
Biomaterials 23 (2002) 491–501
Characteristics of the surface oxides on turned and electrochemically
oxidized pure titanium implants up to dielectric breakdown:
the oxide thickness, micropore configurations, surface roughness,
crystal structure and chemical composition
Young-Taeg Sul
a,b,
*, Carina B. Johansson
a
, Sarunas Petronis
c
, Anatol Krozer
d
, Yongsoo
Jeong
e
, Ann Wennerberg
a
, Tomas Albrektsson
a
a
Department of Biomaterials/Handicap Research, Institute for Surgical Science, University of G
.ooteborg, Sweden
b
Osseointegration Research Institute, Seoul, South Korea
c
Department of Applied Physics, Chalmers University of Technology/GU, Sweden
d
Imego AB, Aschebergsgatan 46 build 11 411 33 G
.ooteborg, Sweden
e
Surface Engineering Department, Korea Institute of Machinery & Materials, 66 Sangnam-dong, Changwon, Kyungnam, South Korea 641 010
Received 16 November 2000; accepted 23 March 2001
Abstract
Titanium implants have been used widely and successfully for various types of bone-anchored reconstructions. It is believed that
properties of oxide films covering titanium implant surfaces are of crucial importance for a successful osseointegration, in particular
at compromized bone sites. The aim of the present study is to investigate the surface properties of anodic oxides formed on
commercially pure (c.p.) titanium screw implants as well as to study ‘native’ oxides on turned c.p. titanium implants. Anodic oxides
were prepared by galvanostatic mode in CH
3
COOH up to the high forming voltage of dielectric breakdown and spark formation.
The oxide thicknesses, measured with Auger electron spectroscopy (AES), were in the range of about 200–1000 nm. Barrier and
porous structures dominated the surface morphology of the anodic film. Quantitative morphometric analyses of the micropore
structures were performed using an image analysis system on scanning electron microscopy (SEM) negatives. The pore sizes were
p8 mm in diameter and had 1.27–2.1 mm
2
opening area. The porosity was in the range of 12.7–24.4%. The surface roughness was in
the range of 0.96–1.03 mm (S
a
), measured with TopScan 3D
s
. The crystal structures of the titanium oxide were amorphous, anatase,
and a mixtures of anatase and rutile type, as analyzed with thin-film X-ray diffractometry (TF-XRD) and Raman spectroscopy. The
chemical compositions
consisted mainly of TiO
2
, characterized with X-ray photoelectron spectroscopy (XPS). The native (thermal)
oxide on turned implants was 17.4 nm (
76.2) thick and amorphous. Its chemical composition was TiO
2
. The surface roughness had
an average height deviation of 0.83 mm (S
a
). The present results are needed to elucidate the influence of the oxide properties on the
biological reaction. The results of animal studies using the presently characterized surface oxides on titanium implants will be
published separately. r 2001 Elsevier Science Ltd. All rights reserved.
Keywords:
Titanium implants; Titanium oxides; Surface oxide properties; Oxide thickness; Micropore configurations
1. Introduction
Since the first scientific documentation of successfully
osseointegrated oral titanium implants based on the
clinical experience by Br
(aanemark et al. [1] in 1977, a
number of long term follow-up results over 10 years
have been documented [2–4]. However, in bone of poor
quantity and quality, inferior clinical results have been
reported by Albrektsson and Johansson [5]. It is possible
that a change in implant surface characteristics will
result in better clinical results. One way to change the
surface characteristics of implants is by varying proper-
ties of the oxide films always present on Ti surfaces.
Oxidized implants represent such a surface change. The
oxide properties of titanium implants probably do play
an important role during the dynamic build up of the
osseointegration process [6].
*Corresponding author. Department of Biomaterials/Handicap
Research, Institute for Surgical Science, G
.ooteborg University,
Medicinaregatan 8b, Box 412, S-405 30 G
.ooteborg, Sweden. Tel.:
+46-31-773-2950; fax: +46-31-773-2941.
E-mail addresses:
young-taeg.sul@hkf.gu.se (Y.-T. Sul).
0142-9612/02/$ - see front matter r 2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 1 3 1 - 4

Titanium is normally covered with a thin protective
oxide film, which largely determines the surface proper-
ties of an implant. Regarding the surface oxide on
commercially available turned c.p. titanium implant
systems, previous spectroscopic studies have reported
that oxide thicknesses are in the range of 1.8–17 nm, and
that the chemical composition consisted mainly of TiO
2
[7–10]. The oxide structure was found to be non-
crystalline as surveyed by Raman spectroscopy [11].
Surface roughness varied from 0.53 to 0.67 mm in Ra
values as measured by TopScan 3D measuring system
[12]. This thin oxide film, naturally formed on a titanium
substrate, is presumably responsible for the excellent
biocompatibility of titanium implants due to a low level
of electronic conductivity [13], a high corrosion resis-
tance and a thermodynamically stable state at physio-
logical pH values [14–16]. In addition, titanium and its
oxide(s) have a low ion-formation tendency in aqueous
environments [17].
In the case of the anodic oxide film, surface properties
have been shown to be sensitive to oxide growth
conditions [18]. We have previously carried out inves-
tigations of electrochemical growth behavior of the
anodic oxide film on c.p. titanium metal in various
electrolytes. The growth behavior strongly depended on
a particular electrolyte used and, for a given electrolyte,
on the employed anodic process parameters such as the
applied current density, the electrolyte concentration,
the electrolyte temperature, agitation speed, and cath-
ode to anode surface area ratios [19]. In general, it has
been shown that various surface characteristics of
anodic films are accompanied with an increase of the
oxide thickness. Previous extensive studies have de-
scribed a variety of properties of anodic oxide films in
detail, e.g. surface morphology, chemical composition,
crystal structures and corrosion resistance [20–23].
Using the higher anodic forming voltage than break-
down voltage, corresponding to Group III–V in the
present study, have been prepared in the Ca- and P-
containing electrolytes [24–27].
The present study is focused on the oxide thickness
and morphology, especially pore configurations. The
crystal structure, chemical composition and surface
roughness are also presented. The biological results
from an animal model using the presently characterized
oxide films on commercially pure titanium implants will
be published separately [28,29].
2. Experimental
2.1. Sample preparation and anodizing apparatus
Screw shaped implants were turned from 5 mm rods
of c.p. titanium (ASTM Grade 1). They had an outer
diameter of 3 mm, a total length of 7 mm with a pitch-
height of 0.5 mm. The implants had a square shaped
head (4 mm 4 mm 2 mm) with an inner threaded
hole of 2 mm which was used as a sample holder during
anodizing. Additionally, a plate type of anode (ASTM
Grade 1) was used, being 30 mm 10 mm 1.0 mm with
a small handle of 30 mm 2.5 mm 1.0 mm. The plate
type was ground on both sides with SiC paper in
successive grades from 300 to 800 grit (Struers, Den-
mark). Each sample was separately put in glass tube and
degreased in trichlorethylene and cleaned in absolute
ethanol in an ultrasonic bath for 2 15 min, respec-
tively, dried in an oven at 501C for 24 h prior to further
surface oxide preparation. Details of the anodizing
apparatus and anodizing procedures employed in this
study have been described previously [19]. In brief, the
anodizing apparatus consisted of an electrolytic cell and
an IBM computer interfaced with a dc power supply. All
the surface oxides were prepared at galvanostatic mode
in 0.1 m acetic acid. Voltage to time characteristic curves
were recorded at intervals of 0.5 s by an IBM computer
interfaced with the power supply. Immediately after
anodic oxidation, all the specimens were thoroughly
rinsed with distilled water and absolute alcohol, again
dried in the same way as mentioned above, and finally
sterilized in a steam autoclaving machine at a maximum
of 1301C for 30 min. The samples were divided into five
groups in accordance with the conditions of the surface
oxide preparation employed.
Group I implants had turned surfaces.
Group II implants; turned and anodized surfaces up
to anodic forming voltage of 100 V with the anodic
oxide forming rate of 16.7 nm/s.
