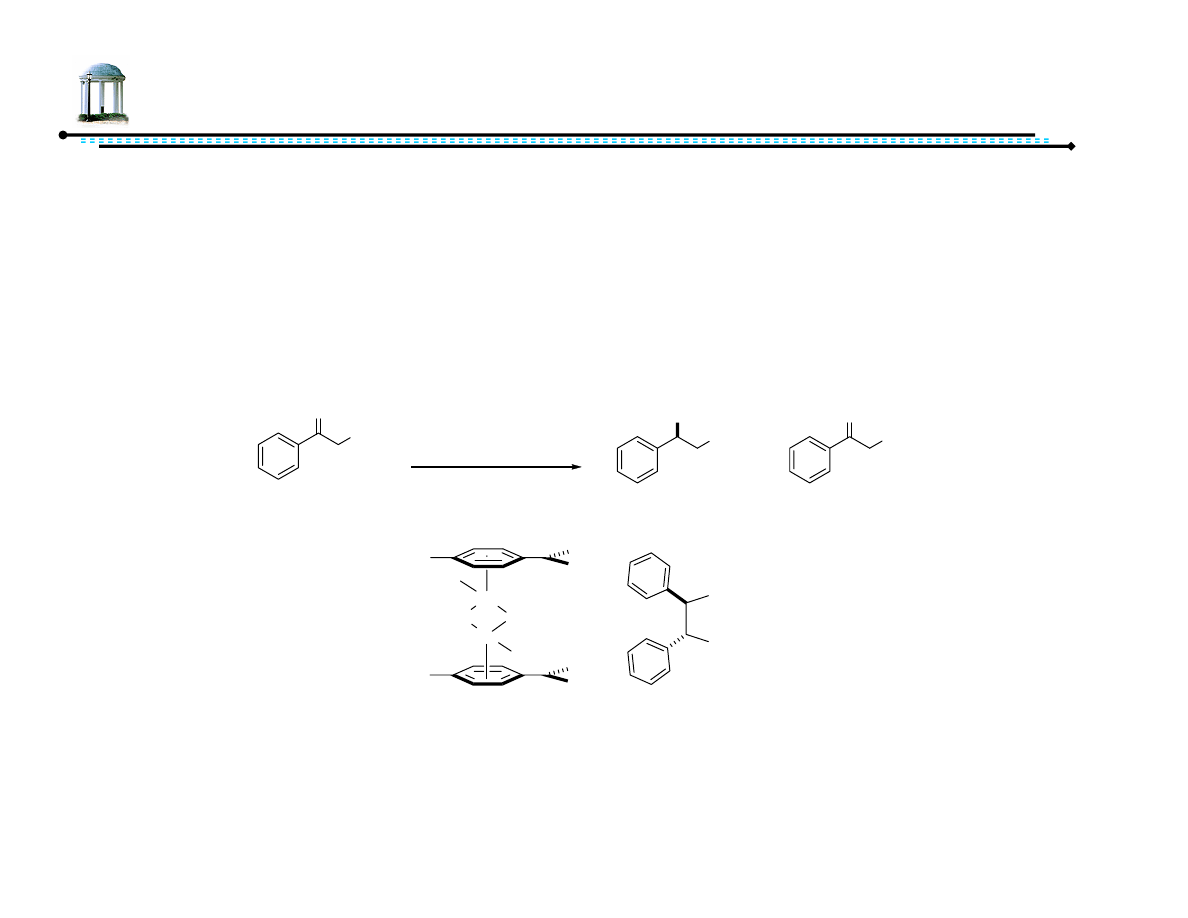
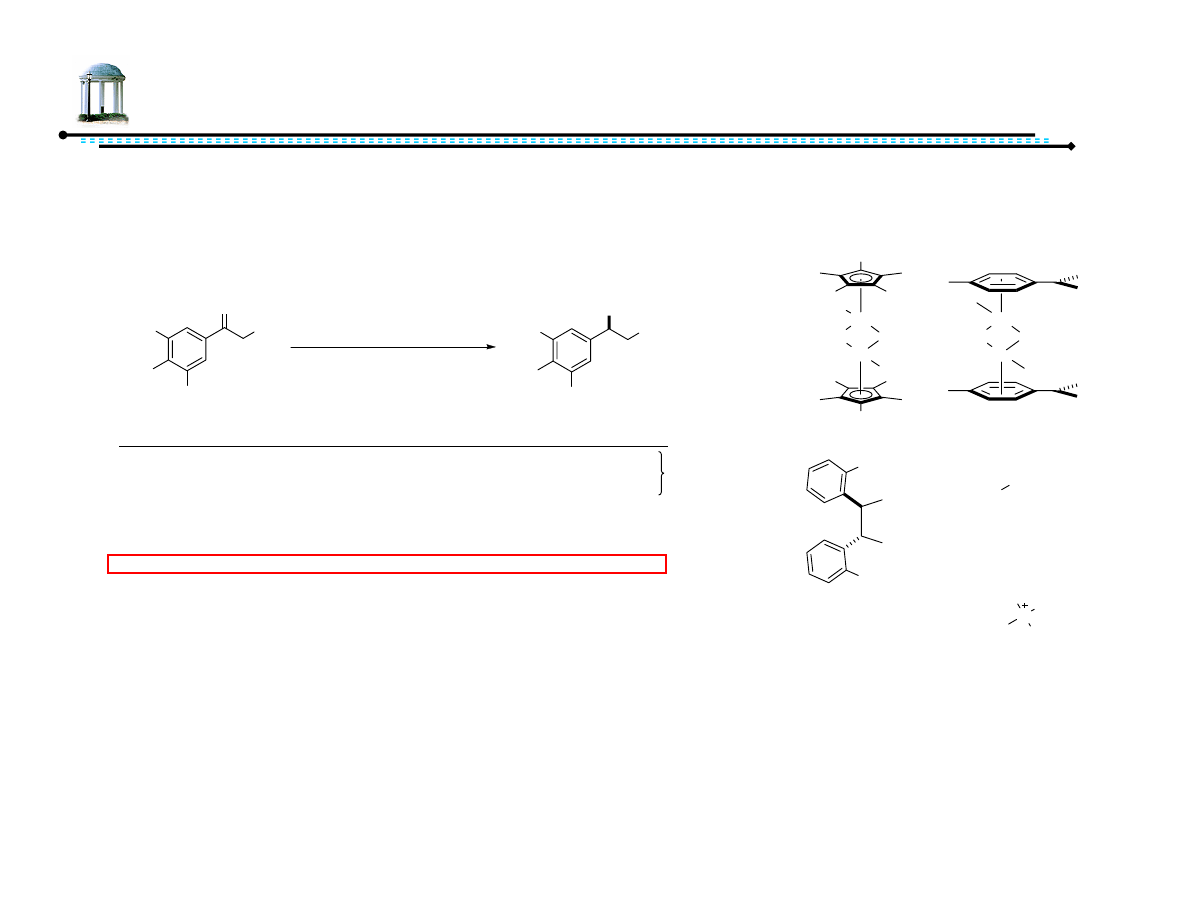
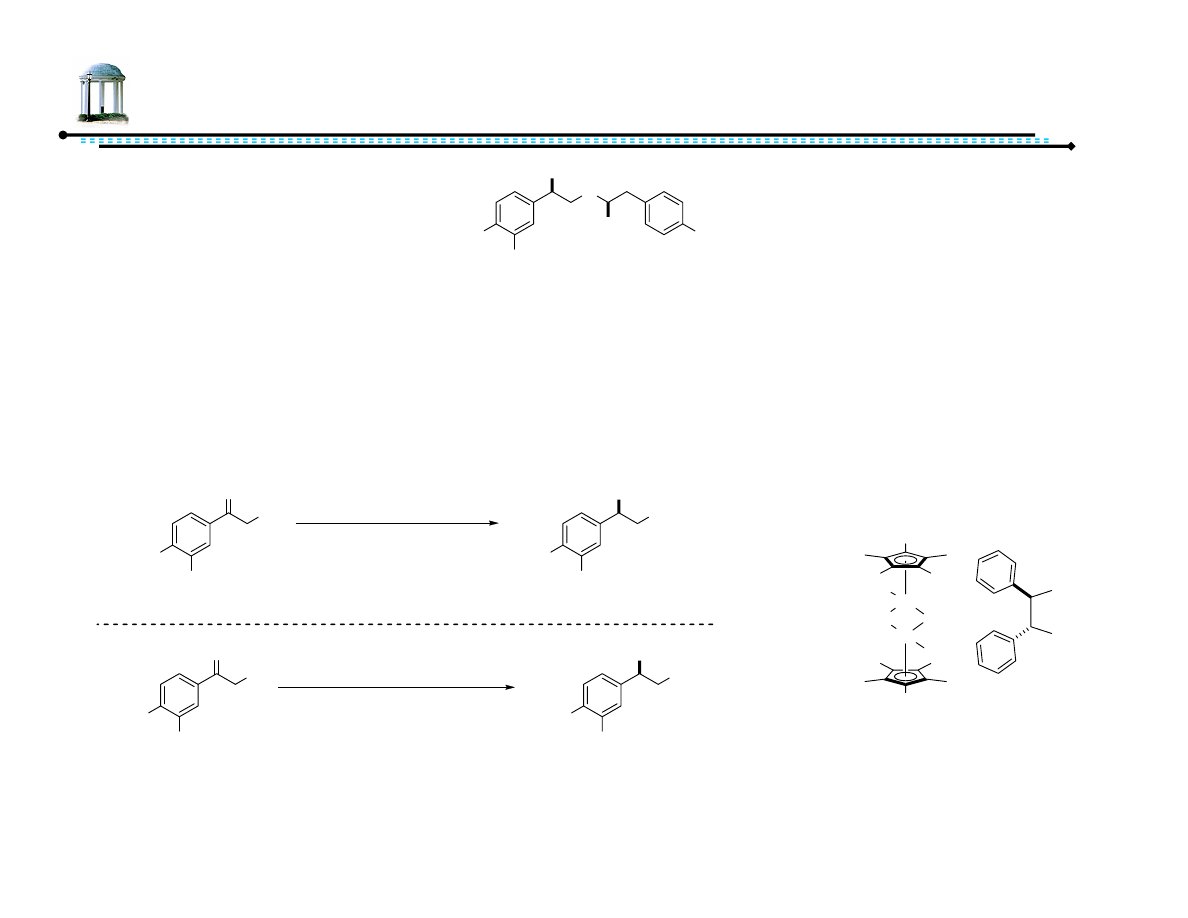
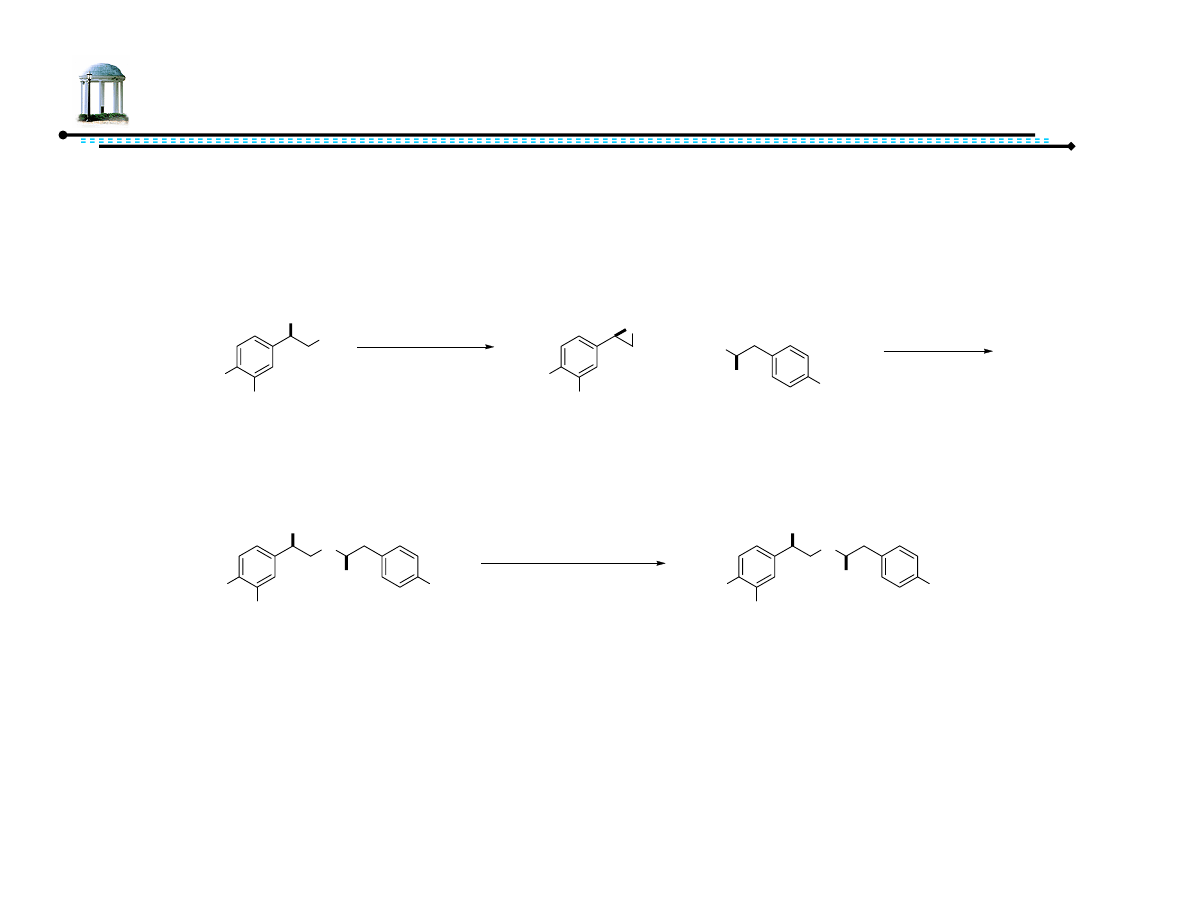
Recent Advances in Asymmetric
Transfer Hydrogenation
Adam M. Azman
8 March 2007
1
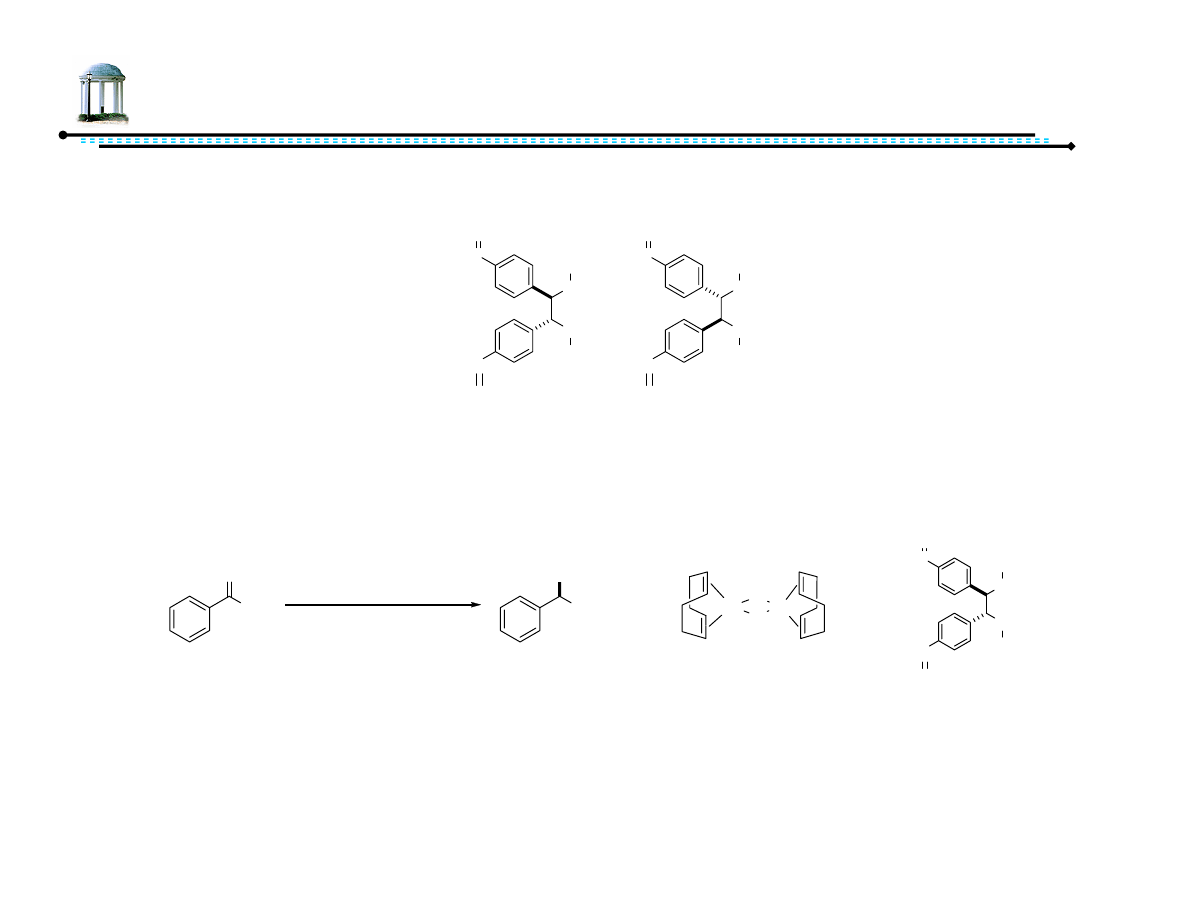
Chiral Secondary Alcohols and Amines
•
Chiral secondary alcohols and amines prevalent
•
Important as intermediates
Me
Me
Me
O
Me
3
Si
Me
Me
Me
HO
Me
3
Si
Me
Me
Me
H
H
HO
H
or
Me
Me
Me
H
H
H
2
C
(−)-β-cubebene
(−)-cubebol
MeO
MeO
N
H
N
Me
H
H
HN
OH
tubulosine
O
N
H
Me
CF
3
HCl
fluoxetine hydrochloride
(Prozac)
HO
OH
OMe
Me
H
N
NHCHO
(R,R)-formoterol
Fürstner, A.; Hannen, P. Chem. Eur. J., 2006, 12, 3006-3019.
