
* Corresponding author. Tel.: #81-88-633-7333; fax: #81-88-633-
9125.
E-mail address: asaoka@dent.tokushima-u.ac.jp (K. Asaoka).
Biomaterials 22 (2001) 2257}2262
Degradation and fracture of Ni}Ti superelastic wire in an oral cavity
Ken'ichi Yokoyama , Kenichi Hamada , Keiji Moriyama
, Kenzo Asaoka *
Department of Dental Engineering, School of Dentistry, Tokushima University, 3-18-15 Kuramoto-cho, Tokushima 770-8504, Japan
Department of Orthodontics, School of Dentistry, Tokushima University, 3-18-15 Kuramoto-cho, Tokushima 770-8504, Japan
Received 21 June 2000; accepted 25 November 2000
Abstract
Superelastic Ni}Ti wire is widely used in orthodontic clinics, but delayed fracture in the oral cavity has been observed. Because
hydrogen embrittlement is known to cause damage to Ti alloy systems, orthodontic wires were charged with hydrogen using an
electro-chemical system in saline. Tensile tests were carried out, and fracture surfaces were observed after hydrogen charging. The
strength of the Co}Cr alloy and stainless steel used in orthodontic treatment, was not a!ected by the hydrogen charging. However,
Ni}Ti wire showed signi"cant decreases in strength. The critical stress of martensite transformation was increased with increasing
hydrogen charging, and the alloy was embrittled. The fractured surface of the alloys with severe hydrogen charging exhibited dimple
patterns similar to those in the alloys from patients. In view of the galvanic current in the mouth, the fracture of the Ni}Ti alloys might
be attributed to the degradation of the mechanical properties due to hydrogen absorption.
2001 Elsevier Science Ltd. All rights
reserved.
Keywords: Hydrogen; Ni}Ti alloy; Superelasticity; Orthodontic wire; Tensile strength
1. Introduction
Many alloy systems show shape memory and/or
superelastic behavior but a few of themhave been de-
veloped on a commercial scale for industrial and medical
use. In these alloys, recent innovations in the processing
of Ni}Ti alloy systems have improved both mechanical
properties and formability [1]. The alloy has shown
excellent ductility and fatigue life, and typical ductile
features such as reduction in the area at fracture and
dimple patterns in its fracture pattern [2]. Because of
their good corrosion resistance and biocompatibility,
Ni}Ti alloys as well as titanium, titanium alloys, Co}Cr-
based alloys and stainless steels have been successfully
used as biomaterials [3}8]. Ni}Ti arch wire is widely
used in orthodontic clinics because it allows the teeth to
move under almost constant force over a long treatment
time, and a much larger displacement of the teeth can be
achieved before the dentist has to retighten the wire.
However, it is known that some wires may break in the
oral cavity after a few months from setting. Some dentists
have recognized that the alloy is pliable during setting
but that the broken wire had lost its deformability [9].
Research on hydrogen fuel as a substitute for fossil fuel
lead to the development of hydrogen storage alloys. In
such research, alloy systems such as titanium, zirconium,
nickel and palladiumalloys were identi"ed as materials
having the ability to absorb hydrogen. Furthermore,
hydrogen absorption was shown to degrade the mechan-
ical properties of those alloys [10]. For example, the
delayed fracture of high-strength steel was attributed to
hydrogen absorption in ambient air [11]. Mechanisms of
hydrogen embrittlement of the alloy are not clearly
understood [12,13]. The absorption of hydrogen easily
occurs in a bioenvironment compared with the ambient
air because hydrogen ions exist in saliva and galvanic
currents may accelerate absorption. Fretting corrosion
might be a contributing factor because hydrogen ions
generally are associated with repassivation of the alloys
with an oxide "lm. The alloys under the same chemical
concentration of hydrogen do not always show brittle-
ness, because hydrogen susceptibility is a unique prop-
erty of the individual alloy.