Group III implants; turned and anodized surfaces up
to anodic forming voltage of 200 V with the anodic
oxide forming rate of 15.2 nm/s.
Group IV implants; turned and anodized surfaces up
to anodic forming voltage of 280 V with the anodic
oxide forming rate of 8.9 nm/s.
Group V implants; turned and anodized surfaces up
to anodic forming voltage of 380 V with the anodic
oxide forming rate of 7.7 nm/s.
A total of 45 samples, 40 screw implants and five plate
samples, were investigated for surface analyses in the
present study.
2.2. Surface analysis
The oxide thickness and surface elements were
measured by continuous sputter etching with 4 KeV Ar
ion in Auger electron microscopy (AES, Physical
Electronics, model PHI 650). Measurements were
performed with a probing beam of 2.5 mm 4.0 mm at
four different locations; one thread-top, one thread-
valley, one thread-flank and in the bottom of the screw
implant. The determination of the oxide thickness was
calculated by the numerical formula d ¼ v
0
t
d
, where d is
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
492
the film thickness, v
0
is the sputter rate, and t
d
is the
sputtering time to make the oxygen peak amplitude
decrease by 50% [30].
Overall surface morphology was characterized by
scanning electron microscopy (SEM, JEOL JSM-5800).
An image analysis system (Bildanalyssystem AB
s
) was
used to describe the pore configurations on SEM
negatives of 3000, e.g. the pore size distributions
(PSD), pore density and porosity, etc. These measure-
ments were obtained from three randomly selected
areas; two flanks and one bottom area of the screw
implant. Scanned areas were 26 mm 20 mm. Pore
characteristics in the present study were confined with
the ‘‘opened pores’’ on the surface (not including the
‘‘buried pores’’ in the anodic oxide film). The pore
density was presented as a total number of opening
pores/scanned area (3 26 mm 20 mm). The porosity
was presented as a total area of opening pores/scanned
area (3 26 mm 20 mm).
For the analyses of the crystal structure of the
titanium oxide, thin-film X-ray diffractometry (TF-
XRD, Rigaku Co.) with PW3020 goniometer was used
on the plate type of specimen, since the screw types
seldom permitted useful X-ray diffraction pattern due to
their geometry. The step size was 0.041 between 201 and
801 of measured scan. Spectra were recorded using Cu
K
a
radiation (1.54 (
A
A). Raman spectroscopy was used for
screw implants. Raman scattering experiments were
performed by using a DILOR LABRAM spectrometer
equipped with a 10 mW helium–neon laser (l¼ 6328 (
A
A)
and 1800 line/mm grating, giving spectral resolution of
about 1 1/cm. Spectra of frequency shifts of scattered
light were measured in 97–115 1/cm energy intervals
averaging three datasets, each accumulated in 100 s.
Probing spots were chosen on one thread-top, one
thread-valley, one thread-flank and one top area of the
screw implant. The probing spot was about 4 mm in
diameter. The spectrometer was calibrated by recording
the spectrum from Si sample with characteristic peak at
520 1/cm.
The chemical composition of titanium surfaces was
analyzed by X-ray photoelectron spectroscopy (XPS).
All the XPS (VG Scientific LTD, model ESCALAB
200R) spectra were recorded using normal Al K
a
radiation (1486.8 eV) with a resolution better than
0.7 eV. Ion gun energy was 5 KeV and ion beam current
was 0.3 mA. Two types of spectra were recorded: wide
energy survey scans and higher resolution narrow scans
of the dominant spectral peaks as detected in the survey
spectra. The latter included Ti 2p, O 1s and C 1s spectral
regions.
The surface roughness was measured with confocal
laser scanning profilometer (TopScan3D
s
) as described
by Wennerberg [31]. The confocal laser scanning (CLS)
method for the evaluation of implant surface roughness
has been compared to other techniques such as a contact
stylus profilometer and an AFM. The CLS equipment
was found to give very reliable data. Maximal vertical
and horizontal resolution of the CLS is 6 nm and 0.5 mm.
Three screws were selected and measured on the 3
thread-tops, 3 thread-valleys, and 3 thread-flanks each,
making 27 measurements for each Group. The measur-
ing area was 245 mm 245 mm for each group. A
Gaussian filter was used to separate roughness from
error of form and waveness. The filter type was set to
50 mm 50 mm.
3. Results and discussion
3.1. Thickness and growth of titanium oxide
The general findings were a great variation in oxide
thickness (from 17 nm up to about 1000 nm) between the
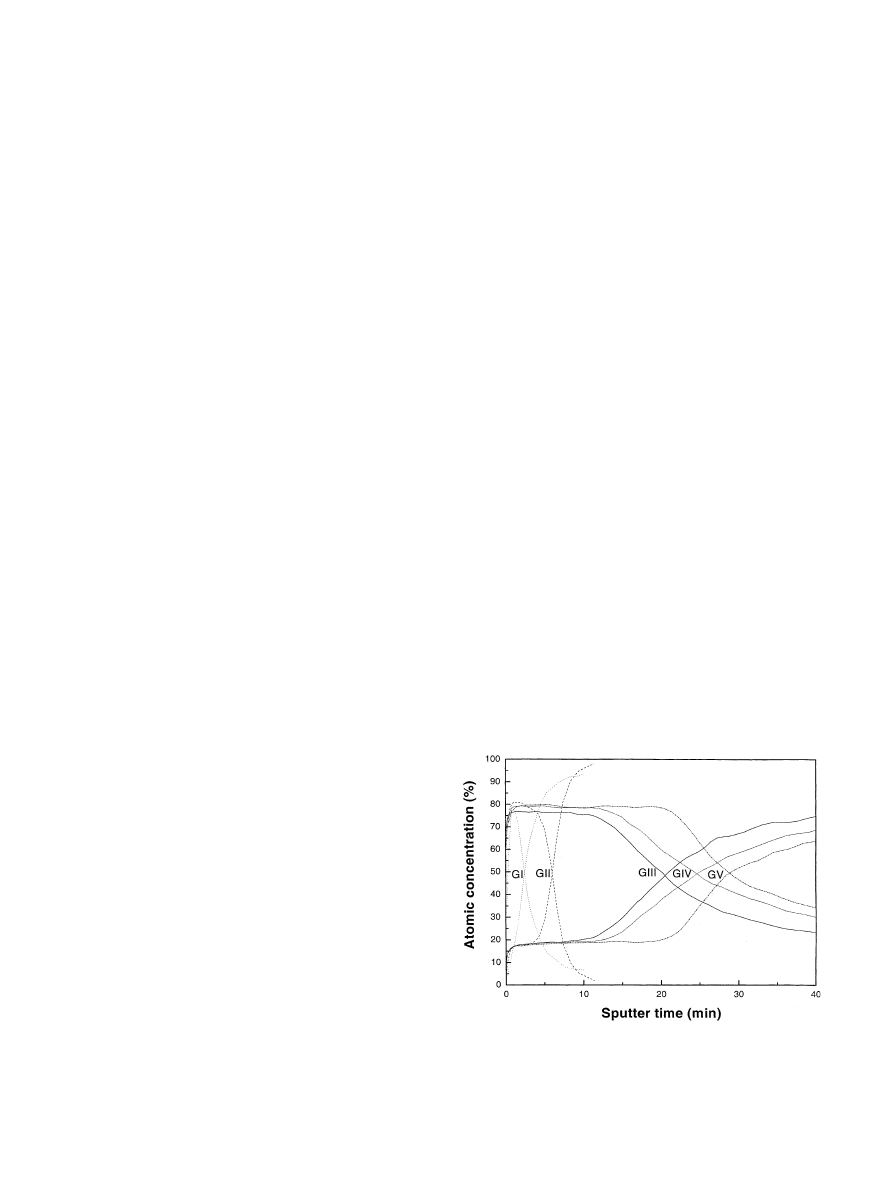
five different groups. Fig. 1 shows the relative atomic
concentration distributions for Ti and O detected in
AES survey spectra from the turned and anodized
Groups. To translate ion sputter profiles into thickness a
calibration standard is needed. We used TiO
2
film
deposited on Si (1 0 0) by metal organic chemical vapor
deposition (MOCVD) as a reference for thickness ion
yield calibration. The film was 91.8 nm thick and had a
refractive index of 2.199 as evaluated by ellipsometry.