Hett, R.; Fang, Q. K.; Gao, Y.; Hong, Y.; Butler, H. T.; Nie, X.; Wald, S. Tetrahedron Lett., 1997, 38, 1125-1128.
2
Formation of Chiral Secondary Alcohols
•
Addition to aldehyde
–
Organometallic Nucleophile
–
Aldol
N
O
•
Epoxide opening
•
Asymmetric carbonyl/imine reduction
Chérest, M.; Felkin, H.; Prudent, N. Tetrahedron Lett., 1968, 18, 2199-2204.
Crimmins, M. T.; King, B. W.; Tabet, E. A.; Chaudhary, K.
J. Org. Chem.
, 2001, 66, 895-902.
R
S
O
Me
L
3
Ti
H
O
R
1
N
O
R
S
O
OH
R
1
"Evans Syn" Product
O
MeLi
Me
OH
Alexakis, A.; Vrancken, E.; Mangeney, P.; Chemla, F.
J. Chem. Soc. Perkin Trans. I
, 2000, 3352.3353.
Ph
Me
O
Ph
(S)
Me
OH
[RuCl
2
(p-cymene)]
2
(1S,2S)-N -(p-toluenesulfonyl)-1,2-
diphenylethylenediamine
i
PrOH, KOH
Hashiguchi, S.; Fujii, A.; Takehara, K.; Ikariya, T.; Noyori, R.
J. Am. Chem. Soc.
, 1995, 117, 7562-7563.
•
Asymmetric alkene oxidation
–
Hydroboration
–
Dihydroxylation
OsO
4
NMO
O
O
O
Me
Me
OH
O
O
O
Me
Me
OH
OH
OH
O
O
O
O
Me
Me
Me
Me
H
H
Brimacombe, J. S.; Hanna, R.; Kabir, A. K. M. S.; Bennett, F.; Taylor, I. D.
J. Chem. Soc. Perkin Trans. I
, 1986, 5, 815-812.
Still, W. C.; Kempf, D.; Hauck, P. Tetrahedron Lett., 1986, 27, 2727-2730.
H
O
R
s
R
L
SnBu
3
OH
R
s
R
L
Felkin-Ahn Product
Me
OH
Me
Me
OH
Me
OH
Me
Me
BH
3
•THF
3
Reduction of C=X π-Bond
•
Meerwein-Ponndorf-Verley Reduction
–
Discovered in 1920s
–
Commonly aluminum or boron metal center
–
Metal-isopropoxide or -alkyl group as reducing agent
•
Metal-based reductions
–
NaBH
4
discovered in 1942 by Brown
–
LiAlH
4
discovered in 1945 by Bond
–
Dissolving metal reduction
•
Transition metal mediated reduction
–
Pioneered by Noyori
•
Asymmetric Transfer Hydrogenation
–
Ru, Rh, Ir hydrides
–
η
6
-arene and diamine ligands
–
No hydrogen atmosphere
–
Isopropanol or formic acid/triethylamine as
stochiometric reducing agent
R
R'
O
O
H
Al
i
PrO
O
i
Pr
R
R'
O
O
H
Al
i
PrO
O
i
Pr
R
R'
OH
O
Ph
2
P
P
Ph
2
Ru
N
H
2
H
2
N
Ph
Ph
Cl
Cl
(S)-BINAP/(S,S)-DPEN-Ru(II) Catalyst
Ar
R
O
H
2
Ru-catalyst
base
Ar
R
OH
Ponndorf, W. Z.; Angew. Chem., 1926, 39, 138.
Ohkuma, T.; Ooka, H.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc., 1995, 117, 2675-2676.
Ph
Me
O
Ph
(S)
Me
OH
[RuCl
2
(p-cymene)]
2
(1S,2S)-N-(p-toluenesulfonyl)-1,2-
diphenylethylenediamine
i
PrOH, KOH
Hashiguchi, S.; Fujii, A.; Takehara, K.; Ikariya, T.; Noyori, R.
J. Am. Chem. Soc.
, 1995, 117, 7562-7563.
Schlesinger, H. I.; Brown, H. C.; Hoekstra, H. R.; Rapp, L. R. J. Am. Chem. Soc., 1953, 75, 199.
Finholt, A. E.; Bond, A. C. Jr.; Schlesinger, H. I. J. Am. Chem. Soc., 1947, 69, 1199.
Barton, D. H. R. J. Chem. Soc., 1953, 1027-1040.

4
Outline
• Mechanism and scope of asymmetric transfer hydrogenation
• Pro-atropisomeric phosphine ligands
• Amino acid-based ligands
• Dendrimer-bound diamine ligands
• Asymmetric transfer hydrogenation in water
• Asymmetric transfer hydrogenation in ionic liquids
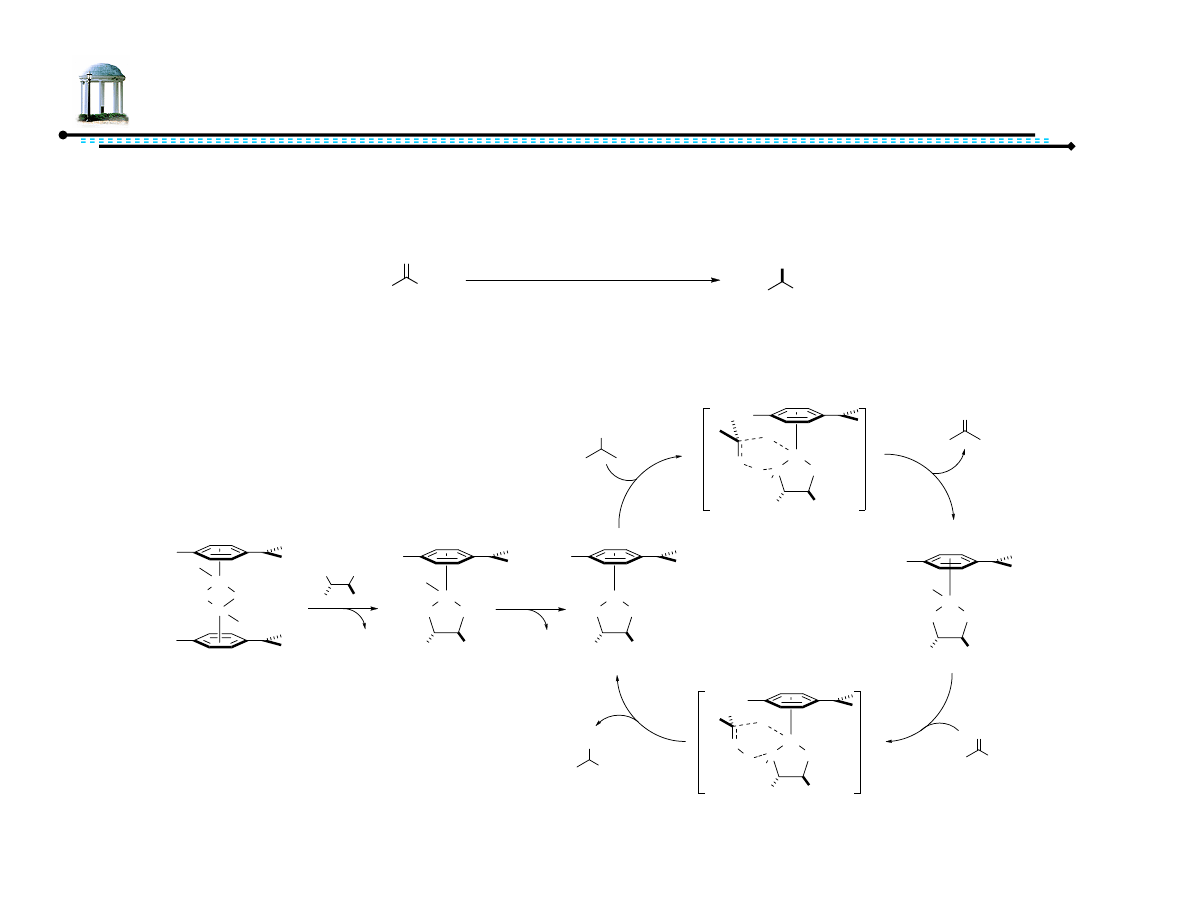
5
Mechanism of ATH
•
Several gas-phase computational studies indicate concerted pathway
NTs
Ru
H
2
N
R
R
H
R
1
O
R
2
NTs
Ru
N
R
R
H
O
H
H
R
1
OH
R
2
*
NTs
Ru
HN
R
R
OH
NTs
Ru
N
R
R
H
O
R
2
R
1
H
H
O
NTs
Ru
H
2
N
R
R
Cl
KOH
Ru
Cl
Cl
Cl
Cl
Ru
(S)(S)
R
H
2
N
NHTs
R
HCl
HCl
Samec, J. S. M.; Bäckvall, J.-E.; Andersson, P. G.; Brandt, P. Chem. Soc. Rev., 2006, 35, 237-248.
Ph
Me
O
Ph
(S)
Me
OH
[RuCl
2
(p-cymene)]
2
(1S,2S)-N-(p-toluenesulfonyl)-1,2-
diphenylethylenediamine
i
PrOH, KOH
substrate:catalyst ~200:1
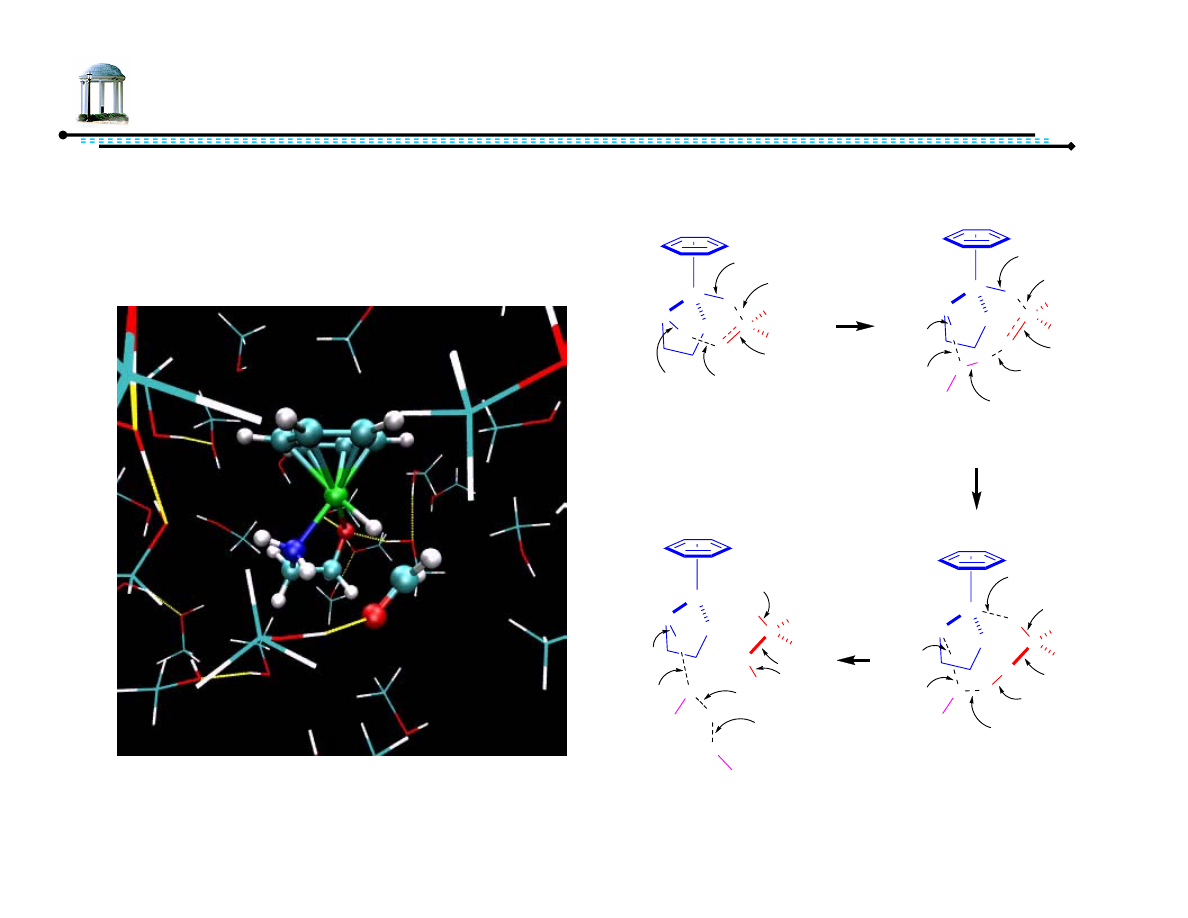
6
Mechanism of ATH
•
Solution phase computational study suggests role of solvent in reduction
Ru
H
O
HN
H
C
O
H
H
1.79 Å
1.32 Å
1.31 Å
1.04 Å
2.51 Å
Ru
H
O
HN
H
C
O
H
H
H
3
C
O H
1.92 Å
1.27 Å
1.34 Å
1.40 Å
1.24 Å
2.02 Å
1.04 Å
Ru
H
O
HN
H
C
O
H
H
H
3
C
O
H
2.06 Å
1.14 Å
1.42 Å
1.02 Å
1.75 Å
1.65 Å
1.11 Å
Ru
H
O
HN
H
C
O
H
H
H
3
C
O
H
H
O
CH
3
1.14 Å
1.42 Å
1.04 Å
1.28 Å
1.27 Å
1.95 Å
1.06 Å
0.00 ps
0.69 ps
0.99 ps
1.08 ps
Handgraaf, J.-W.; Meijer, E. J. J. Am. Chem. Soc. ASAP.