The e!ect of hydrogen uptake on functions of shape
memory and/or superelasticity for Ni}Ti alloys was
studied [14}16]. However, delayed fracture due to hy-
drogen embrittlement has not been studied in the dental
0142-9612/01/$ - see front matter
2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 0 ) 0 0 4 1 4 - 2
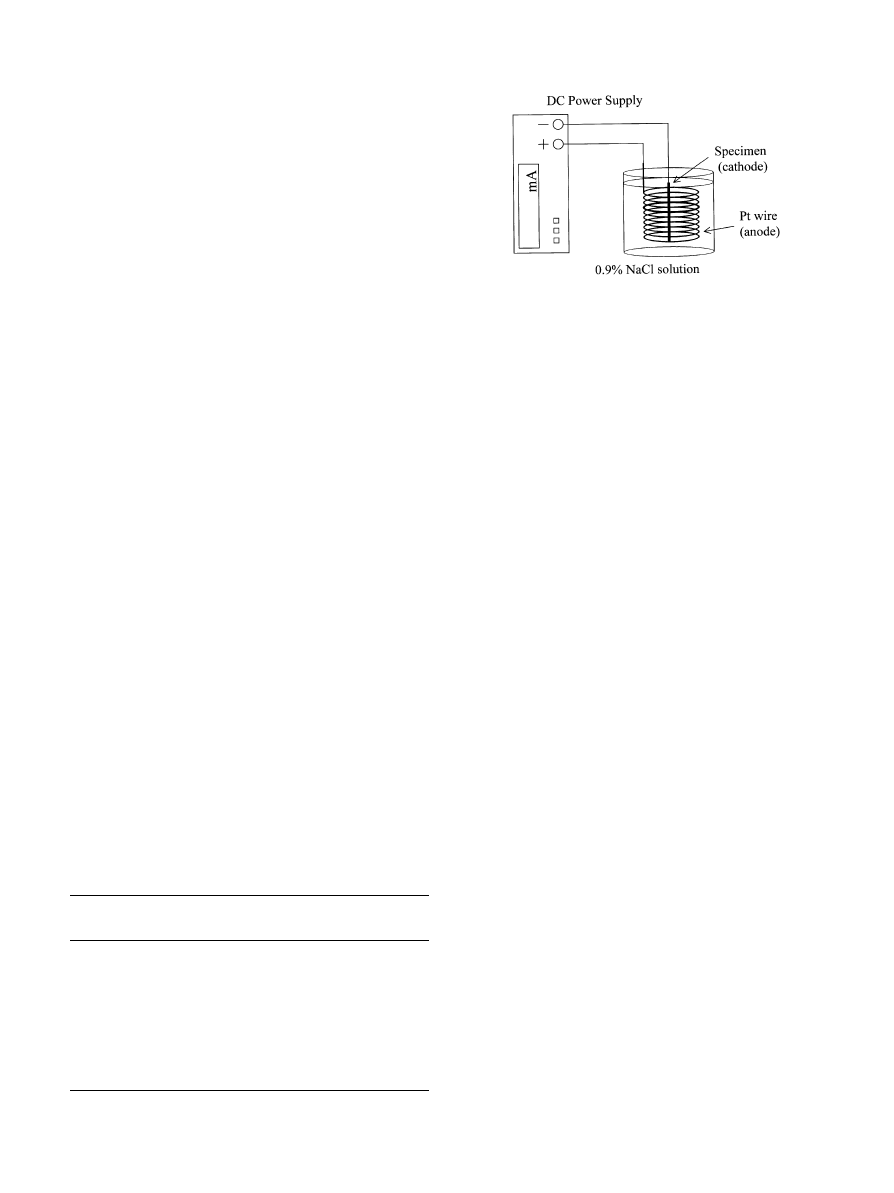
Table 1
Compositions and lot numbers of the commercial orthodontic wires
used
Wire alloy
Brand name
General compositions
(wt%)
Lot number
Ni}Ti
Sentalloy light
55.8% Ni, 44.2% Ti
D4043
Co}Cr
Elgiloy blue
40% Co, 20% Cr,
15% Ni, 15.8% Fe,
7% Mo, 2% Mn,
0.15% C, 0.04% Be
19990614
Stainless
steel
Tomy staineless-
steel red
17}20% Cr, 8}12%
Ni, 0.15% C maxi-
mum, balance
principally Fe
A399
Fig. 1. Scheme of hydrogen charging system by electrolysis.
or medical "eld. Corrosion, fatigue and abrasive wear of
medical/dental devices are reported, but degradation of
its mechanical properties due to microstructural change
has not been touched upon [4,17]. In general, the e!ect of
hydrogen on the mechanical properties of the alloys is
tested using exposure or immersion tests. However, these
tests are not practical because the alloys must be kept in
a speci"c environment for long periods. Hydrogen em-
brittlement of Ni}Ti wires could occur in service. The
aimof this work is to evaluate the conditions of its
occurrence, its extent, and its e!ects on the mechanical
properties of the wires. For this purpose, accelerated
hydrogen embrittlement tests using an electro-chemical
reaction in saline solution were carried out. Stress}strain
curves of the alloys charged with hydrogen were mea-
sured and related to the current density as an indication
of the hydrogen concentration. The fracture surfaces of
the samples were observed.