This value is about 2.0 for amorphous type and 2.5 for
anatase type. The sputtering rate was determined to be
about 7.1 nm/min. Group I implants had a mean oxide
thickness of 17.4 nm (
76.2), which is likely a thermal
oxide film developed mainly during implant manufac-
turing (machine-turning) and during implant steriliza-
tion up to 1341C. The turned implants in the present
study were prepared with no chemical coolants during
Fig. 1. AES depth profiles from thread-top of a screw implant
(GI=Group I) and anodized screw implants (GII–GV=Group II–
V). Thicker oxide films (Group III–V) showed relatively deeper O
diffusion near the interface zone in comparison to thinner oxide films
(Group I and II) (E
p
¼ 4:0 keV, I
p
¼ 300 nA).
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
493
manufacturing. This together with the elevated tem-
peratures during sterilization are the probable reasons
for relatively thick ‘native’ oxide films of the Group I
implants. In previous studies the oxide thickness of
commercially available turned c.p. titanium implants
varied from 1.8 to 17 nm [8–10] and depended on the
preparation history of the implants, in particular the
ultimate temperatures the implants were exposed to, and
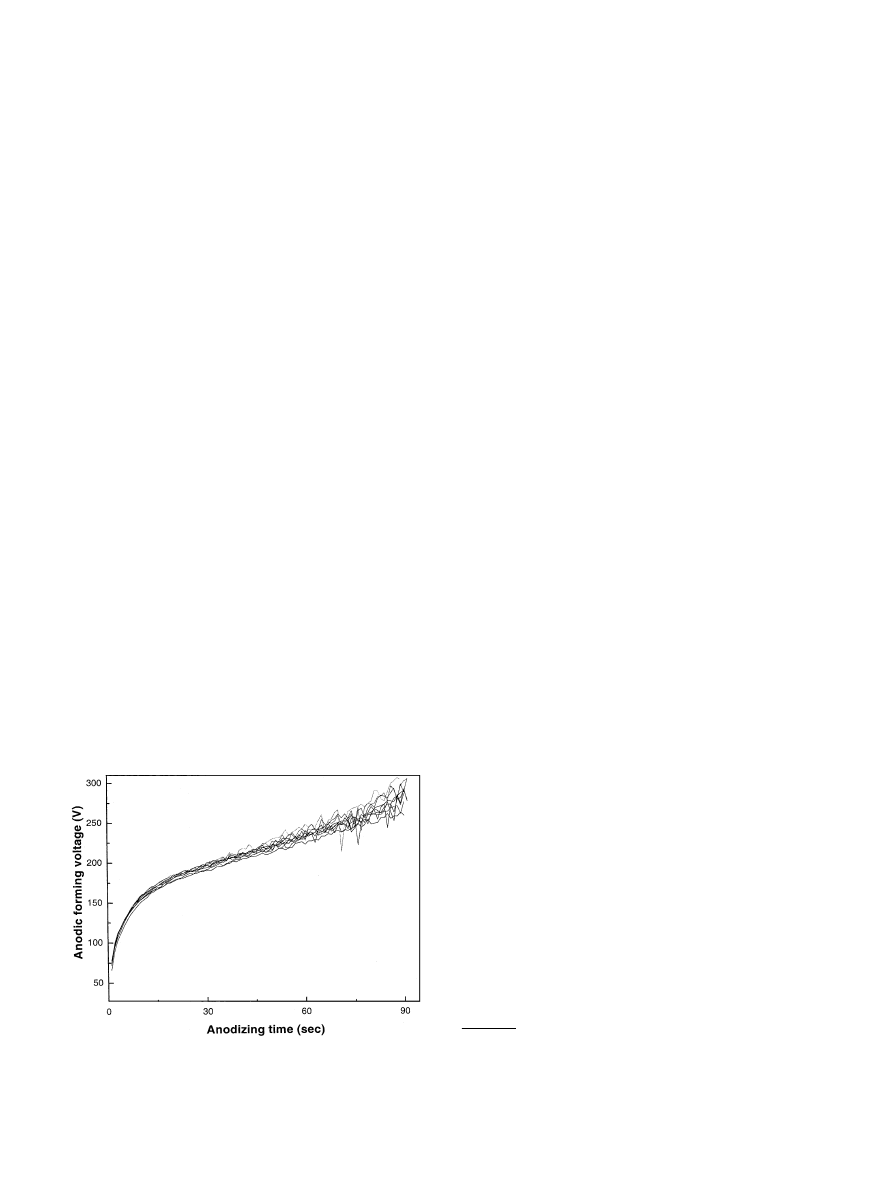
the duration of exposures [22]. Fig. 2 shows the anode-
to-cathode voltage drop (V) as a function of time during
galvanostatic anodization used in the present study.
Group II implants had an oxide thickness of 203 nm
(
753) formed up to 100 V of the anodic forming
voltage. The oxide film grew linearly with the anodic
oxide forming rate of 16.8 nm/s and the average growth
constant, a
E2:0 nm/V for Group II implants. This value
was consistent with 1.92 nm/V found in our previous
study [19]. Group III implants formed up to 200 V
(a
E3:0 nm/V, the anodic oxide forming rate of 15.2 nm/
s) had an oxide thickness of 608 nm (
7127). Group IV
implants formed up to 280 V (a
E2:9 nm/V, the anodic
oxide forming rate of 8.9 nm/s) had an oxide thickness
of 805 nm (
7112). Group V implants formed up to
380 V (a
E2:6 nm/V, the anodic oxide forming rate of
7.7 nm/s) had an oxide thickness of 998 nm (
7200). The
growth behaviors of the anodic films have been
described previously [19]. The anodic parameters
employed in the present study and corresponding oxide
thicknesses measured by AES at four different locations
of the screw implants; one thread-top, one thread-valley,
one thread-flank and on the head of the screw implant
are summarized in Table 1. The oxide thicknesses varied
slightly according to the measuring areas and were
thicker at thread-flanks than at the other measured areas
of the screw implants. The variations of the oxide
thickness are probably due to different geometry of the
measured areas in screw shaped implants, which made it
difficult to position the probing ion beam properly
during depth profiling. Another possible explanation for
these variations is related to the porous structures of the
anodic film (see Section 3.2), which essentially origi-
nated from the breakdown phenomenon of the anodic
oxide film. Fig. 2 shows the electrochemical growth
behavior of the oxide films during galvanostatic
anodizing employed in the present study. Below break-
down voltage involved in the preparation of Group II
implants, oxide growth behavior was very stable and
reproducible, and showed nearly linear relationship
between the anodic forming voltage and time. However,
beyond 200 V of the anodic forming voltage engaged in
the preparation of Group III, IV and V, there was
considerable voltage surge during breakdown (crater
formation) of the anodic oxide films. For instance, in the
case of Group V implants voltage surge was about
730 V. Subsequently during depth profile positioning of
the probing ion beam and the analyzing electron beam
into pores/craters on the oxide surface lead to shadow-
ing effects, which in turn may have resulted in the
observed variations of oxide thickness in Group III, IV
and V.
3.2. Morphology and micropore configuration
In general, with an increase of the anodic forming
voltage (increase of the oxide thickness) the micro arcing
on the screw shaped titanium anode surface (breakdown
phenomenon) became active and vigorous, resulting in
pores/craters
1
being irregular in shape. In addition, pore
size also became larger up to 8 mm in diameter. This is
most likely due to interconnection of some pores to one
another. The turned implants (Group I) were character-
ized by grooves and ridges of widths less than 10 mm and
pits (mechanically induced by machine-turning), and
oriented in the turning direction (Fig. 3a). In case of the
anodic films, morphologies critically depended on oxide
growth behaviors, especially the micro arcing phenom-
enon. Group II implants were anodized before the micro
arcing substantially began. Therefore, the surface oxide
was basically a non-porous barrier (compact and
uniform) film. As seen in Fig. 3b, Group II implants
appeared to have a similar morphology as Group I
implants. In contrast to Group I and II, the surface
morphologies in Group III–V implants had substan-
tially different porous film structures, characterized by
the appearance of craters on mountainous elevations
due to the dielectric breakdown (particularly the micro
Fig. 2. Voltage to time characteristic curves during galvanostatic
anodizing on screw shaped titanium anodes assessed with ten times-
repeated experiment. The range of voltage surge increases with the
anodizing time beyond breakdown voltage.