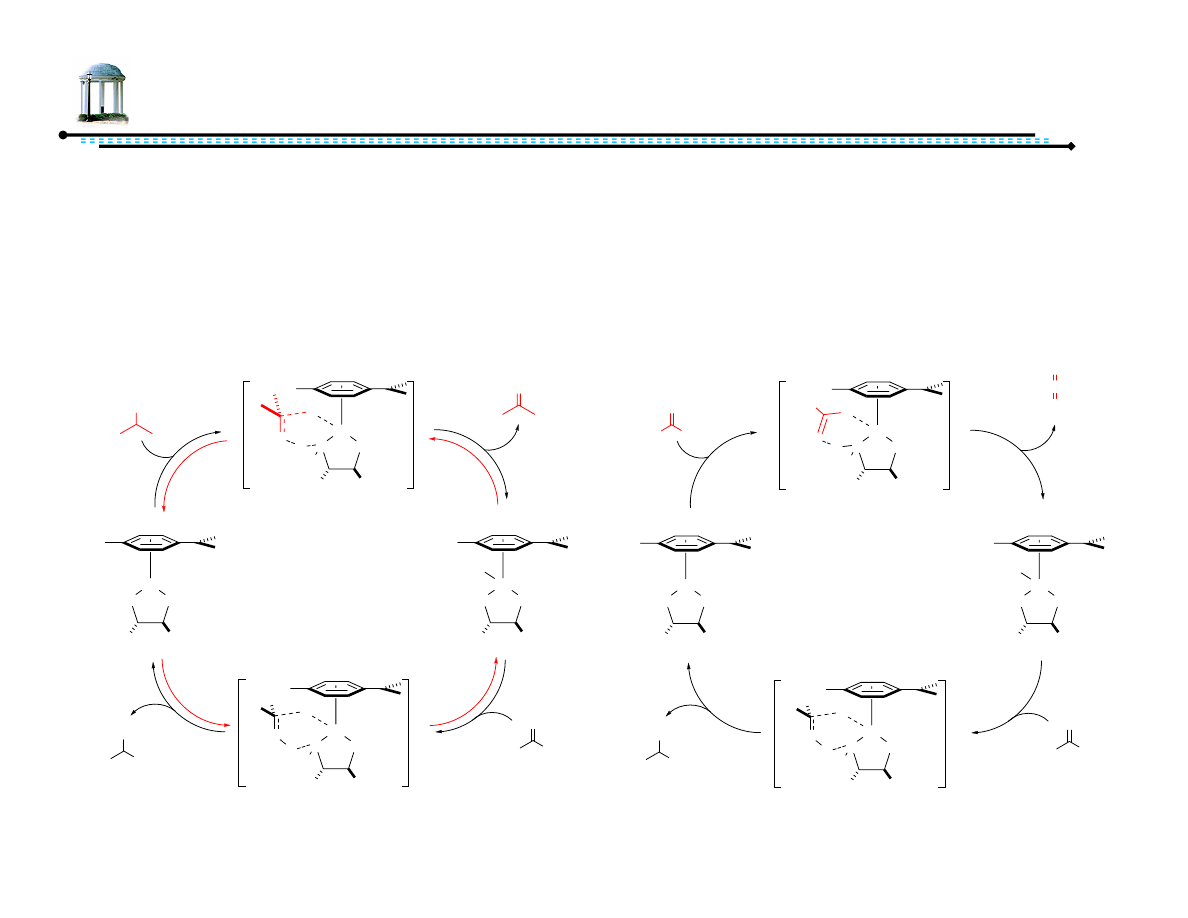
7
Sources of Hydrogen
•
Isopropanol
– Oxidation yields acetone
– Transfer hydrogenation is reversible
– After extended reaction times,
stereoselectivity erodes
•
Formic acid/triethylamine
– Oxidation yields carbon dioxide
– Evolution of carbon dioxide renders
reaction irreversible
– Allows for increase in reaction
concentration
Samec, J. S. M.; Bäckvall, J.-E.; Andersson, P. G.;
Brandt, P. Chem. Soc. Rev., 2006, 35, 237-248.
Koike, T. ;Ikariya, T. Adv. Synth. Catal., 2004, 346, 37-41.
NTs
Ru
H
2
N
R
R
H
R
1
O
R
2
NTs
Ru
N
R
R
H
O
H
H
R
1
OH
R
2
*
NTs
Ru
HN
R
R
OH
NTs
Ru
N
R
R
H
O
R
2
R
1
H
H
O
NTs
Ru
H
2
N
R
R
H
R
1
O
R
2
NTs
Ru
N
R
R
O
O
H
H
R
1
OH
R
2
*
NTs
Ru
HN
R
R
HO
O
H
NTs
Ru
N
R
R
H
O
R
2
R
1
H
H
C
O
O
H
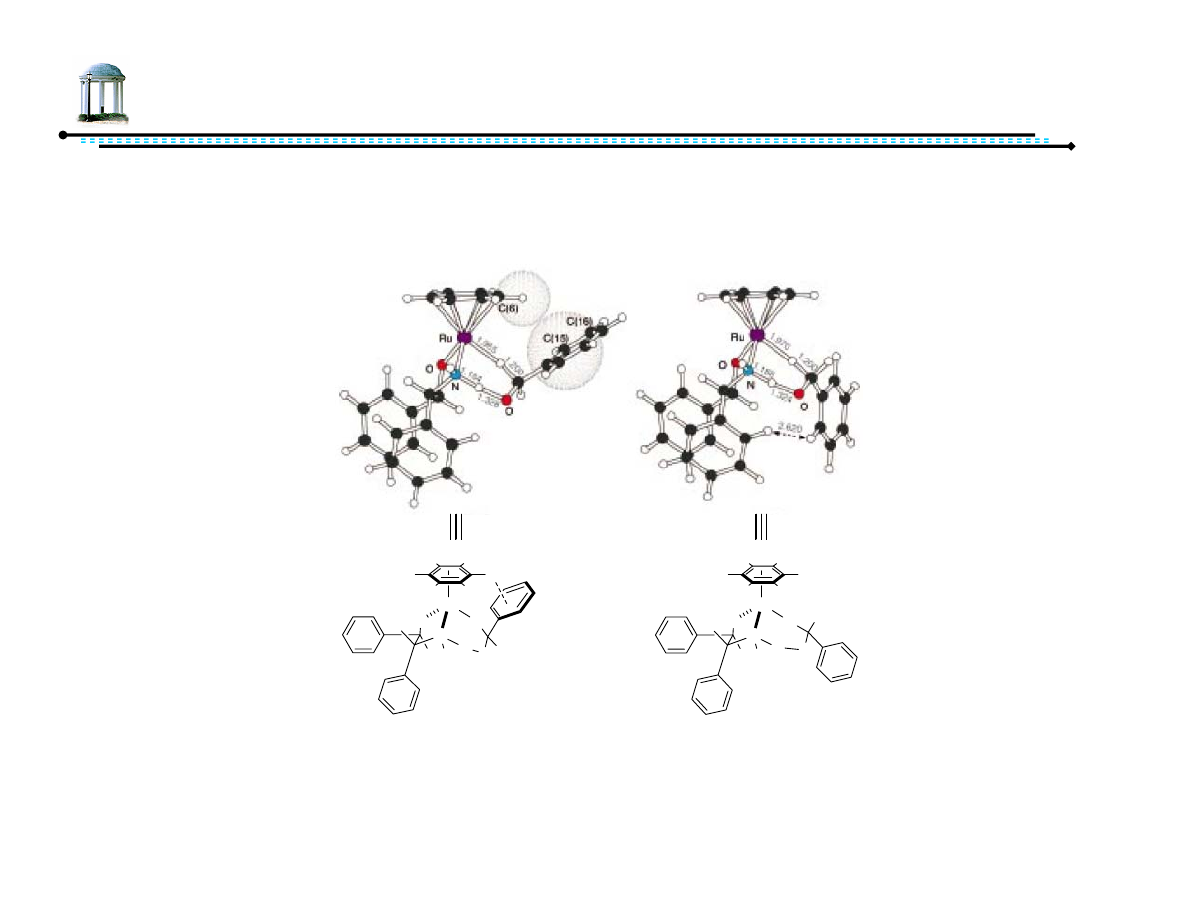
8
Origin of Stereoselectivity
•
Some influence from chiral diamine ligand
•
Significant contribution from arene ligand
– CH-π interaction stabilizes otherwise more-congested transition state
H
Ru
O
N
H
H
R
O
H
H
H
H
H
H
H
H
Ru
O
N
H
H
O
R
H
H
H
H
H
H
H
Addition to Si
face of carbonyl
Addition to Re
face of carbonyl
H
H
Yamakawa, M.; Yamada, I.; Noyori, R. Angew. Chem. Int. Ed., 2001, 40, 2818-2821.
vs.
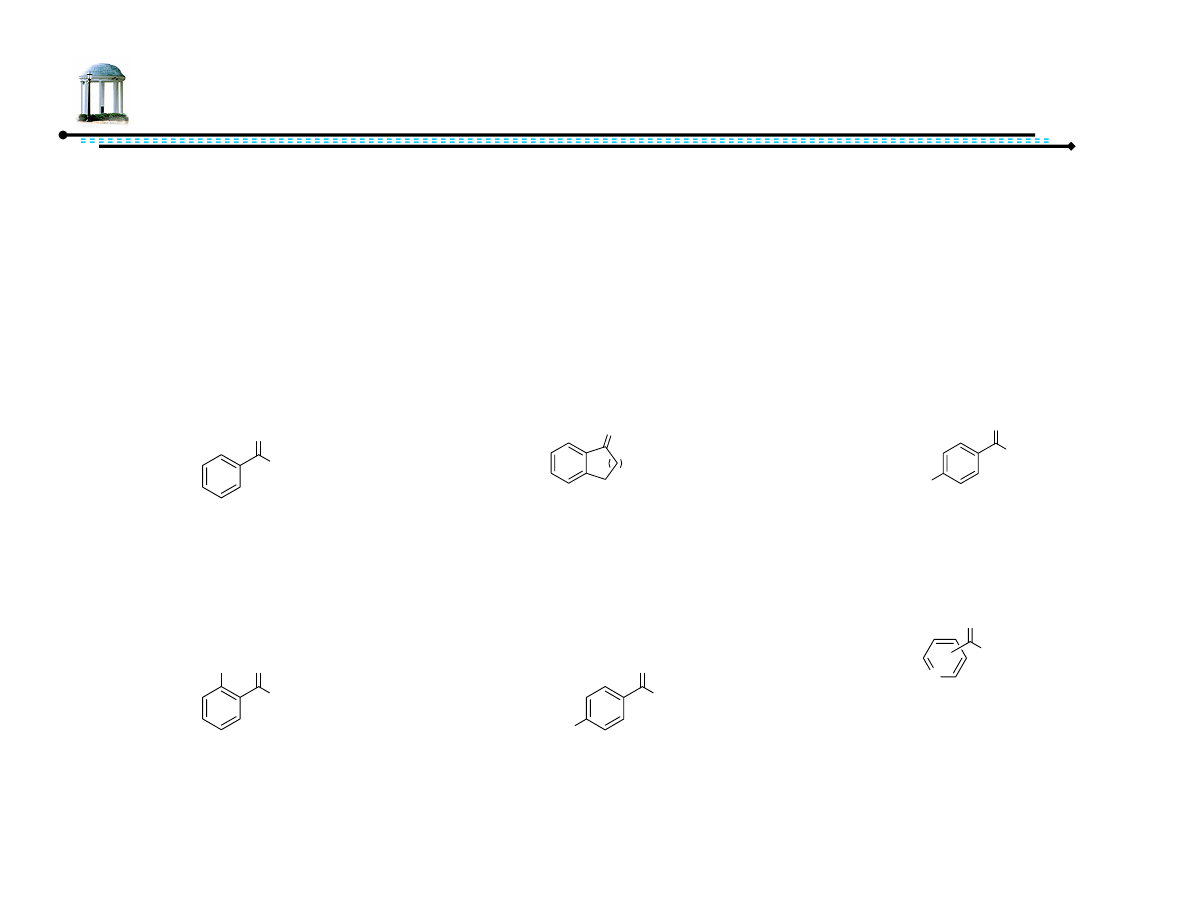
9
Scope of ATH
•
Mainly aryl-alkyl ketones (alkyl-alkynyl ketones)
– Alkyl group
•
Large functional group tolerance (-Cl, -OH, -CN, -N
2
, -NO
2
, -NHBOC)
•
Not sterically bulky
– Aryl group
•
High oxidation potential prefered
•
o-
Subtituted difficult
•
Electron withdrawing groups erode stereoselectivity
•
Heteroaromatic groups tolerated
Noyori, R.; Hashiguchi, S. Acc. Chem. Res., 1997, 30, 97-102.
Okano, K.; Murata, K.; Ikariya, T. Tetrahedron Lett., 2000, 41, 9277-9280.