2. Materials and methods
Three kinds of commercial orthodontic wires with
a diameter of 0.56 mm are used, i.e., Ni}Ti alloy (Sental-
loy Light, Tomy International Inc., Tokyo, Japan),
Co}Cr alloy (Elgiloy Blue, Rocky Mountain Morita Cor-
poration, Tokyo, Japan) and stainless-steel (Tomy Stain-
less-Steel Red, Tomy International Inc., Tokyo, Japan).
Compositions and lot numbers are listed in Table 1.
A direct-current generator (7011DC Signal Source,
Hioki, Ueda, Japan) was used for the charging of hydro-
gen, as shown in Fig. 1. Platinumand the specimen alloy
were based in 0.9% NaCl solution as the anode and
cathode, respectively. Gaseous hydrogen, which was gen-
erated on the surface of the sample, was controlled by the
current density. The alloys were charged with hydrogen
at roomtemperature for 24 h with di!erent current dens-
ities of 100, 200 and 300 A/m
. For Ni
}Ti alloys, speci-
mens with current densities of 1, 2, 5, 7, 10, 15 and
20 A/m
were arranged. The e
!ect of charging time on
the mechanical properties of the alloys was measured
under constant current densities of 1, 2, 5 and 10 A/m
,
up to 144 h. Tensile tests of the charged alloys were
carried out at roomtem
perature on an Instron-type
machine (Autograph AG-100A, Shimadzu, Kyoto, Ja-
pan) at a strain rate of 8.33
;10\/s within a few minutes
after removal from the electrolytic bath. Here, the total
length of the specimen was 50 mm and a gage length of
10 mm was used. Apparent strain was calculated from the
displacement of the cross-head position and initial gage
length. To assure quality of the as-received Ni}Ti wires,
stress}strain curves were measured and statistical analy-
sis was carried out. The fractured surface after rupture
test of each specimen was observed by scanning micros-
copy.
3. Results
Tensile strength of the Co}Cr and stainless-steel alloys
charged with hydrogen for 24 h, was not a!ected by the
charged hydrogen, as shown in Fig. 2. However, the
Ni}Ti superelastic alloys exhibited typical hydrogen em-
brittlement (decrease of strength). Typical superelastic
strain was observed for the as-received Ni}Ti alloys; that
is, after reaching a critical transformation stress
(240 MPa), the alloy starts to transform(fromaustenite
to martensite). During further straining, the stress at
which the transformation occurs is almost constant until
the material is fully transformed. Further straining leads
to elastic loading of the martensite, followed by plastic
deformation and fracture (1164 MPa). Tensile strength