1
It should be noted that the term of pore and/or crater has been used
to describe the surface morphology with holes or cavities during
anodization. The concept of the pore/crater used in this paper also
involves the craters induced with the dielectric breakdown phenom-
enon.
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
494

arcing) of the anodic films during oxide formation
(Fig. 2). As shown in SEM micrographs of Fig. 3c–e,
however, Group III, IV and V implants demonstrated
different surface topographies, especially when quanti-
fying the ‘‘opened pores’’ on the oxide surface (not
including the ‘‘buried pores’’ in the anodic oxide film).
The pores showed very irregular cauliflower-like ap-
pearance, formed on the relatively flat ground oxide
surface. The pore density (a total number of opening
pores/an entire scanning area
F3 mm 26 mm 20 mm)
was 0.1 in Group III implants, 0.16 in Group IV
implants, while it was 0.09 in Group V implants. The
pore size, measured by an opening area of each pore in a
given group, had a mean of 1.27 mm
2
in Group III
implants, 1.53 mm
2
in Group IV implants, and 2.1 mm
2
in
Group V implants, respectively. The pore size, measured
by long axis (diameter) in irregular shape of the opening
pores, was
p8 mm. The porosity (a total area of opening
pores/an entire scanning area
F3 mm 26 mm 20 mm)
was 12.7% in Group III implants, 24.4% in Group IV
implants and 18.7% in Group V implants. The decreases
of the pore density and porosity in Group V are
Table 1
The anodic parameters and oxide thickness of the turned (Group I) and anodized implants (Group II–V). The oxide thickness was measured by Ar
ion sputter etching in AES on four areas of each screw implant; one thread-top, one thread-flank, one thread-valley and one head area
Group I
Group II
Group III
Group IV
Group V
Anodic parameters
Anodic forming voltage (V)
F
100
200
280
380
Anodic oxide forming rate (nm/s)
F
16.8
15.2
8.9
7.7
Oxide growth constant (nm/V)
F
2
3
2.9
2.6
Oxide thickness (nm)
Thread-top
18.2
207.1
649
770.4
893.2
Thread-flank
27.4
274.6
743
898.4
1297.9
Thread-valley
12.8
174.7
436.8
674
893.2
Head of the screw
11.4
154.7
561.6
898.4
893.2
Mean values
17.4
202.7
608.4
805
998.4
Standard deviation
6.24
52.5
127.4
112.3
199.7
Fig. 3. SEM pictures show non-porous (Group I and II) and porous microstructure (Group III–V): a=turned surface
FGroup I; b=anodized
surface at 100 V
FGroup II; c=anodized surface at 200 VFGroup III; d=anodized surface at 280 VFGroup IV; e=anodized surface at
380 V
FGroup V.
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
495

supported by the results of the roughness measurements
by TopScan3D
s
(see Section 3.3). One possible
explanation for the decreases of the pore density and
porosity in Group V may be due to interconnection of
some pores to each other, i.e. the pore size increased
while the number of pores decreased (Fig. 3). The
micropore configurations are summarized in Table 2.
3.3. Surface roughness
Average surface roughness (S
a
) was 0.83 mm for
turned implants (Group I), 0.96 mm for anodized
implants (Group II), 1.03 mm for anodized implants
(Group III), 1.02 mm for anodized implants (Group IV),
and 0.97 mm for anodized implants (Group V). The
values for the space descriptive parameter Scx and
hybrid parameter Sdr are summarized in Table 3. With
multiple comparisons of the Group I to test groups
using one-way ANOVA and Fisher’s PLSD method, S
a
value showed significant difference from Group III and
IV (p ¼ 0:018 and 0.029) and no significance from
Group II and V (p ¼ 0:373 and 0.098) of the present
study. This indicated that average surface roughness
(S
a
) significantly increased with increasing anodic
forming voltage up to 280 V (roughening effect), where-
after it decreased with anodic forming voltage up to
380 V. Eventually, changes of average surface roughness
(S
a
) were consistent with changes of the porosity in the
anodized implants (Group III–V).
3.4. Crystal structure
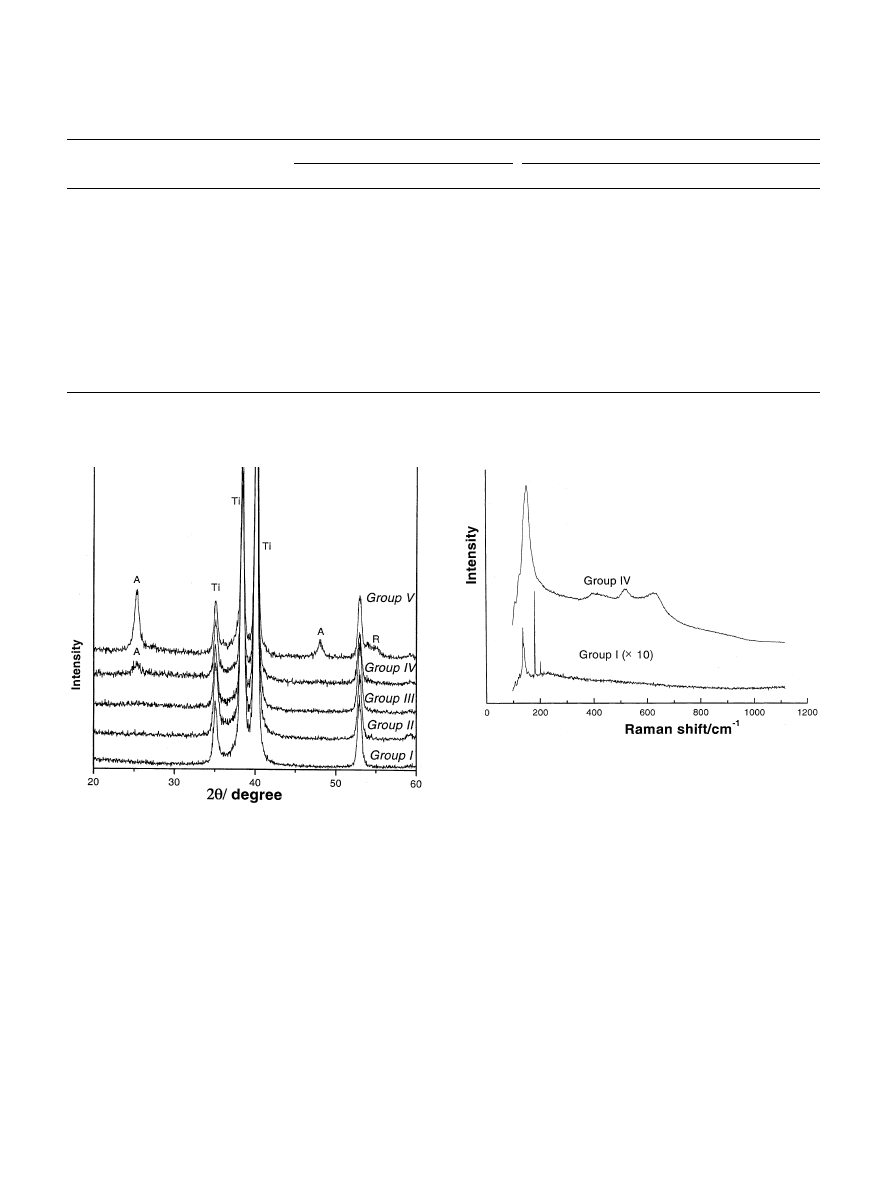
TF-XRD diffraction patterns did not show features of
either anatase or rutile in Group I–III, but showed
anatase peaks in Group IV and a mixture of anatase and
rutile phase in Group V implants. In addition, Raman
spectra demonstrated a mixture of anatase and rutile
phase in Group IV implants. The results are summarized
in Table 4.