Me
O
i
PrOH - 53% yield, 72% ee
HCOOH/NEt
3
- >99% yield, 97% ee
MeO
n = 1
i
PrOH - 45% yield, 91% ee
HCOOH/NEt
3
- >99% yield, 99% ee
n = 2
i
PrOH - 65% yield, 97% ee
HCOOH/NEt
3
- >99% yield, 99% ee
O
n
R
O
R = Me
>99% yield, 98% ee
R = Et
96% yield, 97% ee
R = iPr
41% yield, 83% ee
R = tBu
<1% yield
Me
O
100% yield, 86% ee
O
2
N
Me
O
R = Me
53% yield, 91% ee
R = OMe 24% yield, 89% ee
R
N
Me
O
2-acetylpyridine
99% yield, 91% ee
3-acetylpyridine
99% yield, 89% ee
4-acetylpyridine
99% yield, 92% ee
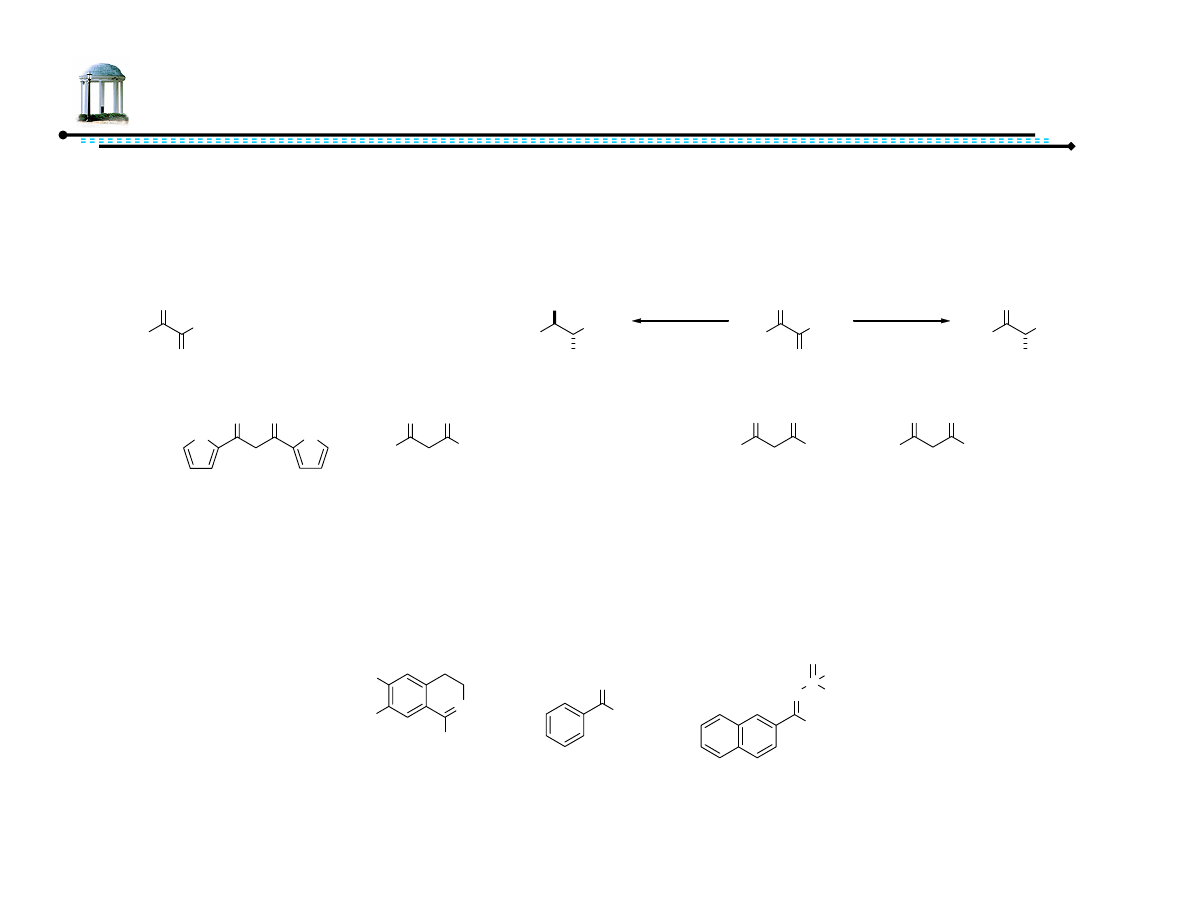
10
Scope of ATH
•
Diketones and β-keto esters
– 1,2-diketone: alkyl ketone preferential at low temp
– 1,3-diketone: symmetrical Æ anti-diol in high ee; unsymmetrical Æ low ee
– β-Keto ester: ketone reduced over ester
•
Imines
– Protic solvents not tolerated
– Cyclic imines more selective than acyclic (except phosphinylimines)
– More reactive than ketones
Ph
O
Ph
O
Ph
O
Me
O
10 °C
87% yield
92% ee
Ph
O
Me
OH
40 °C
78% yield
95% ee
Ph
OH
Me
OH
anti
:sy n - 98.6:1.4
100% yield, >99% ee
MeO
MeO
N
Me
>99% yield
95% ee
NBn
Me
72% yield
77% ee
N
Me
99% ee
P
Ph
O
Ph
Murata, K.; Okano, K.; Miyagi, M.; Iwane, H.; Noyori, R.; Ikaria, T. Org. Lett., 1999, 1, 1119-1121.
Koike, T.; Murata, K.; Ikariya, T. Org. Lett., 2000, 2, 3833-3836.
Cossy, J.; Eustache, F.; Dalko, P. I. Tetrahedron Lett., 2001, 42, 5005-5007.
Everaere, K.; Morteux, A.; Carpentier, J.-F. Adv. Synth. Catal., 2003, 345, 67-77.
Noyori, R.; Hashiguchi, S. Acc. Chem. Res., 1997, 30, 97-102.
Gladiali, S.; Alberico, E. Chem. Soc. Rev., 35, 226-236.
O
O
O
O
anti
:sy n = 95:5
85% yield
Ph
O
O
Me
ant i
:sy n = 56:42
79% yield
Ph
O
O
OEt
Me
O
O
OtBu
99% yield
94% ee
99% yield
68% ee

11
Outline
• Mechanism and scope of asymmetric transfer hydrogenation
• Pro-atropisomeric phosphine ligands
• Amino acid-based ligands
• Dendrimer-bound diamine ligands
• Asymmetric transfer hydrogenation in water
• Asymmetric transfer hydrogenation in ionic liquids
12
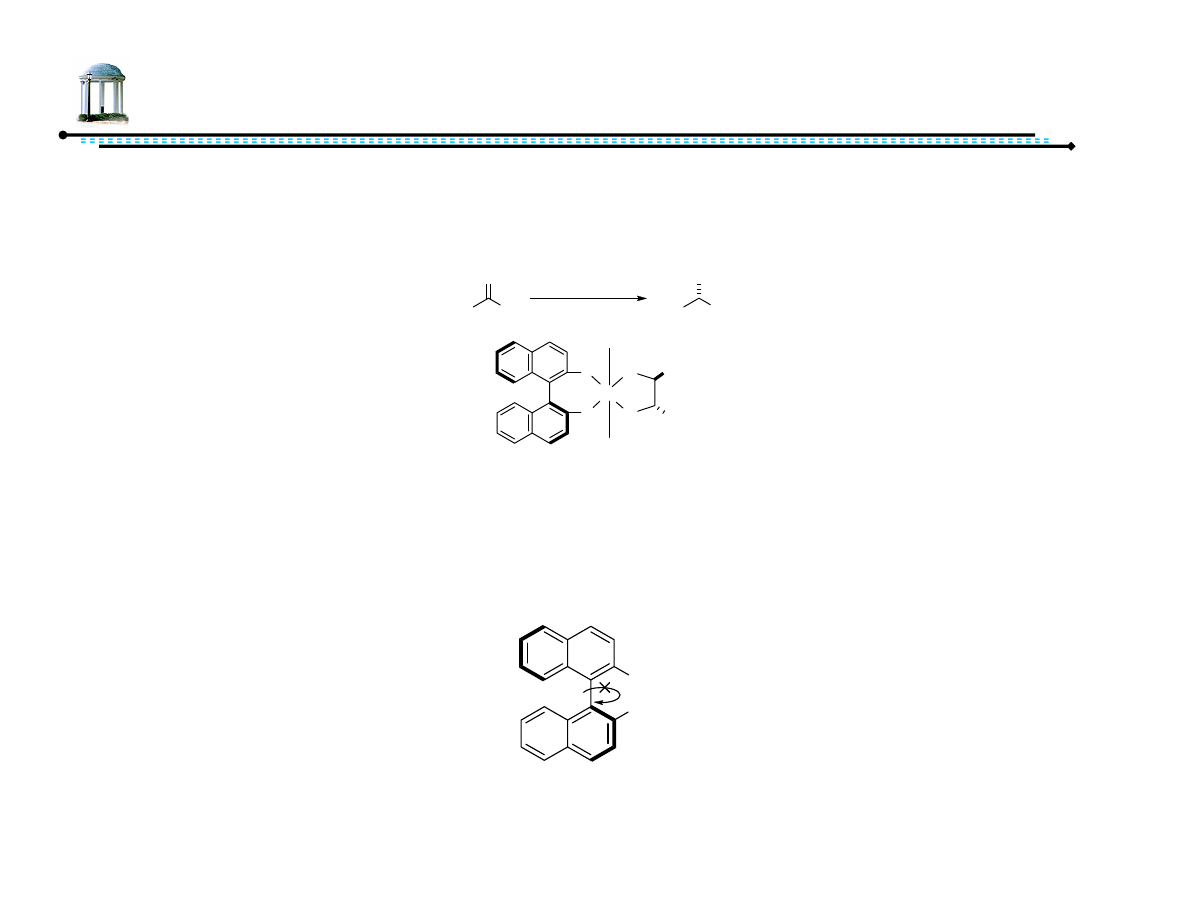
Pro-atropisomeric Phosphine Ligand
•
Noyori received 2001 Nobel Prize for H
2
hydrogenation
•
Utilizes optically pure BINAP ligands
•
BINAP can be resolved into pure (+) and (-) enantiomers due to high barrier
of rotation about the aryl-aryl bond (atropisomeric)
Ph
2
P
P
Ph
2
Ru
N
H
2
H
2
N
Ph
Ph
Cl
Cl
(S)-BINAP/(S,S)-DPEN-Ru(II) Catalyst
Ar
R
O
H
2
Ru catalyst
base
Ar
Me
OH
(R)
PAr
2
PAr
2
(S)-BINAP
Noyori, R.; Asymmetric Catalysis: Science and Opportunities. Nobel Lecture, 8 December 2001.
13
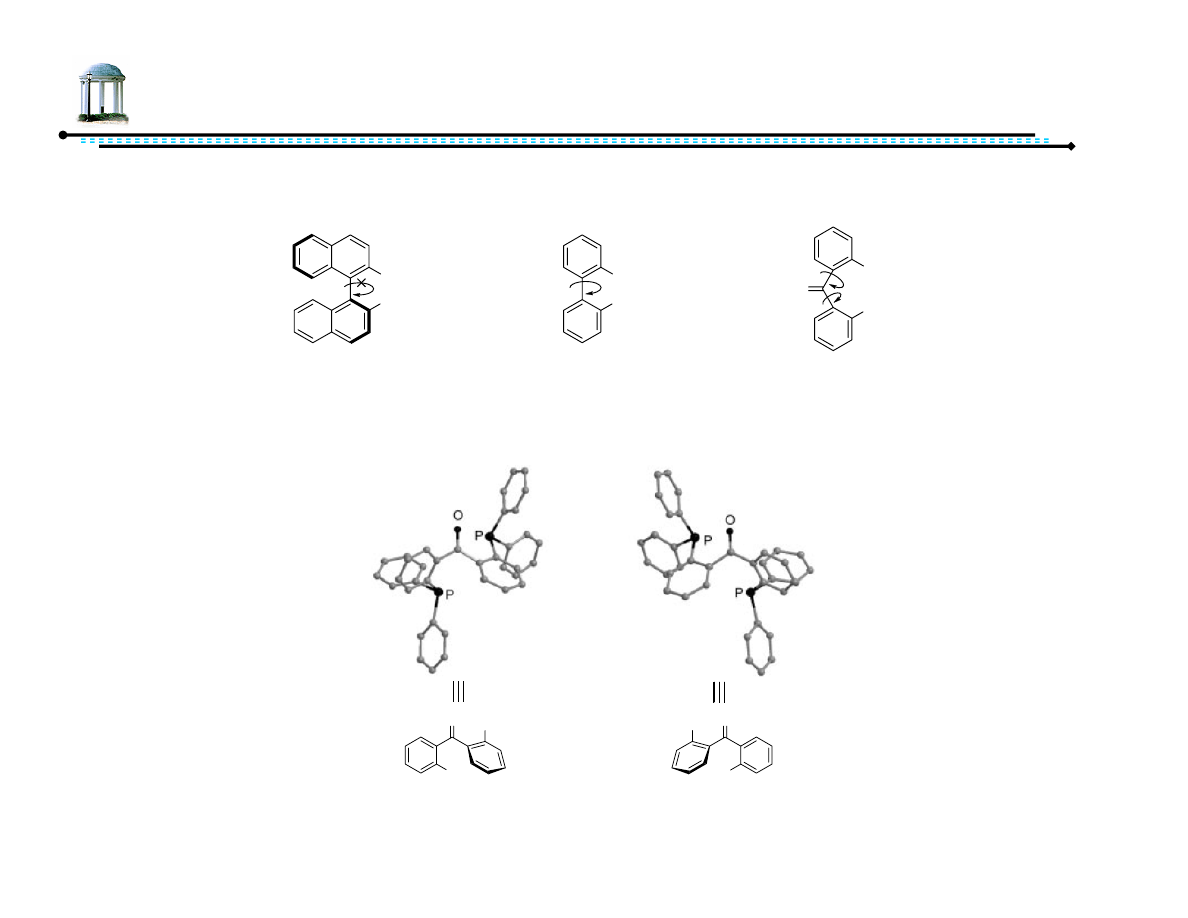
Pro-atropisomeric Phosphine Ligand
•
BIPHEP and DPBP have significantly lower barriers of rotation (tropisomeric
or pro-atropisomeric)
– Optically pure isomers cannot be isolated in solution
– Benzophenone (and derivatives) forms enantiomers in solid state
PAr
2
PAr
2
(S)-BINAP
PAr
2
PAr
2
BIPHEP
O
PAr
2
PAr
2
DPBP
PPh
2
O
PPh
2
Ph
2
P
O
PPh
2
(P)-Conformation
(M)-Conformation
Jing, Q.; Sandoval, C.; Wang, Z.; Ding, K. Eur. J. Org. Chem., 2006, 3606-3616.
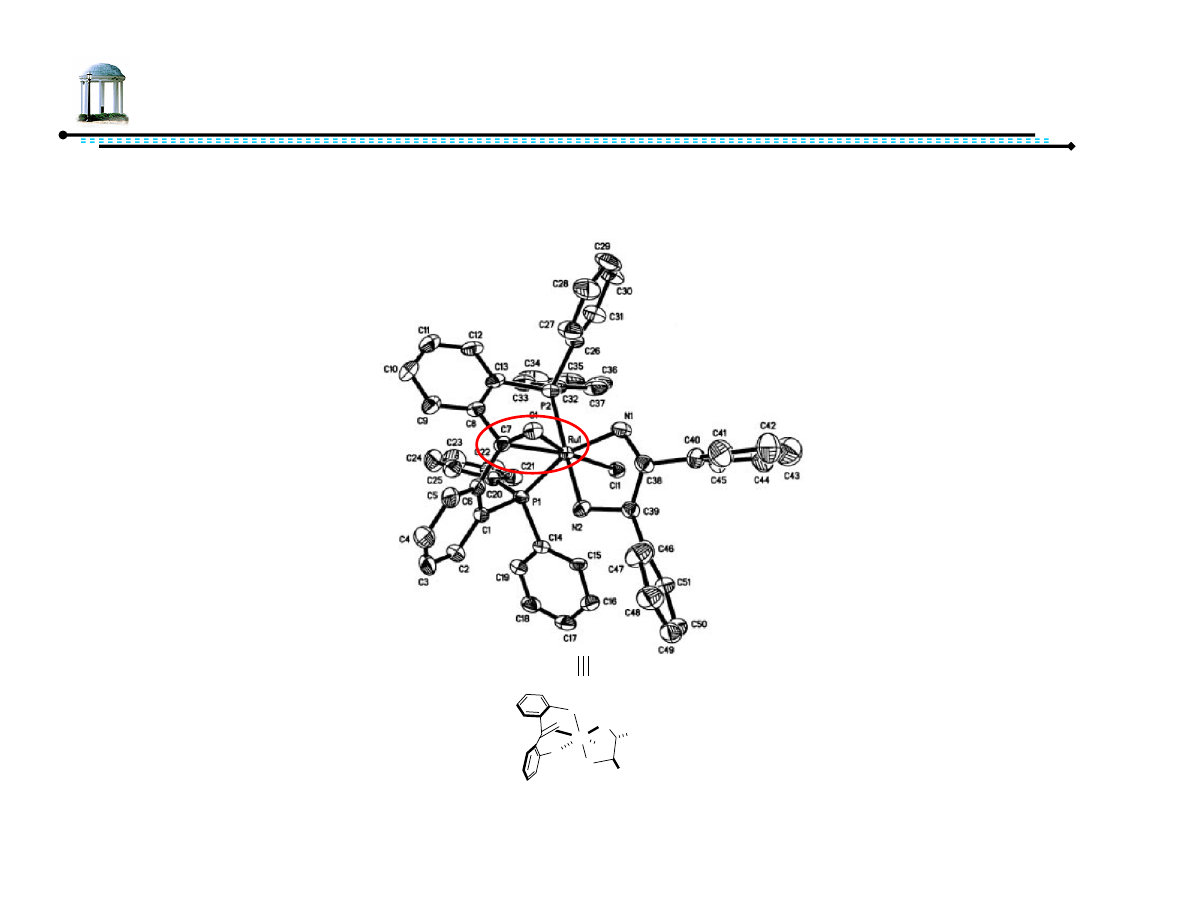
14
(From M enantiomer)
Ru
Ph
2
P
N
H
2
Cl
H
N
P
Ph
2
Ph
Ph
O
Pro-atropisomeric Phosphine Ligand
•
Complexing DPBP to metal with diamine ligand forces single diastereomer
of metal complex
Jing, Q.; Sandoval, C.; Wang, Z.; Ding, K. Eur. J. Org. Chem., 2006, 3606-3616.