and transformation stress of the as-received wires were
1164$6 MPa and 240$5 MPa, respectively, for seven
samples. Here, the standard deviation was calculated
(p(0.05). This result indicated that the wires used were
high-quality products. Thus, the roles of impurities and
scratches were negligible factors in fracture mechanics.
Because measurement of the exact amount of hydrogen
absorbed in the alloy after charging is di$cult, the
stress}strain curves were measured for the alloys with di!er-
ent experimental conditions of charging and compared.
2258
K. Yokoyama et al. / Biomaterials 22 (2001) 2257}2262
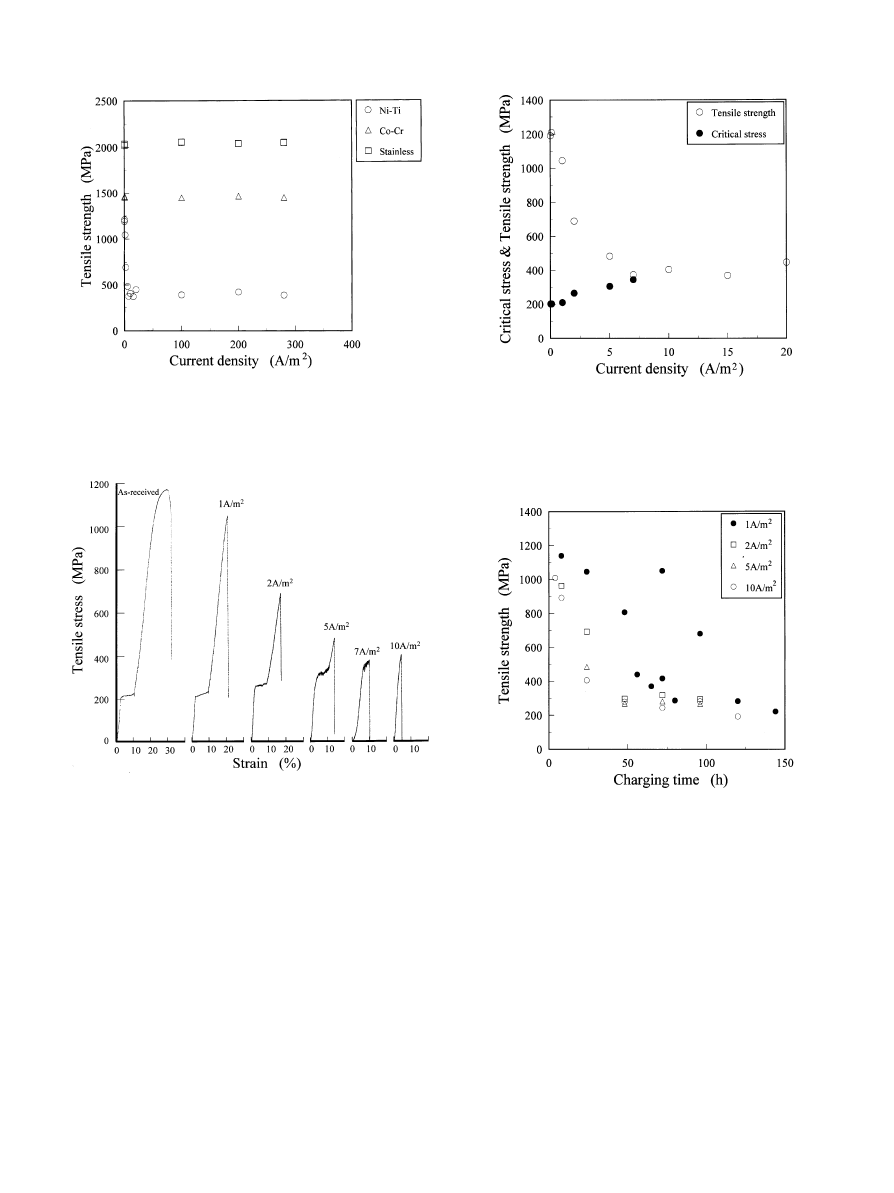
Fig. 2. Each symbol represents the strength of the sample with the
charged hydrogen which was controlled by the current density. Charg-
ing time of 24 h is measured.
Fig. 3. Stress}strain curves of the Ni}Ti superelastic alloy charged with
hydrogen and as-received alloy. Current densities of 1, 2, 5, 7, and
10 A/m
are charged for 24 h. Strain is calculated fromelongation
(displacement of cross-head) and the initial gauge length.
Fig. 4. Each symbol represents the critical stress due to stress-induced
transformation (from austenite to martensite) and tensile strength of the
Ni}Ti superelastic alloy which had been charged with hydrogen with
controlled current density. Charging time of 24 h is used.
Fig. 5. Each symbol represents the tensile strength of Ni}Ti alloys
which had been charged at di!erent current densities and charging
times.
The strength of the superelastic alloys decreased from
1200 to 400 MPa after the absorption of hydrogen. The
stress}strain curves of the Ni}Ti alloys charged with
hydrogen for 24 h are shown in Fig. 3. The lower limit of
the strength was about 400 MPa less when restricted to
current density higher than 7 A/m
, as shown in Fig. 4. At
the higher current density of 10 A/m
, the alloy was
fractured under the critical stress of martensitic trans-
formation. Critical stress for martensitic transformation
increased with from200 to 400 MPa when a high current
density was supplied.
Fig. 5 shows the e!ect of charging time on the strength
of the Ni}Ti alloys. Here, the densities of direct current 1,
2, 5 and 10 A/m
are plotted. In these results, only the
data of 1 A/m
indicated a large spread of values in
tensile strength. The critical stress does not increase with
the charging time for 1 A/m
, and the sample breaks after
stress-induced transformation. Stress-strain curves of the
alloys, which had been charged with 1 and 10 A/m
are
shown in Fig. 6.
