fitzgerald, G. F., & Stanton, C. (2005). Overcoming the
technological hurdles in the development of probiotic foods. Journal of Applied
Microbiology, 98(6), 1410
e1417.
Rudolph, B. R., Chandrasekhar, I., Gaber, B. P., & Nagumo, M. (1990). Molecular
modelling of saccharide-lipid interactions. Chemistry and Physics of Lipids,
53(2
e3), 243e261.
Sadykhov, A., & Kish, S. (1997). Unique ultrasonic vacuum spray dryer
e a new spray
drying concept for high quality powder. The second Israel Conference for
Conveying and Handling of Particulate Solids, Jerusalem, Isreal.
Semyonov, D., Ramon, O., Kaplun, Z., Levin-Brener, L., Gurevich, N., & Shimoni, E.
(2010). Microencapsulation of Lactobacillus paracasei by spray freeze drying.
Food Research International, 43(1), 193
e202.
Shah, N. P., & Ravula, R. R. (2000). Microencapsulation of probiotic bacteria and their
survival in frozen fermented dairy desserts. Australian Journal of Dairy Tech-
nology, 55(3), 139
e144.
Stanton, C., Gardiner, G., Meehan, H., Collins, K., Fitzgerald, G., Lynch, P. B., et al.
(2001). Market potential for probiotics. American Journal of Clinical Nutrition,
73(2), 476S
e483S.
Teixeira, P. C., Castro, M. H., Malcata, F. X., & Kirby, R. M. (1995). Survival of Lacto-
bacillus-delbrueckii ssp bulgaricus following spray-drying. Journal of Dairy
Science, 78(5), 1025
e1031.
Zhang, J., & Steponkus, P. L. (1996). Proposed mechanism for depression of the
liquid-crystalline-to-gel phase transition temperature of phospholipids in
dehydrated sugar-phospholipid mixtures. Cryobiology, 33, 21A.
D. Semyonov et al. / LWT - Food Science and Technology 44 (2011) 1844
e1852
1852
Document Outline
- Using ultrasonic vacuum spray dryer to produce highly viable dry probiotics
- 1 Introduction
- 2 Materials and methods
- 3 Results and discussion
- 4 Conclusions
- Acknowledgment
- References
Wyszukiwarka
Podobne podstrony:
Encapsulating probiotic bacteria by ultrasonic vacuum spray drying (D Semyonov, O Ramon and E Shimon
2003 12 Docbook Using Openoffice Org to Produce Docbook Files
Improving Grape Quality Using Microwave Vacuum Drying Associated with Temperature Control (Clary)
Experimental vacuum spray dryin Nieznany
Improving Grape Quality Using Microwave Vacuum Drying Associated with Temperature Control (Clary)
Kher, Neelam, Molstad Using humor in the classroom to enhance teaching effectiveness in dread cours
Terrorism And Development Using Social and Economic Development to Prevent a Resurgence of Terroris
Wiktorek Smagur, Aneta i inni Green Way of Biomedicine – How to Force Plants to Produce New Importa
Barnett C Helzberg, Jr What I Learned Before I Sold to Warren Buffett [An Entrepreneur’s Guide to D
Using Probabilistic Knowledge And Simulation To Play Poker (Darse Billings)
Ten Secrets to Becoming a Highly Profitable Residential Developer Real Estate Investing Day Trading
Using an open source framework to catch the bad guy
Co to znaczy być biednym, Rok szkolny 2011-2012
NATO to nie tylko USA Nasz Dziennik, 2011 03 18
Odejście tupolewem na drugi krąg to naprawdę nic trudnego Nasz Dziennik, 2011 01 24
więcej podobnych podstron