15
Pro-atropisomeric Phosphine Ligand
•
BINAP not conducive to ATH
•
Pro-atropisomeric DPBP successful for ATH – First pro-atropisomeric
phosphine used in ATH
•
Catalyst for alkyl-alkyl reductions?
Mikami, K.; Wakabayashi, K.; Yusa, Y.; Aikawa, K. Chem. Commun., 2006, 2365-2367.
Noyori, R.; Hashiguchi, S. Acc. Chem. Res., 1997, 30, 97-102.
O
O
O
O
Ligand
= (R)-BINAP
98 % conv. 72 % ee
Ligand
= DPBP
>99 % conv. 99 % ee
Ligand
= (R)-BINAP
61 % conv. 57 % ee
Ligand
= DPBP
99 % conv. 99 % ee
Ligand
= (R)-BINAP
97 % conv. 68 % ee
Ligand
= DPBP
95 % conv. 91 % ee
Ligand
= (R)-BINAP
96 % conv. 71 % ee
Ligand
= DPBP
97 % conv. 89 % ee
R
Me
O
R
Me
OH
Rh-catalyst, ligand, (S,S)-DPEN
KOtBu, iPrOH, rt, 24 h
Rh
Rh-Catalyst
PAr
2
PAr
2
(R)-BINAP
O
PAr
2
PAr
2
DPBP
(S)
(S)
H
2
N
Ph
Ph
H
2
N
+
SbF
6
-
(S,S)-DPEN
Noyori's Catalyst
95% conv. 97% ee
Noyori's Catalyst
53% conv. 91% ee
Noyori's Catalyst
92% conv., 93% ee
NTs
Ru
H
2
N
Ph
Ph
Cl
(R) (R)
Noyori's Catalyst
(R)

16
Outline
• Mechanism and scope of asymmetric transfer hydrogenation
• Pro-atropisomeric phosphine ligands
• Amino acid-based ligands
• Dendrimer-bound diamine ligands
• Asymmetric transfer hydrogenation in water
• Asymmetric transfer hydrogenation in ionic liquids
17
Amino Acid-Based Ligands
•
Initially, amido-oxazoline ligands were targeted
•
Poor yield, stereoselectivity in ATH
•
Noticed synthetic precursor provided better selectivity than target
N
O
O
N
HN
R
1
O
Me
Me
O
HN
R
2
R
1
R
2
N
O
O
N
HN
R
1
O
Me
Me
O
HN
R
2
R
1
R
2
R
1
NHBoc
N
H
O
R
2
OH
Pastor, I. M.; Västilä, P.; Adolfsson, H. Chem. Commun., 2002, 2046-2047.
18
Amino Acid-Based Ligands
•
Boc group & free hydroxyl group crucial
•
Amino acid stereocenter more important than amino alcohol stereocenter
•
1-amino-2-alcohols catalyze ATH with similar yield and ee
Pastor, I. M.; Västilä, P.; Adolfsson, H. Chem. Eur. J., 2003, 9, 4031-4045.
Bøgevig, A.; Pastor, I. M.; Adolfsson, H. Chem. Eur. J., 2004, 10, 294-302.
(S)
Me
OH
(S)
Me
BocHN
N
H
O
(S)
Ph
Me
O
Ru-catalyst, ligand
NaOH, iPrOH
Ru
Cl
Cl
Cl
Cl
Ru-Catalyst
Ru
91% Conversion, 94% ee
OH
(S)
Me
BocHN
N
H
O
(R)
Ph
OH
95% Conversion, 93% ee
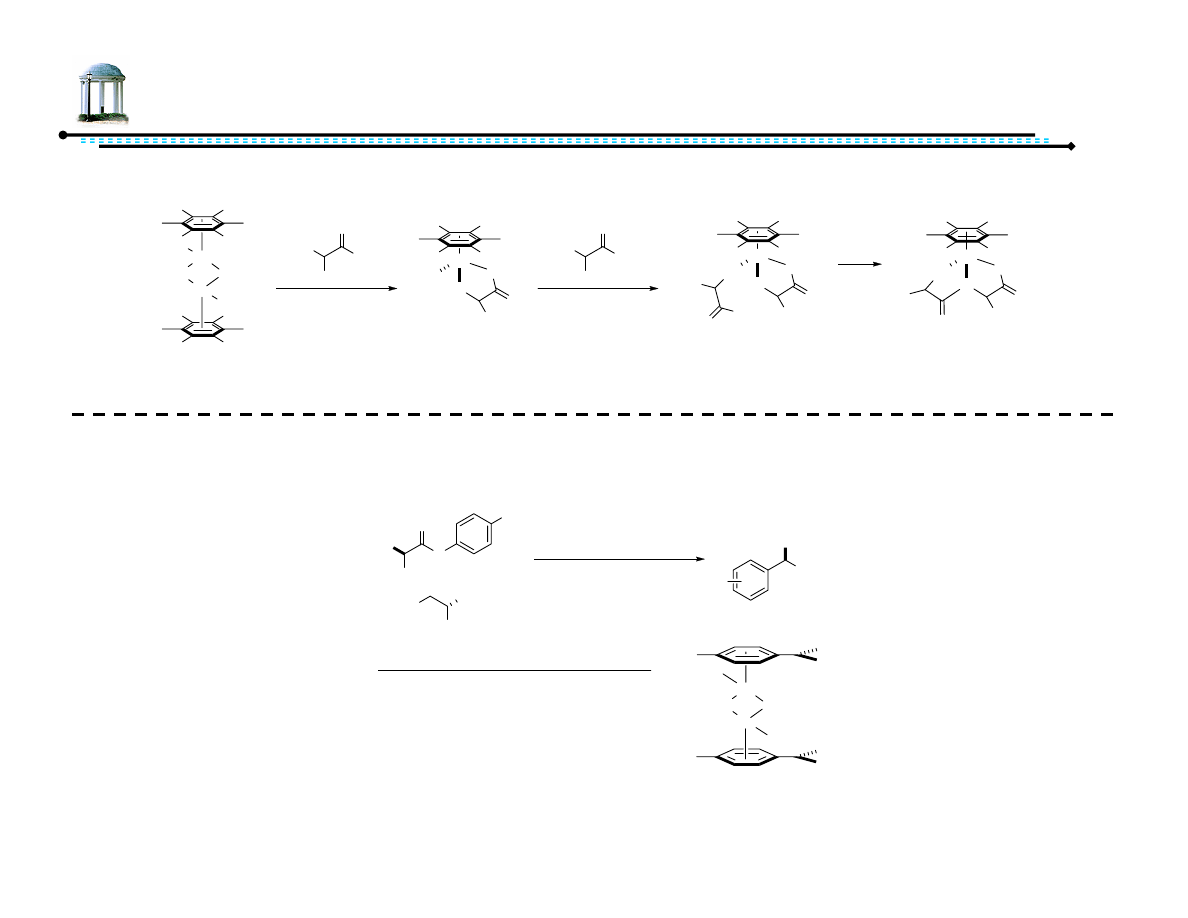
19
Amino Acid-Based Ligands
•
Ru(II)(η
6
-arene) complexes known to facilitate peptide formation
•
Provides template to form ligand in situ, form catalyst in situ, and conduct
ATH in one pot
Ru
H
2
N
Cl
O
O
H
2
N
OMe
O
R
2
-HCl
Ru
HN
H
2
N
O
O
R
2
O
OMe
-MeOH
Ru
N
H
2
N
O
O
R
2
O
R
1
R
1
R
1
Ru
Cl
Cl
Cl
Cl
Ru
H
2
N
OH
O
R
1
-HCl
(S)
NHBoc
Me
O
O
NO
2
H
2
N
(S)
Me
OH
1) iPrOH, Δ, 1h
2) iPrONa, Ru-catalyst,
ketone
i
PrOH, rt, 1h
(S)
Me
OH
Ketone (R=)
Conversion (%)
ee
(%)
R
H
85
97
m
-Me
83
97
o
-F
90
92
m
-OMe
86
97
3,5-OMe
82
97
3,4,5-OMe
45
99
Ru
Cl
Cl
Cl
Cl
Ru
Ru-Catalyst
Haas, K.; Beck, W. Eur. J. Inorg. Chem., 2001, 2485-2488.
Västilä, P.; Wettergren, J.; Adolfsson, H. Chem. Commun., 2005, 4039-4041.
20
Amino Acid-Based Ligands
•
Mechanism elusive for several years
•
Key clues:
– Necessity of NH
Boc
, N
H
C(O)R, O
H
groups
– 3 equivalents of base necessary
– Additives
• Strong Lewis acid additives (Sc(OTf)
3
, Ti(OiPr)
4
) have negative effect on reactivity
• NaCl or KCl additive - similar to nonadditive reactions
• LiCl - higher stereoselectivity (also LiBr, LiI, LiClO
4
, LiOAc)
– Replacing NaOiPr with LiOiPr as base (no additive) increased stereoselectivity to
the same extent as LiCl additive
– No additive effect of LiCl with traditional transfer hydrogenation systems
Västilä, P.; Zaitsev, A. B.; Wettergren, J.; Privalov, T.; Adolfsson, H. Chem. Eur. J., 2006, 12, 3218-3225.
R
1
NHBoc
N
H
O
R
2
OH
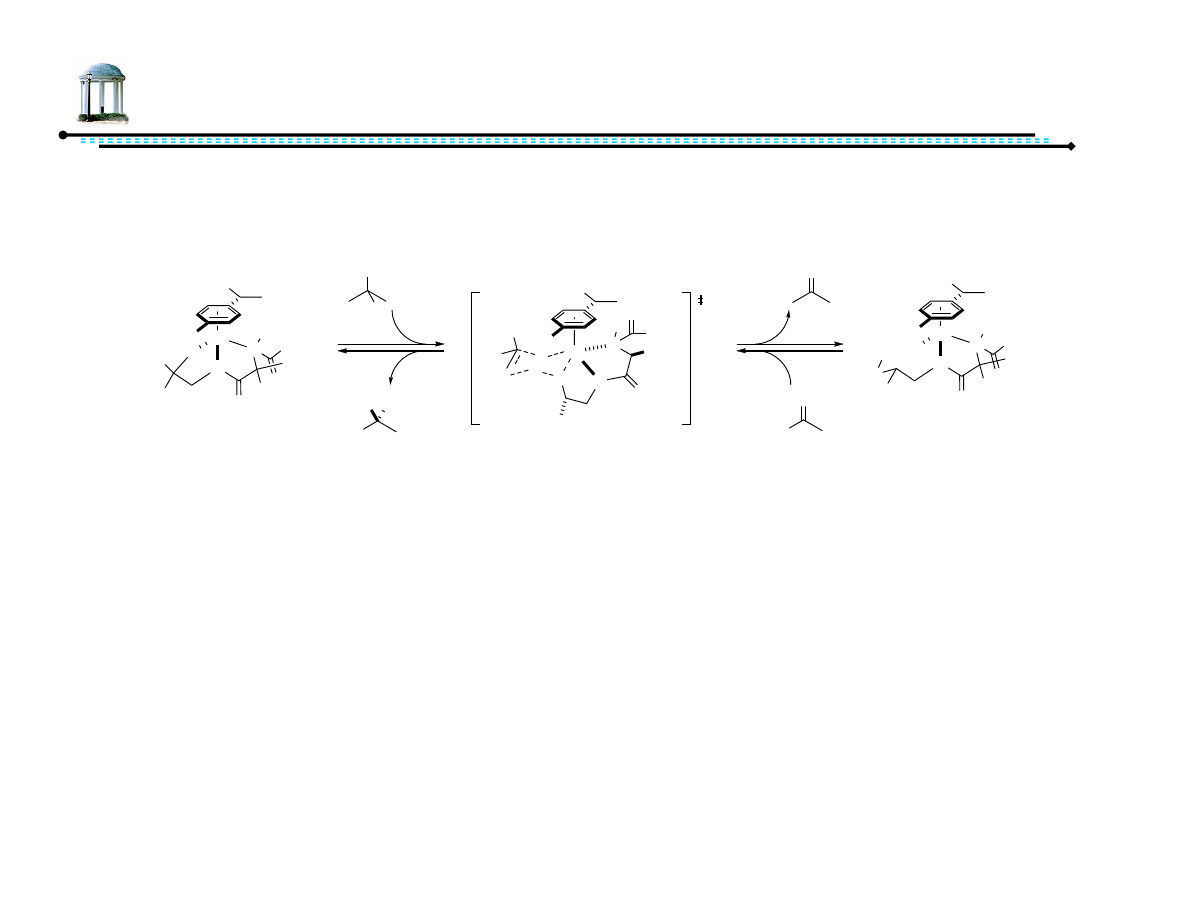
21
Amino Acid-Based Ligands
•
Proposed mechanism:
•
Lithium ion activates and directs incoming ketone
•
Smaller lithium ion forms tighter transition state
•
Crown ethers erode stereoselectivity
•
CH-π interactions not as important
Ru
N
O
N
H
Me
O
H
O
OtBu
Me
OLi
H
O
Ph
Ph
O
H
LiO
Ru
N
H
N
O
Me
O
H
O
OtBu
Me
Li
H
H
O
Li O
Ru
H
N
N
Me
R
H
O
OtBu
Me
O
Me
Västilä, P.; Zaitsev, A. B.; Wettergren, J.; Privalov, T.; Adolfsson, H. Chem. Eur. J., 2006, 12, 3218-3225.