Fig. 7(a) and (b) show the fracture surface of a super-
elastic arch wire, which broke in an oral cavity. The wire,
K. Yokoyama et al. / Biomaterials 22 (2001) 2257}2262
2259
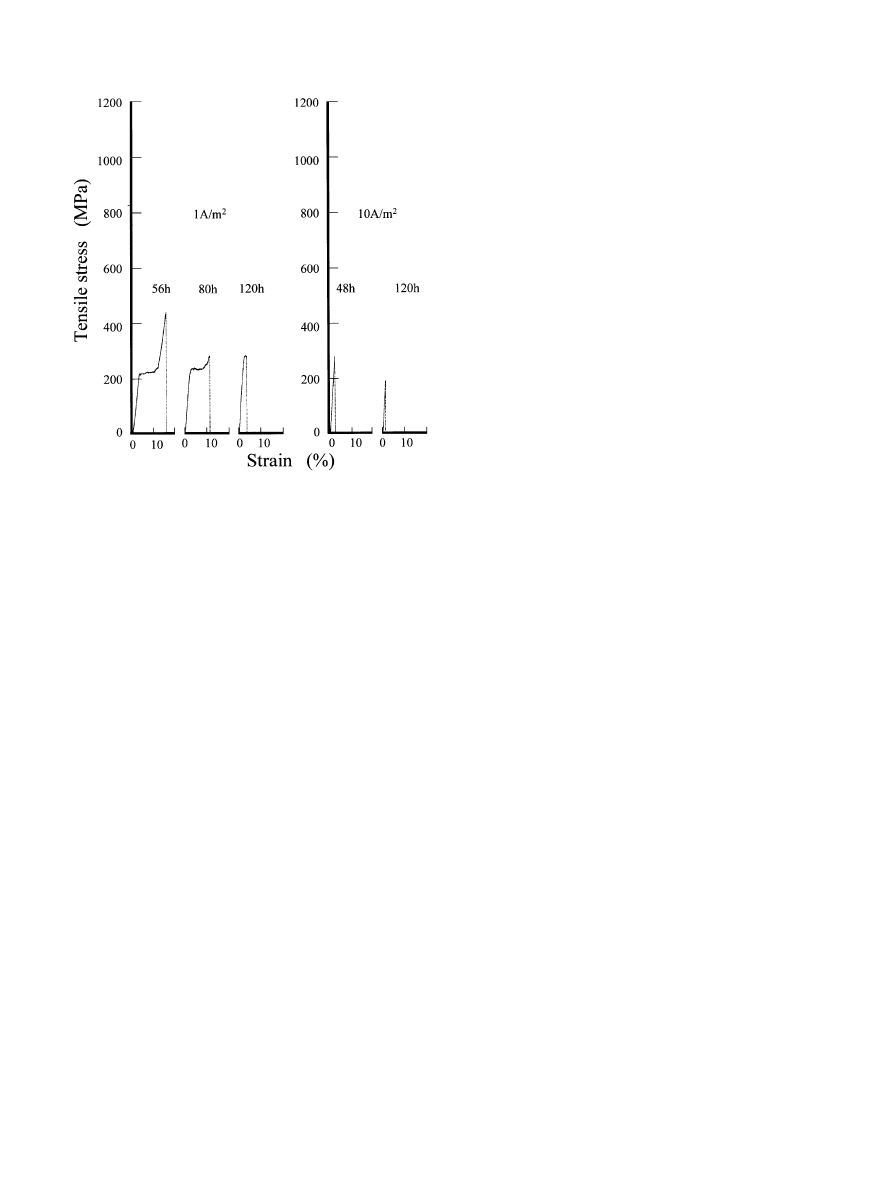
Fig. 6. Typical stress-strain curves of the Ni}Ti superelastic alloy with
hydrogen charging. Current densities of 1 or 10 A/m
in given periods
of time were charged for each alloy.
which had been suspended for about one month by the
orthodontic brackets, was taken o! when the patient
returned for follow-up treatment. The typical reduction
in the area at fracture could not be con"rmed from Fig.
7(a). The alloy was washed in acetone using ultrasonic
cleaning equipment, but accretions were still observed.
The fracture surfaces of the as-received and hydrogen-
charged wires tested are shown in Fig. 7(c), (d) and (e), (f),
respectively. The reduction in the area is observed in
Fig. 7(c) for the as-received alloy. Ductile surfaces are
characterized by dimple morphology, which indicates
local strain. For as-received alloy, small dimples (second-
ary dimples) were observed in the areas between large
dimples (primary dimples), as shown in Fig. 7(d). How-
ever, dimples in Fig. 7(b) and (f ) are only primary and
shallow. These fracture micrographs indicated that the
as-received alloy had ductility but the alloys fractured in
an oral cavity and hydrogen severely charged were frac-
tured without plastic deformation. Dimples that are
hemispherical or equiaxial may form from the in#uence
of uniformplastic strain in the direction of applied stress.
The size of the dimple is determined by factors such as
the size of the inclusions, precipitate particles, and their
densities as well as the amount of plastic growth that
takes place. Secondary dimples may form in association
with the development of primary dimples. A high density
of inclusions (hydrides) may lead to low ductility and no
secondary dimpling.