Group I implants were dominated by peaks of
titanium
substrate
on
X-ray
diffraction
patterns
(Fig. 4). Raman spectra in Group I demonstrated an
intensive double peak near 136–140 1/cm, a very sharp
and strong peak near 180 1/cm, a sharp and less
intensive peak near 201 1/cm, a very broad feature with
a maximum near 230 1/cm (probably due to fluores-
cence) and a weak peak near 285 1/cm. However, these
peak positions of Raman spectra have not been found as
any assignable characteristics of either anatase or rutile
in literature survey data (Table 4). One possible
explanation for the detected structures might be that
these arise from a non-continuous oxide network
formed at the Ti/TiO
2
interface. The thin oxide film is
thus neither amorphous nor cyrstalline but behaves
more like a molecular layer. This is consistent with the
appearance of the Ti1 and Ti
2+
related peaks in the XPS
spectra (see below) from group I implants.
The lack of X-ray diffraction patterns characteristic of
either anatase or rutile in Group II and III may indicate
Table 2
Pore characteristics of screw implants obtained using an image analysis of scanning electron microscopy (SEM) negatives of three randomly selected
areas with scanning area of 20 mm 26 mm
Sample
PSD
a
Porosity (%)
b
Pore density
c
Group I
F
F
F
Group II
Negligible
Negligible
Negligible
Group III
1.27
70.90 mm
2
,
p8 mm
12.7
73.6
0.1
70.01
Group IV
1.53
71.72 mm
2
,
p8 mm
24.4
73.7
0.16
70.03
Group V
2.10
71.96 mm
2
,
p8 mm
18.7
75.2
0.09
70.06
a
Pore size distribution (PSD) was presented by opening area and by diameter, (n ¼ 3, mean
7SD).
b
Porosity presented a total area of the opening pores/a total of the scanned area
F3 mm 20 mm 26 mm in %, (n ¼ 3, mean7SD).
c
The pore density was presented as a total number of opening pores/scanned area (3 mm 26 mm 20 mm), (n ¼ 3, mean
7SD).
Table 3
Three screw sides of each Group were measured, and each screw side was measured on 9 areas. The mean values and standard deviation in brackets
of 27 surface roughness measurements are summarized
a
Samples
S
a
(mm)
Scx (mm)
Sdr (mm)
Ssk (mm)
Group I
0.83 (0.32)
9.78 (1.40)
1.23 (0.11)
1.11 (0.92)
Group II
0.96 (0.34)
10.08 (1.46)
1.25 (0.11)
1.19 (1.0)
Group III
1.03 (0.33)
10.60 (1.02)
1.29 (0.11)
0.71 (0.96)
Group IV
1.02 (0.27)
11.11 (1.23)
1.26 (0.07)
0.54 (1.03)
Group V
0.97 (0.30)
11.16 (1.20)
1.22 (0.07)
0.11 (1.33)
a
S
a
presents the height deviation from the mean plane. Scx presents the average distance between the surface irregularities in spatial direction. Sdr
describes the surface developed ratio. 50 mm 50 mm Gaussian filter was used.
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
496
either amorphous oxide or poorly crystallized anodic
oxides, consisting of ultrafine crystallites beyond the
sensitivity of the used equipment. Previous Raman
spectroscopy studies have reported non-crystalline oxide
[11]. TEM studies, however, showed the presence of
anatase [20]. This discrepancy may be due to differences
in the used electrolyte as previously described [19] or it
may be due to the history of the sample preparation.
Another explanation may be related to the higher
anodic oxide forming rates (nm/s) of the given anodic
films for Group II and III in comparison to Group IV
and V (Table 1). Leach and Pearson [32] have reported
that at higher growth, the appearance of crystalline
oxide was delayed to higher thicknesses.
X-ray diffraction patterns for group IV implants
showed a peak near 25.31, indicative of anatase
structure. In agreement with XRD results, Raman
spectra from Group IV exhibited a very strong peak
near 146 1/cm, and medium peaks near 397, 517 and
630 1/cm, which can be identified as the fingerprint
of anatase structure (Fig. 5). These data are in good
agreement with the reference spectra from anodic
oxide films [33,34]. The peaks at 146 1/cm and at
397 1/cm were accompanied by asymmetrical broad-
ening of the spectra towards higher frequencies while the
peak near 630 1/cm showed asymmetry towards lower
frequencies. These asymmetries are likely due to a
convolution of peaks due to anatase with those arising
from the rutile structure [34,35]. In addition, the peak
Table 4
Analysis of the crystal structures from TF-XRD and Raman spectroscopy
a
Sample
Structure
TF-XRD spectra (1)
Raman spectra (1/cm)
Present
Reference
Present
Reference
Group I
Amorphous
136m, 140m
Group II
Amorphous
201s, 230w
Group III
Amorphous
Group IV
Anatase
25.32
25.28
151s
145s, 440m, 515m, 640m (2)
397m
144s, 197w, 397m, 516m, 641m (4)
518m
144s, 197w, 399m, 516m, 639m (1)
630m
Rutile
143m, 236s, 447s, 612s (4)
143m, 447s, 612s, 826w (5)
Group V
Anatase
25.32s
25.28
48m
48.04
53.8 w
53.8
Rutile
54.4m
54.4
a
s=strong; m=medium; w=weak.
Group II, III and V were not measured by Raman spectroscopy.
Fig. 4. XRD spectra from all five Groups measured on plate types of
c.p. titanium samples.
Fig. 5. Raman spectra measured on the screw implant (Group I) and
anodized implant (Group IV).
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
497
position and sharpness of Raman spectra measured on
top, flank and valley of the thread were similar. The
absence of rutile features in XRD spectra may be due to
the different sensitivities of Raman spectroscopy com-
pared to XRD, but may also be due to the difference
between sample types used for XRD and Raman
analysis, respectively, e.g. Ti plate and screw shaped
implants.
For Group V, X-ray diffraction patterns showed a
strong peak near 25.31 and a medium peak near 481,
indicating an anatase structure. In addition, there was
a broadening near 54.51, which suggested the presence
of rutile phase as well [36]. As a whole, the present
results indicate that the crystal structures of anodic
oxide films consist mainly of (small cyrstallites of)
anatase with some admixture of rutile. The rutile
admixture increases with increasing anodic forming
voltage up to 380 V.