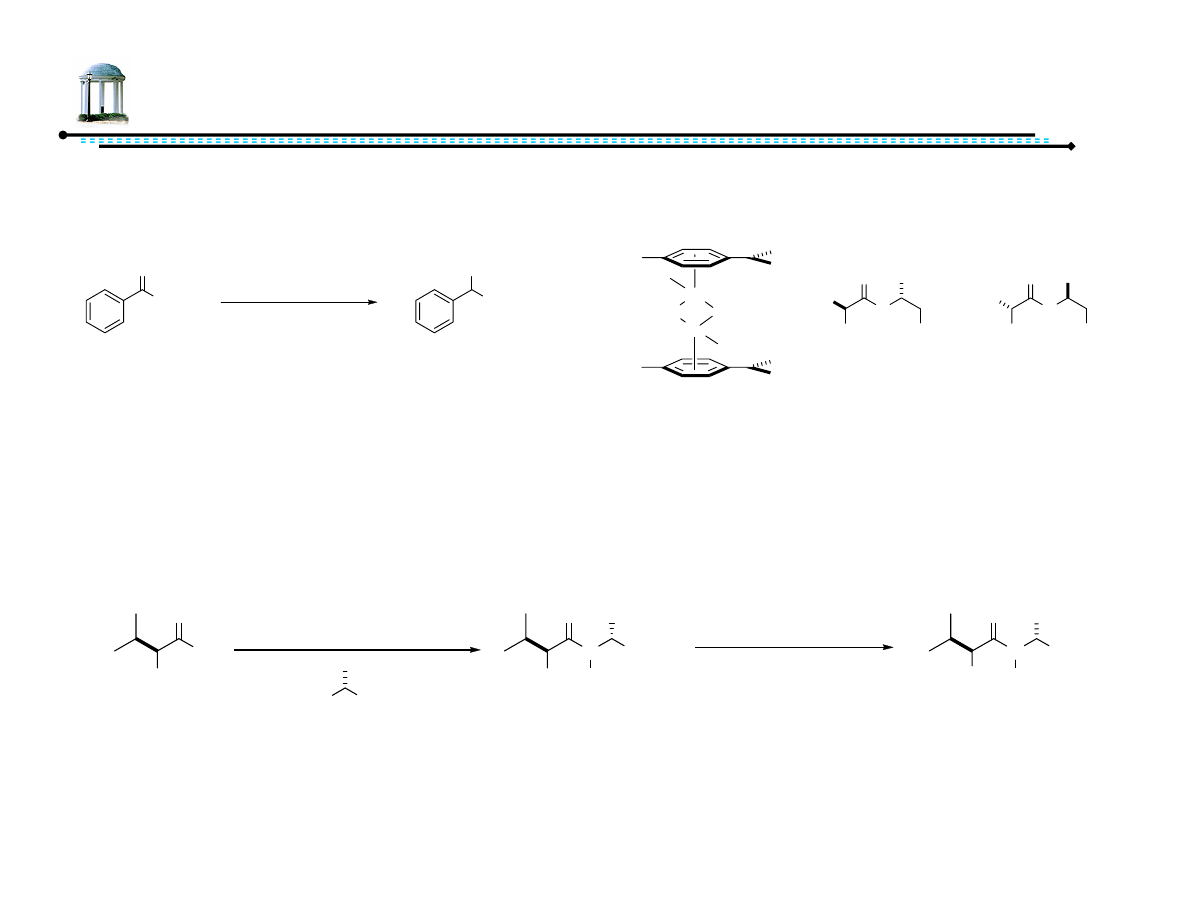
22
Amino Acid-Based Ligands
•
Previously, other product stereoisomer obtained through un-natural amino acid ligand
•
Modification from amide to thioamide, removal of alcohol reverses stereoselectivity
NHBoc
(S)
OH
O
1) N-methylmorpholine
i
-BuOC(O)Cl, -15 °C, 1h
2)
rt, 3 h, THF
BocHN
(S)
N
O
( S)
H
Lawesson's Reagent
THF, 60 °C, 8h
BocHN
(S)
N
S
( S)
H
97%
77%
H
2
N
(S)
Me
Ph
Me
Ph
Me
Ph
Pastor, I. M.; Västilä, P.; Adolfsson, H. Chem. Eur. J., 2003, 9, 4031-4045.
Zaitsev, A. B.; Adolfsson, H. Org. Lett., 2006, 8, 5129-5132.
Me
O
Ru-catalyst, ligand
NaOH, iPrOH
Me
OH
*
Ru
Cl
Cl
Cl
Cl
Ru
Ru-Catalyst
NH
2
(S)
Me
O
N
H
(R)
Ph
OH
NH
2
(R)
Me
O
N
H
(S)
Ph
OH
Ligand A
Ligand B
Ligand A - 95% yield, 93% ee (S)
Ligand B - 91% yield, 93% ee (R)
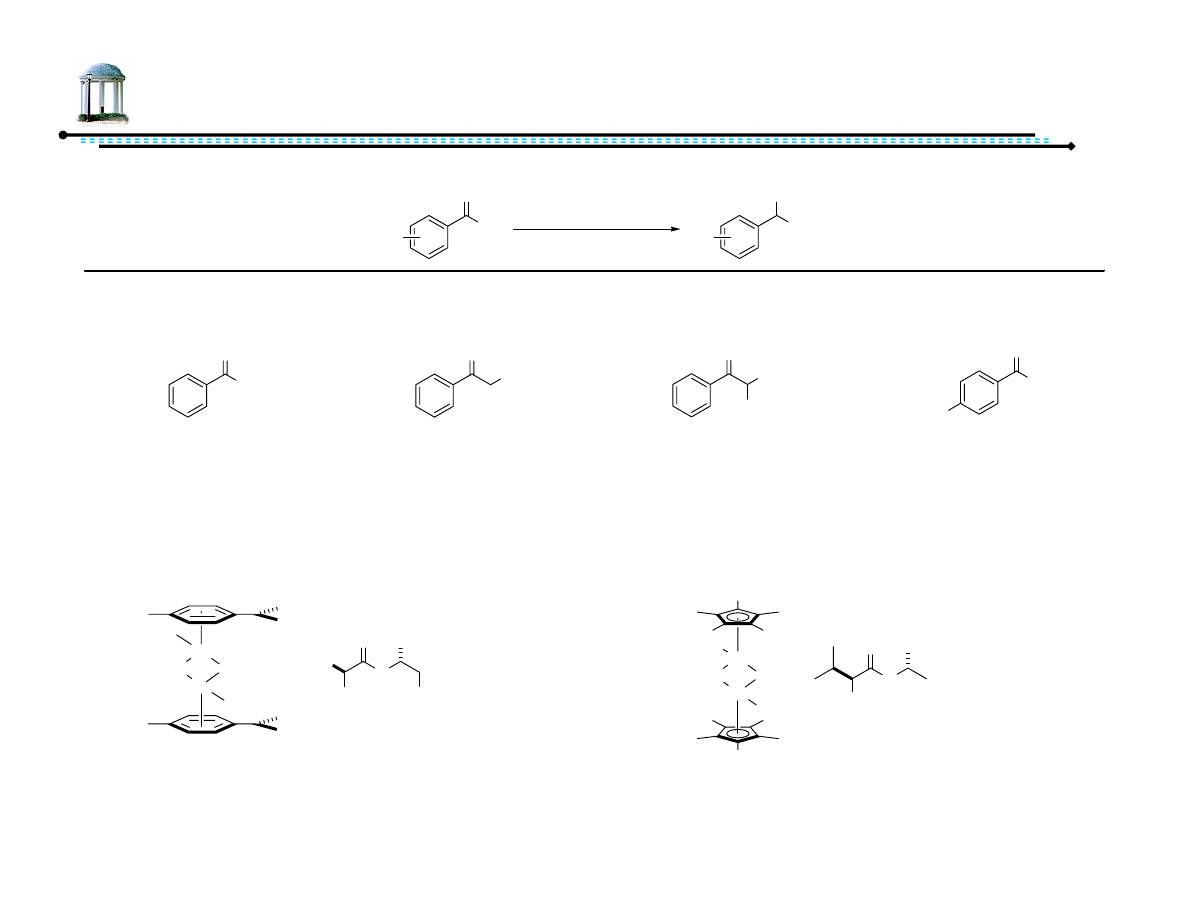
23
Amino Acid-Based Ligands
R
2
O
R
1
Rh-catalyst, ligand
LiCl, iPrONa, iPrOH
R
2
OH
R
1
*
Rh
Cl
Cl
Cl
Cl
Rh
NHBoc
(S)
N
H
S
(S)
Ph
NHBoc
(S)
Me
N
H
O
( R)
OH
Ru
Cl
Cl
Cl
Cl
Ru
Ph
Me
O
O
O
Me
O
Me
Me
Me
MeO
Catalyst A: 95% yield, 93% ee, (S)
Catalyst B: 88% yield, 95% ee, (R)
Catalyst A: 91% yield, 95% ee, (S)
Catalyst B: 88% yield, 96% ee, (R)
Catalyst A: 53% yield, 86% ee, (S)
Catalyst B: 61% yield, 97% ee, (R)
Catalyst A: 63% yield, 95% ee, (S)
Catalyst B: 56% yield, 91% ee, (R)
Catalyst A
Catalyst B
Pastor, I. M.; Västilä, P.; Adolfsson, H. Chem. Eur. J., 2003, 9, 4031-4045.
Zaitsev, A. B.; Adolfsson, H. Org. Lett., 2006, 8, 5129-5132.

24
Outline
• Mechanism and scope of asymmetric transfer hydrogenation
• Pro-atropisomeric phosphine ligands
• Amino acid-based ligands
• Dendrimer-bound diamine ligands
• Asymmetric transfer hydrogenation in water
• Asymmetric transfer hydrogenation in ionic liquids
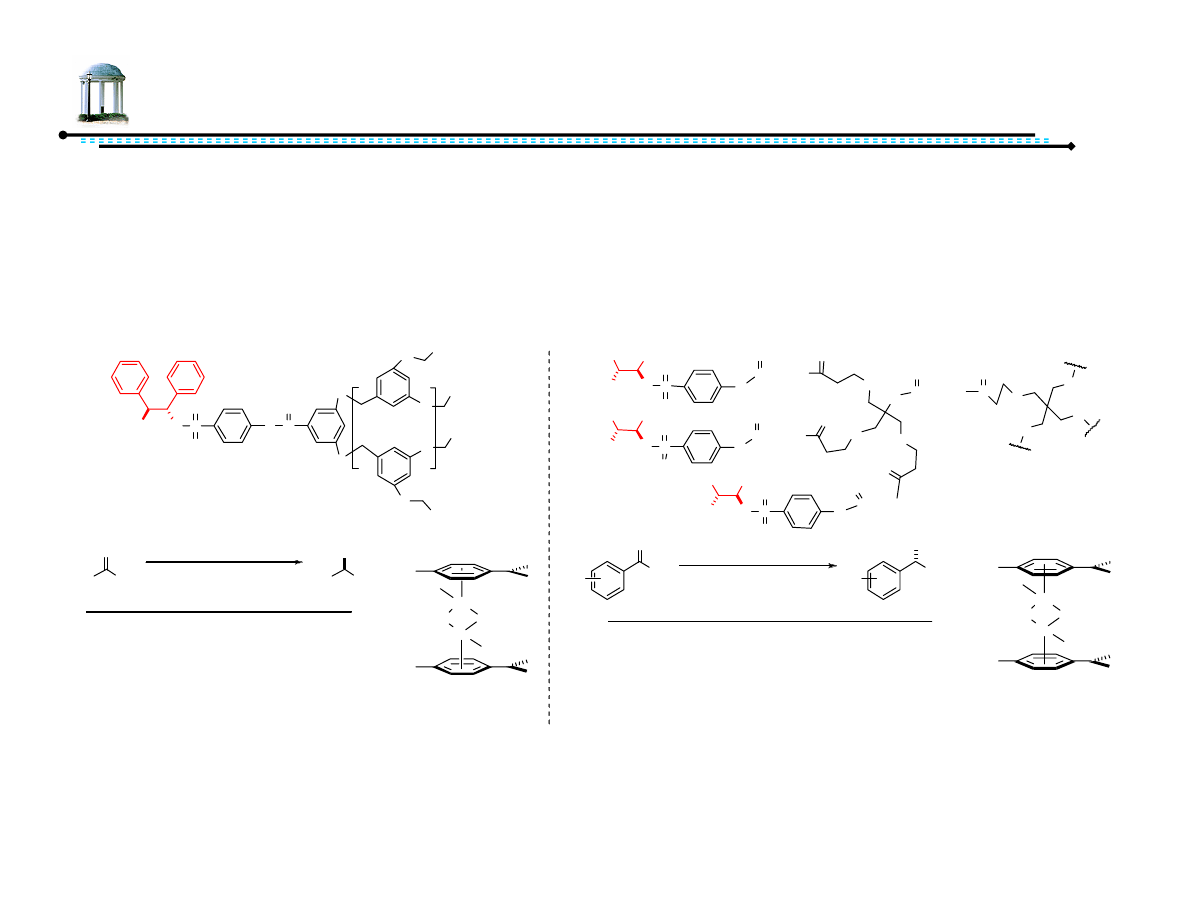
25
Recoverable Diamine Ligands
•
Recoverable systems important with expensive or toxic heavy metal
complexes
•
Immobilization on supporting apparatus can allow for recovery
•
For ATH, two classes of dendrimers initially tested
Chen, Y.-C.; Wu, T.-F.; Deng, J.; Liu, H.; Jiang, Y.-Z.-, Choi, M. C. K.; Chan, A. S. C. Chem. Commun, 2001, 1488-1489.