4. Discussion
4.1. Hydrogen embrittlement of Ni}Ti alloy
Degradation by hydrogen absorption was only ob-
served for Ni}Ti in orthodontic wires (Fig. 2). The prom-
inent features of hydrogen embrittlement [10,12,18,19]
are as follows. (1) Small reduction of the area is exhibited
at fracture. (2) Surface and internal layers have di!erent
patterns of fractography, i.e., the surface layer exhibits
brittle fracture due to hydrogen absorption. (3) For cer-
tain alloys, a high degree of grain re"nement is observed.
Hydrogen absorption of the Ni}Ti alloys tested was
con"rmed from the degradation of mechanical proper-
ties, small reduction of the area and the typical fracture
features. Because the stress state and strain rate di!er
between the alloys in the mouth (bending with tension)
and the specimen in a tensile machine (pure tension),
di!erent dimple morphology such as the shape and size
are shown in Fig. 7(b) and (f ). However, both micro-
graphs show only shallow primary dimples and low duc-
tility. Fromthese common features, it was concluded that
the wire was broken by the occlusion of hydrogen in the
mouth. Titanium alloys are known as hydrogen absorb-
ent alloys [10]. In these alloys, hydrogen absorption can
occur not only in a solution of dissolved hydrogen ions,
but also in ambient air.
4.2. Mechanism of hydrogen embrittlement
The most probable mechanism for the absorption of
hydrogen within the alloy is inter-atomic di!usion from
the surface inward. Grain boundaries, dislocations in the
vicinity of impurity atoms and precipitated particles are
the positions for hydrogen accumulation [12,13]. When
the alloy is loaded, the absorption of hydrogen might be
accelerated because of expanded interatomic space and
increased density of dislocations. In this study, hydrogen
uptake is thought to be due to the following process, i.e.,
the gaseous hydrogen, which was generated by electroly-
sis was absorbed into the alloy, and di!used through it.
The surface layer of the wire was "rst embrittled by the
hydrogen. The hydrogen absorption of the wire, which
was set as an orthodontic attachment was accelerated
because the alloy was under tension. When the wire is
kept under the transformation (critical stress) stress-state,
hydrogen absorption might be accelerated.
4.3. Hydrogen absorption in oral cavity
It is not clear that the Ni}Ti alloy can naturally absorb
the hydrogen in saliva [9]. However, there are several
possible electrode systems in the mouth. One possible
systemis electric current due to electrolytic action in the
mouth. The galvanic currents between gold and amal-
gamwere determined in aerated arti"cial saliva kept at
2260
K. Yokoyama et al. / Biomaterials 22 (2001) 2257}2262
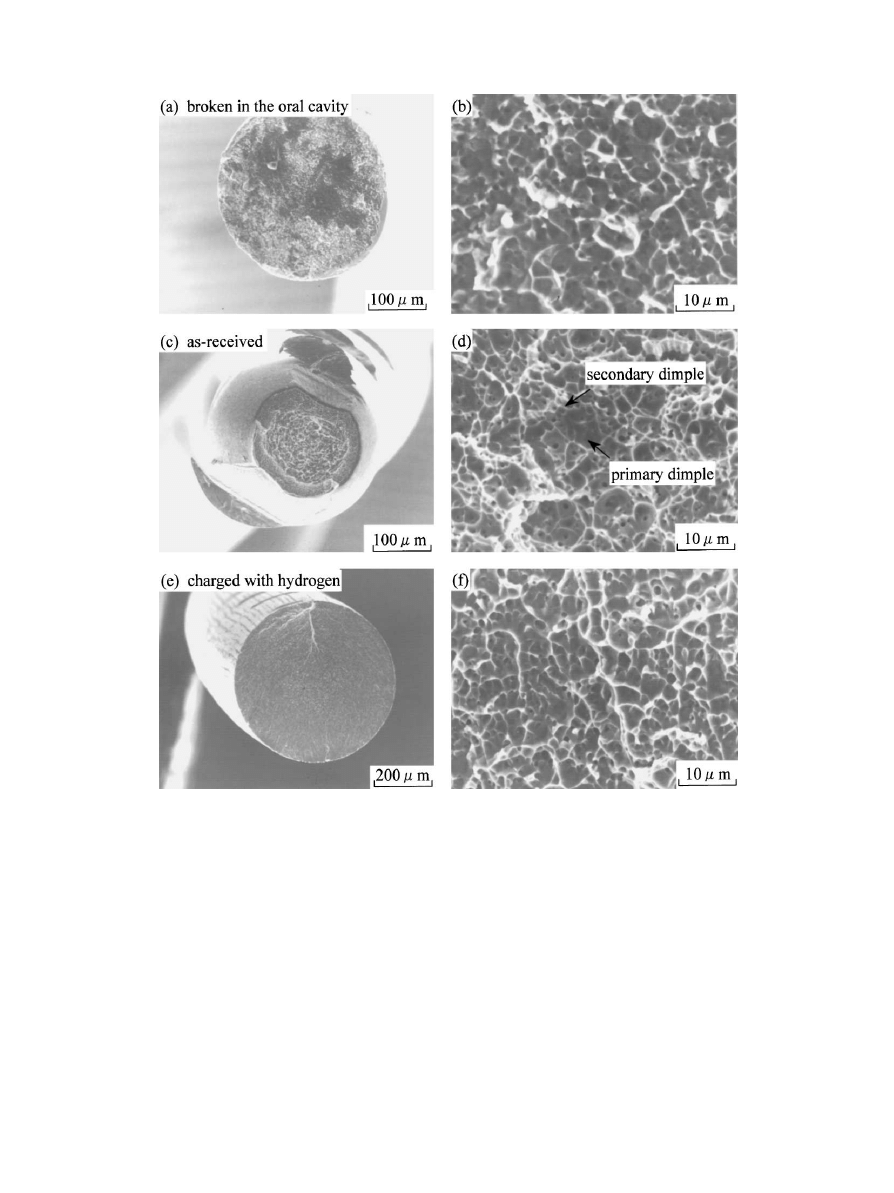