3.5. Surface chemical composition and titanium oxide
stoichiometry
3.5.1. AES survey spectra
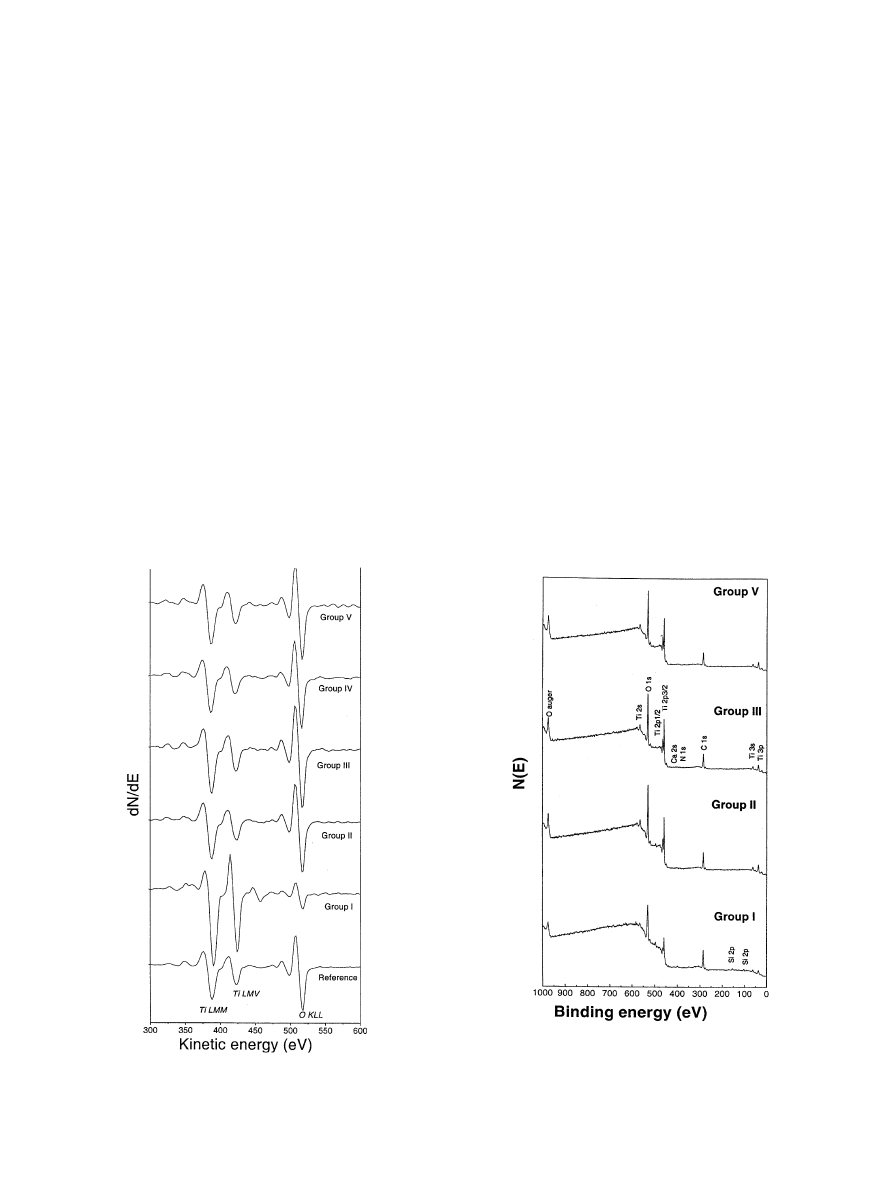
Fig. 6 shows typical AES survey spectra of turned
implants with native oxide (Group I), anodized implants
(Group II–V) and reference TiO
2
after sputter cleaning.
The survey spectra were dominated by strong signals of
Ti, O and C. Anodized implants (Group II–V) and
reference TiO
2
revealed similar shapes of the Auger
peaks. All the given oxide samples revealed Ti LMM
peaks at
E389 eV, Ti LMV at E422 eV and O KLL at
E517 eV. The relative intensity of the C signal and other
trace elements such as Na, Si, Ca, Al and Fe showed
some variation from group to group and from measur-
ing area to area. After argon sputter cleaning, corre-
sponding
approximately
to
1.4 nm
of
the
oxide
thickness, C, Na, Si and Ca almost disappeared.
This suggests that the trace elements are heterogeneous
and appear on the surfaces. In addition, disappearance
of the C signal implies the presence of carbon elements
on oxide surfaces only and the films of Group II–V did
not incorporate CH
3
COO– species into oxide film
during anodizing in CH
3
COOH, but rather from a
monomolecular layer of the contaminated surface
during sample transfer. It has been discussed that any
surface exposed to the ambient is always contaminated
by a chemically heterogeneous hydrocarbon film that
may be as thick as between 10% and 100% of a
monolayer [37].
Fig. 6. AES survey spectra of the surface oxides after sputter-cleaning
to a depth of about 1.4 nm from reference TiO
2
, turned screw implants,
Group I and anodized screw implants of Group II–V.
Fig. 7. Typical XPS spectra from as-received oxide surfaces at the
binding energy up to 1000 eV: turned screw implants, Group I and
anodized screw implants of Group II, Group III and Group V (Group
IV revealed a similar spectrum as the other anodized samples, thus not
shown here).
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
498
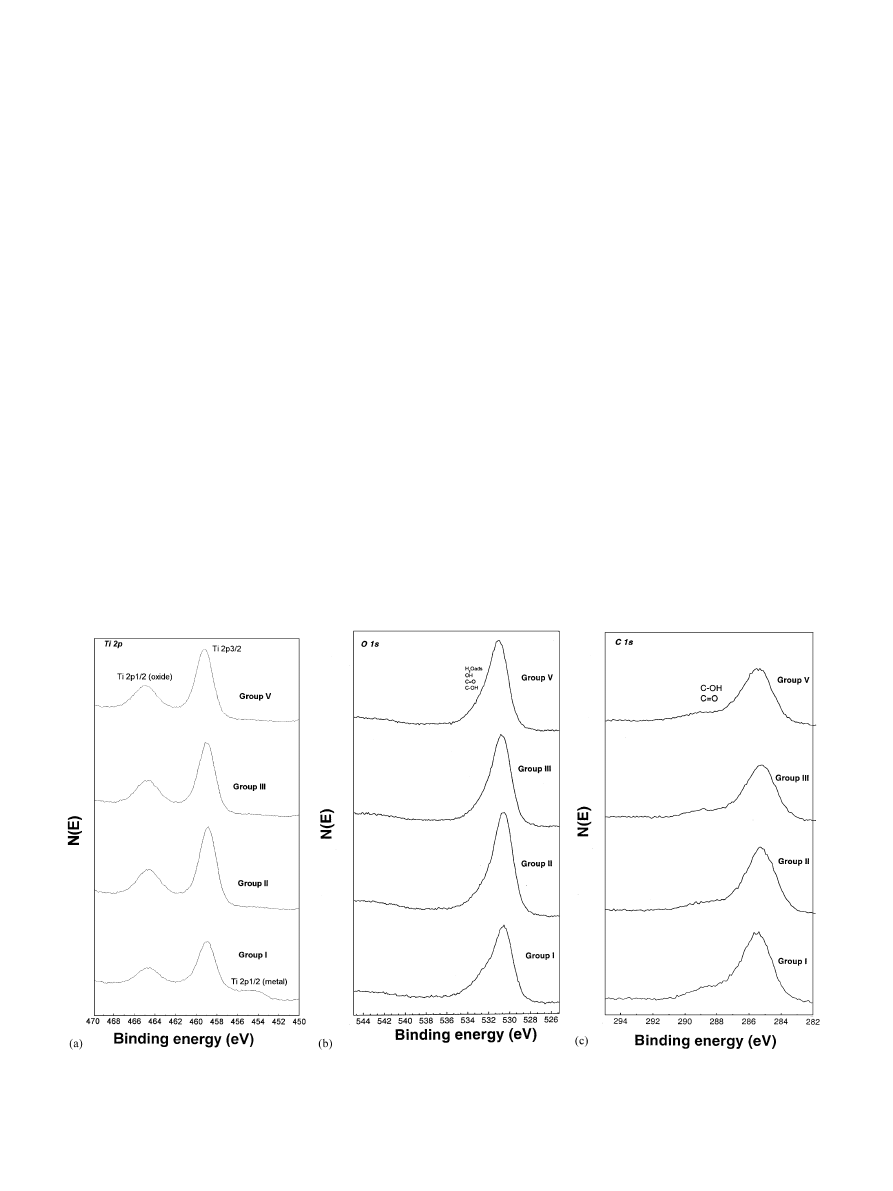
3.5.2. XPS survey spectra
Fig. 7 shows a typical XPS survey spectra from as-
received oxide surface on turned and anodized screw
implants. The detected elements consisted primarily of
Ti, O and C. Traces of Ca, Si, Na and N were also
observed. The detected elements in anodized screw
implants (Group II–V) were nearly the same as those in
turned screw implant (Group I). Surface elements
detected by XPS spectra were generally similar to those
detected by AES, considering that XPS is relatively
more sensitive to elements with a higher atomic number
while AES is relatively more sensitive to elements with a
lower atomic number. Traces of Cr and Ni were detected
at only two implants sterilized using an autoclave
together with surgical instruments, but not for the
others sterilized in another autoclave without surgical
instruments.