Chen, Y.-C.; Wu, T.-F.; Deng, J.; Liu, H.; Xin, C.; Zhu, J.; Jiang, Y.-Z.; Choi, M. C. K.; Chan, A. S. C. J. Org. Chem., 2002, 67, 5301-5306
(S)(S)
H
2
N
HN
S
H
N C
O
O
O
O
O
O
O
O
O
n = 3
Ph
O
Me
Ru-catalyst, ligand
HCOOH/NEt
3
, CH
2
Cl
2
Ph
(S)
OH
Me
Run #
t (h) Conversion (%) ee (%)
1
20
98
96.5
2
20
92
96.6
3
25
87
96.8
4
30
85
96.7
5
40
73
96.3
6
40
52
87
Ru
Cl
Cl
Cl
Cl
Ru
Ru-Catalyst
O
R
2
R
1
Ru-catalyst, ligand
HCOOH/NEt
3
, CH
2
Cl
2
(R)
OH
R
2
R
1
R
1
t (h) Conversion (%) ee (%)
o
-Cl
24
> 99
95.5
p
-tBu
55
98
96.3
R
2
H
H
H
CH
2
C(O)Ph
72
70
>99
H
(CH
2
)
4
C(O)Ph
72
67
>99
Ru
Cl
Cl
Cl
Cl
Ru
Ru-Catalyst
O
O
O
O
C
CCH
2
NH
O
HN
O
O
O
O
CCH
2
NH
O
CCH
2
NH
CCH
2
NH
O
N
H
N
H
N
H
S
S
S
HN
O
O
HN
O
O
HN
O
O
(R)
Ph
( R)
Ph
H
2
N
(R)
Ph
(R)
Ph
H
2
N
(R)
Ph
(R)
Ph
H
2
N
O
O
O
O
Ph
Ph
Ph
Ph
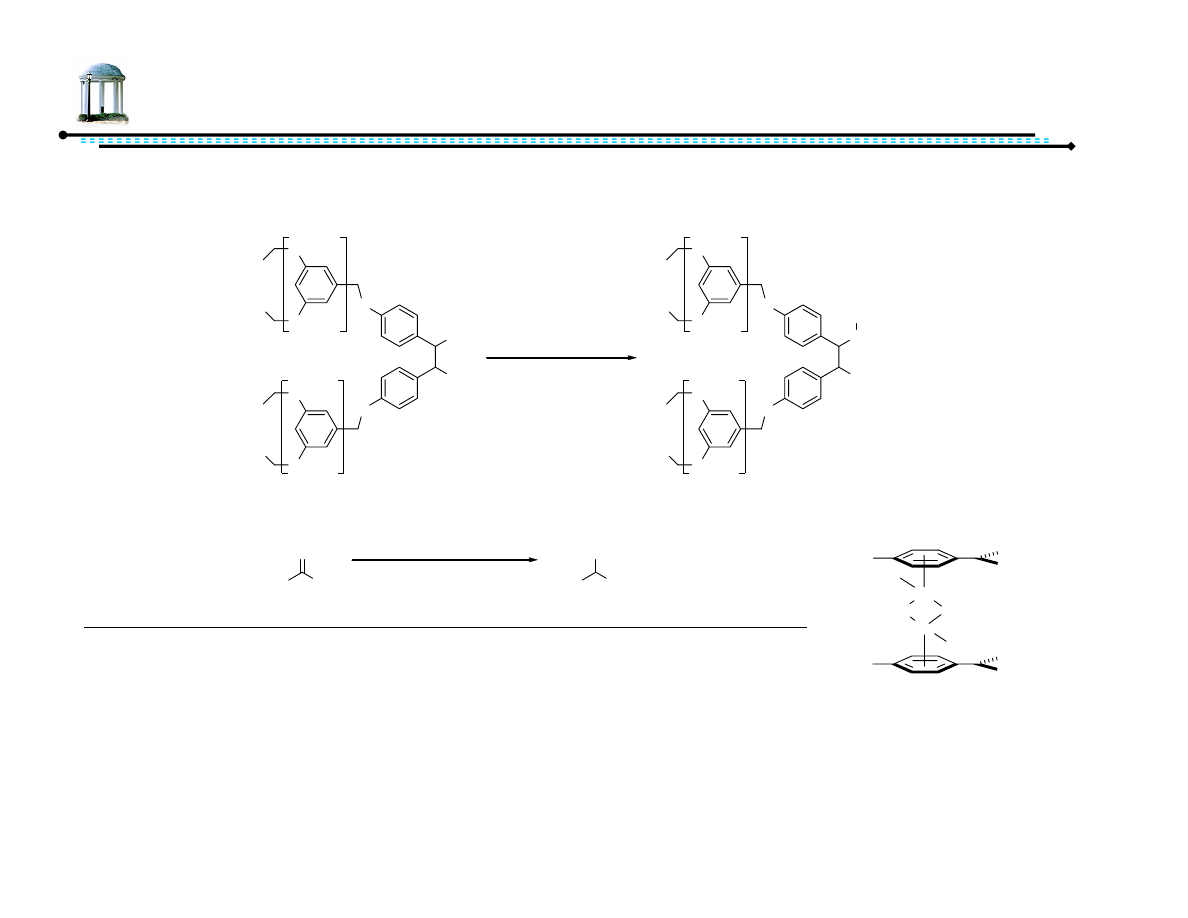
26
Recoverable Diamine Ligands
•
Late-stage tunability of ligand/dendrimer compatible with varying nature of
ketone substrates
•
Recovery procedure:
–
Remove CH
2
Cl
2
in vacuo
–
Precipitate dendrimer with MeOH
–
Filter
Liu, W.; Cui, X.; Cun, L.; Zhu, J.; Deng, J. Tetrahedron: Asymmetry, 2005, 16, 2525-2530.
O
O
O
Ph
Ph
NH
2
NH
2
O
O
O
Ph
Ph
n
n
*
*
Ar
SO
2
Cl, (iPr)
2
NEt
CH
2
Cl
2
, 0 °C - rt
O
O
O
Ph
Ph
NH
NH
2
O
O
O
Ph
Ph
n
n
*
*
SO
2
Ar
Ph
O
Me
Ru-catalyst, ligand
HCOOH/NEt
3
, CH
2
Cl
2
, rt
Ph
OH
Me
*
n
Configuration
Ar
time (h)
Conversion (%)
ee
(%)
Configuration
0
4-CH
3
C
6
H
4
(R,R)
20
95
96.8
R
1
4-CH
3
C
6
H
4
(R,R)
20
>99
96.6
R
2
4-CH
3
C
6
H
4
(R,R)
20
97.1
96.1
R
(95.4, 90.2, 83.7, 71.2) (97.5, 97.2, 97.5, 97.0)
3
4-CH
3
C
6
H
4
(R,R)
20
75
94.6
R
2
2,4,6-Et
3
-C
6
H
2
(S,S)
20
93.0
91.7
S
2
2,4,6-iPr
3
-C
6
H
2
(S,S)
20
91.7
92.8
S
2
1-naphthyl
(S,S)
20
>99
96.3
S
Ru
Cl
Cl
Cl
Cl
Ru
Ru-Catalyst
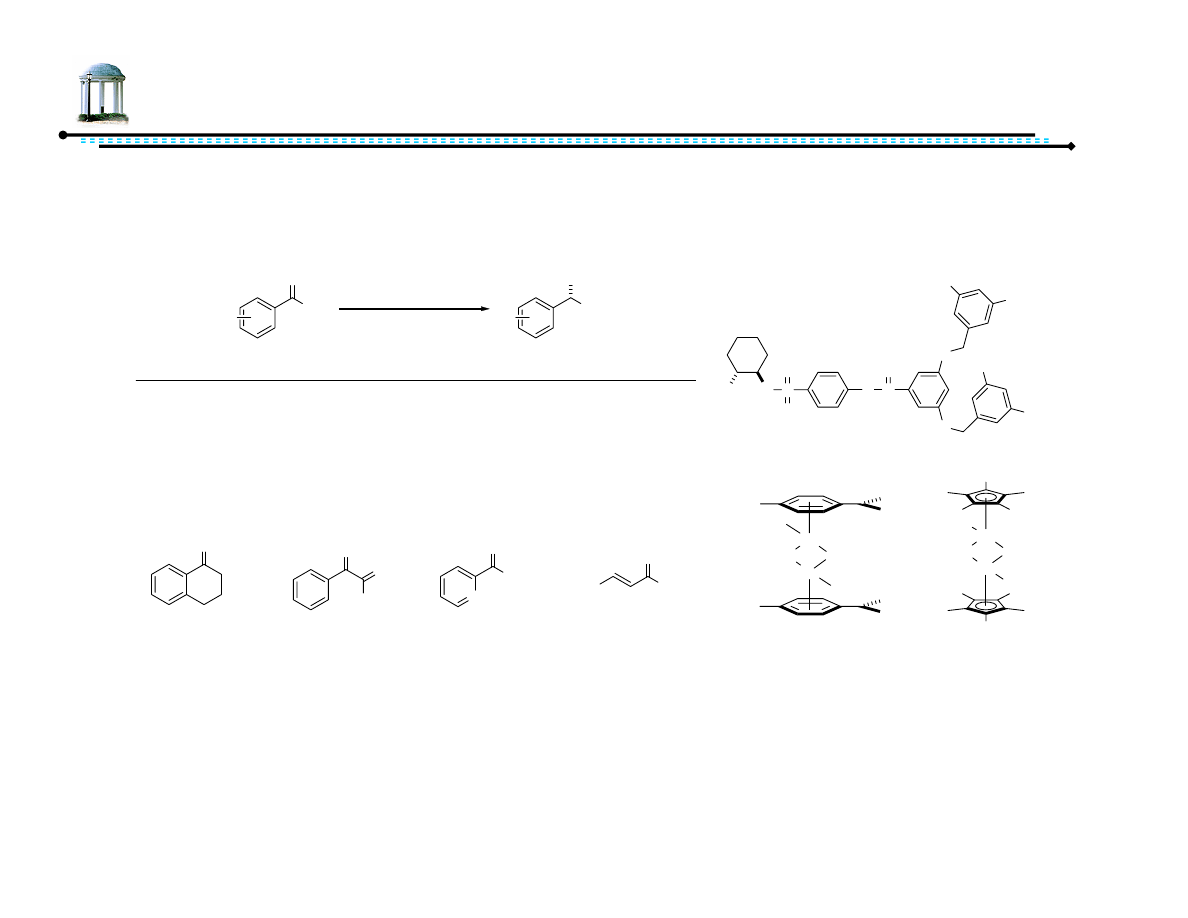
27
Recoverable Diamine Ligands
•
Minimize organic solvent – run ATH in water
– Switch to 1,2-diaminocyclohexane-based ligands and Cp* rhodium catalyst
system
•
Recovery procedure:
– Add hexanes
– Remove organic layer
– Add HCOOH to pH ~7
(R)(R)
H
2
N
S
H
N C
O
O
O
O
O
HN
O
Me
R
Catalyst, ligand
"H
2
"-source Solvent
Catalyst
"H
2
"-source
Solvent
R
Conversion (%)
ee
(%)
(R)
OH
Me
R
Ru
HCOOH/NEt
3
CH
2
Cl
2
H
>99
94
Ru
HCOONa
H
2
O
H
>99
88
Rh
HCOONa
H
2
O
H
>99
96
Rh
HCOONa
H
2
O
p
-OMe
95
94
Rh
HCOONa
H
2
O
o
-OMe
>99
81
(>99, 98, 99, 85, 97) (96, 95, 94, 95, 95, 95)
O
99% Conversion
97% ee
N
Me
O
70% Yield
91% ee
97% Yield
72% ee
94% Yield
52% ee
OEt
O
O
Ph
Me
O
Ru
Cl
Cl
Cl
Cl
Ru
Ru-Catalyst
Rh
Cl
Cl
Cl
Cl
Rh
Rh-Catalyst
BnO
OBn
OBn
BnO
Ligand
Jiang, L.; Wu, T.-F.; Chen, Y.-C.; Zhu, J.; Deng, J. Org. Biomol. Chem., 2006, 4, 3319-3324.

28
Outline
• Mechanism and scope of asymmetric transfer hydrogenation
• Pro-atropisomeric phosphine ligands
• Amino acid-based ligands
• Dendrimer-bound diamine ligands
• Asymmetric transfer hydrogenation in water
• Asymmetric transfer hydrogenation in ionic liquids

29
ATH in Water
• Increases atom economy and environmental friendliness
– Often no organic solvents during reaction
• Allows for ease of product separation, possibility of
catalyst recyclability
– Distillation or extraction
• Vigorously dried solvents and substrates not necessary
30
ATH in Water – Early Work
•
Bujoli group reported phosphonate-substituted diamine ligands with rhodium
metal-catalyzed ATH
•
High conversions (~ 95%), moderate ee (34-60%), cosolvent needed,
catalyst preparation under inert atmosphere
(R)
(R)
NH
NH
Me
Me
(HO)
2
P
(HO)
2
P
O
O
(S)
(S)
NH
NH
Me
Me
(HO)
2
P
(HO)
2
P
O
O
Maillet, C.; Praveen, T.; Janvier, P.; Minguet, S.; Evain, M.; Saluzzo, C.; Tommasion, M. L.; Bujoli, B. J. Org. Chem., 2002, 67, 8191-8196.
Me
O
Rh-catalyst, ligand
t
BuOK, 1:1 H
2
O:iPrOH
(S)
Me
OH
95% yield
46% ee
Rh
Cl
Cl Rh
(R)
(R)
NH
NH
Me
Me
(HO)
2
P
(HO)
2
P
O
O
Rh-Catalyst
Ligand
31
ATH in Water - Advances
•
Deng group developed o-sulfonated ligands with ruthenium catalyst and
HCOONa as hydrogen source
•
Purification difficult
(R)
(R)
NH
2
NH
2
1) 50% SO
3
oleum
0 °C - rt, 22 h
2) BaCO
3
(R)
(R)
NH
2
NH
2
SO
3
-
SO
3
-
Ba
2
+
TsCl, NaOH/SDS
H
2
O/CH
2
Cl
2
, 0 °C - rt, 24 h
then Na
2
SO
4
(R)
(R)
NHTs
NH
2
SO
3
Na
SO
3
Na
C
12
H
25
OSO
3
Na
SDS =
68%
72%
Ma, Y.; Liu, H.; Chen, L.; Cui, X.; Zhu, J.; Deng, J. Org. Lett., 2003,5, 2103-2106.
32
ATH in Water - Advances
•
Recyclability possible – retention of stereoselectivity, loss of conversion
(99% Æ 75%)
•
Surfactant required for satisfactory conversion
Ru
Cl
Cl
Cl
Cl
Ru
O
R
R
Yield (%) ee (%)
p
-Me
94
94
p
-F
88
92
O
n
n
Yield (%) ee (%)
1
66
83
2
21
98
O
R
R Yield (%) ee (%)
87
94
58
84
Br
H
NO
2
Ru-catalyst, ligand
H
2
O, HCO
2
Na, SDS
R
1
R
2
O
R
1
R
2
OH
NHTs
NH
2
(R)
(R)
SO
3
Na
SO
3
Na
Ru-Catalyst
Ligand
C
12
H
25
OSO
3
Na
SDS
(R)
Ma, Y.; Liu, H.; Chen, L.; Cui, X.; Zhu, J.; Deng, J. Org. Lett., 2003,5, 2103-2106.
33
ATH in Water – Recent Work
•
Shift to o-amine ligand and Cp* rhodium catalyst allows for increased yields,
stereoselectivities, and scope over previous work
•
Surfactant no longer necessary
R
1
R
2
O
Rh-catalyst, ligand
HCOONa, H
2
O
R
1
R
2
OH
NHTs
NH
2
(S)
(S)
NH
2
NH
2
Ligand
Rh-catalyst
O
R
2
R
1
R
1
R
2
Yield (%) ee (%)
p
-OMe
H
92
96
o
-OMe
H
90
88
p
-F
H
94
95
p-
Br
H
92
94
H
Br
91
97
p
-NO
2
Br
82
90
O
88% yield
97% ee
S
Me
O
90% yield
98% ee
Rh
Cl
Cl
Cl
Cl
Rh
(S)
Li, L.; Wu, J.; Wang, F.; Liao, J.; Zhang, H.; Lian, C.; Zhu, J.; Deng, J. Green Chem., 2007,9, 23-25.
34
2-Bromo-1-arylethanols
•
Key intermediates in syntheses of β-adrenergic receptor agonists
•
Agonists used as bronchodilators in asthmatics
•
Non-aqueous synthesis by ATH previously hampered
O
Br
(R)
OH
Br
Ru-catalyst, ligand
HCOOH/NEt
3
, 28 °C
Ru
Cl
Cl
Cl
Cl
Ru-Catalyst
Ru
NHTs
NH
2
(R)
(R)
Ligand
O
OCHO
0%
73%
Cross, D. J.; Kenny, J. A.; Houson, I.; Campbell, L.; Walsgrove, T.; Wills, M. Tetrahedron: Asymmetry, 2001, 12, 1801-1806.
35
2-Bromo-1-arylethanols
•
Changing to aqueous system allows for reduction
Ma, Y.; Liu, H.; Chen, L.; Cui, X.; Zhu, J.; Deng, J. Org. Lett., 2003,5, 2103-2106.