Fig. 7. Fractography of the Ni}Ti superelastic wires. (a) and (b) Ni}Ti superelastic wire which was broken in the oral cavity during use. (c) and (d)
as-received Ni}Ti superelastic wire. (e) and (f ) the wire which had been charged with hydrogen at current density of 1 A/m
for 120 h. Primary dimpling
is responsible for plastic deformation and secondary dimpling occurs only in high ductile fracture surfaces.
373C, and were reported to reach 2 A/m
[20]. Relative
position, shape, size and constitution of the restorated
alloys are the factors that determine the current density
in an oral cavity. Other factors are temperature, amount
and quality of saliva and the oral structure. Galvanic
current between the wire and the stainless-steel bracket is
also considered an electrolytic system. From these dis-
cussions, the current density of 1 A/m
used in this study
is not a high current, but is a possible condition in the
mouth. It is reasonable to assume that fretting corrosion
contributes to the hydrogen intake of the alloy with an
oxide "lm. Repassivation of titaniumand titaniumalloys
in water or saline can be represented as follows:
Ti#2HOPTiO#4H>#4e\.
(1)
For nickel and nickel alloys,
Ni#HOPNiO#2H>#2e\.
(2)
In these equations, hydrogen ions might act much in the
same way as does the hydrogen ion generated by the
electro-chemical reaction used in this research.
K. Yokoyama et al. / Biomaterials 22 (2001) 2257}2262
2261

4.4. Process to failure of the wire
In orthodontic treatments, Ni}Ti superelastic ar-
chwire, which was set in the mouth sustains a functional
stress of about 200 MPa. The wire, which was restricted
by brackets, was always kept at about 10% strain if the
wire did not undergo plastic deformation. When the alloy
absorbs hydrogen, the critical stress of martensite trans-
formation becomes higher, as shown in Fig. 3. Galvanic
currents and fretting corrosion can accelerate the absorp-
tion of hydrogen. This result suggested that the ortho-
dontic force increased with the increase of the critical
stress. At the same time, ductility of the alloy decreased
with the increase of hydrogen absorption. When the
strain restriction exceeds the fracture strain, the wire,
which had become brittle, may often fracture. The exact
process leading to the fracture of Ni}Ti wire, including
placement of the wire and skill of the orthodontist will be
considered further in a future paper.
Acknowledgements
This study was supported by the University of
Tokushima.
References
[1] Humbeeck JV, Stalmans R, Besselink PA. Shape memory alloys.
In: Helsen JA, Breme HJ, editors. Metals as biomaterials. Chi-
chester: Wiley, 1988. p. 73}100.
[2] Melton KN, Mercier O. Fatigue of NiTi thermoelastic marten-
sites. Acta Metall 1979;27:137}44.
[3] Oshida Y, Miyazaki S. Corrosion and biocompatibility of shape
memory alloys. Zairyo-to-Kankyo 1991;40:834}44.