3.5.3. XPS line shapes of high resolution spectra
Fig. 8a–c shows typical XPS high resolution spectra
of Ti 2p, O 1s, and C 1s energy region from turned and
anodized implants. None of the Groups showed an
essential difference in terms of titanium oxide stoichio-
metry. Spectra of Ti 2p energy region consisted
dominantly of a doublet peak of at
E459 eV and
E464.8 eV (Fig. 8a). These two major peaks in each
sample were attributed to the tetravalent titanium form,
i.e., Ti
4+
2p3/2 and Ti
4+
2p1/2, respectively. Generally,
the present peak positions and line shapes are in good
agreement with those observed for TiO
2
reference
spectra [38]. Turned implants (Group I) showed a broad
shoulder of the spectrum at
E453.5 and E455.1 eV,
corresponding to Ti12p peak and Ti
2+
2p peak, respec-
tively. However, this broadening was not detected in all
the anodized implants (Group II–V). This may be due to
the relatively thin oxide thickness of 17 nm in Group I in
comparison to 200–1000 nm in Group II–V. In addition,
minor contributions between Ti
4+
2p3/2 peak and
Ti12p3/2 peak for turned implants may suggest the
presence of admixtures of other (hydro-) oxides.
Previous studies have reported non-stoichiometries of
titanium dioxide such as TiO and Ti
2
O
3
[39,40] and the
presence of carbide (TiC
x
) or nitride (TiN
x
) during
machining [7]. A three-layer model [41], i.e. an
intermediate suboxide TiO
2
@x
layer between a TiO
layer contacting with the titanium substrate and the
TiO
2
being in the outermost layer of the titanium oxide
has been proposed which is in rough agreement with our
results.
The typical high resolution XPS spectrum of the O 1s
peak shows a main peak at
E530.4 eV and asymmetrical
broadening in the range of 530.4–535.7 eV (Fig. 8b). The
O 1s peak at
E530.4 eV for both turned and anodized
implants is most certainly ascribed to oxygen in TiO
2
.
Fig. 8. Typical XPS high resolution spectra from as-received oxide surfaces of turned screw implants, Group I and anodized screw implants of
Group II, Group III and Group V (Group IV revealed similar spectra as the other anodized samples, thus not shown here) at: a=the Ti 2p region;
b
=the O 1s region; c=the C 1s region.
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
499

However, asymmetrical broadening of the O 1s spectra
may be deconvoluted into a peak at
E533 eV for the
contaminated O
2
@
organic molecules such as C=O, O–
C=O, etc. [7,42] and a peak at
E532.4 eV related to OH
(for example from chemisorbed H
2
O or Ti–OH) [43–45].
Another explanation for the oxygen asymmetry in
anodized screw implants may be due to the appearance
of the patches of non-stoichiometric Ti oxides. It has
been reported that Ti–OH formation during anodic
oxidation [46] and defect concentration profile were
markedly dependent on the growth rate of the anodic
oxide film. Oxygen overstoichiometry in the presence of
hydrogen was also observed in the outer part of the
anodic oxide layer, using Rutherford back scattering
[47].
Fig. 8c shows high resolution spectra of the C 1s
region from turned implants and anodized implants.
The main peak of all Groups was dominated at 285 eV.
There was also an asymmetrical broadening of the
major peak toward the higher binding energy for all
group implants. This indicates that the presence of
various carbon species is essentially not a constituent of
the surface oxide layer but rather attributable to surface
contamination, by absorbed organic carbon-containing
molecules during sample handling.
4. Summary and conclusions
The thickness of the anodic oxide films prepared on
turned screw implants in CH
3
COOH at galvanostatic
mode was estimated to be 200 nm (
753) at 100 V,
600 nm (
7127) at 200 V, 800 nm (7112) at 280 V, and
1000 nm (
7200) at 380 V. With such an increase of the
oxide thickness, the microstructural properties and
crystallinity of the titanium oxide varied substantially
with the oxide thickness. In oxides thicker than 600 nm,
porous microstructures appeared due to voltage surge
and micro arcing (breakdown phenomenon). Quantita-
tive morphometric analysis of the micropore structures
for the anodized screw implants (Group III–IV) was
performed. The pore sizes were
p8 mm in diameter and
were about 1.27–2.1 mm
2
by opening area. The porosity
(a total area of opening pores/an entire scanning
area
F3 mm 26 mm 20 mm) ranged between 12.7%
and 24.4%. The crystal structures of the titanium oxide
revealed different oxide structures at different thick-
nesses: thermal oxide was amorphous (the films in
Group I and II may be too thin to be characterized by
XRD measurement) while anodic oxidation produced
mainly the anatase phase. Increasing oxide thickness
(voltage) resulted in the increase % of admixture of the
rutile into a main anatase phase. The chemical
compositions were essentially similar for all studied
implant Groups, consisting mainly of TiO
2
. The
contamination level of the surface elements was strongly
dependent on the handling procedures of the samples. In
conclusion, the present results describe native/anodic
oxide characteristics of titanium implants that may be
analyzed in future studies on how surface oxide
characteristics influence biological reactions.
Acknowledgements
This study was supported by grants from the Hjalmar
Svensson
Research
Foundation
and
the
Swedish
Medical Research Council.
References
[1] Br
(aanemark PI, Hansson BO, Adell R, Breine U, Lindstr.oom J,
Hall
!een O, .O
Ohman A. Osseointegrated implants in the treatment
of the edendulous jaw. Experience from a 10-year period. Scan
J Plast Reconstr Surg 1977;Suppl 16(1):7–127.
[2] Wyatt CCL, ZarbGA. Treatment outcomes of patients with
implant-supported fixed prostheses. Int J Oral Maxillofac
Implants 1998;13:204–11.
[3] Lekholm U, Gunne J, Henry P, Higuchi K, Lind
!een U, Bergstr.oom
C, Steenberghe DV. Survival of the Br
(aanemark implant in
partially edentulous jaws: a 10-year prospective multicenter study.
Int J Oral Maxillofac Implants 1999;14:639–45.
[4] Ivanoff CJ, Gr
.oodahl K, Bergstr.oom C, Lekholm U, Br(aanemark PI.
Influence
of
bicortical
or
monocortical
anchorage
on
maxillary implant stability: a 15-year retrospective study of
Br
(aanemark system implants. Int J Oral Maxillofac Implants
2000;15:103–10.
[5] Albrektsson T, Johansson CB. Experimental and clinical studies
of different ways to improve the outcome of implants placed in
bone of deficient quality and quality. J Parodontol a
D’Implantol Orale 2000;19:271–88.
[6] Albrektsson T, Br
(aanemark PI, Hansson HA, Kasemo B, Larsson
K, Lundstr
.oom I, McQueen D, Skalak R. The interface zone of
inorganic implants in vivo: titanium implants in bone. Ann
Biomed Eng 1983;11:1–27.
[7] Lausmaa J, Kasemo B. Surface spectroscopic characterization
of titanium implant materials. Appl Surf Sci 1990;44:133–46.
[8] Binon PP, Weir DJ, Marshall SJ. Surface analysis of an original
Br
(aanemark implant and three related clones. Int J Oral Maxillofac
Implants 1992;7:168–74.
[9] Olefjord I, Hansson S. Surface analysis of four dental implant
system. Int J Oral Maxillofac Implants 1993;8:32–40.
[10] Machnee CH, Wagner WC, Jaarda MJ, Lang BR. Identification
of oxide layers of commercially pure titanium in response to
cleaning procedures. Int J Oral Maxillofac Implants 1993;
8:529–33.
[11] W
.aalivaara B, Aronsson BO, Rodahl M, Lausmaa J, Tengvall P.
Titanium with different oxides: in vitro studies of protein
adsorption and contact activation. Biomaterials 1994;9:827–34.
[12] Wennerberg A, Albrektsson T, Andersson B. Design and surface
characteristics of 13 commercially available oral implant systems.
Int J Oral Maxillofac Implants 1993;8:622–33.