Wang, F.; Liu, H.; Cun, L.; Zhu, J.; Deng, J.; Jiang, Y. J. Org. Chem., 2005, 70, 9424-9429.
Li, L.; Wu, J.; Wang, F.; Liao, J.; Zhang, H.; Lian, C.; Zhu, J.; Deng, J. Green Chem., 2007,9, 23-25.
(R)
OH
Br
R
1
R
2
R
3
R
1
R
2
R
3
Yield (%)
ee
(%)
Surfactant
H
H
H
SDS
87
94
H
H
SDS
47
92
OBn
H
H
H
2:1 SDS:CTAB
97
98
H
OMe
H
2:1 SDS:CTAB
80
90
NO
2
OBn
2:1 SDS:CTAB
H
87
93
H
H
H
−
91
97
H
OMe
H
−
70
96
OBn
H
OBn
−
82
95
O
Br
R
1
R
2
R
3
catalyst, ligand
Surfactant, HCOONa, H
2
O
CH
2
Cl
2
cosolvent
Rh
Cl
Cl
Cl
Cl
Rh
NHTs
NH
2
(R)
(R)
R
4
R
4
Ligand
Rh-Catalyst
Catalyst Ligand
Ru
Cl
Cl
Cl
Cl
Ru-Catalyst
C
12
H
25
OSO
3
Na
SDS
Ru
C
16
H
33
N
Me
Me
Me
Br
-
CTAB
A - R
4
= SO
3
Na
B - R
4
= H
C - R
4
= NH
2
Ru
A
Ru
A
Rh
B
Rh
B
Rh
B
Rh
C
Rh
C
Rh
C
36
(R,R)-Formoterol
•
Long-acting, β
2
-agonist
•
Bronchodilator in treatment of patients with asthma and chronic bronchitis
•
(R,R)-enantiomer more active than other 3 possible stereoisomers
Wilkinson, H. S.; Tanoury, G. J.; Wald, S. A.; Senanayake, C. H. Org. Process Res. Dev., 2002, 6, 146-148.
Li, L.; Wu, J.; Wang, F.; Liao, J.; Zhang, H.; Lian, C.; Zhu, J.; Deng, J. Green Chem., 2007,9, 23-25.
O
Br
BnO
BH
3
-diethylaniline,
(R,S)-aminoindanol
THF
(R)
OH
Br
BnO
NO
2
NO
2
O
Br
BnO
Rh-catalyst, ligand
2:1 SDS:CTAB, HCOONa, H
2
O
(R)
OH
Br
BnO
87% yield, 93% ee
2.3 kg, 80% yield, 88% ee
Rh
Cl
Cl
Cl
Cl
Rh
NHTs
NH
2
(R)
(R)
Ligand
Rh-Catalyst
NO
2
NO
2
HO
OH
OMe
Me
H
N
NHCHO
(R,R)-formoterol
37
(R,R)-Formoterol
OH
Br
BnO
MeOH, aq NaOH
98%
BnO
O
OMe
BnHN
Me
1) neat, 90 °C
2) PtO
2
, H
2
3) HCOOH
BnO
OH
OMe
Me
Bn
N
NHCHO
NO
2
NO
2
1) Pd-C, H
2
, EtOH
2)
L
-tartaric acid, iPrOH
85%
HO
OH
OMe
Me
H
N
NHCHO
(R,R)-formoterol
45%, 3 steps
+
Hett, R.; Fang, Q. K.; Gao, Y.; Hong, Y.; Butler, H. T.; Nie, X.; Wald, S. Tetrahedron Lett., 1997, 38, 1125-1128.
38
ATH in Water - Imines
•
Noyori reported ATH of imines not conducive to protic solvents
•
Deng achieved reduction in water with o-sulfonated diamine ligands
– Acyclic imines unsuccessful
•
Recovery procedure
–
Extract (3x) with 1:1 Et
2
O:hexanes
–
Add 1 eq. HCOOH
–
Ready for reuse
MeO
MeO
N
R
R
Yield (%)
ee
(%)
Me
97
95
Et
68
92
i
Pr
90
90
MeO
MeO
S
N
R
Yield (%)
ee
(%)
Me
97
65
t
Bu
95
94
O
O
R
97
94
94
95
96
94
85
94
Recycle
Experiments
MeO
MeO
N
+
R
Bn
R
Yield (%)
ee
(%)
Me
85
90
Ph
94
95
N
R
Ru-catalyst, ligand, CTAB
HCOONa, H
2
O, 28 °C
NH
R
C
16
H
33
N
Me
Me
Me
Br
-
CTAB
NHTs
NH
2
(R)
(R)
SO
3
Na
SO
3
Na
Ru-Catalyst
Ligand
Ru
Cl
Cl
Cl
Cl
Ru
( S)
Wu, J.; Wang, F.; Ma, Y.; Cui, X.; Cun, L.; Zhu, J.; Deng, J.; Yu, B. Chem. Commun., 2006,1766-1768.

39
Outline
• Mechanism and scope of asymmetric transfer hydrogenation
• Pro-atropisomeric phosphine ligands
• Amino acid-based ligands
• Dendrimer-bound diamine ligands
• Asymmetric transfer hydrogenation in water
• Asymmetric transfer hydrogenation in ionic liquids
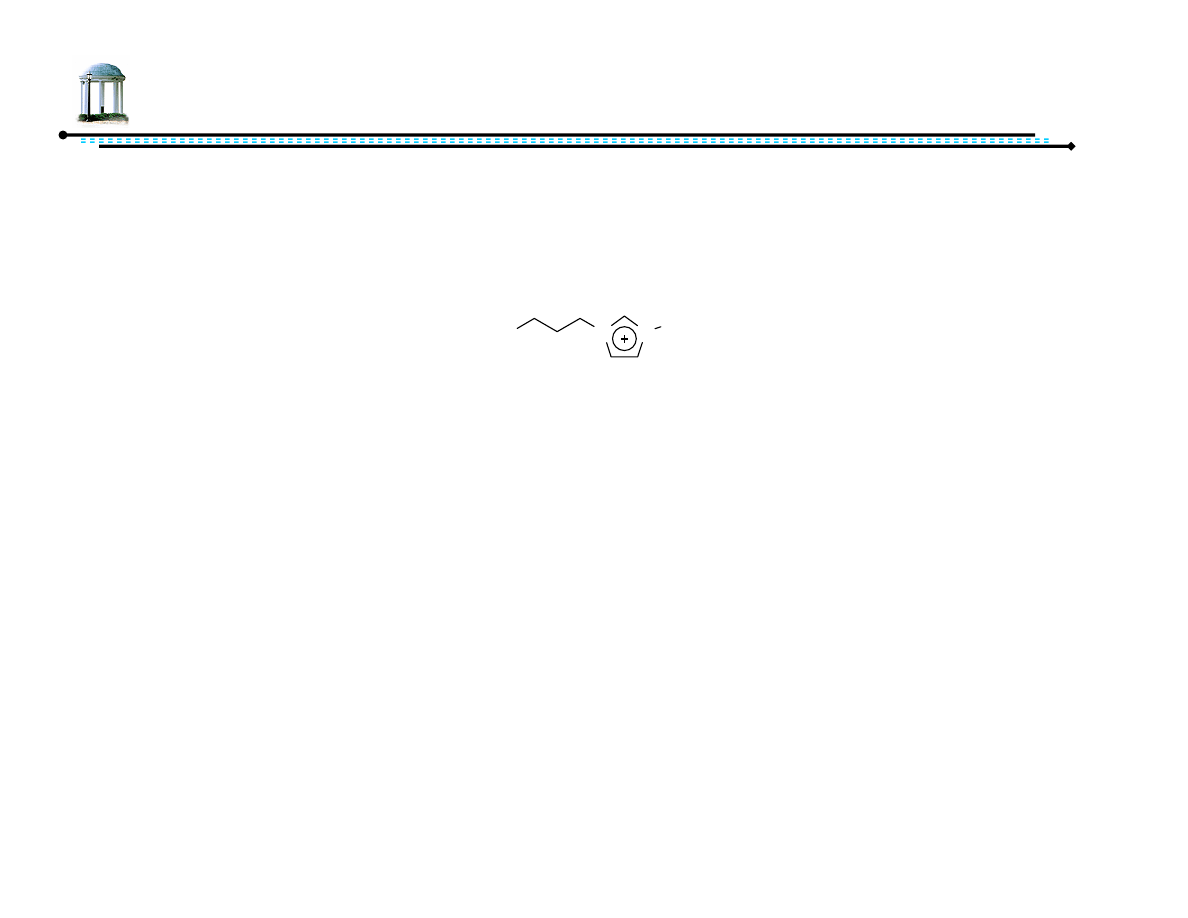
40
ATH in Ionic Liquids
• Ionic liquid: salt of organic cation with melting point near
ambient temperature
• Can stabilize/immobilize transition metal catalysts
• Negligible vapor pressure
• Tunable miscibility
• Easy recyclability
N
N
Me
BF
4
-
Me
[bmim][BF
4
] =
butylmethylimidazolium tetrafluoroborate
41
ATH in Ionic Liquids
•
Synthesis of ionic liquid-supported precursor
H
2
N
Cl
NHTs
Ph
Ph
Ru
N
N
Me
Me
Cl
N
N
Me
Me
toluene, 110 °C
1)
2) NaBF
4
, CH
2
Cl
2
N
N
BF
4
-
Me
Me
RuCl
3
MeOH, 80 °C
BF
4
-
BF
4
-
Ru
Cl
Cl
Cl
Ru
Cl
N
N
Me
Me
Ph
NHTs
H
2
N
Ph
DMF, rt
N
N
Me
Me
BF
4
-
Geldbach, T. J.; Dyson, P. J. J. Am. Chem. Soc., 2004, 126, 8114-8115.
42
ATH in Ionic Liquids
•
Recovery procedure:
–
Extract – Et
2
O or hexanes
–
Wash – water
–
Dry in vacuo
H
2
N
Cl
NHTs
Ph
Ph
Ru
(R)
(R)
Me
O
catalyst, [bmmim][PF
6
]
HCOOH/NEt
3
, 40 °C, 24 h
(R)
Me
OH
Cycle #
Catalyst A
(% yield,
% ee)
Catalyst B
(% yield,
% ee)
1
>99%, 99% >99%, 99%
2
>99%, 99% >99%, 99%
3
>99%, 99%
80%, 99%
4
99%, 99%
45%, 99%
5
96%, 99%
H
2
N
Cl
NHTs
Ph
Ph
Ru
(R)
(R)
BF
4
-
N
N
Me
Me
Catalyst A
Catalyst B
Cycle #
R =
Conversion (%)
ee
(%)
1
o
-Me
72%
97%
2
p
-Cl
99%
95%
3
H
99%
99%
4
H
98%
99%
1
Acetophenone
99%
97%
2
Tetralone
99%
97%
3
Benzaldehyde
90%
N/A
N
N
Me
Me
n
Bu
PF
6
-
[bmmim][PF
6
]
Geldbach, T. J.; Dyson, P. J. J. Am. Chem. Soc., 2004, 126, 8114-8115.
43
ATH in Ionic Liquids
•
Library of ionic liquids screened
– Hydrophilic ILs inhibit reaction
– Hydrophobic ILs slow reaction, but good ee
Joerger, J.-M.; Paris, J.-M.; Vaultier, M. Arkivoc, 2006, 152-160.
Me
O
Ionic
Liquid
Cycle #
Time (h) Conversion (%)
ee
(%)
[bmim][PF
6
]
1
31
97
96
2
50
92
95
3
95
46
89
[bmim][BF
4
]
40
<1
-
[bmim][MeSO
4
]
48
19
85
[emim][OTf]
24
0
-
Hydrophilic
Ionic Liquids
[bmim][NTf
2
]
1
27
98
96
2
21
58
96
[tmba][NTf
2
]
1
26
98
97
2
41
99
97
3
94
99
97
4
50
56
96
Hydrophobic
Ionic Liquids
(S)
Me
OH
catalyst, ionic liquid
HCOOH/NEt
3
N
N
Me
n
Bu
BF
4
-
[bmim][BF
4
]
N
N
Me
Et
[emim][OTf]
-
O
CF
3
S
O
O
N
+
Me
Me
Me
n
Bu
N
-
CF
3
S
O
O
F
3
C
S
O
O
[tmba][NTf
2
]
H
2
N
Cl
NHTs
Ph
Ph
Ru
(S)
(S)
Catalyst

44
Conclusions
• Role of solvent in transition state may be significant
• Pro-atropisomeric phosphine ligands can impart
stereocontrol
• Ligands based on naturally-occurring amino acids
suitable for ATH
• Catalyst can be recovered and reused
• ATH can be run in water or ionic liquid
• Future directions:
– Increase substrate:catalyst ratio
– Expand scope
– Improve recoverability further

45
Acknowledgments
Acknowledgments
•
Professor Crimmins
•
Crimmins Group Members
•
UNC Chemistry
Document Outline
- Recent Advances in Asymmetric Transfer Hydrogenation
- Chiral Secondary Alcohols and Amines
- Formation of Chiral Secondary Alcohols
- Reduction of C=X π-Bond
- Outline
- Mechanism of ATH
- Mechanism of ATH
- Sources of Hydrogen
- Origin of Stereoselectivity
- Scope of ATH
- Scope of ATH
- Outline
- Pro-atropisomeric Phosphine Ligand
- Pro-atropisomeric Phosphine Ligand
- Pro-atropisomeric Phosphine Ligand
- Pro-atropisomeric Phosphine Ligand
- Outline
- Amino Acid-Based Ligands
- Amino Acid-Based Ligands
- Amino Acid-Based Ligands
- Amino Acid-Based Ligands
- Amino Acid-Based Ligands
- Amino Acid-Based Ligands
- Amino Acid-Based Ligands
- Outline
- Recoverable Diamine Ligands
- Recoverable Diamine Ligands
- Recoverable Diamine Ligands
- Outline
- ATH in Water
- ATH in Water – Early Work
- ATH in Water - Advances
- ATH in Water - Advances
- ATH in Water – Recent Work
- 2-Bromo-1-arylethanols
- 2-Bromo-1-arylethanols
- (R,R)-Formoterol
- (R,R)-Formoterol
- ATH in Water - Imines
- Outline
- ATH in Ionic Liquids
- ATH in Ionic Liquids
- ATH in Ionic Liquids
- ATH in Ionic Liquids
- Conclusions
- Acknowledgments
Wyszukiwarka
Podobne podstrony:
recent developments in cannabinoid ligands life sci 77 1767 1798 (2005)
Fill in blanks transforming the words in brackets
recent developments in the med chem of cannabimimetic indoles pyrroles and indenes curr med chem 12
Stepanova Terrorism in asymmetrical conflict
Bernays, Edward L , Recent Trends in Public Relations Activities (1937)
Miller Recent Developments In Slab A Software Based System For Interactive Spatial Sound Synthesis
1998 Recent developments in superstring theory Schwarz
In festo transfigurationis Domini nostri Jesu Christi, S 188 (Liszt, Franz)
Recent Developments in Cosmology
CEREBRAL VENTICULAR ASYMMETRY IN SCHIZOPHRENIA A HIGH RESOLUTION 3D MR IMAGING STUDY
Transformations in Suniti Namyoshiĺs Snapshots of?liban
Effects of Clopidogrel?ded to Aspirin in Patients with Recent Lacunar Stroke
więcej podobnych podstron