[4] Shabalovskaya SA. On the nature of the biocompatibility and on
medical applications of NiTi shape memory and superelastic
alloys. Bio Med Mater Eng 1996;6:267}89.
[5] KimH, Johnson JW. Corrosion of stainless steel, nickel}titanium,
coated nickel}titanium, and titanium orthodontic wires. Angle
Orthod 1999;69:39}44.
[6] Rondelli G, Vicentini B. Localized corrosion behaviour in
simulated human body #uids of commercial Ni}Ti orthodontic
wires. Biomaterials 1999;20:785}92.
[7] Wever DJ, Veldhuizen AG, Sanders MM, Schakenraad JM, Horn
van
JR.
Cytoxicity,
allergic
and
genotoxic
activity
of
a nickel}titaniumalloy. Biomaterials 1997;18:1115}20.
[8] Wever DJ, Veldhuizen AG, Vries de J, Busscher HJ, Uges DRA,
Horn van JR. Electrochemical and surface characterization of
a nickel}titaniumalloy. Biomaterials 1998;19:761}9.
[9] Harris EF, Newman SM, Nicholson JA. Nitinol arch wire in
a simulated oral environment; Changes in mechanical properties.
AmJ Orthod Dentofac Orthop 1988;93:508}13.
[10] Nelson HG, Williams DP, Stein JE. Environmental hydrogen
embrittlement of
}
titaniumalloy: E
!ect
of microstructure.
Metall Trans 1972;3:469}75.
[11] BeachemCD. A new model for hydrogen-assisted cracking: hy-
drogen embrittlement. Metall Trans 1972;3A:437}51.
[12] Hirth JP. E!ects of hydrogen on the properties of iron and steel.
Metall Trans 1980;11A:861}90.
[13] Nagumo M. Fundamental aspects of hydrogen embrittlement of
iron. Materia Jpn 1994;33:914}21.
[14] Maslenkov SB, Budigina NB, Shorshorov MKH, Flomenblit
YUM. Shape memory e!ects, phase and structural transforma-
tions caused by hydrogen in alloys of the Ti}Ni system. Phys Met
Metallogr 1988;66:90}6.
[15] Adachi Y, Wade N, Hosoi Y. E!ect of hydrogen on the shape
memory e!ect and transformation behavior of Ti}Ni alloy. J Jpn
Inst Metals 1990;54:525}31.
[16] Asaoka T, Yamashita H, Saito H, Ishida Y. E!ect of a small
amount of hydrogen on pseudo-elastic properties of Ti}Ni alloy.
J Jpn Inst Metals 1993;57:1123}9.
[17] Ryhanen J, Niemi E, Serlo W, Niemela E, Sandvik P, Pernu H,
Salo T. Biocompatibility of nickel}titanium shape memory metal
and its corrosion behavior in human cell cultures. J Biomed
Mater Res 1997;35:451}7.
[18] Kerr WR. The e!ect of hydrogen as temporary alloying element
on the microstructure and tensile properties of Ti}6Al}4 V. Metall
Trans 1985;16A:1077}87.
[19] Yoshimura H, Kimura K, Hayashi M, Ishii M, Takamura J.
Ultra-"ne equiaxed grains obtained by process of hydrogenation:
aging and dehydrogenation in
# type titaniumalloys. J Jpn
Inst Metals 1991;55:1375}81.
[20] Holland RI. Galvanic currents between gold and amalgam. Scand
J Dent Res 1980;88:269}72.
2262
K. Yokoyama et al. / Biomaterials 22 (2001) 2257}2262
Wyszukiwarka
Podobne podstrony:
Shock Wave Deformation and Fracture of Zirconium Dioxide Ceramics of Various Fractional Composition
Degradable Polymers and Plastics in Landfill Sites
Legg Calve Perthes disease The prognostic significance of the subchondral fracture and a two group c
Degradable Polymers and Plastics in Landfill Sites
Szewczyk, Rafał; Długoński, Jerzy Pentachlorophenol and spent engine oil degradation by Mucor ramos
Unexplained Fractures in Infants and Child Abuse The Case for Re
Postmodernity and Postmodernism ppt May 2014(3)
Scoliosis and Kyphosis
L 3 Complex functions and Polynomials
4 Plant Structure, Growth and Development, before ppt
Osteoporosis ľ diagnosis and treatment
05 DFC 4 1 Sequence and Interation of Key QMS Processes Rev 3 1 03
Literature and Religion
lec6a Geometric and Brightness Image Interpolation 17
Historia gry Heroes of Might and Magic
Content Based, Task based, and Participatory Approaches
Lecture10 Medieval women and private sphere
więcej podobnych podstron