[13] Zitter H, Plenk HJ. The electrochemical behaviour of metallic
implant materials as indicator of their biocompatibility. J Biomed
Mater Res 1987;21:881–96.
[14] Jackson JD, Boyd WK. Stress corrosion cracking in titanium and
alloys. In: Jaffee RI, Promisel NE, editors. The science,
technology and application of titanium. Proceeding of an
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
500

International
Conference.
Oxford:
Pergamon
Press,
1968.
p. 267–81.
[15] William DF. Corrosion of implant materials. Ann Rev Mater Sci
1976;6:237–65.
[16] Solar RJ, Pollack SR, Korostoff E. In vitro corrosion testing of
titanium surgical implant alloys: an approach to understanding
titanium release from implants. J Biomed Mater Res 1979;
13:217–50.
[17] Tengvall P, Lundstr
.oom I. Physico-chemical considerations of
titanium as a biomaterial. Clin Mater 1992;9:115–34.
[18] Blackwood DJ, Petter LM. The influence of growth rate on the
properties of anodic oxide films on titanium. Electrochim Acta
1989;34:1505–11.
[19] Sul YT, Johansson CB, Jeong Y, Albrektsson T. The electro-
chemical oxide growth behaviour on titanium in acid and alkaline
electrolytes. Medical Engineering and Physics 2001, accepted for
publication.
[20] Mattsson L, Rolander U. Structure and morphology of anodic
oxide films on titanium. G
.ooteborg Institute of Physics Report
GIPR-264, Chalmers University of Technology, G
.ooteborg,
Sweden, 1985.
[21] Tabrizi R. Surface treatments of titanium and its alloy. PhD
Thesis, University of Manchester, Institute of Science and
Technology, Corrosion and Protection Centre, UK, 1989.
[22] Lausmaa J. Surface oxides on titanium: preparation, character-
ization and biomaterial applications. PhD Thesis, Chalmers
University of Technology, G
.ooteborg, Sweden, 1991.
[23] Jeong Y. The structural chemistry of anodic alumina. PhD Thesis,
The University of Manchester Institute of Science and Technol-
ogy, Corrosion and Protection Centre, UK, 1993.
[24] Ishizawa H, Ogino M. Formation and characterization of anodic
titanium oxide films. J Biomed Mater Res 1995;29:65–72.
[25] Ishizawa H, Ogino M. Characterization of thin hydroxyapatite
layers formed on anodic titanium-oxide films containing C and
P by hydrothermal treatment. J Biomed Mater Res 1995;29:
1071–9.
[26] Ishizawa H, Ogino M. Thin hydroxyapatite layers formed
on porous titanium using electrochemical and hydrothermal
reaction. J Mater Sci 1996;31:6279–84.
[27] Schreckenbach J, Schlottig F, Textor M, Spencer ND, Marx G.
Characterization of anodic spark-converted titanium surfaces for
biomedical applications. J Mater Sci Med 1999;10:453–7.
[28] Sul YT, Johansson CB, Jeong Y, Wennerberg A, Albrektsson T.
Resonance frequency- and removal torque analysis of implants
with turned and anodized surface oxides. Clin Oral Impl Res
2001, accepted for publication.
[29] Sul YT, Johansson CB, R
.ooser K, Albrektsson T. Qualitative and
quantitative observations of bone tissue reactions to anodised
implants. A histologic, enzyme histochemical and histomorpho-
metric analysis. Biomaterials, submitted.
[30] Mathieu HJ, Mathieu JB, McClure DE, Landolt D. Beam effects
in Auger electron spectroscopy analysis of titanium oxide films.
J Vac Sci Technol 1977;14:1023–8.
[31] Wennerberg A. On surface roughness and implant incorporation.
PhD Thesis, University of G
.ooteborg, Sweden, 1966.
[32] Leach JSL, Pearson BR. Crystallization in anodic oxide films.
Corros Sci 1988;28:43–56.
[33] Ohtuka T, Guo J, Sato N. Raman spectra of the anodic oxide film
on titanium in acidic sulfate and neutral phosphate solutions.
J Electrochem Soc 1986;133:2473–6.
[34] Arsov LD, Kormann C, Plieth W. Electrochemical synthesis and
in situ Raman spectroscopy of thin films of titanium oxide.
J Raman Spectrosc 1991;22:573–5.
[35] Felske A, Plieth WJ. Raman spectroscopy of titanium dioxide
layers. Electrochim Acta 1988;34:75–7.
[36] JCPDS
FInternational Centre for Diffraction Data, 1994.
[37] Kasemo B, Lausmaa J. The biomaterial–tissue interface and its
analogues in surface science and technology. In: David JE, editor.
The bone–biomaterials interface. Toronto: University of Toronto
Press, 1991. p. 19–29.
[38] Chastain J, King RC. Handbook of X-ray photoelectron
spectroscopy. Eden Prairie, MN: Perkin-Elmer, 1992.
[39] Lewis G. X-ray photoelectron study of surface layers on
orthophedic alloy. J Vac Sci Technol A 1993;11:325–35.
[40] Pouilleau P, Devillier D, Garrido F, Durand-Vidal S, Mah
!ee E.
Structure and composition of passive titanium oxide films. Mater
Sci Eng 1997;B47:235–43.
[41] Blackwood DJ, Greef R, Peter LM. An ellipsometric study of the
growth and open-circuit dissolution of the anodic oxide film on
titanium. Electrochim Acta 1989;34:875–80.
[42] Briggs D. Applications of XPS in polymer technology. In: Briggs
D, Seah MP, editors. Practical surface analysis, vol. 1: auger and
X-ray photoelectron spectroscopy, 2nd ed. New York: Wiley,
1996. p. 437–83.
[43] Gopel W, Anderson JA, Frankel D, Jaehnig M, Phillips K,
Schafer JA, Rocker G. Surface defects of TiO
2
(1 1 0): a combined
XPS, XAES and ELS study. Surf Sci 1984;139:333–46.
[44] Shirkhanzadeh M. XRD and XPS characterization of super-
plastic TiO
2
coatings prepared on Ti6Al4 V surgical alloy by
an electrochemical method. J Mater Sci: Mater Med 1995;6:
206–10.
[45] Shibata T, Zhu YC. The effect of film formation conditions on the
structure and composition of anodic oxide films on titanium.
Corros Sci 1995;37:253–70.
[46] Ohtsuka T, Nomura N. The dependence of the optical property of
Ti anodic oxide film on its growth rate by ellipsometry. Corros Sci
1997;39:1253–63.
[47] Serruys Y, Sakout T, Gorse D. Anodic oxidation of titanium in
1M H
2
SO
4
, studied by Rutherford backscattering. Surf Sci
1993;282:279–87.
Y.-T. Sul et al. / Biomaterials 23 (2002) 491–501
501
Wyszukiwarka
Podobne podstrony:
In silico characterization of the family of PARP like
Fricke Visual assessments of the surface diusion properties of concert halls
Ultrastructural characterization of the implant
Characterization of titanium surfaces with calcium and phosp
analytical characterisation of the routes by thermolytic decarboxylation from tryptophan to tryptami
Crime and Punishment Analysis of the Character Raskol
Lord of the Flies Character Changes in the Story
65 935 946 Laser Surface Treatment of The High Nitrogen Steel X30CrMoN15 1
Empire of the Petal Throne Generating Pe Choi Characters in Gardasiyal
Death of a Salesman Analysis of the Character Willy
16 Changes in sea surface temperature of the South Baltic Sea (1854 2005)
Best Available Techniques for the Surface Treatment of metals and plastics
Crime and Punishment Analysis of the Character Raskolnikov
Detection and Molecular Characterization of 9000 Year Old Mycobacterium tuberculosis from a Neolithi
The characteristics of japanese tendai
Bondeson; Aristotle on Responsibility for Ones Character and the Possibility of Character Change
How to Have the Character of a Champion
0198752091 Oxford University Press USA The Character of Mind An Introduction to the Philosophy of Mi
Corrosion behavior and surface characterization of titanium
więcej podobnych podstron